Abstract
The treatment of peripheral nerve injuries with nerve gaps largely consists of autologous nerve grafting utilizing sensory nerve donors. Underlying this clinical practice is the assumption that sensory autografts provide a suitable substrate for motoneuron regeneration, thereby facilitating motor endplate reinnervation and functional recovery. This study examined the role of nerve graft modality on axonal regeneration, comparing motor nerve regeneration through motor, sensory, and mixed nerve isografts in the Lewis rat. A total of 100 rats underwent grafting of the motor or sensory branch of the femoral nerve with histomorphometric analysis performed after 5, 6, or 7 weeks. Analysis demonstrated similar nerve regeneration in motor, sensory, and mixed nerve grafts at all three time points. These data indicate that matching of motor-sensory modality in the rat femoral nerve does not confer improved axonal regeneration through nerve isografts.
Keywords: Femoral nerve, preferential motor regeneration, nerve architecture, motor graft, sensory graft, modality-specific regeneration
Introduction
Peripheral nerve injuries represent a significant source of morbidity and disability in the United States and abroad, accounting for nearly 3% of all traumatic injuries, with two-thirds of these occurring in the upper extremity (Mackinnon, 1988). Peripheral nerves can also be injured during surgery, either iatrogenically or intentionally during tumor resection (Kreiger, et al., 1981). In situations where end-to-end repair is not possible, autologous nerve grafting remains the gold standard for reconstruction of a irreducible nerve gap. Such grafts are typically taken from superficial sensory nerves in an effort to minimize donor-site morbidity (i.e. sural nerve, medial antebrachial cutaneous nerve, lateral antebrachial cutaneous nerve, superficial and deep peroneal nerves, intercostal nerves, and posterior and lateral cutaneous nerves of the thigh) (Kline, 1990, Mackinnon, 1988, Mackinnon, et al., 2001), yet recently several reports have demonstrated improved motor nerve regeneration with motor grafts, and likewise for sensory, a phenomenon referred to as modality-specific regeneration (MSR) (Brenner, et al., 2006, Hoke, et al., 2006).
The concept of MSR evolved from the body of knowledge accumulated over the past two decades on the phenomenon known as preferential motor regeneration (PMR). PMR describes the tendency for regenerating motor axons to choose motor pathways when offered a choice between motor and sensory modalities. In a landmark investigation, Brushart demonstrated that the motor axons of the rat femoral nerve exhibited preference for motor pathways after proximal transection (Brushart, 1988). The putative mechanism for this specificity is described in the “pruning hypothesis”, which proposes that regenerating neurons project multiple axonal collaterals to sample distal pathways (Brushart, 1993). Motor collaterals projecting to motor pathways are preserved while those projecting to the sensory pathways are pruned, conferring preference to motor regeneration.
However, central to the pruning hypothesis is the notion of comparison and choice; an axon has to “see” both motor and sensory modalities in order to choose the correct path. It follows, then, that pruning only suffices to explain PMR in the setting of a branched nerve or conduit. This hypothesis does not operate in the absence of branching, in unifascicular nerve graft as would be performed in a human patient, since axons have no opportunity to compare and choose between modalities.
More recent investigations have sought to address this problem by examining MSR in nerve grafts that do not branch. We previously demonstrated significantly higher number of regenerating mixed motor and sensory nerve fibers from the transected rat tibial nerve in motor nerve grafts (femoral motor branch) compared to sensory (femoral sensory branch) or mixed grafts (tibial nerve) at short time points (4 weeks) (Brenner, et al., 2006). When varying the number of nerve graft cables, even a single cable of motor graft supported a higher number of regenerating fibers than four cables of sensory (Brenner, et al., 2006). However, this experiment investigated the reconstruction of the tibial nerve a mixed motor and sensory nerve at a relatively short time point and was unable to distinguish between regenerating motor and sensory fibers. Using a femoral motor/sensory model where the injury to each branch is distal to the bifurcation, Brushart and associates recently demonstrated a significantly higher number of regenerating motor neurons in ventral root grafts in the motor branch than in femoral cutaneous nerve grafts placed in the motor branch. Sensory nerve regeneration in the sensory branch was likewise superior in sensory grafts (Hoke, et al., 2006). However, the direct clinical application of this data is limited by the inability to harvest ventral roots for use as nerve grafts in human patients. In addition, while ventral roots contain a pure population of motor axons in the absence of sensory afferents, they may not accurately model axonal regeneration in a peripheral nerve graft.
Recently, multiple experiments have demonstrated modality matching between donor and recipient nerves results in improved peripheral nerve regeneration (Brushart, 1988, Brushart, 1993, Brushart, et al., 1998, Brushart and Seiler, 1987, Le, et al., 2001, Madison, et al., 1996, Redett, et al., 2005). While the notion that motor nerve defects are best repaired with motor grafts is intuitive, it challenges the existing clinical dogma of repairing motor nerves with sensory autografts. The purpose of this study was to determine whether modality matching improves axonal regeneration when using unifascicular grafts to reconstruct primarily motor or sensory nerves in a manner similar to clinical reconstruction.
Experimental Procedures
Experimental animals
Adult male Lewis rats (Harlan Sprague-Dawley, Indianapolis, IN) weighing 250–350g were utilized in this study. All interventions were performed in accordance with the National Institutes of Health guidelines under a study protocol approved by the Washington University Animal Studies Committee.
Experimental design
Rats were randomized into six groups based on graft type and recipient nerve as described in Table 1. The rat femoral nerve bifurcates below the inguinal ligament into a motor branch to the quadriceps muscles and a terminal sensory branch, the femoral cutaneous nerve (Brushart, 1988). The motor branch was utilized as a source of motor nerve graft, and the sensory branch was utilized for harvesting of sensory grafts. Mixed nerve grafts were obtained from the peroneal nerve, as this nerve provides an easily accessible source of mixed nerve grafts with adequate length and is a good size match to the motor and sensory branches of the femoral nerve.
Table 1.
Experimental design for each group
| Group | Graft type | Recipient Nerve | n | ||
|---|---|---|---|---|---|
| 5 week | 6 week | 7 week | |||
| I | Motor | Motor | 6 | 10 | 8 |
| II | Mixed | Motor | 6 | 0 | 7 |
| III | Sensory | Motor | 7 | 10 | 6 |
| IV | Motor | Sensory | 6 | 0 | 6 |
| V | Mixed | Sensory | 7 | 0 | 9 |
| VI | Sensory | Sensory | 6 | 0 | 6 |
In recipient animals, motor, sensory, or mixed grafts were used to repair defects in either the motor or sensory branch of the rat femoral nerve (Figure 1). In all animals, the recipient nerve was transected and immediately repaired with a 1-cm nerve graft. For the 5 week group, the motor branch of the femoral nerve distal to the bifurcation was transected and grafted with either a motor (n = 6), a mixed (n = 6), or a sensory (n = 7) graft (Figure 1A). In a different set of animals from the 5 week group, the sensory branch of the femoral nerve was transected distal to the bifurcation and either a motor (n = 6), a mixed (n = 7), or a sensory (n = 6) graft was sutured in place (Figure 1B). Similarly at 7-weeks, the motor branch of the femoral nerve distal to the bifurcation was transected and grafted with either a motor (n = 8), a mixed (n = 7), or a sensory (n = 6) graft (Figure 1A). In a different set of animals from the 7 week group, the sensory branch of the femoral nerve was transected distal to the bifurcation and either a motor (n = 6), a mixed (n = 9), or a sensory (n = 6) graft was sutured in place (Figure 1B). Because of the importance of timing in rodent models of peripheral nerve injury, the intermediate time point of 6 weeks was added to the study to supplement the results that were seen at 5 and 7 weeks. At the 6 week time point, the motor branch of the femoral nerve distal to the bifurcation was transected and grafted with either a motor (n = 10), or a sensory (n = 10) graft.
Figure 1.
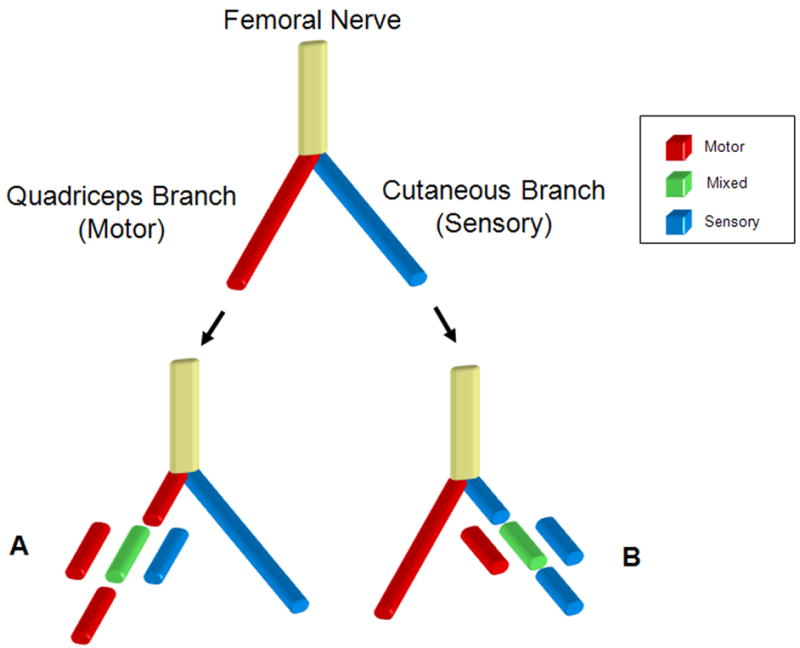
Rodent femoral nerve model used to study modality specific regeneration. Below the femoral bifur cation, either the motor branch to the quadriceps (A) or the cutaneous sensory branch (B) was cut and reconstructed with a motor (red), mixed (green), or sensory (blue) nerve graft harvested from the femoral motor branch, peroneal nerve or femoral sensory branch respectively. Grafts from each group were then harvested at 5, and 7 weeks post transplantation and evaluated using histomorphometry. For the 6 week time point motor grafts or sensory grafts were placed in the transected motor branch of the femoral nerve.
Surgical Technique
All surgical procedures were performed under general anesthesia utilizing an intramuscular injection of 75 mg/kg ketamine hydrochloride (Fort Dodge Animal Health, Ford Dodge, IA) and 0.5 mg/kg medetomidine hydrochloride (Orion Corporation, Espoo, Finland). All surgeries were performed under aseptic conditions with the animals shaved over the groin and prepped with betadine solution.
In donor animals, nerves were harvested through a longitudinal groin incision to expose the femoral neurovascular bundle. Under 16x magnification using a Wild M61 operating microscope (Leica Microsystems, Deerfield, IL), the motor branch to the quadriceps muscles and the femoral cutaneous nerve were identified and dissected proximally to the femoral bifurcation. An internal neurolysis of the common femoral nerve was typically performed in order to obtain a 1-cm length of donor nerve. The proximal ends were marked with ink and the donor nerves sharply transected with microscissors proximally and distally. Donor nerves were wrapped in a saline-moistened gauze until engraftment. A gluteal muscle-splitting incision was then made to harvest a 1-cm length of peroneal nerve distal to the sciatic trifurcation. The harvesting procedure was then repeated on the contralateral side, after which the donor animal was sacrificed with an intracardiac injection of Euthasol (Delmarva Laboratories, Des Moines, IA). The use of an isograft in a Lewis rat model of peripheral nerve injury has been shown to illicit no adverse immune response and has no adverse effect on peripheral nerve regeneration (Evans, et al., 1998).
In recipients, the femoral neurovascular bundle was exposed through a groin incision, and the femoral motor and sensory branches were neurolysed proximally and distally. The motor or sensory branch was then transected sharply with microscissors and a 7 mm segment of nerve was removed to allow for a tension-free repair with out excessive redundancy following graft placement. The cut nerve was then repaired with a 10 mm long motor, sensory, or mixed nerve graft under 20x magnification using four, 11-0 nylon epineurial microsutures for each coaptation (Figure 2). The wound was irrigated and closed with interrupted 4-0 nylon sutures. Animals were reversed with atipamezole HCl (Pfizer Animal Health, Exton, PA) and allowed to recover in a heated environment prior to transfer to the animal care facility.
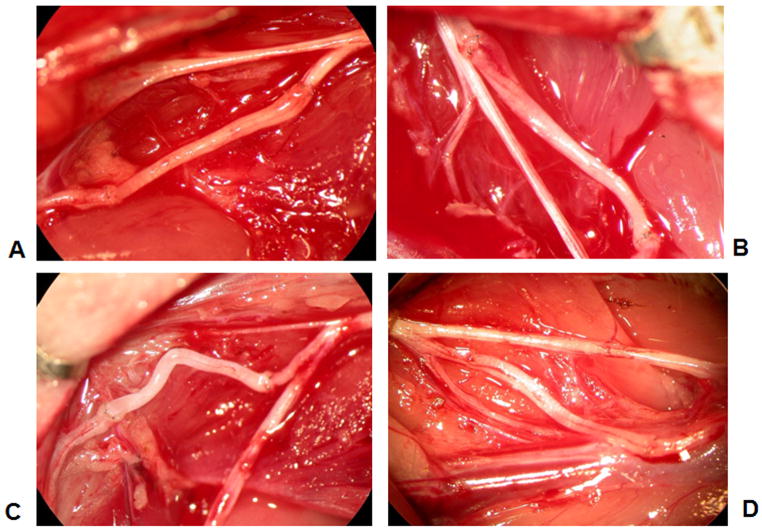
Figure 2.
Gross appearance of surgical nerve grafts. A) Motor nerve graft in femoral motor branch. B) Sensory nerve graft in femoral motor branch. C) Sensory nerve graft in femoral sensory branch. D) Mixed nerve graft in femoral sensory branch.
Histomorphometry
After 5, 6, or 7 weeks, donor nerves were harvested 5-mm proximal and distal to the repair sites. Specimens were fixed overnight in 3% glutaraldehyde, postfixed in 1% osmium tetroxide, dehydrated in graduated ethanol concentrations (50, 70, 90, and 100%), and embedded in Araldite 502 resin (Polysciences, Warrington, PA). An LKB III Ultramicrotome (LKB-Produkter, Sweden) was used to generate 1 μm-thick cross-sections of the midgraft and distal nerve segments. Sections were stained with 1% toluidine blue and viewed under light microscopy. Histomorphometry of the nerve cross-section was performed using a digital image analysis system with linked morphometry software (Leco Instruments, St. Joseph, MI) as previously described (Hunter, et al., 2007).
Statistical analysis
Statistical analyses were performed in Statistica (Version 7.1, StatSoft, Tulsa, OK). All results are represented as the average ± standard deviation unless otherwise stated. Statistical differences in fiber counts, fiber width, nerve density, percent nerve and percent debris were identified using one-way analysis of variance with an additional post hoc Student-Newman-Keuls test. Differences were considered significant when p < 0.05. A minimum of 6 animals for each experimental group was chosen prior to the study to obtain a power of 0.80 for detection of 50% differences between groups. The power analysis was performed based upon results from previous studies in the rat model of peripheral nerve injury performed by our group and also based on levels of significance that, in our experience, make an observed difference when clinically translated (Brenner, et al., 2006, Brenner, et al., 2008, Evans, et al., 1991, Evans, et al., 1995, Evans, et al., 1998, Hunter, et al., 2007, Kasukurthi, et al., 2009, Mackinnon, et al., 2001, Mackinnon, et al., 1992, Midha, et al., 1993, Moradzadeh, et al., 2008).
Results
Histomorphometry
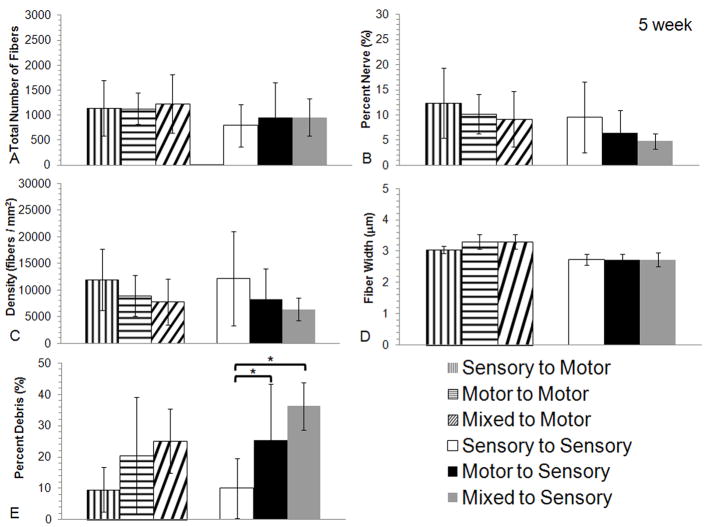
Histomorphometry was utilized to quantify difference in the regeneration characteristics of nerve from each experimental group. After 5 weeks, there were few regenerating fibers seen in the recipient nerves distal to the graft. Analyses through the mid-graft, however, demonstrated robust axonal regeneration with motor, mixed, and sensory nerve grafts in both the sensory and motor groups (Figure 3A–C). Quantification of the regenerating motor branch revealed no statistically significant differences between any of the groups for all measured parameters (Figure 4A–E). Similarly, evaluation of regeneration in the femoral sensory branch demonstrated similar regeneration in motor, mixed, and sensory nerve grafts (Figure 3D–F). Quantification of the regenerating sensory branch revealed no statistically significant differences in fiber counts, fiber width, nerve density, or percent nerve (Figure 4A–D). The percent debris of the sensory graft compared to the motor and mixed in the femoral cutaneous nerve was significantly different (Figure 4E, p < 0.05).
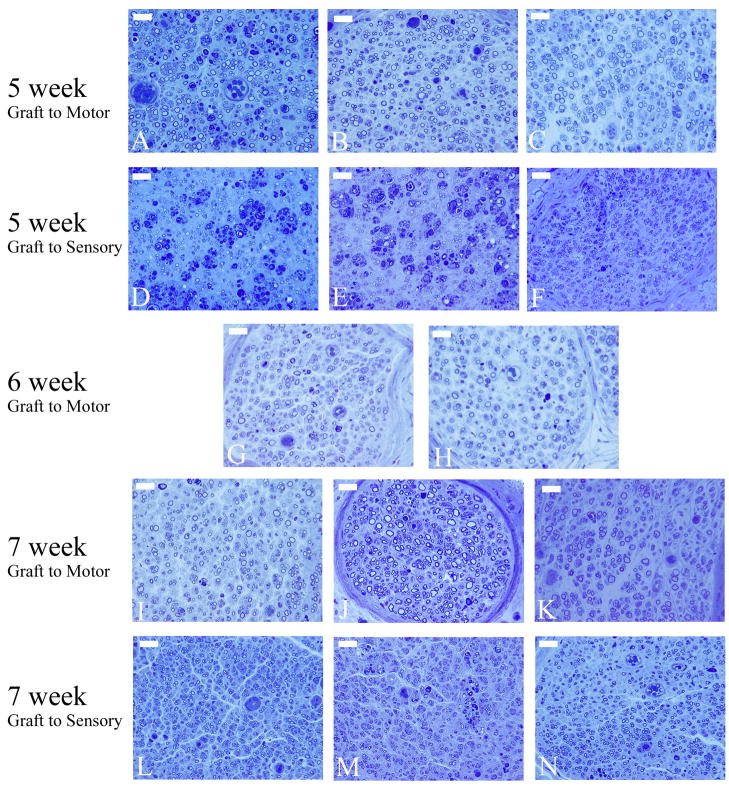
Figure 3.
Representative toluidine blue histological sections used for histomorphometric analysis from each experimental group at each time point. The first two rows of images are mid-graft photomicrographs of motor (A&D), mixed (B&E), and sensory(C&F) grafts bridging the transected femoral motor (A–C) and transected femoral sensory branch (D–F) at the 5 week time point. The third row of images is photomicrographs of the distal stump of the transected femoral motor branch with either a motor graft (G) or sensory graft (H) 6 weeks after grafting. The final two rows of images display photomicrographs from the distal stump of the transected femoral motor (I–K) and femoral sensory (L–N) branches treated with either a motor (I&L), mixed (J&M), or sensory graft (K&N) 7 weeks after grafting. (scale bar = 5 μm)
Figure 4.
Histomorphometric comparisons of nerve regeneration in the mid-graft of the different grafts (motor, mixed or sensory) in the femoral motor and sensory branch after 5 weeks. No differences were seen in the quantification of A) total number of fibers, B) percent nerve, C) fiber density, or D) fiber width between any of the experimental groups regardless of modality match or mismatch. E) Quantification of the percent debris revealed a difference between sensory grafts used in femoral sensory branch when compared to motor or mixed grafts used in the femoral sensory branch (* indicate p < 0.05 vs. sensory graft to the femoral sensory branch, bars are averages, error bars are standard deviation, Motor to Motor n = 6, Mixed to Motor n = 6, Sensory to Motor n = 7, Motor to Sensory n = 6, Mixed to Sensory n = 7, and Sensory to Sensory n = 6)
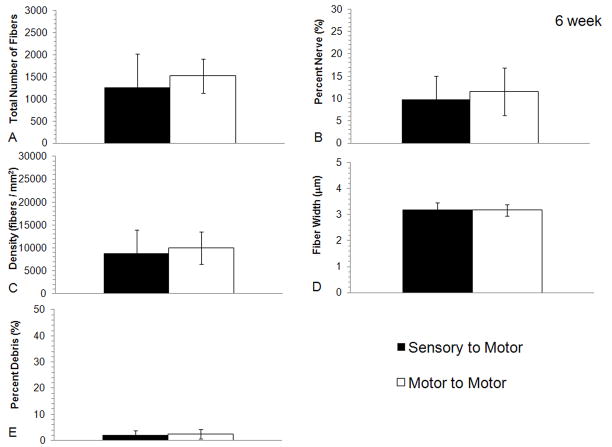
The intermediate time point of 6 weeks was added to the study to supplement the results that were seen at 5 and 7 weeks. This time point evaluated repair of the femoral motor branch with either motor or sensory grafts. Analysis distal to the graft after 6 weeks demonstrated axonal regeneration across both sensory and motor grafts (Figure 3G & H). The quantification of regeneration distal to the graft demonstrated similar histomorphometry between the motor and sensory graft groups (Figure 5A–E). Fiber counts were similar between the groups and no statistically significantly differences were identified (p > 0.05).
Figure 5.
Histomorphometric comparisons of nerve regeneration in the distal stump of the transected femoral motor branch using either sensory or motor graft modalities after 6 weeks. No differences were seen in the quantification of A) total number of fibers, B) percent nerve, C) fiber density, D) fiber width or E) percent debris between either of the experimental groups regardless of modality match or mismatch (bars are averages, error bars are standard deviation, Motor to Motor n = 10, Sensory to Motor n = 10).
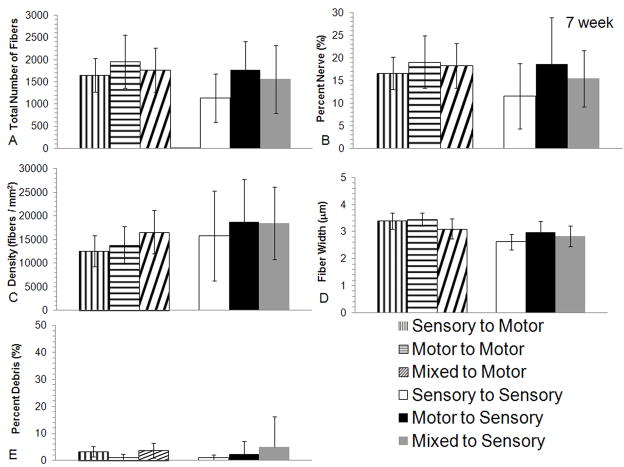
Analysis was undertaken at a later, 7-week time point in an attempt to identify late differences between groups after axons had been allowed to regenerate through the entire graft and into the distal stump. After 7-weeks of regeneration, excellent nerve regeneration was seen in the distal stump of the femoral motor branch with all three graft types (Figure 3I–K). Total fiber counts distal to the graft in the regenerating femoral motor branch were greater than 1600 for all groups, and no significant differences were identified in any of the histomorphometric parameters evaluated (Figure 6A–E). Similarly, there was excellent sensory regeneration seen in the distal stump of the femoral cutaneous nerve regardless of graft type at 7 weeks (Figure 3L–N and Figure 6A–E).
Figure 6.
Histomorphometric comparisons of nerve regeneration in the distal stump of the transected femoral motor or sensory branch using different graft modalities (motor, mixed or sensory) after 7 weeks. No differences were seen in the quantification of A) total number of fibers, B) percent nerve, C) fiber debris, D) fiber width or E) percent density between any of the experimental groups regardless of modality match or mismatch (bars are averages, error bars are standard deviation, Motor to Motor n = 8, Mixed to Motor n = 7, Sensory to Motor n = 6, Motor to Sensory n = 6, Mixed to Sensory n = 9, and Sensory to Sensory n = 6).
A distribution of the caliber of regenerating fibers of both the motor branch of the femoral and sensory branch of the femoral was performed to support the assertion that mostly motor fibers are regenerating in the motor branch and mostly sensory fibers are regenerating in the sensory branch (Supplemental Figure 1). As would be expected from the regeneration of the femoral motor branch, the distribution of regenerating fibers is shifted to larger caliber fibers. In contrast, the distribution of regenerating fibers in the sensory branch is shifted towards smaller caliber fibers.
Discussion
In clinical nerve reconstruction, a primary goal is to maximize the speed of motor axon regeneration in order to reinnervate motor endplates before they degenerate. In the current study, we investigated whether the modality of nerve graft affects axonal regeneration in both motor and sensory nerves. The results have demonstrated that after 5, 6, or 7 weeks, there were no observed significant difference in the histomorphometry of regenerating axons of motor or sensory nerves when using motor or sensory nerve grafts in this rodent model of peripheral nerve regeneration.
As mentioned in the methods, the power of the study was designed such that 50% differences should be detected 80% of the time (power of 0.8). In our research experience, we have found that only very substantial treatment effects in the rodent model translate to clinically observable improvement in humans. Smaller differences in nerve regeneration that may be observed in a rodent model are less likely to translate because of the well documented superlative regenerative power of the rodent peripheral nervous system (Brenner, et al., 2008, Mackinnon, et al., 1985). Following the completion of the study, post-hoc power analysis demonstrated desired power at the 6- and 7-week endpoints (calculated power 7 week = 0.80, and calculated power 6 week = 0.836). In contrast, the 5 week group exhibited increased variation and did not retain the desired power (calculated power of 5 week = 0.41). Any interpretation of the data presented in this study should keep these factors in mind.
Regardless of modality, at 5 weeks in all experimental groups regeneration had not yet crossed the distal coaptation and was in the process of regenerating across the nerve graft. During this period of regeneration across the motor, mixed, and sensory grafts, analysis in the femoral motor branch demonstrated no differences in histomorphometric parameters. Similarly for grafts in the sensory branch, there were no differences between the different grafts, except for percent debris. In the grafts of the sensory branch the percent debris in the sensory graft was significantly different than the mixed or motor graft. This has previously been shown to be due to architectural differences inside the grafts (Moradzadeh, et al., 2008). Moradzadeh et al. demonstrated that compared to sensory grafts both mixed and motor grafts exhibit larger areas of debris as a consequence of the larger endoneurial tubes of the motor nerves.
Our 5 week data indicated, as axons cross the graft, regenerating neurons project axonal processes in a manner that is not hindered by mismatched modalities. This is consistent with the finding that following proximal femoral nerve transection, motor axons initially project collateral branches in equal numbers down the motor and sensory pathways (Brushart, 1993). However, given the incomplete regeneration seen at 5 weeks, these data alone were not taken as conclusive evidence that motor, mixed, and sensory grafts were similar in their ability to support motor or sensory axonal regeneration.
Recent studies have demonstrated the importance of timing when assessing peripheral nerve regeneration in a rodent model (Brenner, et al., 2008). Evaluating regeneration at later time points, allows more time for axons to regenerate. However, this additional time can also allow the superlative regenerative capacity of rat peripheral nerves to mask real differences between groups (Brenner, et al., 2008). To account for these effects, we chose to further evaluate femoral motor nerve regeneration through motor and sensory grafts at the 6 and 7 week time points. Histological examination at 6 and 7 weeks demonstrated no significant differences in the regeneration of motor and sensory axons through all of the graft types. Combined with the 5 week data, these results demonstrate that motor, mixed, and sensory grafts were similar in their ability to support motor or sensory axonal regeneration. Given the existing body of literature supporting modality-specific axonal regeneration, it was our expectation that the current study would demonstrate a similar preference of motor axons for motor grafts and likewise for sensory when evaluated at a later time point (5, 6, or 7 weeks) and with longer grafts (10mm). In contrast, our results suggest that in the absence of motor and sensory “choice”(Brushart, 1988, Brushart, 1993), motor and sensory axons have no demonstrable preference for motor or sensory grafts at the 5, 6, and 7 week time points in a rat model.
Recently, Höke, et al, evaluated motoneuron regeneration in a deafferented rat sciatic nerve following grafting with a unifascicular graft derived from a pure sensory (femoral cutaneous graft) or motor (ventral root graft) source (Hoke, et al., 2006). Likewise, they evaluated sensory nerve regeneration in the rat femoral cutaneous (sensory) nerve with pure motor and sensory nerve grafts. After 2 weeks, they demonstrated a significantly higher number of labeled motoneurons with ventral root grafts than with femoral cutaneous grafts. Similarly, they found a significantly higher number of labeled dorsal root ganglia (DRG) neurons with sensory grafts than with motor grafts. This apparent preference of motor axons for motor pathways and sensory axons for sensory pathways was believed to reflect a purely neurotropic effect provided by modality-matched grafts, as no distal coaptation had been made. The combination of Schwann cells, basal lamina architecture, expression of growth factors, and absence of end organ signaling was shown to favor regeneration of modality-matched axons (Hoke, et al., 2006). However, the importance of both neurotropism and neurotrophism to modality-specific regeneration has previously been demonstrated by Brushart et al. (Brushart, 1993, Brushart, et al., 2005). From this study the authors concluded that preferential motor reinnervation depends on both pathway (neurotropic) and end-organ (neurotrophic) influences. Neurotropic effects occur early (2–4 weeks in the current study) as regenerating axons sample and scrutinize their environment, and neurotrophic effects occur later as axons reestablish contact with their end-organ targets in muscle or skin ( > 6 weeks in the current study).
In the current study the axons of the regenerating nerve are exposed to the modality matched or mismatched graft environment for a finite time (5, 6 or 7 weeks) as axonal regeneration proceeds across the graft to a modality matched distal nerve stump. These series of events are similar to the progression of regeneration through a nerve graft clinically and are fundamentally different than what was seen experimentally by Höke et. al. In addition, while a ventral root graft may be less conducive to sensory regeneration than a cutaneous nerve graft, our data indicate that motor grafts obtained from peripheral sources such as the femoral motor branch are suitable for sensory nerve regeneration. Since ventral roots are not used as a source of autograft in humans and grafting without a distal coaptation is not clinically relevant in most cases. Our data, which may be more relevant to clinical nerve reconstruction, suggests modality matched grafts do not enhance regeneration across a 10mm gap.
It is possible that the differences in caliber of the nerve grafts used in this study affected regeneration of axons through the graft. Previously we have characterized the caliber of the sensory and motor branch of the femoral nerve and found that on average the motor branch has a diameter of 0.289 mm and the sensory branch an average diameter 0.264 mm (Brenner et al 2006). For the current study, we found that the peroneal nerve is a reasonable size match for both the sensory and motor branches of the femoral nerve. However, despite the small differences in diameter it is possible that a mismatch in nerve caliber could have affected regeneration in the current study. If excessive caliber mismatch of the grafted nerve had occurred, we would have expected to observe excessive extra-fascicular fibers or neuroma formation at the suture site. These characteristics often accompany such a mismatch, and in the current study these conditions were not observed in any of our experimental groups. Previously, it has been shown by Siemionow et al. that large differences in nerve cable caliber of unifascicular nerves has a minimal effect on the regeneration of axons through the graft and into the distal stump (Siemionow, et al., 2004). Based on these observations, it is unlikely that a caliber mismatch negatively affected our reported findings.
Despite the results of the current study our group has previously demonstrated a significantly higher level of axonal regeneration in the mixed rat tibial nerve with motor nerve grafts compared to sensory. These experiments involved reconstruction of a 5mm nerve gap with assessments carried out at 3 (Brenner, et al., 2006, Nichols, et al., 2004) or 4 weeks (Moradzadeh, et al., 2008). For these studies, the diameter of the motor and sensory fascicle of the femoral nerve varied in size and to control for graft cable size the number of cables was varied. When the number of graft cables was varied, it was found that even a single cable of motor graft supported a higher number of regenerating fibers than four cables of sensory graft (Brenner, et al., 2006). It was further determined that motor nerve grafts that had been decellularized by cold-preservation still supported more regenerating fibers than either fresh or cold-preserved sensory grafts (Moradzadeh, et al., 2008). This suggested that the architecture of the endoneurial tubes provided by motor-derived nerve grafts favored axonal regeneration.
Recently, Madison et al. published a study investigating PMR in which they outline evidence for and proposed a hierarchy of trophic support that regulates PMR with muscle contact being the most potent, followed by the number or density of Schwann cells in the distal nerve branches (Madison, et al., 2009). The results from our current study support the evidence for this “hierarchy” and previous results from our group mentioned above (Moradzadeh, et al., 2008) would suggest that a third tier of PMR, in the absence of differential trophic support, involves the geometric configuration of the extracellular matrix.
In the current study, sectioning the mid-graft level at a later time point (5 weeks) in a longer unifascicular nerve graft (10 mm) reveals no differences in regeneration between matched and mismatched groups. In contrast to the previous investigations carried out by this laboratory, the current study utilized unifascicular nerve grafts to eliminate the technical complexity of cable grafting and the potentially confounding variable of cable number. This variability, though accounted for in the previous study, may have an effect on the comparison of regeneration between mismatched modalities that would not have been seen in the current study. For the current study we also studied regeneration in primarily motor and sensory nerve branches which leaves little doubt as to the modality of axons regenerating through the nerve graft. Though we did not incorporate experimental maneuvers to create truly pure populations of motor or sensory axons (Hoke, et al., 2006), the quadriceps branch of the femoral nerve and sensory nerve utilized in this study do provide nerve grafts of predominately motor or sensory origin. The use of these nerves as sources of graft material was intended to more closely mirror the clinical situation in which peripheral motor (e.g., obturator nerve branch to the gracilis muscle) or sensory (e.g., sural) nerves are utilized for reconstruction.
Finally, it is possible that differences in analysis timing (3 or 4 weeks previously vs. 5 weeks in the current study) in the relatively long graft (10 mm) resulted in no detection of disparity in the advancing axonal fronts. However, it is unlikely that a disparity would not be brought out over the subsequent time points (6 and 7 weeks) which demonstrated no differences in the regeneration of nerve fibers in the distal stump of all experimental groups. The trend toward higher fiber counts with motor grafts in both motor and sensory nerve regeneration at 7 weeks may be explained by the finding that motor grafts provide larger endoneurial conduits for axonal growth that could affect fiber number (Moradzadeh, et al., 2008). In the case of sensory regeneration, this architectural advantage of motor grafts could ultimately trump the neurotropic advantage conferred by modality matching. The use of retrograde labeling techniques with injection of tracer molecules directly into muscle or skin could support the histomorphometry data in the current study. However, utilizing the model of mostly pure population of motor or sensory axons used in the current study would make it unlikely that the retrograde labeling would change our findings.
As outlined above, previous work in our laboratory using the mixed population motor and sensory tibial nerve has shown that regeneration across a motor graft was superior to that across a sensory graft. When these motor and sensory grafts were minced, this benefit was not realized and we concluded that it was the architecture of the larger endoneurial tubes in the motor graft that facilitated more robust nerve regeneration (Lloyd, et al., 2007). Thus, we were surprised to see the results from this study that show when a pure motor or sensory nerve was used, this advantage of the motor graft was lost. This led us to repeat the study at two other time points for a total of 100 animals to verify that there was no advantage with sensory-motor graft matching. We are considering now that the advantage of the motor graft in the mixed nerve is that the larger endoneurial tubes allow for more efficient regeneration when motor and sensory axons are in the same environment and determining directional regeneration. By contrast, in a pure population of motor or sensory axons where there is no interaction/competition at the onset of regeneration, the larger diameter endoneurial tubes do not offer any advantage. In 1991, Evans et al (Evans, et al., 1991) published a manuscript which may have application to this above hypothesis. In the rat sciatic nerve, which is mixed, a short diameter (5 mm) conduit allowed for appropriate reorganization of misaligned peroneal and tibial nerve fascicles with equal regeneration as to when these fascicles were appropriately aligned. By contrast, when a direct suture repair misaligned the fascicles, functional recovery was not forthcoming. From the Evans et al. paper it would suggest that in a situation where the regenerating motor and sensory nerves have some issue with respect to topographical alignment, there is an advantage to having room for maneuvering.
In summary, our study demonstrates that injured motor and sensory axons can regenerate satisfactorily through grafts of motor, mixed, or sensory origin to reinnervate the distal nerve stump. No significant differences were identified in motor or sensory nerve regeneration with variations in graft origin. The study was designed to obtain a power of 0.80 for detection of 50% differences between groups. The power was chosen based on levels of significance that, in our experience, make an observed difference when translating experimental results in the rodent model to larger animals and humans (Brenner, et al., 2005, Brenner, et al., 2008, Jensen, et al., 2005, Matsuyama, et al., 2000, Tung, et al., 2006). Our results corroborate previous observations that axons can regenerate down modality mismatched pathways (Robinson and Madison, 2006, Weiss and Edds, 1945). Although previous data from our laboratory have supported the notion of MSR, there are confounding factors (timing, cable number, length of the graft, and modality of the regenerating nerve) that make the comparison of our previous studies with the current results difficult. However, taken as a whole our results suggest that regeneration of a mixed nerve, which exhibit interactions/competition between regenerating populations of both motor and sensory axons, that the more space permissive environment of the motor nerve graft provides a more suitable environment for regeneration based solely on architecture (Brenner, et al., 2006, Moradzadeh, et al., 2008, Nichols, et al., 2004). In contrast, in the regeneration of a nerve containing pure populations of either motor or sensory axons, which exhibit no interaction/competition between different regenerating axon populations, that both motor and sensory graft are suitable for regeneration. The findings of this study have implications for clinical nerve reconstruction, as they support the experience of clinicians who utilize sensory nerve grafts for reconstruction of motor nerve defects with good clinical results.
Supplementary Material
Supplemental Figure 1. Comparison of the fiber caliber distribution of each modality matched and mismatched at each time point. A) 5 week following graft implantation, mid graft evaluation of the fiber caliber distribution of regenerating motor axons is shifted to the right as would be expected for a purely motor axon. B) 7 week following graft implantation, distal evaluation of the fiber caliber distribution of regenerating axons is similarly shifted to the right. Again, these results are consistent with a mostly motor regenerating nerve and mostly sensory regenerating axons of the motor and sensory branches. (bars are averages and error bars are standard deviation)
Acknowledgments
We would like to thank Dr. Gregory Borschel for his input in the preparation of this manuscript.
This work funded in part by grants from the American Association for Hand Surgery, the Plastic Surgery Educational Foundation and the National Institute of Health (5R01NS05170604).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brenner MJ, Hess JR, Myckatyn TM, Hayashi A, Hunter DA, Mackinnon SE. Repair of motor nerve gaps with sensory nerve inhibits regeneration in rats. Laryngoscope. 2006;116:1685–1692. doi: 10.1097/01.mlg.0000229469.31749.91. [DOI] [PubMed] [Google Scholar]
- 2.Brenner MJ, Lowe JB, 3rd, Fox IK, Mackinnon SE, Hunter DA, Darcy MD, Duncan JR, Wood P, Mohanakumar T. Effects of Schwann cells and donor antigen on long-nerve allograft regeneration. Microsurgery. 2005;25:61–70. doi: 10.1002/micr.20083. [DOI] [PubMed] [Google Scholar]
- 3.Brenner MJ, Moradzadeh A, Myckatyn TM, Tung TH, Mendez AB, Hunter DA, Mackinnon SE. Role of timing in assessment of nerve regeneration. Microsurgery. 2008;28:265–272. doi: 10.1002/micr.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brushart TM. Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci. 1988;8:1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brushart TM. Motor axons preferentially reinnervate motor pathways. J Neurosci. 1993;13:2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brushart TM, Gerber J, Kessens P, Chen YG, Royall RM. Contributions of pathway and neuron to preferential motor reinnervation. J Neurosci. 1998;18:8674–8681. doi: 10.1523/JNEUROSCI.18-21-08674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brushart TM, Jari R, Verge V, Rohde C, Gordon T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp Neurol. 2005;194:221–229. doi: 10.1016/j.expneurol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Brushart TM, Seiler WAt. Selective reinnervation of distal motor stumps by peripheral motor axons. Exp Neurol. 1987;97:289–300. doi: 10.1016/0014-4886(87)90090-2. [DOI] [PubMed] [Google Scholar]
- 9.Evans PJ, Bain JR, Mackinnon SE, Makino AP, Hunter DA. Selective reinnervation: a comparison of recovery following microsuture and conduit nerve repair. Brain Res. 1991;559:315–321. doi: 10.1016/0006-8993(91)90018-q. [DOI] [PubMed] [Google Scholar]
- 10.Evans PJ, Mackinnon SE, Best TJ, Wade JA, Awerbuck DC, Makino AP, Hunter DA, Midha R. Regeneration across preserved peripheral nerve grafts. Muscle Nerve. 1995;18:1128–1138. doi: 10.1002/mus.880181009. [DOI] [PubMed] [Google Scholar]
- 11.Evans PJ, Mackinnon SE, Levi AD, Wade JA, Hunter DA, Nakao Y, Midha R. Cold preserved nerve allografts: changes in basement membrane, viability, immunogenicity, and regeneration. Muscle Nerve. 1998;21:1507–1522. doi: 10.1002/(sici)1097-4598(199811)21:11<1507::aid-mus21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Hoke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter DA, Moradzadeh A, Whitlock EL, Brenner MJ, Myckatyn TM, Wei CH, Tung TH, Mackinnon SE. Binary imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. J Neurosci Methods. 2007;166:116–124. doi: 10.1016/j.jneumeth.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen JN, Brenner MJ, Tung TH, Hunter DA, Mackinnon SE. Effect of FK506 on peripheral nerve regeneration through long grafts in inbred swine. Ann Plast Surg. 2005;54:420–427. doi: 10.1097/01.sap.0000151461.60911.c0. [DOI] [PubMed] [Google Scholar]
- 15.Kasukurthi R, Brenner MJ, Moore AM, Moradzadeh A, Ray WZ, Santosa KB, Mackinnon SE, Hunter DA. Transcardial perfusion versus immersion fixation for assessment of peripheral nerve regeneration. J Neurosci Methods. 2009 doi: 10.1016/j.jneumeth.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Kline DG. Surgical repair of peripheral nerve injury. Muscle Nerve. 1990;13:843–852. doi: 10.1002/mus.880130911. [DOI] [PubMed] [Google Scholar]
- 17.Kreiger N, Kelsey JL, Harris C, Pastides H. Injuries to the upper extremity: patterns of occurrence. Clin Plast Surg. 1981;8:13–19. [PubMed] [Google Scholar]
- 18.Le TB, Aszmann O, Chen YG, Royall RM, Brushart TM. Effects of pathway and neuronal aging on the specificity of motor axon regeneration. Exp Neurol. 2001;167:126–132. doi: 10.1006/exnr.2000.7538. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd BM, Luginbuhl RD, Brenner MJ, Rocque BG, Tung TH, Myckatyn TM, Hunter DA, Mackinnon SE, Borschel GH. Use of motor nerve material in peripheral nerve repair with conduits. Microsurgery. 2007;27:138–145. doi: 10.1002/micr.20318. [DOI] [PubMed] [Google Scholar]
- 20.Mackinnon SaDL. Surgery of the Peripheral Nerve. Thieme; New York: 1988. [Google Scholar]
- 21.Mackinnon SE, Dellon AL. Nerve repair and nerve grafting. In: Mackinnon SE, Dellon AL, editors. Surgery of the Peripheral Nerve. Thieme Medical Publishing; New York: 1988. pp. 89–121. [Google Scholar]
- 22.Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419–1429. doi: 10.1097/00006534-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Mackinnon SE, Hudson AR, Hunter DA. Histologic assessment of nerve regeneration in the rat. Plast Reconstr Surg. 1985;75:384–388. doi: 10.1097/00006534-198503000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Mackinnon SE, Midha R, Bain J, Hunter D, Wade J. An assessment of regeneration across peripheral nerve allografts in rats receiving short courses of cyclosporin A immunosuppression. Neuroscience. 1992;46:585–593. doi: 10.1016/0306-4522(92)90146-s. [DOI] [PubMed] [Google Scholar]
- 25.Madison RD, Archibald SJ, Brushart TM. Reinnervation accuracy of the rat femoral nerve by motor and sensory neurons. J Neurosci. 1996;16:5698–5703. doi: 10.1523/JNEUROSCI.16-18-05698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madison RD, Sofroniew MV, Robinson GA. Schwann cell influence on motor neuron regeneration accuracy. Neuroscience. 2009;163:213–221. doi: 10.1016/j.neuroscience.2009.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuyama T, Midha R, Mackinnon SE, Munro CA, Wong PY, Ang LC. Long nerve allografts in sheep with Cyclosporin A immunosuppression. J Reconstr Microsurg. 2000;16:219–225. doi: 10.1055/s-2000-7556. [DOI] [PubMed] [Google Scholar]
- 28.Midha R, Mackinnon SE, Evans PJ, Best TJ, Hare GM, Hunter DA, Falk-Wade JA. Comparison of regeneration across nerve allografts with temporary or continuous cyclosporin A immunosuppression. J Neurosurg. 1993;78:90–100. doi: 10.3171/jns.1993.78.1.0090. [DOI] [PubMed] [Google Scholar]
- 29.Moradzadeh A, Borschel GH, Luciano JP, Whitlock EL, Hayashi A, Hunter DA, Mackinnon SE. The impact of motor and sensory nerve architecture on nerve regeneration. Exp Neurol. 2008;212:370–376. doi: 10.1016/j.expneurol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nichols CM, Brenner MJ, Fox IK, Tung TH, Hunter DA, Rickman SR, Mackinnon SE. Effects of motor versus sensory nerve grafts on peripheral nerve regeneration. Exp Neurol. 2004;190:347–355. doi: 10.1016/j.expneurol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Redett R, Jari R, Crawford T, Chen YG, Rohde C, Brushart TM. Peripheral pathways regulate motoneuron collateral dynamics. J Neurosci. 2005;25:9406–9412. doi: 10.1523/JNEUROSCI.3105-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson GA, Madison RD. Developmentally regulated changes in femoral nerve regeneration in the mouse and rat. Exp Neurol. 2006;197:341–346. doi: 10.1016/j.expneurol.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Siemionow M, Zielinski M, Meirer R. The single-fascicle method of nerve grafting. Ann Plast Surg. 2004;52:72–79. doi: 10.1097/01.sap.0000097258.99511.ad. [DOI] [PubMed] [Google Scholar]
- 34.Tung TH, Doolabh VB, Mackinnon SB, Mohanakumar T, Hicks ME. Survival of long nerve allografts following donor antigen pretreatment: a pilot study. J Reconstr Microsurg. 2006;22:443–449. doi: 10.1055/s-2006-947699. [DOI] [PubMed] [Google Scholar]
- 35.Weiss P, Edds MVJR. Senosory-motor nerve crosses in the rat. 1945:173–193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Comparison of the fiber caliber distribution of each modality matched and mismatched at each time point. A) 5 week following graft implantation, mid graft evaluation of the fiber caliber distribution of regenerating motor axons is shifted to the right as would be expected for a purely motor axon. B) 7 week following graft implantation, distal evaluation of the fiber caliber distribution of regenerating axons is similarly shifted to the right. Again, these results are consistent with a mostly motor regenerating nerve and mostly sensory regenerating axons of the motor and sensory branches. (bars are averages and error bars are standard deviation)