Abstract
Fever is common in critically ill patients and is associated with worse clinical outcomes, including increased intensive care unit mortality. In animal models, febrile-range hyperthermia (FRH) worsens acute lung injury, but the mechanisms by which this occurs remain uncertain. We hypothesized that FRH augments the response of the alveolar epithelium to TNF-α receptor family signaling. We found that FRH augmented LPS-induced lung injury and increased LPS-induced mortality in mice. At 24 h, animals exposed to hyperthermia and LPS had significant increases in alveolar permeability without changes in inflammatory cells in bronchoalveolar lavage fluid or lung tissue as compared with animals exposed to LPS alone. The increase in alveolar permeability was associated with an increase in alveolar epithelial apoptosis and was attenuated by caspase inhibition with zVAD.fmk. At 48 h, the animals exposed to hyperthermia and LPS had an enhanced lung inflammatory response. In murine lung epithelial cell lines (MLE-15, LA-4) and in primary type II alveolar epithelial cells, FRH enhanced apoptosis in response to TNF-α but not Fas ligand. The increase in apoptosis was caspase-8 dependent and associated with suppression of NF-κB activity. The FRH-associated NF-κB suppression was not associated with persistence of IκB-α, suggesting that FRH-mediated suppression of NF-κB occurs by means other than alteration of IκB-α kinetics. These data show for the first time that FRH promotes lung injury in part by increasing lung epithelial apoptosis. The enhanced apoptotic response might relate to FRH-mediated suppression of NF-κB activity in the alveolar epithelium with a resultant increase in susceptibility to TNF-α–mediated cell death.
Fever is common in critically ill patients, with an estimated prevalence at admission to the intensive care unit of 30–70% (1–3). However, the effect of fever in critically ill patients is not fully understood. Some evidence suggests that fever augments innate immune responses and benefits humans and animals by enhancing antimicrobial defenses (4–8). Yet, clinical studies suggest that fever in critically ill patients is associated with worse outcomes, including increased mortality (1, 2, 9). Moreover, animal studies suggest that febrile-range hyperthermia (FRH) has a direct effect on critical illness-related end-organ damage, worsening acute renal failure and acute lung injury (ALI) (10–15).
Several groups have shown that mild hyperthermia in the range of clinically observed fever augments lung injury in animal models (11–14). However, the biological mechanisms by which FRH enhances lung injury are incompletely understood. Inflammatory and apoptotic responses in the alveolar epithelium are critical contributors to the development of ALI (16). The TNF-α receptor (TNFR) superfamily, a group of receptors related by the extracellular expression of cysteine-rich repeats and that includes the TNF-α, Fas ligand, and TRAIL receptors, has been implicated in both inflammation and apoptosis in ALI (17, 18). Matute-Bello et al. (19–23) showed that activation of the Fas pathway in the lungs of mice causes experimental ALI through a mechanism that is associated with alveolar epithelial apoptosis. In addition, activation of the Fas receptor yields inflammation. Intratracheal (IT) administration of Fas ligand or a Fas-activating Ab results in a neutrophilic alveolitis, and blocking the Fas receptor results in attenuated neutrophil recruitment and tissue injury after IT exposure to LPS or bacteria (24–29). Others have shown that TNF-α mediates both type II cell alveolar epithelial apoptosis and inflammation and participates in the pathogenesis of ALI in lung injury models (30). The concentration of TRAIL is increased in the bronchoalveolar lavage (BAL) fluid of patients with ALI, and this ligand has been implicated in respiratory syncytial virus-associated ALI in children (31, 32). In nonpulmonary organ systems, mild hyperthermia alone or as a concomitant exposure with a death receptor ligand, such as TNF-α, Fas ligand, or TRAIL, enhances TNFR signaling, including apoptotic cell death (33–37). Collectively, these data suggest that modification of TNFR family signaling could be an important mechanism by which FRH augments both apoptosis and inflammation in ALI.
Therefore, we hypothesized that FRH augments the response of the alveolar epithelium to TNFR family signaling. We studied the effect of FRH on the severity of LPS-induced lung injury in vivo and the response of alveolar epithelial cells to the two major members of the TNFR family, TNF-α and Fas ligand. We found that FRH augments the innate immune response after lung injury by enhancing alveolar epithelial apoptosis. The data show that enhanced caspase-dependent apoptosis contributes to FRH-augmented lung injury. We observed that FRH enhances TNF-α–mediated apoptosis of alveolar epithelial cells, which occurs as a result of FRH-mediated suppression of NF-κB activity. These data provide new insights into the mechanisms by which FRH augments lung injury.
Materials and Methods
Reagents
Proteins
Recombinant murine TNF-α was obtained from R&D Systems (Minneapolis, MN). Human Fas ligand was obtained from Alexis Biochemicals (San Diego, CA). Total IκB-α Ab was purchased from Cell Signaling Technology (Danvers, MA). Peroxidase-conjugated goat anti-rabbit IgG Ab was obtained from Pierce (Rockford, IL). Peroxidase-conjugated donkey anti-goat IgG Ab, Abs to TNF-α receptors 1 and 2, and FITC-labeled anti-rabbit IgG were obtained from Abcam (Cambridge, MA). Biotin-conjugated anti-mouse CD16/32 Ab was purchased from Biolegend (San Diego, CA). Collagen IV, Dispase, and biotin-conjugated anti-mouse CD45 and anti-mouse TER-119 Abs were purchased from BD Biosciences (San Jose, CA). DNase I from bovine pancreas was obtained from Sigma-Aldrich (St. Louis, MO).
Chemicals
The broad caspase inhibitor zVAD.fmk, caspase-8 inhibitor zIETD.fmk, and the inactive chemical analog of the caspase inhibitors, FA. fmk, were purchased from BD Pharmingen (San Jose, CA). The caspase-9 inhibitor, zLEHD.fmk, was purchased from Biovision (Mountain View, CA). Inhibitors of p38, MEK1/2, and JNK (SB-202190, U0126, and SP-600125, respectively) were obtained from Biomol International (Plymouth Meeting, PA). The IκB-α kinase inhibitor III, BMS 345541, was obtained from Calbiochem (La Jolla, CA).
Plasmids and RT-PCR
PCR probe primers for KC and TNF-α were purchased from Applied Biosciences (Foster City, CA). The NF-κB–inducible firefly luciferase plasmid was obtained from Invivogen (San Diego, CA). The renilla luciferase plasmid was obtained from Promega (Madison, WI).
Animal protocols
The animal protocols were approved by the Animal Care Committee of the VA Puget Sound Medical Center, Seattle, WA. Briefly, male C57BL/6 mice aged 6–8 wk (20–30 g) (The Jackson Laboratory, Bar Harbor, ME) were treated with IT bacterial LPS (Escherichia coli O111: B4 LPS, List Biological Laboratories, Campbell, CA) or PBS at either normal or febrile-range core body temperature. The core body temperature was monitored using a telemetry system composed of an implantable temperature transponder and an external receiver (Respironics Mini-mitter E-mitter G2 probe and receiver, Mini-Mitter, Bend, OR). The transponders were placed in the peritoneal cavity of anesthetized mice via a small abdominal incision. Following the transponder implantation, mice were monitored daily to ensure abdominal wound healing, and then studied 7 d or later post-implantation. On the day of the study, and immediately after the IT instillation of PBS with or without LPS (below), the mice were placed in cages at room temperature (23°C) or inside a heated, humidified infant incubator (35°C) (Airshields C2000, Hill-Rom, Batesville, IN). These ambient temperatures resulted in core temperatures of 37°C or between 39.5 and 40°C in sentinel animals, respectively. At least one sentinel animal carrying an implantable temperature transponder was placed in each temperature-exposure cage to confirm that the target core temperature had been reached. The core temperature data from the sentinel animals were transmitted at 1-min intervals from the i.p. probes to receivers located below each cage, and the signal was recorded using digital telemetry software (VitalView, Mini-Mitter). The core body temperatures of the sentinel mice were considered to be representative of all of the mice within the same cage.
To induce lung injury, mice were anesthetized with inhaled 4% isoflurane in 0.5 l/min O2 and endotracheally intubated with a gavage tube as previously described (38). Once tracheal intubation had been confirmed, the mice were treated with E. coli LPS or PBS in a volume of 50 µl. Following instillation, the gavage tube was removed, and the mice were immediately transferred to their designated temperature exposure environments and allowed to recover from anesthesia with free access to food and water. The animals were monitored hourly for the initial 6 h and afterward every 6 h until the end of the experiment. At each monitoring time, the mice were evaluated for the following criteria: panting or other respiratory difficulty, abnormal posture, ruffled fur, pale eyes, or loose stools. Mice were euthanized if they met three or more endpoint criteria or one endpoint criterion if severe.
To evaluate the role of caspase activity in FRH-augmented lung injury, we administered zVAD.fmk, a broad caspase inhibitor, to animals exposed to a sublethal dose of IT LPS (100 ng) at normal or febrile-range core body temperatures. Mice received either zVAD.fmk (10 mg/kg in 10% DMSO) or vehicle s.c. every 16 h for 48 h or until meeting euthanasia criteria. The experimental conditions, including induction of lung injury, modification and monitoring of core body temperature, and euthanasia criteria were as described above.
At the end of each experiment, the mice were euthanized with an i.p. injection of pentobarbital (120 mg/kg) and exsanguinated by closed cardiac puncture. Blood was collected in heparinized syringes, and the plasma fraction was stored in individual aliquots at −80°C. Immediately after death, the thorax was opened, and the left hilum was clamped. The left lung was removed, weighed, flash frozen, and stored at −80°C. At a later date, the frozen lung was homogenized in sterile water, and aliquots were stored at −80°C. BAL was performed in the right lung with four separate 0.5 ml aliquots of 0.9% NaCl containing 0.6 mM EDTA. For histological processing, the right lung was fixed in 4% paraformaldehyde at 15 cm H2O transpulmonary pressure. An aliquot of BAL fluid was used immediately for cell counts and differential. The remaining BAL fluid was spun for 10 min at 200 × g, 4°C, and supernatants were stored at −80°C.
Experimental design
The main experimental variables were the core temperature, dose of LPS, and duration of the exposure following LPS instillation. We tested two core temperatures, 37°C (euthermic) and 39.5 to 40°C (hyperthermic), doses of LPS (0, 5, 25, or 50 µg), and two different exposure times (24 or 48 h). The resulting groups are shown in Table I. To evaluate the effect of caspase inhibition, zVAD.fmk 10 mg/kg or vehicle alone was administered s.c. every 16 h to mice exposed to IT LPS (100 ng), at normal or elevated body temperature, for 24 or 48 h.
Table I.
Experimental groups
| IT Instillationa |
||||
|---|---|---|---|---|
| LPS |
||||
| Temperature Exposure | PBS Only | 5 µg | 25 µg | 50 µg |
| Euthermia 24 h | 8 | 4 | 6 | 6 |
| Euthermia 48 h | 2 | 10 | 5 | |
| Hyperthermia 24 h | 8 | 5 | 10 | 7 |
| Hyperthermia 48 h | 2 | 6 | 4 | |
All IT instillations performed in 50 µl of sterile PBS.
Measurements
Histological examination
Postfixation, lungs were paraffin-embedded, cut at 4-µm thickness, and stained with H&E.
Lung injury
The alveolar permeability was assessed by measurement of BAL fluid total protein and BAL IgM concentrations. BAL fluid IgM was assessed on BAL fluid using an immunoassay (Bethyl Laboratories, Montgomery, TX). BAL fluid total protein was assessed using the bicin-choninic acid method (Pierce).
Lung inflammation
The lung inflammatory response was assessed using BAL cell counts and differentials performed in a blinded manner. Total cell counts were performed on an aliquot of BAL fluid using a hemacytometer. The cell differentials were counted on cytospin preparations using the Quick-diff method. Tissue polymorphonuclear cells (PMNs) were assessed by myeloperoxidase (MPO) activity measured in lung homogenates using the Amplex Red Peroxidase Assay Kit (Invitrogen, Carlsbad, CA).
Apoptosis
The TUNEL assay was performed on 4-µm thickness sections of paraffin-embedded lung tissue according to the manufacturer’s instructions (Roche Diagnostics, Mannheim, Germany). Fluorescence and differential interference contrast microscopy were performed using a Nikon Eclipse 80i microscope (Nikon, Melville, NY). Measurement of TUNEL-positive cells was performed in a blinded manner on 10 randomly generated visual fields at ×400 magnification.
Tissue culture and protocols
MLE-15 cells (provided by J. Whitsett, Cincinnati Children’s Hospital, Cincinnati, OH) were grown in RPMI 1640 media (Cellgro, Manassas, VA) supplemented with 4% heat-inactivated FCS at 37°C, 5% CO2. LA-4 cells, purchased from American Type Culture Collection (ATCC number CCL-196; Manassas, VA), were grown in F12K media (American Type Culture Collection) supplemented with 15% heat-inactivated FCS at 37°C, 5% CO2. The cells were kept in passage for 4–6 wk. Prior to any experimental exposure, cells were grown to 80% confluence at 37°C, 5% CO2. Unless otherwise noted, experimental conditions lasted 18 h. For temperature modification, cells were incubated at 34, 37, or 39.5°C with 5% CO2.
Cell survival
MLE-15 cells and LA-4 cells were exposed to 34, 37, or 39.5°C and to TNF-α at 1, 10, or 100 ng/ml or to Fas ligand at 2, 55, or 500 ng/ml. Cell survival was assessed with alamar Blue (Biosource, Camarillo, CA), which fluoresces in response to the reduction of culture media caused by cell growth. At the conclusion of cell survival experiments, alamar Blue was added to the media of the cells to be evaluated, and plates were incubated at 37°C for 4 h. The fluorescence produced was quantified using a Cytofluor II plate reader (PerSeptive Biosystems, Foster City, CA) at an excitation of 530 nm and emission of 590 nm. Viability was expressed as the fluorescence of treated cells divided by the fluorescence of untreated cells.
Isolation of primary murine type II cells
Type II alveolar epithelial cells were isolated from C57BL/6 mice, aged 6–8 wk, using a protocol based on the method of Corti et al. (21, 39). Animals were euthanized with i.p. Beuthanasia-D (Schering-Plough, Kenilworth, NJ). The ventral surface of the mouse was cleansed with 70% ethanol and betadine and the mouse exsanguinated by aortic transsection. The right ventricle was exposed and perfused with PBS/0.5 mM EDTA to clear the pulmonary vasculature. Dispase was then instilled into the airspaces via a tracheal cannula and followed by 1% low-melting agarose. The lungs were then removed, briefly covered with crushed ice, and incubated in Dispase at room temperature for 45 min with rotation. The lungs were then placed in media-I (Ham’s F12, American Type Culture Collection) containing DNase I 0.01% and manually teased from the trachea and major airways using forceps. The resulting slurry was then filtered through 100 µM and 25 µM Millipore mesh filters (Millipore, Billerica, MA). The filtered suspension was then spun at 130 × g for 10 min at 8°C, the pellet resuspended in media-I, and cells quantified. Biotinylated anti-CD16/32 (0.65 µg/million cells), anti-CD45 (1.5 µg/million cells) and anti-mouse TER-119 (1 µg/million cells) were added to the cell suspension and incubated at 37°C, 5% CO2 for 30 min. The cells were then pelleted at 200 × g, resuspended in media-I, and combined with PBS-washed streptavidin beads for 30 min at room temperature while rocking. The suspension was placed in a magnetic bead separator, the supernatant was removed and pelleted, and the cells were resuspended in media-II (Ham’s F12 supplemented with 2% FCS). The cell suspension was then placed in a tissue culture Petri dish and incubated for 16 h at 37°C, 5% CO2. Postincubation, the nonadherent cells were collected, pelleted at 200 × g, and resuspended in media-II. The cells were then plated in collagen IV– coated 96-well plates at a seeding density of 2 × 105 cells/cm2 and incubated at 37°C, 5% CO2, until the cells reached 80% confluence. The type II alveolar epithelial cell phenotype from this protocol has been confirmed with Papanicolaou staining showing lamellar bodies and RT-PCR studies showing mRNA for surfactant protein C, but not for vimentin, fibroblast-specific protein 1, podoplanin, or cytokeratin-8/18. The isolated cells were exposed to TNF-α or Fas ligand and 34, 37, or 39.5°C, 5% CO2, for 18 h, and viability was evaluated with the alamar Blue assay in the same manner used for the MLE-15 and LA-4 cell lines.
Caspase activity
For caspase-3/7, 8, and 9 activity assays, MLE-15 cells were exposed to TNF-α at 34, 37, or 39.5°C, 5% CO2, for 2 h. Caspase 3/7, −8, and −9 activity assays were obtained from Promega. For caspase-3/7 activity, a DEVD cleavage assay was performed according to the manufacturer’s instructions on MLE-15 cells. The fluorescence was measured at excitation of 485 nm and emission of 530 nm using a Cytofluor II plate reader. To evaluate caspase-8 and caspase-9 activities, luminescence assays were performed according to the manufacturer’s instructions. Luminescence was determined using a Veritas microplate luminometer (Promega).
Caspase inhibitor treatment
MLE-15 cells were exposed to TNF-α 5 ng/ml and incremental doses of inhibitors of caspase-8 (zIETD.fmk), caspase-9 (zLEHD.fmk), a broad caspase inhibitor (zVAD.fmk), or the inactive chemical analog of the inhibitors (FA.fmk) at 34, 37, or 39.5°C, 5% CO2. Control cells were exposed to concentrations of DMSO equivalent to experimental exposures. Viability was assessed using the alamar Blue assay.
Flow cytometry
To measure apoptosis, MLE-15 cells were dual stained for Annexin V-FITC and propidium iodide postexposure to 34, 37, or 39.5°C, 5% CO2, for 4 h in the presence or absence of TNF-α 100 ng/ml. Staining was performed according to the manufacturer’s instructions (BD Biosciences).
For evaluation of surface expression of TNF-α receptors on MLE-15 cells, the cells were exposed to 34, 37, or 39.5°C. The cells then released from the culture plate using trypsin 0.05%/0.53 mM EDTA, spun, and then 1 × 106 cells were incubated first with anti-TNFR1 or 2 Abs and then with FITC-labeled secondary Ab for 30 min each. All flow cytometry analysis was performed using a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ).
RT-PCR
Quantitative mRNA evaluation was performed by RT-PCR. Posttreatment with TNF-α 50 ng/ml and incubation temperature of 34, 37 or 39.5°C for 1 or 3 h, MLE-15 cells were lysed. After RNA extraction using the Absolutely RNA Microprep kit (Stratagene, La Jolla, CA), PCR was performed using primers for KC and TNF-α and an ABI Prism 7000 Taqman machine (Applied Biosciences). The results were quantified using the δ-δ Ct method (40).
Signaling pathways
MLE-15 cells were exposed to one of the MAPK inhibitors at 1 µM, TNF-α 5 ng/ml, and 34, 37, or 39.5°C. Survival was assessed by alamar Blue assay. NF-κB activity was assessed using transient transfection with an NF-kB–driven luciferase reporter construct. MLE-15 cells were dually transfected with a plasmid containing an NF-κB–inducible promoter for firefly luciferase and a plasmid containing constitutively active renilla luciferase. Postincubation with the plasmids in lipofectamine for 4 h at 37°C, 5% CO2, the MLE-15 cells were treated with TNF-α. The luminescence was measured using the Dual-Luciferase Reporter Assay System (Promega) according to manufacturer’s instructions using a PerkinElmer Victor3 V fluorescence plate reader (PerkinElmer, Waltham, MA). NF-κB activity was expressed as firefly luminescence divided by renilla luminescence to correct for transfection efficiency and cell viability.
An IκB-α kinase inhibitor was added to MLE-15 cells at concentrations of 1 or 33 µM treated with or without TNF-α 5 ng/ml and 34, 37, or 39.5°C. Survival was assessed at 18 h.
The effect of temperature on TNF-α–induced depletion of IκB-α was assessed with Western blotting for total IκB-α in cell lysates, which were not treated with a proteasome inhibitor. MLE-15 cells were exposed to TNF-α 100 ng/ml and 34, 37, or 39.5°C for 5, 15, 30, or 60 min. Given the brief temperature exposure time, the media and TNF-α solution were kept in water baths at the intended temperature, and the plates were placed in direct contact with heat blocks at 34, 37, or 39.5°C. The cells were then lysed in a solution of 50 mM Tris (pH 8), 150 mM NaCl, and 1% Tergitol-type NP-40 with protease inhibitors and spun for 15 min at 200,000 × g at 4°C. The supernatants were collected, and proteins were separated using SDS-PAGE. Densitometry to correct for differences in protein loading was performed using Image J software (National Institutes of Health, Bethesda, MD).
Statistics
All experiments were performed at least three times. The data were analyzed using GraphPad Prism 4.0 software (GraphPad, San Diego, CA). Among multiple groups, comparisons of means were performed using one-way ANOVA. Comparisons between the means of two groups were performed using an unpaired t test. Survival curves were compared with a log-rank test. Data are shown in the figures as the mean with notation of the SE in a bar. A p value <0.05 was considered significant.
Results
FRH enhances LPS-induced lung injury
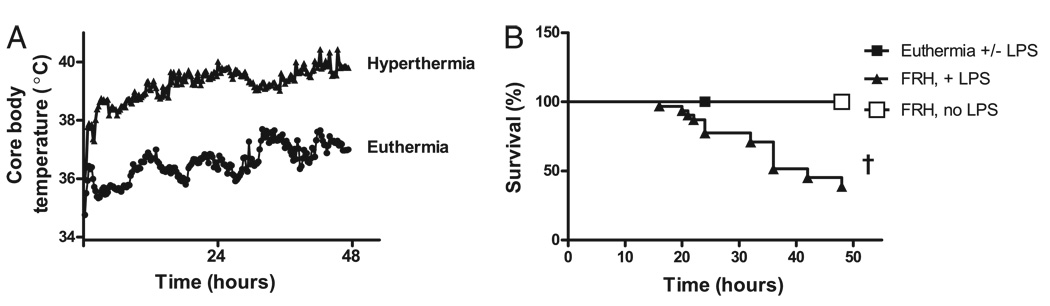
The effect of FRH was evaluated in a mouse model of LPS-induced lung injury. Animals were treated with IT LPS 5, 25, or 50 µg in 50 µl sterile PBS and then exposed to room temperature (23°C) or FRH (35°C) (Table I). Independent of LPS instillation, exposure to ambient hyperthermia caused an elevation in the animals’ core body temperature to between 39.5–40°C, which was maintained for up to 48 h (Fig. 1A). Animals treated with IT LPS and hyperthermia had an elevated mortality rate compared with those animals treated with LPS or hyperthermia alone (Fig. 1B). Mice with febrile-range core body temperature that were treated with IT LPS 50 µg had a mortality rate of 62%, whereas euthermic animals treated with 50 µg IT LPS had a mortality rate of 0% over 48 h. Similar increases in mortality were seen in hyperthermic animals treated with IT LPS doses of 5 or 25 µg (data not shown), whereas all euthermic animals exposed to LPS survived.
FIGURE 1.
FRH is associated with increased LPS-induced mortality in an animal model. A, Exposure to ambient hyperthermia (35°C) generates febrile-range core body temperatures (39.5–40°C) in mice as compared with animals that were maintained at room temperature (23°C) independent of LPS exposure. B, Exposure to IT LPS and FRH results in increased mortality in mice. Animals treated with LPS alone or FRH alone had no mortality by 48 h, whereas animals treated with FRH plus LPS had 62% mortality. The FRH plus LPS survival curve shown reflects an LPS dose of 50 µg. Data from 5 and 25 µg doses LPS plus FRH are not shown but also demonstrate increased mortality. †p< 0.0001.
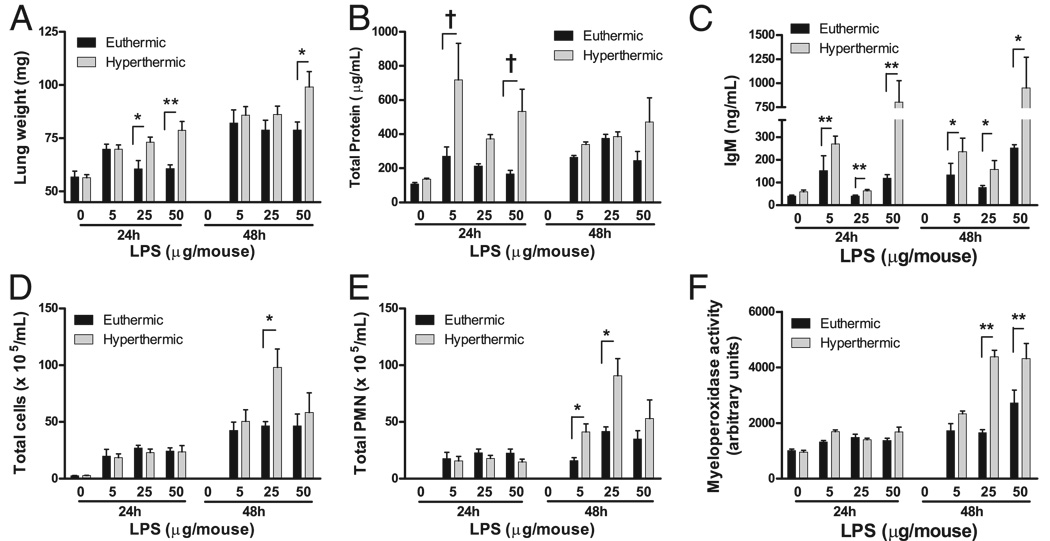
Hyperthermic animals treated with LPS had elevated lung weight as well as elevated BAL concentrations of IgM and total protein at 24 h (Fig. 2A–C). However, the total cell and PMN counts in the lavage fluid were not different between the hyperthermic and euthermic groups at 24 h. These data show that hyperthermia increases LPS-induced alveolar permeability at 24 h without changing PMN recruitment as compared with euthermic animals. At 48 h, the accumulation of PMNs in BAL fluid and the lung tissue MPO activity, which is considered representative of total lung PMN content, were significantly increased in the mice exposed to LPS and hyperthermia as compared with mice treated with LPS alone, suggesting that the hyperthermia enhances late PMN responses (Fig. 2D, 2F).
FIGURE 2.
FRH augments alveolar permeability and delayed neutrophil recruitment in an LPS injury model. Lung weight (A), total protein in BAL fluid (B), and IgM concentration in BAL fluid (C) are increased in animals exposed to FRH and LPS as compared with animals exposed to LPS and euthermia. The changes in alveolar permeability precede FRH-associated changes in LPS-induced inflammation. Total cells in BAL fluid (D), total BAL fluid neutrophils (E), and tissue MPO activity in lung homogenates (F) are increased in animals exposed to FRH and LPS as compared with LPS alone after 48 h. For all groups, *p< 0.05; **p< 0.01; †p< 0.001.
FRH augments alveolar epithelial apoptosis in vivo
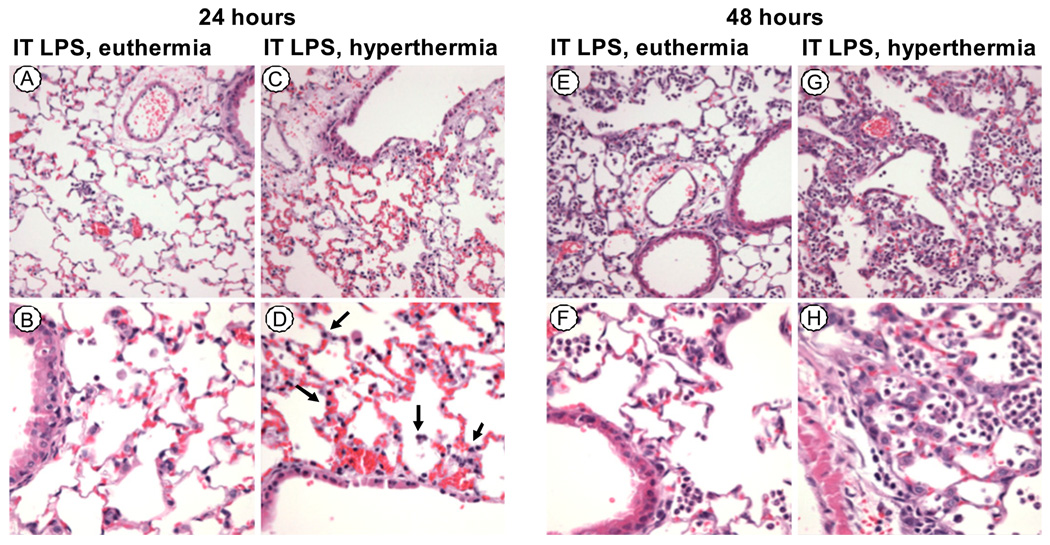
The lungs of mice treated with LPS and hyperthermia for 24 h showed thickening of the alveolar septa, with increased pyknotic nuclei in the epithelium and microvascular congestion as compared with euthermic LPS-treated animals (Fig. 3). At 24 h, the lung inflammatory infiltrates were similar in LPS-treated animals exposed to either hyperthermic or euthermic conditions. In contrast, by 48 h, the lungs of the hyperthermic LPS-treated animals had increased inflammatory cells, predominantly neutrophils, as compared with the euthermic LPS-treated animals.
FIGURE 3.
FRH causes histologic evidence of LPS-induced lung injury prior to neutrophil recruitment. H&E stains of paraffin-embedded lungs of animals exposed to LPS 50 µg and euthermia for 24 h, original magnifications ×200 (A) and ×400 (B), or hyperthermia, ×200 (C) and ×400 (D). The animals exposed to LPS and hyperthermia have more airway edema, alveolar septal thickening, and pyknotic nuclei (arrows) in the alveolar walls as compared with LPS alone. After 48 h, the lungs of animals exposed to LPS 50 µg and euthermia, original magnifications ×200 (E) and ×400 (F), or hyperthermia, ×200 (G) and ×400 (H) show that hyperthermia exaggerates delayed neutrophil recruitment.
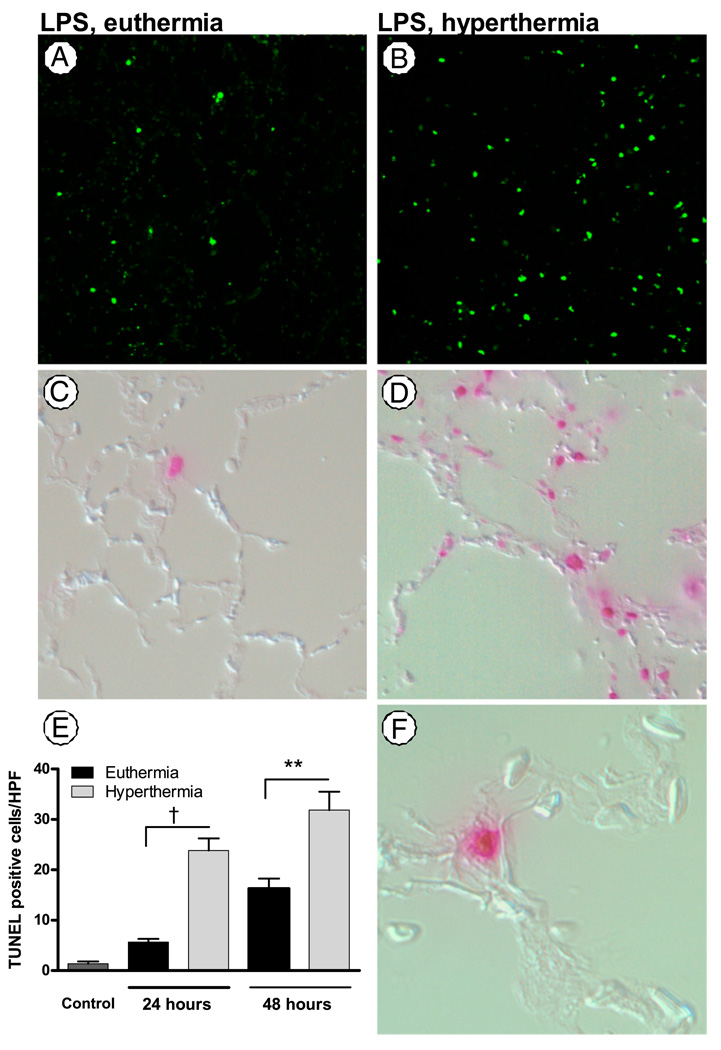
At 24 h, the animals treated with LPS and hyperthermia had more TUNEL-positive cells in the lungs as compared with animals treated with LPS and euthermia (hyperthermic 23.88 ± 2.36 versus euthermic 5.65 ± 0.64; p < 0.0001) (Fig. 4). The TUNEL-positive cells localized to corners of alveoli, suggesting that the TUNEL-positive population largely represents apoptotic type II alveolar epithelial cells. This suggests that increased alveolar epithelial apoptosis is an initial pathophysiological event in FRH-augmented lung injury. After 48 h, the hyperthermic animals continued to have more TUNEL-positive cells than euthermic animals.
FIGURE 4.
FRH enhances alveolar epithelial apoptosis. The lungs of mice exposed to LPS 50 µg and FRH (B) show an increase in TUNEL-positive cells as compared with LPS alone (A), original magnification ×100. Differential interference contrast microscopic images merged with the fluorescent TUNEL images (original magnification ×400) in euthermic (C) or hyperthermic (D) mice show that the fluorescence localizes to the alveolar walls. E, At 24 and 48 h, the number of TUNEL-positive cells per high-power field in hyperthermic animals exposed to LPS is significantly higher than in euthermic animals exposed to LPS. F, Many of the TUNEL-positive cells localize to the corners of alveoli, original magnification ×1000. †p< 0.0001; **p< 0.01.
To determine whether caspase-dependent apoptosis is important in the augmented lung injury seen in the hyperthermic mice, we administered a broad caspase inhibitor, zVAD.fmk, or vehicle alone to animals exposed to a sublethal dose of IT LPS at either normal or febrile-range core body temperature. Animals exposed to LPS and hyperthermia had enhanced alveolar permeability as compared with animals exposed to LPS and euthermia. The administration of zVAD.fmk attenuated the FRH-enhanced alveolar permeability induced with IT LPS, as evaluated by BAL total protein and IgM, but did not affect the cellular inflammatory response, as reflected by total cells and total PMNs in lung BAL fluids (Table II). Treatment with zVAD.fmk did not, however, attenuate FRH-augmented lung injury induced by high-dose IT LPS (50 µg).
Table II.
Effect of caspase inhibition on FRH-augmented lung injury
| Measurements of Lung Injury in BAL |
||||
|---|---|---|---|---|
| Total Cells (× 105 cells/ml) | Total PMN (× 105 cells/ml) | Total Protein (µg/ml) | IgM (ng/ml) | |
| Euthermia | 8.5 ± 1.1 | 1.9 ± 0.46 | 133.8 ± 13.9 | 44.4 ± 12.2 |
| FRH | 7.3 ± 0.53 | 2.53 ± 0.41 | 481 ± 143.4a | 364.4 ± 162b |
| FRH plus caspase inhibitorc | 6.6 ± 0.54 | 1.7 ± 0.37 | 129.4 ± 25.1d | 47.1 ± 10.5e |
Lung injury induced by LPS IT 100 ng.
LPS plus FRH versus LPS; p = 0.02.
LPS plus FRH versus LPS; p< 0.01.
zVAD.fmk 10 mg/kg s.c. every 16 h used as broad caspase inhibitor.
With zVAD versus without zVAD; p = 0.02.
With zVAD versus without zVAD; p< 0.01.
These findings suggest that FRH-augmented lung injury is associated with increased epithelial apoptosis prior to FRH-generated changes in the neutrophilic inflammatory response in the lungs and that caspase-dependent apoptosis participates in the pathogenesis of FRH-augmented lung injury.
Incubation temperature modifies TNF-α–induced cell death
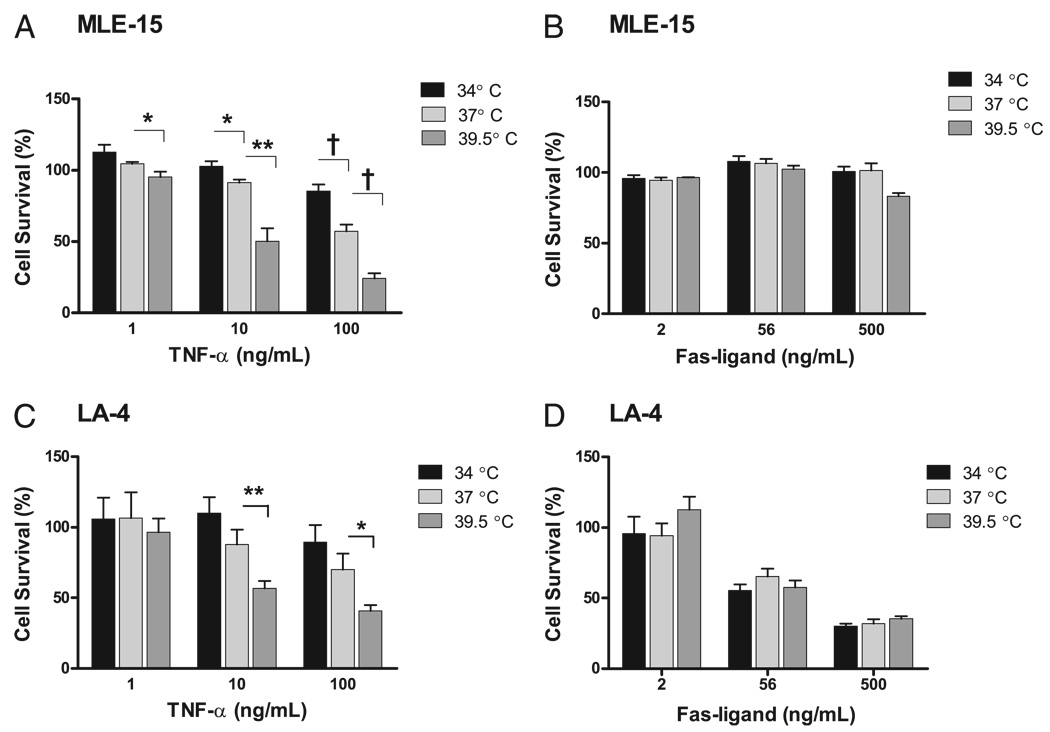
Next, we investigated whether temperature modifies apoptosis of epithelial cells exposed to the TNFR family members, TNF-α or Fas ligand. MLE-15 and LA-4 cells were exposed to TNF-α or Fas ligand at 34, 37, or 39.5°C for 18 h; survival was assessed by the alamar Blue assay. Cell death induced by TNF-α was enhanced in both cell lines as the incubation temperature increased (Fig. 5A, 5C). In contrast, mild hypothermia protected MLE-15 cells from TNF-α–mediated cell death, and this trend was seen in LA-4 cells, although the change did not reach statistical significance. Interestingly, the MLE-15 cells were resistant to death mediated by Fas ligand. Increased incubation temperature did not sensitize the MLE-15 cells to Fas ligand-mediated death (Fig. 5B). In contrast, LA-4 cells were sensitive to Fas ligand-mediated death. However, as with MLE-15 cells, incubation temperature had no effect on the LA-4 cell response to Fas ligand (Fig. 5D). To determine the relevance of the cell line findings for primary cells, we isolated type II cells from normal mice. The cells were exposed to TNF-α or Fas ligand and 34, 37, or 39.5°C for 18 h. As seen with the MLE-15 cells, hyperthermia enhanced cell death in response to TNF-α (viability after TNF-α 100 ng/ml as compared with cells exposed to media only at the same incubation temperature: at 34°C, 105 ± 23.2%; at 37°C, 76.6 ± 13.4%; at 39.5°C, 41.7 ± 4.8%; p < 0.01). As with MLE-15 cells, Fas ligand did not cause cell death in murine primary type II alveolar epithelial cells at any of the temperatures studied. Therefore, the modification of cellular responses to TNF-α by temperature is seen with several different types of lung epithelial cells, including primary type II alveolar epithelial cells, and appears to be specific to the TNF-α receptors.
FIGURE 5.
Incubation temperature modifies TNF-α, but not Fas-mediated, death in alveolar epithelial cell lines. Both MLE-15 (A) and LA-4 cells (C) exposed to TNF-α show increased sensitivity to TNF-α mediated death as incubation temperatures increase. B, MLE-15 cells are insensitive to Fas ligand-mediated cell death, and incubation temperatures do not affect the sensitivity. D, LA-4 cells are sensitive to Fas-mediated cell death, and this response is not affected by incubation temperatures. *p< 0.05; **p< 0.01; †p< 0.001.
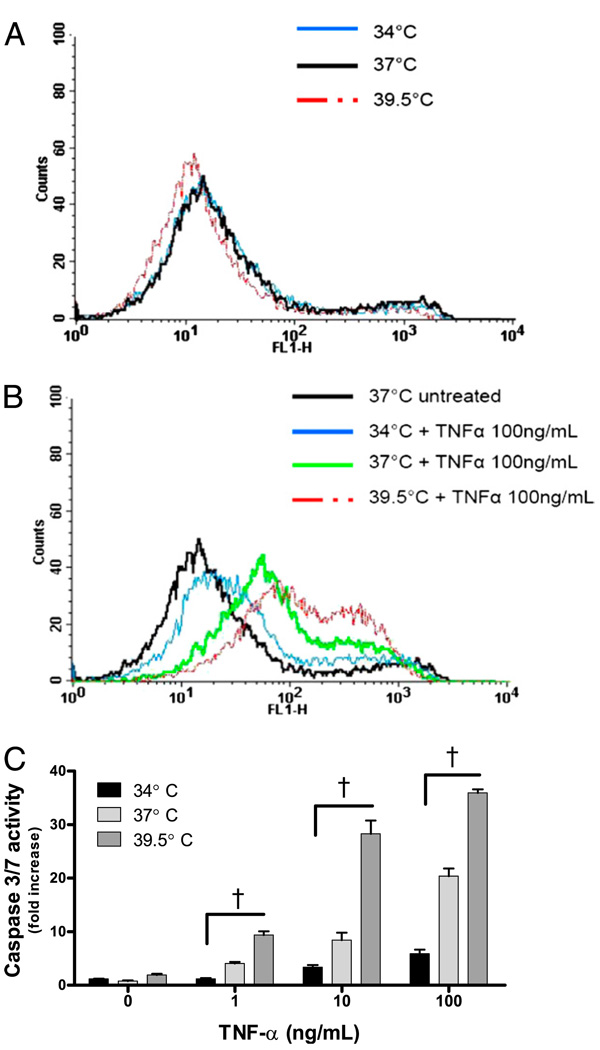
To confirm that the mechanism of cell death was apoptosis, MLE-15 cells exposed to TNF-α and temperatures of 34, 37, or 39.5°C for 6 h were evaluated for membrane exposure of phosphatidylserine, a marker of apoptosis, by labeling with Annexin V-FITC. In the absence of TNF-α, the MLE-15 cells did not change phosphatidylserine expression as a function of temperature (Fig. 6A). The Annexin V-FITC labeling of MLE-15 cells exposed to TNF-α increased as a function of temperature and was highest at 39.5°C as compared with cells at 37°C or 34°C (Fig. 6B). As an additional measure of apoptotic pathways, we also determined caspase-3/7 activity in MLE-15 cells exposed to TNF-α at various incubation temperatures. Cells exposed to TNF-α at 39.5°C had a 2-fold increase in caspase-3/7 activity compared with cells incubated at 37°C, and a 6-fold increase over cells treated with TNF-α at 34°C (Fig. 6C). These data suggest that FRH enhances cell death by augmenting TNF-α–induced apoptosis.
FIGURE 6.
Febrile-range temperature enhances apoptosis in MLE-15 cells exposed to TNF-α. A, Temperature alone does not affect Annexin V-FITC binding in MLE-15 cells. B, MLE-15 cells exposed to TNF-α 100 ng/ml and 34, 37, or 39.5°C for 6 h demonstrate Annexin V-FITC binding increases as a function of temperature. C, Caspase-3/7 activity in MLE-15 cells exposed to TNF-α and 34, 37, or 39.5°C for 2 h shows that caspase-3/7 activity generated by cells exposed to TNF-α increases as a function of incubation temperature. †p< 0.05.
FRH enhances TNF-α–mediated cell death in a caspase-dependent manner
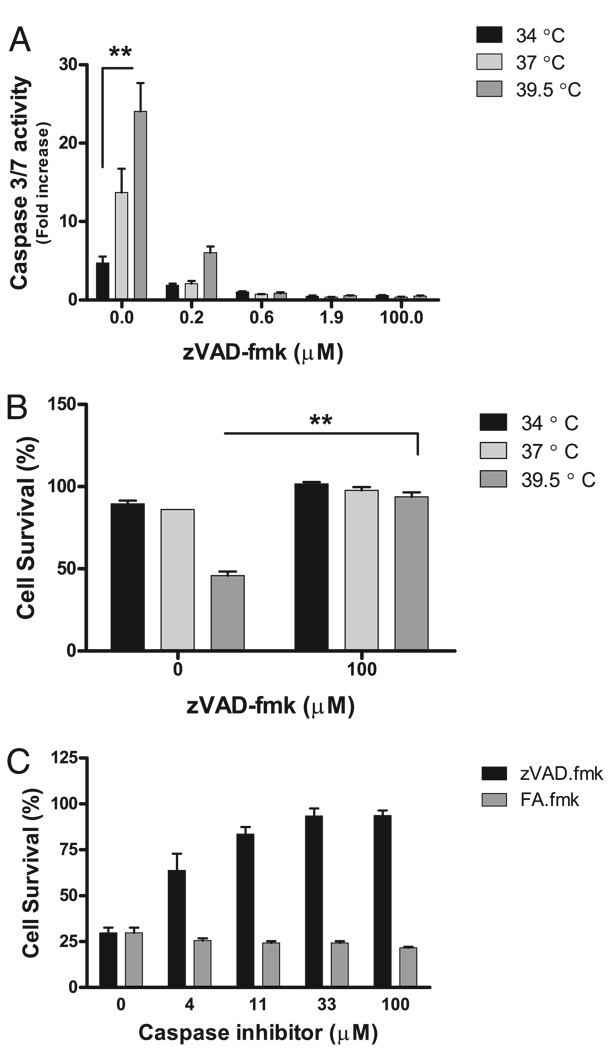
We used zVAD.fmk, a broad caspase inhibitor, to evaluate the role of caspases in temperature-dependent TNF-α–mediated cell death. Low concentrations of zVAD.fmk suppressed caspase-3/7 activity, as assessed by DEVD cleavage (Fig. 7A). FA.fmk, an inactive chemical analog of zVAD.fmk, did not affect DEVD cleavage (data not shown). Next, the alamar Blue assay was used to assess the effect of zVAD.fmk on MLE-15 cell survival postexposure to TNF-α and FRH. zVAD.fmk protected cells from the augmented cell death associated with hyperthermia and TNF-α (Fig. 7B). There was a strong dose-response relationship between zVAD.fmk and cell survival in MLE-15 cells exposed to TNF-α and 39.5°C (Fig. 7C). FA.fmk did not affect cell survival (Fig. 7C). Therefore, hyperthermia augments MLE-15 cell death postexposure to TNF-α, and this effect depends upon enhanced caspase activity.
FIGURE 7.
FRH enhances TNF-α–mediated cell death in a caspase-dependent manner. A, zVAD.fmk suppresses caspase-3/7 activity in MLE-15 cells exposed to TNF-α 5 ng/ml, 34, 37, or 39.5°C at low doses. B, zVAD.fmk improves MLE-15 survival posttreatment with TNF-α 5 ng/ml and 39.5°C. C, A strong dose response exists between zVAD.fmk and MLE-15 cell survival at 39.5°C. FA.fmk does not improve MLE-15 cell survival after TNF-α exposure at 39.5°C. **p< 0.01.
Hyperthermia augments TNF-α–induced cell death via the death-receptor pathway
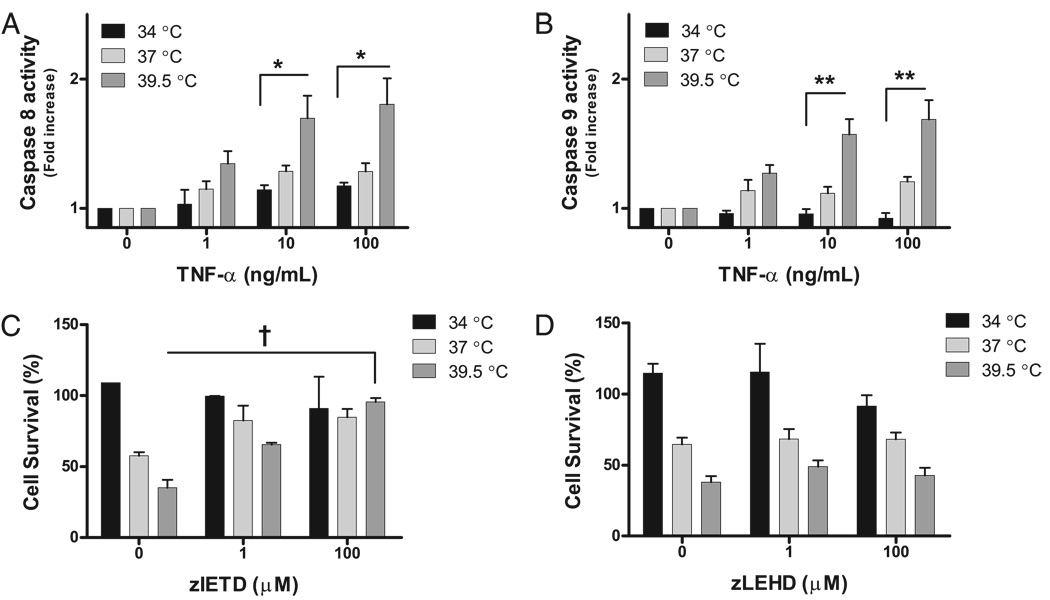
Ambient phenomena such as temperature are commonly thought to effect apoptosis by modulating the mitochondrial pathway. However, our studies showed modulation of the receptor-mediated pathway. Therefore, we next investigated the effect of hyperthermia on caspase-8 and caspase-9 activity. Caspase-8 activity is associated with death receptor (e.g., TNF-α, TRAIL, Fas receptor) activation; caspase-9 activity increases as a consequence of direct mitochondrial injury or by caspase-8–dependent cleavage of Bid. Therefore, the pattern of proximal caspase activity during exposure to hyperthermia and TNF-α reflects whether hyperthermia enhances TNF-α–mediated cell death by enhancing signaling through death receptors or by causing direct mitochondrial injury. Caspase-8 and −9 activities were greatest in MLE-15 cells exposed to TNF-α 5 ng/ml and 39.5°C (Fig. 8A, 8B). In contrast, mild hypothermia decreased the amount of caspase-8 and −9 activities in MLE-15 cells after TNF-α exposure as compared with 37°C. To demonstrate that hyperthermia-enhanced TNF-α–mediated cell death depends on caspase-8 activity, chemical inhibitors of caspase-8 or −9 were added to MLE-15 cells exposed to TNF-α 5 ng/ml and 34, 37, or 39.5°C. The caspase-8 inhibitor, zIETD.fmk, protected MLE-15 cells from hyperthermia-enhanced cell death after TNF-α exposure (Fig. 8C). Neither the caspase-9 inhibitor, zLEHD.fmk (Fig. 8D), nor the inactive chemical analog of these inhibitors, FA.fmk (Fig. 7C), protected MLE-15 cells during TNF-α exposure at 37 and 39.5°C. Therefore, hyperthermia enhances TNF-α–mediated apoptosis by increasing death-receptor signaling via caspase-8, whereas the mitochondrial pathway, in which cas-pase-9 is an effector, is less important.
FIGURE 8.
FRH augments TNF-α–mediated cell death via the death-receptor pathway. Caspase-8 (A) and caspase-9 (B) activity increase as a function of TNF-α dose and incubation temperature. C, The chemical caspase-8 inhibitor, zIETD, rescued MLE-15 cells from FRH-augmented death at TNF-α 5 ng/ml. Neither the chemical caspase-9 inhibitor, zLEHD (D), nor the inactive chemical analog of the inhibitors, FA.fmk (Fig. 7C), improved MLE-15 survival postexposure to TNF-α 5 ng/ml and FRH. *p< 0.05; **p< 0.01; †p< 0.001.
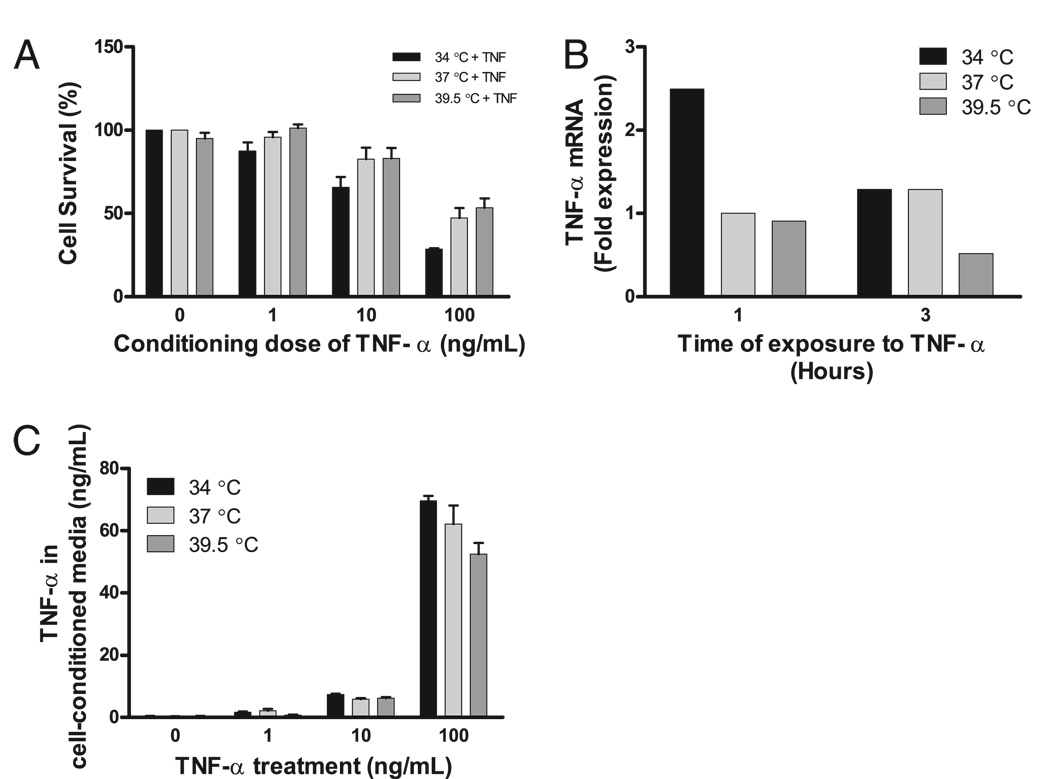
The mechanisms by which hyperthermia enhances the death receptor pathway were then examined. First, to determine whether TNF-α acted in trans by causing the release of a soluble factor that then enhanced cell death, the media of MLE-15 cells exposed to TNF-α and 34, 37, or 39.5°C for 18 h were collected and transferred to MLE-15 cells, which were then incubated at 37°C for 18 h. The cell-conditioned media did not cause significant differences in recipient cell mortality. However, supernatants of cells exposed to 34°C and TNF-α tended to cause more cell death in the recipient MLE-15 cells than cell-conditioned media of cells treated with 37 or 39.5°C (Fig. 9A). The conditioned media of MLE-15 cells treated with TNF-α at 34°C contained a higher TNF-α concentration than the media of cells treated with TNF-α at 37 or 39.5°C, although this also did not reach statistical significance (Fig. 9B, 9C). The data suggest the induction of cell death by hyperthermia is not mediated by a temperature-dependent autocrine factor.
FIGURE 9.
FRH-augmented cell death is not mediated by a soluble factor. A, The media of MLE-15 cells exposed to TNF-α and various incubation temperatures for 18 h was collected and transferred to MLE-15 cells and then incubated at 37°C for 18 h. No difference in the cell survival at each conditioning dose of TNF-α exists, although a trend for increased mortality from the cell-conditioned media of cells at 34°C exists. This reflects increased production (B) of and increased stability (C) of the TNF-α at 34°C compared with 39.5°C.
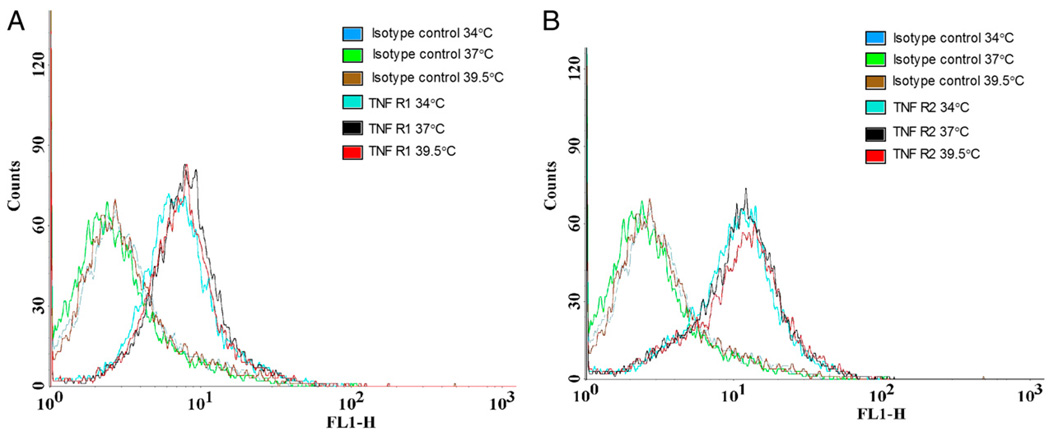
Next, the effect of incubation temperature on surface expression of TNFR1 and TNFR2 in MLE-15 cells was examined by flow cytometry. Neither mild hypothermia nor mild hyperthermia affected the surface expression of TNFR1 or TNFR2 in MLE-15 cells (Fig. 10).
FIGURE 10.
Temperature does not modify expression of TNFRs on MLE-15 cells. MLE-15 cells were exposed to 34, 37, or 39.5°C, 5% CO2, and then evaluated for surface expression of TNFR1 and 2. No difference in surface expression of TNFR1 (A) or 2 (B) exists.
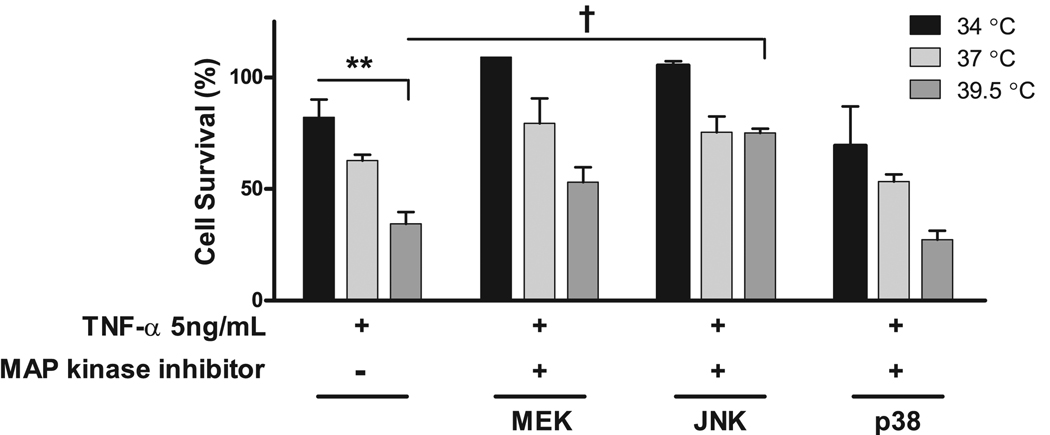
The role of the MAPKs in the temperature modification of TNF-α–induced cell death was then examined using chemical inhibitors of p38 (SP202190), MEK1/2 (U0126), and JNK (SP600125). Neither MEK1/2 nor p38 inhibitors significantly altered the response of the MLE-15 cells to TNF-α and various incubation temperatures (Fig. 11). However, the JNK inhibitor significantly improved survival.
FIGURE 11.
JNK, but not other MAPKs, contributes to FRH-augmented cell death by TNF-α. Chemical inhibitors of the MAPKs, MEK1/2 (U0126), JNK (SP600125), and p38 (SB202190) at 1 µM concentration were added to MLE-15 cells exposed to TNF-α 5 ng/ml and 34, 37, or 39.5°C. The MAPK inhibitors for MEK1/2 and p38 did not affect cell survival. The JNK inhibitor protected MLE-15 cells from exposure to TNF-α and 39.5°C. **p< 0.01; †p< 0.001.
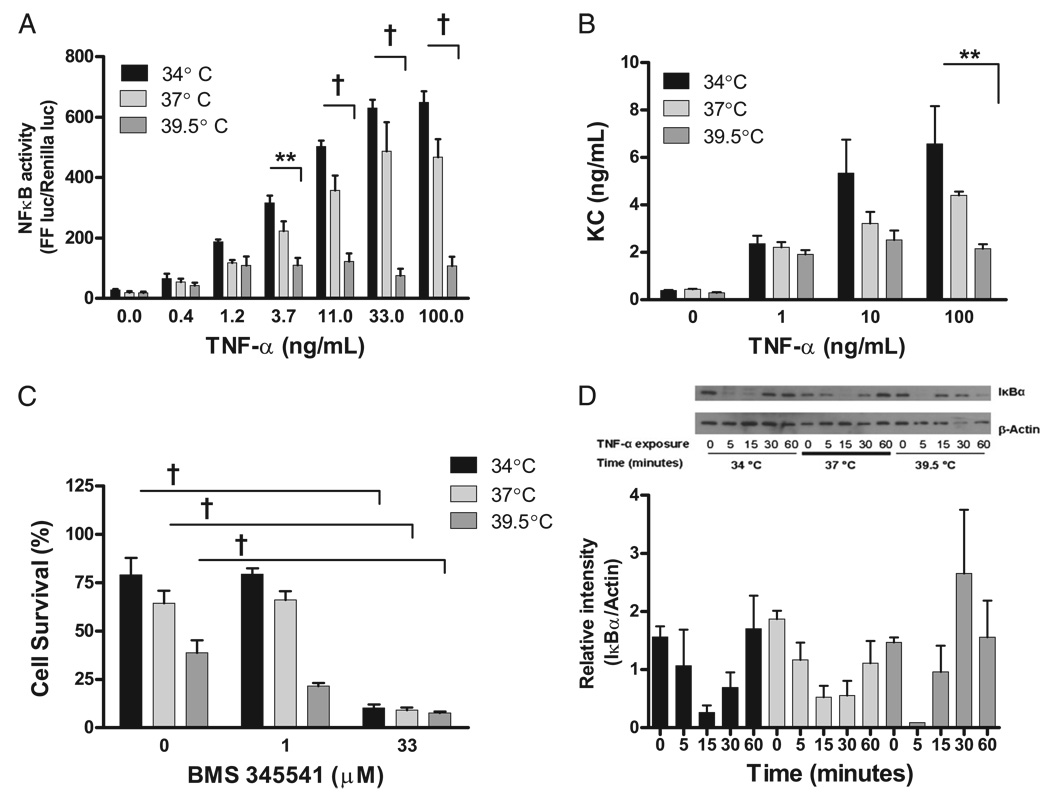
Finally, the role of NF-κB activity in the temperature dependence of TNF-α–induced cell death was examined using transient transfection of MLE-15 cells with an NF-κB–inducible firefly luciferase. The TNF-α–dependent NF-κB activity was enhanced by mild hypothermia and suppressed by hyperthermia (Fig. 12A). To confirm that temperature modified the NF-κB pathway, we measured KC production, a chemokine produced under control of NF-κB. KC production was assessed in the cells by RT-PCR and in cell-conditioned media by ELISA. The KC concentration in cell supernatants decreased as a function of incubation temperature at each concentration of TNF-α (Fig. 12B), and KC mRNA transcript copy number was also suppressed by increasing incubation temperature (data not shown). To evaluate whether changes in NF-κB activity modified cell survival after TNF-α exposure, the effect of an IκB-α kinase inhibitor (BMS 345541) on cell survival was evaluated using the alamar Blue assay. The IκB-α kinase inhibitor prevents the dissociation of IκB-α and NF-κB and therefore inhibits nuclear translocation and activity of NF-κB. The addition of an IκB-α kinase inhibitor (33 µM) enhanced TNF-α–mediated cell death at all temperatures (Fig. 12C). Therefore, when NF-κB activity is inhibited, cells exposed to TNF-α die regardless of incubation temperature. In the absence of TNF-α, the IκB-α kinase inhibitor did not affect MLE-15 cell viability (data not shown). Finally, the total IκB-α level in MLE-15 cells treated with TNF-α 100 ng/ml at 34, 37, or 39.5°C for 5, 15, 30, or 60 min without a proteasome inhibitor was evaluated by Western blot (Fig. 12D). Total IκB-α levels are typically inversely proportional to NF-κB nuclear translocation and activity. Interestingly, the total IκB-α levels in MLE-15 cells exposed to TNF-α were similar in all incubation temperatures. Therefore, hyperthermia enhances MLE-15 cell death after TNF-α exposure in part by reducing NF-κB activity. The mechanism of altered NF-κB activity appears independent of changes in IκB-α kinase activity or cellular concentrations of IκB-α.
FIGURE 12.
FRH suppresses NF-κB activity, which increases MLE-15 susceptibility to TNF-α–mediated death. A, MLE-15 cells were transiently dually transfected with an NF-κB–inducible firefly luciferase and a constitutively active renilla luciferase and then exposed to TNF-α at 34, 37, or 39.5°C. NF-κB activity in MLE-15 cells generated by TNF-α exposure is strongly suppressed as a function of increasing incubation temperature. B, KC production from MLE-15 cells after TNF-α exposure is suppressed by increasing incubation temperatures. C, The IκB-α kinase inhibitor BMS 345541 renders the MLE-15 cells at all incubation temperatures more sensitive to TNF-α–mediated cell death. In the absence of TNF-α, the IκB-α kinase inhibitor does not affect cell viability (data not shown). D, Total IκB-α levels in MLE-15 cells exposed to TNF-α 50 ng/ml fluctuate similarly in all temperature exposures. A representative Western blot is shown with densitometry from three Western blots for total IκB-α normalized for protein loading. **p< 0.01; †p< 0.001.
Discussion
The primary goal of this study was to evaluate the effect of FRH on LPS-induced lung injury and to define the mechanisms of FRH-enhanced injury. We hypothesized that FRH augmented lung injury by enhancing apoptotic and inflammatory signaling through the TNFR superfamily. We found that 24 h after LPS exposure, FRH enhanced alveolar permeability without affecting inflammatory cell responses. This was associated with a dramatic increase in TUNEL-positive cells in the alveolar epithelium, which indicates DNA fragmentation, suggesting apoptosis. Additionally, we observed that treatment with a broad caspase inhibitor attenuated FRH-augmented alveolar permeability induced with a sublethal dose of LPS. Interestingly, caspase inhibition with zVAD.fmk did not attenuate lung injury induced by FRH at a high dose of LPS (50 µg). We infer from these data that low-dose LPS activates apoptosis pathways in the alveolar epithelium in vivo, whereas the higher dose LPS combined with FRH results in activation of multiple injurious pathways, so that caspase inhibition alone is not protective in this circumstance. The ability of zVAD.fmk to suppress the alveolar permeability effects of FRH at a lower dose of LPS shows that caspase-dependent pathways are involved in FRH-augmented lung injury. After 48 h, the animals exposed to LPS and hyperthermia had enhanced inflammatory cell recruitment to the lungs as compared with animals exposed to LPS alone. Additionally, FRH increased the mortality associated with LPS-induced lung injury when a higher dose of LPS was used. Notably, in the absence of LPS, FRH did not affect measures of lung injury or mortality of mice.
In lung epithelial cell lines and in primary type II alveolar epithelial cells, we found that hyperthermia enhanced TNF-α–mediated apoptosis. The hyperthermia-enhanced TNF-α–mediated death occurs by a mechanism involving augmented caspase-8 signaling and reduced NF-κB activity. Interestingly, these findings were specific to the TNF-α receptors, as temperature-dependent modification of Fas-mediated death was not observed. These findings suggest that FRH and the innate immune system act synergistically to enhance lung injury and that increased alveolar epithelial apoptosis is one mechanism by which FRH causes enhanced tissue injury. The augmented apoptotic response in the alveolar epithelium may occur in part by FRH-mediated suppression of NF-κB activity, which sensitizes epithelial cells to TNF-α–mediated apoptosis.
Other groups also have shown that FRH augments lung injury in animal models, although the mechanisms that drive FRH-enhanced lung injury are not fully understood (11–14). Hasday et al. (12) examined the relationship between hyperthermia and ALI by comparing the severity of lung injury in hyperthermic versus euthermic mice exposed to hyperoxia, live bacteria, or exogenous bacterial products. In each model, the hyperthermic animals showed higher mortality and more severe lung injury than the euthermic animals. PMN recruitment was enhanced in hyper-thermic animals, and PMN recruitment continued even after the decline of MIP2a and KC in BAL fluid, leading to the speculation that increased PMN-induced inflammation and necrosis caused worsening lung injury. The cellular mechanisms by which FRH augmented total neutrophil recruitment and generated a delayed neutrophilic alveolitis were not clarified.
Suzuki et al. (13) and Akinci et al. (14) studied the effect of core body temperature on ventilator-induced lung injury. The latter group ventilated temperature-controlled rabbits (33, 37, or 41°C) with normal or high-pressure ventilation for 2 h (13). The results showed that in both an in vivo model and in an isolated-perfused lung model, the development of pathologic alveolar permeability as a result of an injurious ventilator strategy was significantly worse in hyperthermic rabbits. Changes in inflammatory cell accumulation in the BAL fluid were not evaluated. However, the development of increased edema in the isolated-perfused lung model at higher temperature suggests that augmented ventilator-induced lung injury associated with FRH is not entirely dependent on circulating leukocytes.
To integrate observations about FRH-enhanced alveolar permeability related to resident pulmonary cells and FRH-enhanced PMN recruitment, we hypothesized that members of the TNFR superfamily, such as TNF-α and Fas ligand, participate in FRH-enhanced lung injury via their proapoptotic and proinflammatory effects in the lungs. The TNFR family is central to the development of alveolar epithelial apoptosis and to neutrophilic alveolitis in ALI. Matsuda et al. (41) showed the importance of the TNFR superfamily signaling in experimental lung injury using an small interfering RNA approach to knock down Fas-associated death domain protein, a signaling protein required for caspase-8 recruitment to the death domain of the TNFR superfamily. This protected mice from the effects of cecal ligation and puncture, including lung injury, alveolitis, and death (41). Matute-Bello et al. (19–23) showed that activation of the Fas pathway in the lungs of mice causes ALI through a mechanism that is dependent upon alveolar epithelial apoptosis. Although TNF-α is known to have both proinflammatory and proapoptotic functions, data regarding the proinflammatory capacity of Fas are still accumulating. Fas ligand is capable of causing neutrophil infiltration independent of caspase-1 activity in a peritoneal exudate model (42), and the Fas pathway can also be proinflammatory in the lungs. The IT administration of Fas ligand or a Fas-activating Ab results in a neutrophilic alveolitis within 24 h postinstillation (18, 21, 23, 24, 27–29). The Fas receptor is also critical to the development of the delayed neutrophilic alveolitis after LPS exposure, as lpr mice, which lack a functioning Fas receptor, have a less robust delayed PMN recruitment after IT LPS compared with Fas-normal mice, and less tissue injury (25, 26). Thus, TNFR superfamily signaling in the alveolar epithelium generates both apoptotic and inflammatory responses that are critical to the development of lung injury.
Therefore, we hypothesized that FRH augments lung injury by enhancing TNFR family signaling in the alveolar epithelium, with increases in both inflammatory and apoptotic events. The exposure of animals to IT LPS and FRH yielded an increase in BAL concentrations of IgM and total protein at 24 h without increasing BAL inflammatory cells or tissue MPO activity. Animals exposed to LPS and hyperthermia developed microvascular congestion by 24 h that was not observed in euthermic animals. This may reflect FRH-enhanced thrombosis, as described by Hernandez-Espinoza et al. (43) in addition to FRH-enhanced lung injury. Our study did not address this issue directly. The TUNEL assay on lung sections revealed a large increase in the number of TUNEL-positive alveolar epithelial cells and caspase inhibition via administration of zVAD.fmk attenuated FRH-augmented alveolar permeability. This suggests that FRH enhanced TNFR superfamily signaling in the alveolar epithelium, which increased alveolar epithelial cell death and increased alveolar permeability. At 48 h, animals exposed to LPS and hyperthermia demonstrated an exaggerated neutrophilic alveolitis as compared with mice exposed to LPS alone. As TNFR family members are required for delayed neutrophil recruitment, the observed increases in the inflammatory response at 48 h might also reflect FRH-enhanced TNFR signaling. These data suggest that the pathogenesis of FRH-augmented lung injury includes not only enhanced PMN-associated injury, as described previously, but also FRH-enhanced alveolar epithelial apoptosis. These findings significantly extend prior work and provide new mechanistic insight into pathogenesis of FRH-augmented lung injury.
Interestingly, FRH is not directly cytotoxic. However, FRH has been associated with increased sensitivity of death receptors in lymphocytes as well as a number of malignant cell lines (33, 36, 37, 44–47). The mechanisms by which fever enhances apoptosis are cell-type dependent and include enhanced clustering of membrane receptors due to alterations in membrane fluidity, enhanced activity of various caspases, and alterations in intracellular proteins and kinases that regulate caspase activity. Kokura et al. (48) found that hyperthermia (42°C) induces TNF-α–mediated apoptosis in a human gastric cell line and suggested that this is related to suppression of NF-κB activity. NF-κB activity determines cellular sensitivity to apoptosis by TNF-α receptor activation (49–55). The ability of NF-κB to suppress apoptosis has been linked to the transcription of a number of antiapoptotic proteins, such as X-linked inhibitor of apoptosis protein and FLIP, as well as to suppression of TNF-α–mediated JNK activation (49, 53, 56–63). JNK-mediated apoptosis is not completely understood, but both the intrinsic (mitochondrial) and extrinsic (TNFR family-mediated) pathways have been implicated (64–66). Hence, NF-κB– deficient cells are particularly sensitive to TNF-α–mediated apoptosis, but inhibitors of JNK rescue these cells from TNF-α–mediated death (49, 52, 53, 57, 59). Moreover, chronic signaling via JNK, modeled by transfection of Chinese hamster ovary cells with a constitutively active MKK7-JNK fusion protein is sufficient to induce cell death without modification of NF-κB activity (67). JNK has been linked to cell death following stress signals, including heat shock, but has not previously been associated with modification of apoptosis by FRH (68, 69).
We examined the effect of FRH on the response of alveolar epithelial cells to two of the major representatives of the TNFR family, TNF-α and Fas. Interestingly, the receptors showed different responses to hyperthermia. Hyperthermia enhanced TNF-α–mediated death in two different cell lines and in primary type II alveolar epithelial cells, whereas FRH neither sensitized cells to Fas-mediated death nor enhanced Fas-mediated death in cells that are susceptible to Fas stimulation. This suggests that the effects of hyperthermia on cell death are conserved and are specific to the TNF-α receptor(s). We found that hyperthermia-enhanced cell death depends upon caspase signaling through the receptor-mediated pathway and that caspase-8 is more important than caspase-9. In addition, NF-κB activity is strongly suppressed in cells exposed to FRH and TNF-α. Through inhibitor studies, we demonstrated that increased JNK activity participates in enhanced cell death and that NF-κB activity is protective against hyperthermia-enhanced TNF-α–mediated cell death. However, FRH did not cause preservation of total IκB-α levels in cells exposed to TNF-α. Therefore, the suppression of NF-κB appears to be more complex than a simple alteration of the kinetics of IκB-α. We speculate that FRH-mediated suppression of NF-κB results in an alteration of the balance between pro- and antiapoptotic proteins and involves an increase in JNK activity, which favors apoptosis following TNF-α stimulation. The mechanism by which NF-κB activity is suppressed appears to occur after dissociation from IκB-α in the cytoplasm, as FRH does not markedly affect the cytoplasmic concentrations of IκB-α. The suppression of NF-κB has been linked with enhanced apoptosis in lymphocytes and other cell lines (50–52, 55). However, our data provide the first evidence that hyperthermia suppresses NF-κB activity in the alveolar epithelium and provide important new insights about the mechanisms of FRH-induced tissue damage. Interestingly, NF-κB activation and the resultant inflammatory response have been identified as central pathophysiologic mechanisms in critical illness-related end-organ damage (69). Suppression of NF-κB activation ameliorates systemic hypotension, disseminated intravascular coagulation, and other end-organ dysfunction caused with experimental sepsis and has been proposed as an intervention to improve outcomes from sepsis and septic shock (70, 71). Therefore, our data not only provide a new understanding of the mechanism of FRH-enhanced tissue damage, but also support a dual role for NF-κB in the pathogenesis as well as protection from critical illness-associated end-organ damage.
Our study has several limitations. First, more information is needed to determine whether the observed increase in TNF-α sensitivity in the lung epithelial cells is the mechanism of the increased alveolar epithelial apoptosis in our animal model. Moreover, alternative mechanisms of increased epithelial apoptosis, including alterations in proximal signaling patterns generated by fever, have not been explored in this model. Lastly, the animal model of FRH allows direct study of the effects of hyperthermia without the complicating cluster of cytokines that generate clinical fever. This system is necessarily simplified to evaluate the effects of fever and not pyrogens and therefore may overlook the effect of pyrogenic cytokines on the in vivo responses to hyperthermia.
In summary, the results show that FRH acts synergistically with innate immune system activation by LPS to enhance lung injury. In this model, FRH is associated with increased mortality and increased lung injury after LPS exposure. Hyperthermia causes an early increase in alveolar permeability, which is mediated by caspase-dependent epithelial apoptosis, and a later increase in neutrophil recruitment in LPS-treated mice. In lung epithelial cell lines, FRH enhances caspase-dependent cell death in response to TNF-α, but not Fas, stimulation. This is associated with suppression of NF-κB activity without significant alterations in IκB-α levels and is associated with increased JNK activity. We conclude that FRH-enhances lung injury by a mechanism involving increased TNFR family signaling that results in augmented alveolar epithelial apoptosis and that this is related to FRH-enhanced sensitivity of alveolar epithelial cells to TNF-α–mediated apoptosis.
Acknowledgments
We thank Dr. Jeffrey Hasday for help and scientific advice and Alex Farnand and Sucheol Gil for technical assistance.
This work was supported in part by National Institutes of Health Grants HL096348 (to A.B.L.), HL083044 (to G.M.-B.), and HL081764 (to T.R.M.), the Mills Endowed Fund Career Development Award (to A.B.L.), and the Medical Research Service of the Department of Veterans Affairs.
Abbreviations used in this paper
- ALI
acute lung injury
- BAL
bronchoalveolar lavage
- FRH
febrile-range hyperthermia
- IT
intratracheal
- MPO
myeloperoxidase
- PMN
polymorphonuclear cell
- TNFR
TNF-αreceptor
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Laupland KB, Shahpori R, Kirkpatrick AW, Ross T, Gregson DB, Stelfox HT. Occurrence and outcome of fever in critically ill adults. Crit. Care Med. 2008;36:1531–1535. doi: 10.1097/CCM.0b013e318170efd3. [DOI] [PubMed] [Google Scholar]
- 2.Peres Bota D, Lopes Ferreira F, Mélot C, Vincent JL. Body temperature alterations in the critically ill. Intensive Care Med. 2004;30:811–816. doi: 10.1007/s00134-004-2166-z. [DOI] [PubMed] [Google Scholar]
- 3.Circiumaru B, Baldock G, Cohen J. A prospective study of fever in the intensive care unit. Intensive Care Med. 1999;25:668–673. doi: 10.1007/s001340050928. [DOI] [PubMed] [Google Scholar]
- 4.Swenson BR, Hedrick TL, Popovsky K, Pruett TL, Sawyer RG. Is fever protective in surgical patients with bloodstream infection? J. Am. Coll. Surg. 2007;204:815–821. doi: 10.1016/j.jamcollsurg.2007.01.033. discussion 822-813. [DOI] [PubMed] [Google Scholar]
- 5.Kluger MJ, Vaughn LK. Fever and survival in rabbits infected with Pasteurella multocida. J. Physiol. 1978;282:243–251. doi: 10.1113/jphysiol.1978.sp012460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaughn LK, Bernheim HA, Kluger MJ. Fever in the lizard Dipsosaurus dorsalis. Nature. 1974;252:473–474. doi: 10.1038/252473a0. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein MP, Iannini PB, Stratton CW, Eickhoff TC. Spontaneous bacterial peritonitis. A review of 28 cases with emphasis on improved survival and factors influencing prognosis. Am. J. Med. 1978;64:592–598. doi: 10.1016/0002-9343(78)90578-8. [DOI] [PubMed] [Google Scholar]
- 8.Bryant RE, Hood AF, Hood CE, Koenig MG. Factors affecting mortality of gram-negative rod bacteremia. Arch. Intern. Med. 1971;127:120–128. [PubMed] [Google Scholar]
- 9.Barie PS, Hydo LJ, Eachempati SR. Causes and consequences of fever complicating critical surgical illness. Surg. Infect. (Larchmt) 2004;5:145–159. doi: 10.1089/sur.2004.5.145. [DOI] [PubMed] [Google Scholar]
- 10.Zeledon JI, McKelvey RL, Servilla KS, Hofinger D, Konstantinov KN, Kellie S, Sun Y, Massie LW, Hartshorne MF, Tzamaloukas AH. Glomerulonephritis causing acute renal failure during the course of bacterial infections. Histological varieties, potential pathogenetic pathways and treatment. Int. Urol. Nephrol. 2008;40:461–470. doi: 10.1007/s11255-007-9323-6. [DOI] [PubMed] [Google Scholar]
- 11.Rice P, Martin E, He JR, Frank M, DeTolla L, Hester L, O’Neill T, Manka C, Benjamin I, Nagarsekar A, et al. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. J. Immunol. 2005;174:3676–3685. doi: 10.4049/jimmunol.174.6.3676. [DOI] [PubMed] [Google Scholar]
- 12.Hasday JD, Garrison A, Singh IS, Standiford T, Ellis GS, Rao S, He JR, Rice P, Frank M, Goldblum SE, Viscardi RM. Febrile-range hyperthermia augments pulmonary neutrophil recruitment and amplifies pulmonary oxygen toxicity. Am. J. Pathol. 2003;162:2005–2017. doi: 10.1016/S0002-9440(10)64333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki S, Hotchkiss JR, Takahashi T, Olson D, Adams AB, Marini JJ. Effect of core body temperature on ventilator-induced lung injury. Crit. Care Med. 2004;32:144–149. doi: 10.1097/01.CCM.0000098857.14923.44. [DOI] [PubMed] [Google Scholar]
- 14.Akinci OI, Celik M, Mutlu GM, Martino JM, Tugrul S, Ozcan PE, Yilmazbayhan D, Yeldandi AV, Turkoz KH, Kiran B, et al. Effects of body temperature on ventilator-induced lung injury. J. Crit. Care. 2005;20:66–73. doi: 10.1016/j.jcrc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Zager RA, Altschuld R. Body temperature: an important determinant of severity of ischemic renal injury. Am. J. Physiol. 1986;251:F87–F93. doi: 10.1152/ajprenal.1986.251.1.F87. [DOI] [PubMed] [Google Scholar]
- 16.Martin TR, Hagimoto N, Nakamura M, Matute-Bello G. Apo-ptosis and epithelial injury in the lungs. Proc. Am. Thorac. Soc. 2005;2:214–220. doi: 10.1513/pats.200504-031AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s) Microsc. Res. Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Perl M, Lomas-Neira J, Chung CS, Ayala A. Epithelial cell apoptosis and neutrophil recruitment in acute lung injury-a unifying hypothesis? What we have learned from small interfering RNAs. Mol. Med. 2008;14:465–475. doi: 10.2119/2008-00011.Perl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matute-Bello G, Frevert CW, Liles WC, Nakamura M, Ruzinski JT, Ballman K, Wong VA, Vathanaprida C, Martin TR. Fas/Fas ligand system mediates epithelial injury, but not pulmonary host defenses, in response to inhaled bacteria. Infect. Immun. 2001;69:5768–5776. doi: 10.1128/IAI.69.9.5768-5776.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matute-Bello G, Lee JS, Liles WC, Frevert CW, Mongovin S, Wong V, Ballman K, Sutlief S, Martin TR. Fas-mediated acute lung injury requires fas expression on nonmyeloid cells of the lung. J. Immunol. 2005;175:4069–4075. doi: 10.4049/jimmunol.175.6.4069. [DOI] [PubMed] [Google Scholar]
- 21.Matute-Bello G, Liles WC, Frevert CW, Nakamura M, Ballman K, Vathanaprida C, Kiener PA, Martin TR. Recombinant human Fas ligand induces alveolar epithelial cell apoptosis and lung injury in rabbits. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L328–L335. doi: 10.1152/ajplung.2001.281.2.L328. [DOI] [PubMed] [Google Scholar]
- 22.Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS) J. Immunol. 1999;163:2217–2225. [PubMed] [Google Scholar]
- 23.Matute-Bello G, Winn RK, Jonas M, Chi EY, Martin TR, Liles WC. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am. J. Pathol. 2001;158:153–161. doi: 10.1016/S0002-9440(10)63953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wortinger MA, Foley JW, Larocque P, Witcher DR, Lahn M, Jakubowski JA, Glasebrook A, Song HY. Fas ligand-induced murine pulmonary inflammation is reduced by a stable decoy receptor 3 analogue. Immunology. 2003;110:225–233. doi: 10.1046/j.1365-2567.2003.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matute-Bello G, Winn RK, Martin TR, Liles WC. Sustained lipopolysaccharide-induced lung inflammation in mice is attenuated by functional deficiency of the Fas/Fas ligand system. Clin. Diagn. Lab. Immunol. 2004;11:358–361. doi: 10.1128/CDLI.11.2.358-361.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matute-Bello G, Liles WC, Frevert CW, Dhanireddy S, Ballman K, Wong V, Green RR, Song HY, Witcher DR, Jakubowski JA, Martin TR. Blockade of the Fas/FasL system improves pneumococcal clearance from the lungs without preventing dissemination of bacteria to the spleen. J. Infect. Dis. 2005;191:596–606. doi: 10.1086/427261. [DOI] [PubMed] [Google Scholar]
- 27.Neff TA, Guo RF, Neff SB, Sarma JV, Speyer CL, Gao H, Bernacki KD, Huber-Lang M, McGuire S, Hoesel LM, et al. Relationship of acute lung inflammatory injury to Fas/FasL system. Am. J. Pathol. 2005;166:685–694. doi: 10.1016/S0002-9440(10)62290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perl M, Chung CS, Perl U, Lomas-Neira J, de Paepe M, Cioffi WG, Ayala A. Fas-induced pulmonary apoptosis and inflammation during indirect acute lung injury. Am. J. Respir. Crit. Care Med. 2007;176:591–601. doi: 10.1164/rccm.200611-1743OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of Fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am. J. Pathol. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An S, Hishikawa Y, Liu J, Koji T. Lung injury after ischemia-reperfusion of small intestine in rats involves apoptosis of type II alveolar epithelial cells mediated by TNF-alpha and activation of Bid pathway. Apoptosis. 2007;12:1989–2001. doi: 10.1007/s10495-007-0125-1. [DOI] [PubMed] [Google Scholar]
- 31.Lee KS, Choi YH, Kim YS, Baik SH, Oh YJ, Sheen SS, Park JH, Hwang SC, Park KJ. Evaluation of bronchoalveolar lavage fluid from ARDS patients with regard to apoptosis. Respir. Med. 2008;102:464–469. doi: 10.1016/j.rmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Bem RA, Bos AP, Wösten-van Asperen RM, Bruijn M, Lutter R, Sprick MR, van Woensel JB. Potential role of soluble TRAIL in epithelial injury in children with severe RSV infection. Am. J. Respir. Cell Mol. Biol. 2009 doi: 10.1165/rcmb.2009-0100OC. In press. [DOI] [PubMed] [Google Scholar]
- 33.Tran SE, Meinander A, Holmström TH, Rivero-Müller A, Heiskanen KM, Linnau EK, Courtney MJ, Mosser DD, Sistonen L, Eriksson JE. Heat stress downregulates FLIP and sensitizes cells to Fas receptor-mediated apoptosis. Cell Death Differ. 2003;10:1137–1147. doi: 10.1038/sj.cdd.4401278. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe N, Niitsu Y, Umeno H, Sone H, Neda H, Yamauchi N, Maeda M, Urushizaki I. Synergistic cytotoxic and antitumor effects of re-combinant human tumor necrosis factor and hyperthermia. Cancer Res. 1988;48:650–653. [PubMed] [Google Scholar]
- 35.Yamauchi N, Watanabe N, Maeda M, Okamoto T, Sasaki H, Tsuji N, Tsuji Y, Umeno H, Akiyama S, Niitsu Y. Mechanism of synergistic cytotoxic effect between tumor necrosis factor and hyperthermia. Jpn. J. Cancer Res. 1992;83:540–545. doi: 10.1111/j.1349-7006.1992.tb01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo J, Kim HR, Lee YJ. Hyperthermia enhances tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human cancer cells. Int. J. Hyperthermia. 2006;22:713–728. doi: 10.1080/02656730601074052. [DOI] [PubMed] [Google Scholar]
- 37.Moulin M, Carpentier S, Levade T, Arrigo AP. Potential roles of membrane fluidity and ceramide in hyperthermia and alcohol stimulation of TRAIL apoptosis. Apoptosis. 2007;12:1703–1720. doi: 10.1007/s10495-007-0096-2. [DOI] [PubMed] [Google Scholar]
- 38.Bem RA, Farnand AW, Wong V, Koski A, Rosenfeld ME, van Rooijen N, Frevert CW, Martin TR, Matute-Bello G. Depletion of resident alveolar macrophages does not prevent Fas-mediated lung injury in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:L314–L325. doi: 10.1152/ajplung.00210.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 1996;14:309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Matsuda N, Yamamoto S, Takano K, Kageyama S, Kurobe Y, Yoshihara Y, Takano Y, Hattori Y. Silencing of fas-associated death domain protects mice from septic lung inflammation and apoptosis. Am. J. Respir. Crit. Care Med. 2009;179:806–815. doi: 10.1164/rccm.200804-534OC. [DOI] [PubMed] [Google Scholar]
- 42.Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat. Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 43.Hernández-Espinosa D, Mota R, Miñano A, Ordóñez A, Yélamos J, Vicente V, Corral J. In vivo effects of hyperthermia on the functional and conformational characteristics of antithrombin. J. Thromb. Haemost. 2007;5:963–970. doi: 10.1111/j.1538-7836.2007.02479.x. [DOI] [PubMed] [Google Scholar]
- 44.Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 45.Klostergaard J, Barta M, Tomasovic SP. Hyperthermic modulation of tumor necrosis factor-dependent monocyte/macrophage tumor cytotoxicity in vitro. J. Biol. Response Mod. 1989;8:262–277. [PubMed] [Google Scholar]
- 46.Klostergaard J, Leroux ME, Auzenne E, Khodadadian M, Spohn W, Wu JY, Donato NJ. Hyperthermia engages the intrinsic apoptotic pathway by enhancing upstream caspase activation to overcome apoptotic resistance in MCF-7 breast adenocarcinoma cells. J. Cell. Biochem. 2006;98:356–369. doi: 10.1002/jcb.20729. [DOI] [PubMed] [Google Scholar]
- 47.Cippitelli M, Fionda C, Di Bona D, Piccoli M, Frati L, Santoni A. Hyperthermia enhances CD95-ligand gene expression in T lymphocytes. J. Immunol. 2005;174:223–232. doi: 10.4049/jimmunol.174.1.223. [DOI] [PubMed] [Google Scholar]
- 48.Kokura S, Yoshida N, Ueda M, Imamoto E, Ishikawa T, Takagi T, Naito Y, Okanoue T, Yoshikawa T. Hyperthermia enhances tumor necrosis factor alpha-induced apoptosis of a human gastric cancer cell line. Cancer Lett. 2003;201:89–96. doi: 10.1016/s0304-3835(03)00463-4. [DOI] [PubMed] [Google Scholar]
- 49.Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 50.Kucharczak J, Simmons MJ, Fan Y, Gélinas C. To be, or not to be: NF-kappaB is the answer—role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22:8961–8982. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- 51.Bubici C, Papa S, Pham CG, Zazzeroni F, Franzoso G. NF-kappaB and JNK: an intricate affair. Cell Cycle. 2004;3:1524–1529. doi: 10.4161/cc.3.12.1321. [DOI] [PubMed] [Google Scholar]
- 52.De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 53.Franzoso G, Zazzeroni F, Papa S. JNK: a killer on a transcriptional leash. Cell Death Differ. 2003;10:13–15. doi: 10.1038/sj.cdd.4401154. [DOI] [PubMed] [Google Scholar]
- 54.Javelaud D, Besançon F. NF-kappa B activation results in rapid inactivation of JNK in TNF alpha-treated Ewing sarcoma cells: a mechanism for the anti-apoptotic effect of NF-kappa B. Oncogene. 2001;20:4365–4372. doi: 10.1038/sj.onc.1204570. [DOI] [PubMed] [Google Scholar]
- 55.Stathopoulos GT, Sherrill TP, Cheng DS, Scoggins RM, Han W, Polosukhin VV, Connelly L, Yull FE, Fingleton B, Blackwell TS. Epithelial NF-kappaB activation promotes urethane-induced lung carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:18514–18519. doi: 10.1073/pnas.0705316104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 57.Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 58.Kanetaka Y, Hayashida M, Hoshika A, Yanase N, Mizuguchi J. Interferon-alpha induces transient upregulation of c-FLIP through NF-kappaB activation. Exp. Cell Res. 2008;314:246–254. doi: 10.1016/j.yexcr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Lamb JA, Ventura JJ, Hess P, Flavell RA, Davis RJ. JunD mediates survival signaling by the JNK signal transduction pathway. Mol. Cell. 2003;11:1479–1489. doi: 10.1016/s1097-2765(03)00203-x. [DOI] [PubMed] [Google Scholar]
- 60.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 61.Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat. Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 62.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 63.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 64.Abreu-Martin MT, Palladino AA, Faris M, Carramanzana NM, Nel AE, Targan SR. Fas activates the JNK pathway in human colonic epithelial cells: lack of a direct role in apoptosis. Am. J. Physiol. 1999;276:G599–G605. doi: 10.1152/ajpgi.1999.276.3.G599. [DOI] [PubMed] [Google Scholar]
- 65.Faris M, Kokot N, Latinis K, Kasibhatla S, Green DR, Koretzky GA, Nel A. The c-Jun N-terminal kinase cascade plays a role in stress-induced apoptosis in Jurkat cells by up-regulating Fas ligand expression. J. Immunol. 1998;160:134–144. [PubMed] [Google Scholar]
- 66.Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 67.Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol. Cell. Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zanke BW, Boudreau K, Rubie E, Winnett E, Tibbles LA, Zon L, Kyriakis J, Liu FF, Woodgett JR. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr. Biol. 1996;6:606–613. doi: 10.1016/s0960-9822(02)00547-x. [DOI] [PubMed] [Google Scholar]
- 69.Gaitanaki C, Mastri M, Aggeli IK, Beis I. Differential roles of p38-MAPK and JNKs in mediating early protection or apoptosis in the hyper-thermic perfused amphibian heart. J. Exp. Biol. 2008;211:2524–2532. doi: 10.1242/jeb.018960. [DOI] [PubMed] [Google Scholar]
- 70.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 71.Ding J, Song D, Ye X, Liu SF. A pivotal role of endothelial-specific NF-kappaB signaling in the pathogenesis of septic shock and septic vascular dysfunction. J. Immunol. 2009;183:4031–4038. doi: 10.4049/jimmunol.0900105. [DOI] [PMC free article] [PubMed] [Google Scholar]