Abstract
Adipose tissue-derived stem cells (ADSC) are routinely isolated from the stromal vascular fraction (SVF) of homogenized adipose tissue. Freshly isolated ADSC display surface markers that differ from those of cultured ADSC, but both cell preparations are capable of multipotential differentiation. Recent studies have inferred that these progenitors may reside in a perivascular location where they appeared to coexpress CD34 and smooth muscle actin (α-SMA) but not CD31. However, these studies provided only limited histological evidence to support such assertions. In the present study, we employed immunohistochemistry and immunofluorescence to define more precisely the location of ADSC within human adipose tissue. Our results show that α-SMA and CD31 localized within smooth muscle and endothelial cells, respectively, in all blood vessels examined. CD34 localized to both the intima (endothelium) and adventitia neither of which expressed α-SMA. The niche marker Wnt5a was confined exclusively to the vascular wall within mural smooth muscle cells. Surprisingly, the widely accepted mesenchymal stem cell marker STRO-1 was expressed exclusively in the endothelium of capillaries and arterioles but not in the endothelium of arteries. The embryonic stem cell marker SSEA1 localized to a pericytic location in capillaries and in certain smooth muscle cells of arterioles. Cells expressing the embryonic stem cell markers telomerase and OCT4 were rare and observed only in capillaries. Based on these findings and evidence gathered from the existing literature, we propose that ADSC are vascular precursor (stem) cells at various stages of differentiation. In their native tissue, ADSC at early stages of differentiation can differentiate into tissue-specific cells such as adipocytes. Isolated, ADSC can be induced to differentiate into additional cell types such as osteoblasts and chondrocytes.
Introduction
Isolated from the SVF of adipose tissue, adipose tissue-derived stem cells (ADSC) bear a strong resemblance to bone marrow stem cells (BMSC) as demonstrated by their expression of common cell surface markers, their similar gene expression profiles, and their similar differentiation potentials [1–3]. Unlike BMSC, however, ADSC can be obtained in large quantities at low risks [4]. In addition to being more abundant and easily accessible, the adipose tissue yields far more stem cells than bone marrow on a per gram basis (5,000 vs. 100–1,000) [5]. Therefore, it is reasonable to expect that ADSC will become the preferred choice of adult stem cells for future clinical applications.
Despite the importance of ADSC and the publication of more than 200 articles on their characterization, the cellular origin of ADSC within adipose tissue remains unknown. Recently, Yamamoto et al. [6] used immunofluorescence (IF) staining of mouse adipose tissue to identify cells expressing CD90, CD105, Sca-1, and/or p75NTR. The results showed widespread distribution of each of these markers suggesting that they are not specific for ADSC. In another recent study, Zannettino et al. [7] attempted to identify ADSC in human adipose tissue by employing IF staining for cellular markers 1A6.12, 1B5, STRO-1, CD146, and 3G5. Although these markers were detected in two large blood vessels of unknown identity (arteries or veins?), their location in the adipose tissue cannot be inferred due to the lack of adipocytes or any other landmarks in the neighborhood of these two blood vessels. Furthermore, the study did not examine the small vessels (arterioles, venules, or capillaries) in adipose tissue although the authors did acknowledge that mesenchymal stem cells (MSC), such as ADSC, likely reside in specialized niches within the microvascular networks.
Several lines of evidence indicate that the vascular network plays a critical role in the development and expansion of adipose tissue. First, during embryonic development, the formation of capillary convolutions is a decisive and specific phase in the development of fat lobules [8]. Second, extensive vascularization is necessary for the optimal function of adipose tissue as a metabolic and endocrine organ [9]. Third, cells of adipose lineage have been shown to secrete potent angiogenic factors [10–13]. Finally, antiangiogenic agents promote adipose tissue loss thus underlining the importance of angiogenesis for maintaining adipogenesis [14].
Several lines of evidence suggest that ADSC are vascular precursor cells. First, several studies have shown that SVF contains progenitor cells that are able to differentiate into endothelial cells and participate in blood vessel formation [15–19]. Second, a recent study demonstrated that SVF cells expressing both pericyte and mesenchymal markers reside in a periendothelial location and stabilize endothelial networks [20]. Finally, another recent study showed that ADSC transplanted into ischemic cortex preferentially migrate toward microvessels where they differentiate into vascular smooth muscle cells [21].
In the present study, we identified cells that express markers of vascular smooth muscle, endothelia, and stem cells in human adipose tissues, particularly in the adipose vasculature. The results show that ADSC are likely vascular stem cells (VSC) at various stages of differentiation toward becoming vascular smooth muscle and endothelial cells.
Materials and Methods
Human adipose tissues
A total of four adipose tissue samples were obtained from patients during routine abdominoplasty following informed patient consent and according to the guidelines set by our institution's Committee on Human Research. Whole intact portions of adipose tissue and associated skin were transported in sterile saline and immediately processed for cell culture or in a fixative and subsequently processed for histological examination.
Cell isolation and culture
The procedure of ADSC isolation has been described previously [22]. Briefly, the adipose tissue was rinsed with PBS containing 1% penicillin and streptomycin, minced into small pieces, and then incubated in a solution containing 0.075% collagenase type IA (Sigma-Aldrich, St. Louis, MO) for 1 h at 37°C with vigorous shake. The top lipid layer was removed and the remaining liquid portion was centrifuged at 220g for 10 min at room temperature. The pellet was treated with 160 mM NH4Cl for 10 min to lyse red blood cells. The remaining cells were suspended in DMEM supplemented with 10% fetal bovine serum (FBS), filtered through a 40-μm cell strainer (BD Biosciences, Bedford, MA), and plated at a density of 1 × 106 cells in a 10-cm dish. After reaching 80% confluence, the cells were harvested and stored in liquid nitrogen at a density of 5 × 105 cells per ml of freezing media (DMEM, 20% FBS, and 10% DMSO). The frozen cells were thawed for experiments as needed.
Flow cytometry
Freshly isolated ADSC were analyzed by flow cytometry for cell surface and intracellular antigen expression. The cells were incubated with primary antibody (Table 1) in 50 μL wash buffer (PBS containing 1% FBS and 0.1% Na3N) for 30 min on ice followed by another incubation with FITC-conjugated secondary antibody. The cells were then rinsed twice with wash buffer, fixed with 1% paraformaldehyde in PBS, and analyzed by a fluorescence-activated cell sorter (FACSVantage SE System, BD Biosciences, San Jose, CA). The raw data were further analyzed with FlowJo software (Tree Star, Inc., Ashland, OR).
Table 1.
Antibodies Used in this Study
| Target protein | Supplier | Catalog No. |
|---|---|---|
| CD34 | Santa Cruz Biotech, Santa Cruz, CA, USA | sc-7324 |
| SSEA1 | Abcam Inc, Cambridge, MA, USA | ab16285 |
| OCT4 | Abcam Inc, Cambridge, MA, USA | ab19857 |
| CD31 | Chemicon, Temecula, CA, USA | CBL468 |
| Wnt5a | Abcam Inc, Cambridge, MA, USA | ab50632 |
| Telomerase | Abcam Inc, Cambridge, MA, USA | ab32020 |
| α-SMA | Sigma-Aldrich, St. Louis, MO, USA | A-5228 |
| STRO-1 | Invitrogen, Carlsbad, CA, USA | 398401 |
| CD140b | Abcam Inc, Cambridge, MA, USA | ab32570 |
Immunohistochemical and immunofluorescence staining
Tissue samples were fixed in cold 2% formaldehyde and 0.002% saturated picric acid in 0.1 M phosphate buffer, pH 8.0, for 4 h followed by overnight immersion in buffer containing 30% sucrose. The specimens were then embedded in OCT Compound (Sakura Finetic USA, Torrance, CA) and stored at −70°C until use. Fixed frozen tissue specimens were cut at 10 microns, mounted onto SuperFrost-Plus charged slides (Fisher Scientific, Pittsburgh, PA), and air dried for 5 min. The slides were then placed in 0.3% H2O2/methanol for 10 min, washed twice in PBS for 5 min and incubated with 3% horse serum in PBS/0.3% Triton X-100 for 30 min at room temperature. After draining this solution from the tissue section, the slides were incubated overnight at 4°C with primary antibodies (Table 1). Control tissue sections were similarly prepared except that no primary antibody was added. Staining of the tissue was performed with the Elite ABC kit (Vector Labs, Burlingame, CA) followed by hematoxylin counterstain. For image analysis, five randomly selected fields per tissue were photographed and recorded using a Retiga Q Image digital still camera and ACT-1 software (Nikon Instruments Inc., Melville, NY). For immunofluorensence staining, the tissue was incubated with primary antibody (Table 1) followed by secondary antibody conjugated with FITC or Texas Red (Vector Labs, Burlingame, CA). Nuclear staining was performed with 4′,6-diamidino-2-phenylindole (DAPI). Stained tissues were examined by fluorescence microscopy and confocal microscopy. Subsequent image analysis was done as described above.
Results
Abdominal subcutaneous adipose tissue samples were obtained from four patients. They were examined by immunohistochemistry (IH) and IF for the expression of α-smooth muscle actin (α-SMA), endothelial marker CD31, hematopoietic marker CD34, niche marker Wnt5a [23], and stem cell markers OCT4, SSEA1, telomerase, and STRO-1. Because ADSC are derived from the stromal vascular fraction (SVF) of adipose tissue, the localization of these cellular markers in the blood vessels is the focus of the presentation below. The first section will provide an overview of the distribution of these markers, while three subsequent sections will provide detailed analyses based on the sizes of blood vessels.
Distribution of vascular and stem cell markers
Consistent with their anatomical origin, all four tissue samples of this study were white adipose tissues composed of adipocytes and stroma (Fig. 1). The adipocytes measured 40 to 80 μm in diameter and the stroma as small as having a single capillary (∼10 μm in diameter) or as big as having bundles of blood vessels (∼100 μm in diameter). The adipocytes were not stained for any of the cellular markers used in this study, whereas the stroma were heterogeneously stained. Specifically, α-SMA was detected in the smooth muscle of all small blood vessels, arterioles, and venules, and both CD31 and CD34 were positively identified in the endothelium of the same blood vessels (Fig. 1). However, although CD31 was restricted to the endothelium, CD34 was more widely distributed. SSEA1 staining was observed in some smooth muscle cells of some blood vessels but not in endothelial cells (Fig. 1). Wnt5a staining largely followed that of α-SMA (Figs 2–4). STRO-1 expression was strictly endothelial and only in certain blood vessels (Figs 2–4). Positive staining for OCT4 or telomerase was rarely detected; the few positively stained cells were either smooth muscle or some unknown cell types but not endothelial cells (Figs 2–4).
FIG. 1.
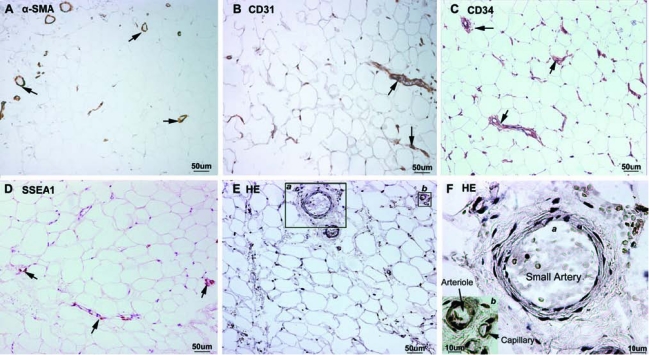
Immunohistochemical localization of vascular and stem cell markers in the adipose tissue. Human adipose tissues were examined by immnuohistochemistry for the expression of α-SMA (A), CD31 (B), CD34 (C), and SSEA1 (D). Positively stained structures are marked with arrows. Control specimen stained with HE (without antibody) is shown in (E), and two areas of which (a and b) are enlarged and displayed in (F). The small artery, the arteriole, and the capillary are approximately 70, 25, and 10 μm in diameter, respectively.
FIG. 2.
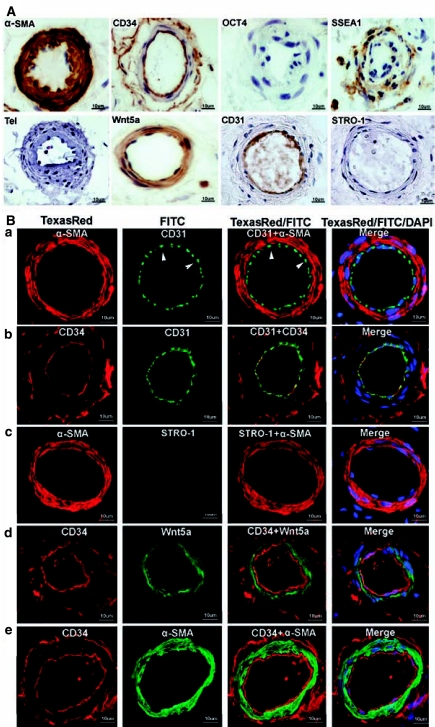
Immunohistochemical and immunofluorescence localization of vascular and stem cell markers in small arteries of adipose tissue. Human adipose tissues were immunostained for the indicated markers and a small artery (∼70 μm in diameter) from each stained specimen was chosen for display. (A) Note the absence of staining for OCT4, telomerase, and STRO-1. Also note the double-ring staining pattern of CD34 (intima and adventitia), as opposed to the single-ring staining of CD31 (intima only). (B) Each row (a, b, c, d, and e) displays a selected artery, while the columns are arranged as follows: From left to right, columns 1 and 2 show images of arteries stained for the indicated markers. Column 3 shows merged images of columns 1 and 2. Column 4 shows merged images of columns 1 and 2 plus an image of DAPI (nuclear) staining. Arrowheads indicate the punctuated staining pattern of CD31.
FIG. 4.
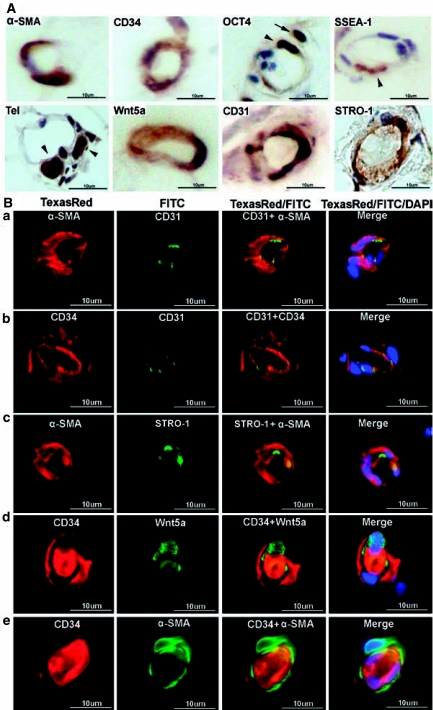
Immunohistochemical and immunofluorescence localization of vascular and stem cell markers in capillaries of adipose tissue. Human adipose tissues were immunostained for the indicated markers and a capillary in each stained specimen was chosen for display. In (A) the arrowhead and arrow in OCT4 point to two positively stained cells in the capillary's inner and outer regions, respectively. These cells are probably precursors of endothelial cells and pericytes, respectively. Cells positively stained for SSEA1 and telomerase are also indicated by arrowheads. In (B) each row (a, b, c, d, and e) displays a selected capillary, while the columns are arranged as follows: From left to right, columns 1 and 2 show images of capillaries stained for the indicated markers. Column 3 shows merged images of columns 1 and 2. Column 4 shows merged images of columns 1 and 2 plus an image of DAPI (nuclear) staining. The luminal materials are blood clots.
Small arteries and veins
Small blood vessels are defined in the present study as having a diameter of 75 to 150 μm. They were always positively stained for α-SMA and Wnt5a in the smooth muscle and for CD31 and CD34 in the endothelium (Fig. 2). Although CD31 and CD34 were both localized to the endothelium, their staining patterns were different. Although CD34 staining appeared homogeneous and contiguous throughout the entire endothelium, CD31 staining was mainly concentrated at cell-cell junctions. Furthermore, CD34 was localized to both the endothelium and adventitia thus giving the vessels a double-ring appearance. Both the cell junction staining and double-ring appearance have been reported previously (see Discussion).
In contrast to the above mentioned four markers, stem cell marker OCT4, telomerase, or STRO-1 were never found in arteries or veins (Fig. 2). On the other hand, the other stem cell marker, SSEA1, was often seen in smooth muscle although its sporadic and discrete staining pattern was different from the homogeneous staining pattern of α-SMA and Wnt5a (Fig. 2A). It appears that SSEA1 was expressed in not all but certain smooth muscle cells.
Arterioles and venules
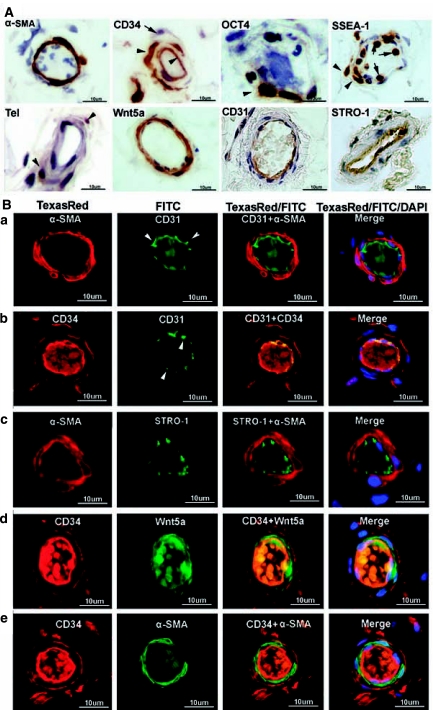
Arterioles and venules are defined as having a diameter of 25 to 75 μm. Similar to above mentioned small vessels, arterioles and venules were always positively stained for α-SMA and Wnt5a in the smooth muscle and for CD31 and CD34 in the endothelium (Fig. 3). Positive CD34 staining in the adventitia was still evident although some adventitia cells were not stained (Fig. 3). The sporadic staining pattern of SSEA1 in the smooth muscle continued to hold, while STRO-1 staining was now clearly visible (Fig. 3). Surprisingly, the STRO-1 staining occurred specifically in the endothelium (Fig. 3) and its punctuated pattern was reminiscent of CD31 (Fig. 3B). OCT4 staining was detected only once in the nuclei of two cells of unknown identity (Fig. 3A). Telomerase staining was faintly visible in only one cell of one blood vessel (Fig. 3A).
FIG. 3.
Immunohistochemical and immunofluorescence localization of vascular and stem cell markers in arterioles of adipose tissue. Human adipose tissues were immunostained for the indicated markers and an arteriole (∼25 μm in diameter) from each stained specimen was chosen for display. (A) The two arrowheads in CD34 point to positively stained cells in the endothelium and adventitia, respectively, while the lone arrow points to an unstained cell in the adventitia. The arrowheads in SSEA1 point to two positively stained cells in the muscle layer, while the arrows point to two white blood cells. The arrowheads in telomerase point to two positively stained cells. Also note the specific STRO-1 staining in the endothelium possibly of a venule. (B) Each row (a, b, c, d, and e) displays a selected arteriole, while the columns are arranged as follows: From left to right, columns 1 and 2 show images of arteries stained for the indicated markers. Column 3 shows merged images of columns 1 and 2. Column 4 shows merged images of columns 1 and 2 plus an image of DAPI (nuclear) staining. Arrowheads indicate the punctuated staining pattern of CD31. The luminal materials are blood clots.
Capillaries
Capillaries are approximately 10 μm in diameter and their lumen is encircled by 1 to 3 endothelial cells as seen in cross sections. Despite lacking smooth muscle, capillaries were stained positive for α-SMA (Fig. 4) suggesting the presence of pericytes. Similar to the situations with arteries and arterioles in which smooth muscle cells were always positively stained for α-SMA and Wnt5a (Figs 2 and 3), capillary cells (possibly pericytes) that were positively stained for α-SMA were also stained positive for Wnt5a (Fig. 4B). Likewise, capillary endothelial cells were always stained positively for CD31 and CD34 (Fig. 4). However, cells stained positive for α-SMA and Wnt5a were stained negative for CD34 (Fig. 4B) suggesting that the probable pericytes are CD34−. There were nevertheless CD34+ cells outside the endothelium as they were stained negative for CD31 (Fig. 4B). To more precisely locate pericytes, we stained adipose tissue with an antibody against pericyte marker CD104/PDGF-Rβ, and the results show that this marker did not colocalize with CD34 (Fig. 5). Thus it does not appear that CD34+ cells of the adipose vasculature are pericytes (see Discussion for more details).
FIG. 5.
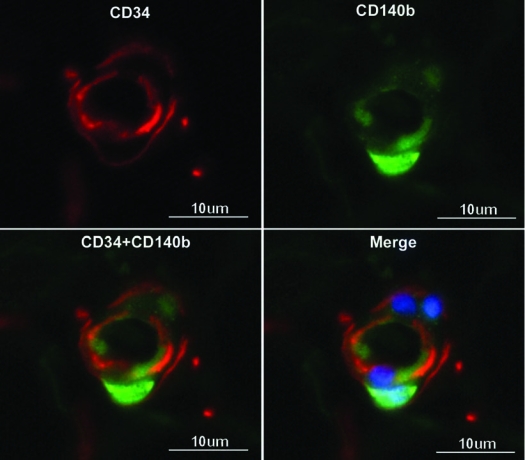
Immunofluorescence localization of pericytes in capillaries of adipose tissue. Human adipose tissues were immunostained for pericyte marker CD140b and CD34. Cell nucleus was stained with DAPI.
Cells stained positive for OCT4 were still rare and their locations suggest both smooth muscle and endothelial cell lineages (Fig. 4A). Cells stained positive for telomerase were also rare and their morphology and locations suggest their being undifferentiated cells. Although STRO-1 staining continued to be primarily endothelial, overlaps with α-SMA staining became evident (Fig. 4B), suggesting the existence of cells bearing both smooth muscle and endothelial identities.
Expression of stem cell markers in primary ADSC
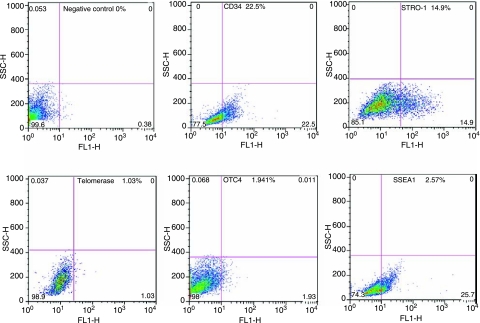
The expression of stem cell markers OCT4, telomerase, SSEA1, and STRO-1 were further analyzed by flow cytometry in primary ADSC, i.e., freshly isolated SVF cells that were allowed to attach to the plastic culture dish only once. In these analyses, the expression of CD34 was also determined and used for comparison. The results showed that CD34 was expressed in 22.5% of cells, STRO-1 14.9%, telomerase 1.03%, OCT4 1.941%, and SSEA1 25.7% (Fig. 6).
FIG. 6.
Cytometric analysis of primary ADSC. SVF cells were isolated from human adipose tissue, allowed to attach to a plastic culture dish, and then harvested for flow cytometric analysis for the indicated markers. The results are presented next to the markers as a percent of positively stained cells.
Discussion
Although there is ample evidence that ADSC are bona fide adult stem cells, the location of these cells in adipose tissue remained unknown. Recently, Yamamoto et al. [6] used IF staining on mouse adipose tissue to locate cells positive for CD90, CD105, Sca-1, and/or p75NTR. Their results showed widespread distribution of each of these four markers, thus casting doubts on the suitability of these markers for the identification of ADSC. Another study by Zannettino et al. [7] attempted to identify ADSC in human adipose tissue by employing IF staining for cellular markers 1A6.12, 1B5, STRO-1, CD146, and 3G5. In the histology images presented, each of the two large blood vessels was shown without any reference landmarks, thus making it impossible to know their relationship with the rest of the adipose tissue. Furthermore, although the images show that all of the tested markers localized to the vessel wall, they do not display sufficient details as to tell whether the markers are located in the intima or in the adventitia. Thus, this latter study does not provide us a better understanding of the identity of ADSC within adipose tissue.
Another study [20], which was published online while this manuscript was in preparation, focused on CD34+ cells in adipose tissue. The results showed that CD34+ cells are widely distributed among adipocytes and predominantly associated with vascular structures. Although these observations appear to be similar to ours, their histological analyses show only the longitudinal aspect of two blood vessels of unknown sizes and thus are of limited usefulness in terms of delineating the cellular composition of the adipose vasculature. In spite of these limitations, the study further showed that the majority of the CD34+ cells expressed pericytic markers, and this appears to suggest that ADSC are CD34+ pericytes. Whether CD34+ cells are ADSC and whether ADSC are CD34+ will be discussed below.
As pointed out by Zannettino et al. [7], mesenchymal stem cells, including ADSC, likely reside in perivascular niches. The fact that ADSC are isolated from adipose SVF further points to a close relationship between ADSC and blood vessels. Thus, in the present study, we sought to locate ADSC by employing vascular smooth muscle marker α-SMA, endothelial marker CD31, hematopoietic marker CD34, niche marker Wnt5a, and stem cell markers OCT4, telomerase, SSEA1, and STRO-1. Because IH staining provides optimal histology whereas IF enables colocalization (with double or triple staining), both techniques were used throughout this study to localize these markers except for OCT4, telomerase, and SSEA1 whose antibodies produced poor IF staining.
Due to their specificity for smooth muscle and the endothelium, respectively, anti-α-SMA and anti-CD31 antibodies enabled us to visualize the distribution of blood vessels in the adipose tissue specimens. Anti-CD31 antibody was not expected to stain ADSC as it is generally agreed that ADSC lack CD31 expression [1–3]. Anti-α-SMA antibody clearly stained cells in capillaries (Fig. 4), thus implicating these cells as pericytes. However, these cells were invariably CD34−, suggesting that pericytes in the adipose vasculature do not express CD34 and thus contradicting the findings by Traktuev et al. [20]. Despite of this disagreement, our results do not exclude the possibility that ADSC are pericytes and vice versa.
Wnt signaling has been shown to regulate the self-renewal and differentiation of both hematopoietic and BMSC, and importantly Wnt5a has been localized to the bone marrow niche environment [23, 24]. In the present study, Wnt5a localized to the smooth muscle of small blood vessels, arterioles, and venules. It does not appear that Wnt5 was expressed in the endothelium, based on the lack of colocalization with CD34 (Fig. 2). In capillaries Wnt5a staining was still evident (Fig. 4), and which again was distinct from CD34 staining. Therefore, it appears that, similar to α-SMA, Wnt5a was expressed in smooth muscle cells of small vessels and in pericytes of capillaries, and neither cell types expressed CD34. This expression pattern suggests that neither vascular smooth muscle cells nor pericytes are ADSC but rather niche cells of ADSC. However, since “stem cell niche” is still a poorly defined biological entity, this Wnt-niche-ADSC hypothesis can only be substantiated in future studies. Interestingly, a recent study showed that recombinant Wnt5a protein could induce the differentiation of ADSC into beating cardiomyocyte colonies in a dose-dependent manner [25].
CD34 has long been regarded a reliable marker for hematopoiectic stem cells (HSC), but recent studies have demonstrated the existence of CD34-negative HSC and that the two populations of HSC (CD34+ and CD34−) can differentiate into one another [26]. Several papers, including ours, have shown that CD34 is highly expressed in freshly isolated ADSC (SVF cells), but its expression is quickly lost in cultured ADSC within the first few (<3) passages [1,2,22]. This loss of expression is probably due to downregulation of CD34 expression rather than death of CD34+ cells (unpublished observation). In any event, the abundance of CD34 expression in SVF cells can perhaps be explained by the abundance of CD34+ cells in adipose tissue, as reported both in the present study and in a recent study [20]. Although CD34 localized to the endothelium, its staining was homogeneous while that of CD31 was more intense at intercellular junctions (Figs 2–4). This differential staining of the endothelium by anti-CD31 and anti-CD34 antibodies has been previously observed in blood and lymphatic vessels [27–29]. In addition, CD34 staining was different from that of CD31 in that it was visible in the adventitia of all blood vessels; thus, the cross section of these CD34-stained vessels had the appearance of two concentric circles (endothelium and adventitia) sandwiching the unstained smooth muscle layer (Figs 2 and 3). A similar staining pattern in subcutaneous tissue has been previously reported [30].
Although the CD34+CD31+ inner circle of blood vessels is undoubtedly the endothelium, the CD34+CD31− outer circle is an entity of uncertain cellular identity. The outer circle, being outside of the muscle layer, is by definition the adventitia. Although the identification of CD34+CD31− cells in the adventitia of blood vessels in adipose tissue is a novel contribution by the present study, similar cells have in fact been observed previously in the aortic adventitia, and, interestingly, these cells have been shown to be vascular progenitor cells [31]. In regard to the CD34+CD31− cells in adipose tissue, two previous studies have shown that this particular cell population possess both endothelial and adipocytic differentiation potentials [16, 32]. With a focus on in vitro differentiation, these studies presented no data on the localization of the CD34+ CD31− cells in adipose tissue. More recently, as mentioned above, a recent study [20] showed that CD34+CD31− cells in adipose tissue are pericytes that also express α-SMA, and the authors went on to suggest that these CD34+CD31−α-SMA+ cells are ADSC. However, our staining data clearly showed that, while CD34+CD31− cells resided in the adventitia, the α-SMA+ cells were found exclusively in the media of arteries and arterioles (Figs 2 and 3). Thus, while we agree that ADSC are generally CD34+CD31−, we disagree that ADSC are CD34+CD31−α-SMA+. We also dispute the contention that pericytes are CD34+, as we clearly show that CD34 did not colocalize with CD140/PDGFRβ (Fig. 5). However, as pericytes remain an ill-defined entity, we cannot exclude the possibility that ADSC are certain types of pericytes.
OCT4 is a widely accepted marker of embryonic stem cells, but its expression in adult stem cells is less certain [33]. To our knowledge, only one paper has provided experimental data concerning OCT4 expression in ADSC and it showed that OCT4 was abundantly expressed in cultured ADSC [34]. Similar to this study, we performed RT-PCR and western blot analysis on OCT4 expression in human adipose tissue and in cultured human ADSC, but our results were different from the above study in that, while clearly detectable, OCT4 expression was low in all tested tissue and cell samples (data not shown). In the present study, histological and flow cytometric analyses again respectively showed that OCT4+ cells were rarely detectable in the adipose tissue and cultured ADSC. These results are consistent with the well-known fact that OCT4 is an embryonic transcription factor whose expression persists in only a limited number of cells in adult tissues [33].
Similar to OCT4, telomerase is better accepted as a stem cell marker for ESC than for adult stem cells [35]. In cultured ADSC, telomerase was reported to be present [36–39] or absent [40]. In adipose tissue, our present study appears to be the first to examine telomerase expression, and which was detectable in two capillary cells. Their location and morphology suggest they are undifferentiated cells.
SSEA1 is a marker for ESC and BMSC [41, 42]. Its expression was reported to be negative in cultured human ADSC by flow cytometric analysis [38]. However, in the present study, both cytometric and histological analyses showed that SSEA1 was expressed at rather high levels. In adipose tissue SSEA1 was expressed at a lower level than CD34 (Fig. 1), but in cultured ADSC of passage 0, it was expressed at a higher level than CD34 (25.7% vs. 22.5%, Fig. 6). This is most likely due to the rapid downregulation of CD34 in cultured ADSC.
STRO-1 is by far the best-known MSC marker [43] but whether or not it is expressed in ADSC is controversial [1]. Although it was reported to be negative by Gronthos et al. in 2001 [44], it was nevertheless used in the author's recent paper as an ADSC marker [7]. In our previous and present studies, we showed that STRO-1 was detectable both in cultured ADSC [22] and in adipose tissue. However, its expression pattern in adipose tissue was surprising—highly specific for endothelial cells in arterioles and capillaries but not in arteries. In capillaries, some of the positively stained cells were also stained positive for α-SMA (Fig. 4), suggesting the existence of vascular progenitor cells. Positive staining of endothelial cells by anti-STRO-1 antibody has been reported before, but other cell types were also positively stained [45]. One of the reasons why immunostaining with the STRO-1 antibody produced conflicting results is that, despite its widespread use in hundreds of published studies, this antibody remains an orphan with no known antigen. In our current research, we have found that the STRO-1 antibody produced rather complicated staining patterns in various tissues. Thus, prolonged efforts will be required to solve the problems.
Based on our experimental results and the above-discussed evidence, we believe that ADSC exist in adipose tissue as a mixed population of “vascular stem cells (VSC)”, as opposed to “fat stem cells”, which is casually but incorrectly used as a synonym for ADSC. This “vascular stem cell theory” suggests (1) ADSC and mesenchymal stem cells in general are VSC, (2) the differentiation potential of VSC is proportional to the angiogenic potential of the vasculature and ranges from pluri to multi to uni in a continuous rather than discrete fashion, (3) in addition to vascular smooth muscle and endothelial cells, VSC are capable of differentiating in situ into host tissue-specific cell types (e.g., adipocytes in adipose tissue), and (4) depending on its differentiation potential at the time of isolation from the host tissue, an individual VSC can be experimentally induced to differentiate into various cell types. The theory thus helps explain many perplexing issues in regard to ADSC's cellular properties, such as (1) why ADSC and BMSC are virtually identical, (2) why conflicting evidence persists in regard to ADSC surface marker expression and differentiation potential, and (3) why ADSC exhibit various differentiation potentials in a clonal fashion. Furthermore, by equating ADSC to VSC, the theory helps explain why the yield of ADSC is much greater than BMSC [5] as adipose tissue is known to be highly vascularized and have angiogenic properties [9,14,46]. Specifically, the extensive capillary network that surrounds each adipocyte [14] and the angiogenic (differentiation) potential are additional attributes of the adipose tissue, which has been known to possess many advantages over the other tissues (see Introduction), as an optimal source of adult stem cells. Thus, by examining the stem cell characteristics of adipose tissue and proposing the concept of “vascular stem cells”, the present study helps clarify several perplexing issues surrounding ADSC research and further elevates the status of ADSC as a legitimate and optimal stem cell source.
Conclusions
ADSC are likely VSC at various stages of differentiation toward becoming smooth muscle and endothelial cells. Those at earlier stages of differentiation can also differentiate into tissue-specific cells such as adipocytes in their native tissue, and when isolated, can be induced to differentiate into several additional cell types.
Acknowledgments
This work was supported by grants from the California Urology Foundation, Mr. Arthur Rock and the Rock Foundation, and the National Institutes of Health. We are grateful to Dr. Mary McGrath of University of California San Francisco for providing tissue specimens for this study.
References
- 1.Gimble JM. Katz AJ. Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helder MN. Knippenberg M. Klein-Nulend J. Wuisman PI. Stem cells from adipose tissue allow challenging new concepts for regenerative medicine. Tissue Eng. 2007;13:1799–1808. doi: 10.1089/ten.2006.0165. [DOI] [PubMed] [Google Scholar]
- 3.Schaffler A. Buchler C. Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 4.Housman TS. Lawrence N. Mellen BG. George MN. Filippo JS. Cerveny KA. DeMarco M. Feldman SR. Fleischer AB. The safety of liposuction: results of a national survey. Dermatol Surg. 2002;28:971–978. doi: 10.1046/j.1524-4725.2002.02081.x. [DOI] [PubMed] [Google Scholar]
- 5.Strem BM. Hicok KC. Zhu M. Wulur I. Alfonso Z. Schreiber RE. Fraser JK. Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto N. Akamatsu H. Hasegawa S. Yamada T. Nakata S. Ohkuma M. Miyachi E. Marunouchi T. Matsunaga K. Isolation of multipotent stem cells from mouse adipose tissue. J Dermatol Sci. 2007;48:43–52. doi: 10.1016/j.jdermsci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Zannettino AC. Paton S. Arthur A. Khor F. Itescu S. Gimble JM. Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 8.Wassermann P. The Development of Adipose Tissue. American Physiological Society; Washinton, DC: 1965. [Google Scholar]
- 9.Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Dobson DE. Kambe A. Block E. Dion T. Lu H. Castellot JJ., Jr. Spiegelman BM. 1-Butyryl-glycerol: a novel angiogenesis factor secreted by differentiating adipocytes. Cell. 1990;61:223–230. doi: 10.1016/0092-8674(90)90803-m. [DOI] [PubMed] [Google Scholar]
- 11.Claffey KP. Wilkison WO. Spiegelman BM. Vascular endothelial growth factor. Regulation by cell differentiation and activated second messenger pathways. J Biol Chem. 1992;267:16317–16322. [PubMed] [Google Scholar]
- 12.Bouloumie A. Drexler HC. Lafontan M. Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 13.Sierra-Honigmann MR. Nath AK. Murakami C. Garcia-Cardena G. Papapetropoulos A. Sessa WC. Madge LA. Schechner JS. Schwabb MB. Polverini PJ. Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 14.Rupnick MA. Panigrahy D. Zhang CY. Dallabrida SM. Lowell BB. Langer R. Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Planat-Benard V. Silvestre JS. Cousin B. Andre M. Nibbelink M. Tamarat R. Clergue M. Manneville C. Saillan-Barreau C. Duriez M. Tedgui A. Levy B. Penicaud L. Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 16.Miranville A. Heeschen C. Sengenes C. Curat CA. Busse R. Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y. Sun Z. Liao L. Meng Y. Han Q. Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 18.Wosnitza M. Hemmrich K. Groger A. Graber S. Pallua N. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation. 2007;75:12–23. doi: 10.1111/j.1432-0436.2006.00110.x. [DOI] [PubMed] [Google Scholar]
- 19.Grenier G. Scime A. Le Grand F. Asakura A. Perez-Iratxeta C. Andrade-Navarro MA. Labosky PA. Rudnicki MA. Resident endothelial precursors in muscle, adipose, and dermis contribute to postnatal vasculogenesis. Stem Cells. 2007;25:3101–3110. doi: 10.1634/stemcells.2006-0795. [DOI] [PubMed] [Google Scholar]
- 20.Traktuev DO. Merfeld-Clauss S. Li J. Kolonin M. Arap W. Pasqualini R. Johnstone BH. March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 21.Kubis N. Tomita Y. Tran-Dinh A. Planat-Benard V. Andre M. Karaszewski B. Waeckel L. Penicaud L. Silvestre JS. Casteilla L. Seylaz J. Pinard E. Vascular fate of adipose tissue-derived adult stromal cells in the ischemic murine brain: A combined imaging-histological study. Neuroimage. 2007;34:1–11. doi: 10.1016/j.neuroimage.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Ning H. Lin G. Lue TF. Lin CS. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 2006;74:510–518. doi: 10.1111/j.1432-0436.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 23.Nemeth MJ. Bodine DM. Regulation of hematopoiesis and the hematopoietic stem cell niche by Wnt signaling pathways. Cell Res. 2007;17:746–758. doi: 10.1038/cr.2007.69. [DOI] [PubMed] [Google Scholar]
- 24.Baksh D. Tuan RS. Canonical and non-canonical Wnts differentially affect the development potential of primary isolate of human bone marrow mesenchymal stem cells. J Cell Physiol. 2007;212:817–826. doi: 10.1002/jcp.21080. [DOI] [PubMed] [Google Scholar]
- 25.Palpant NJ. Yasuda S. MacDougald O. Metzger JM. Non-canonical Wnt signaling enhances differentiation of Sca1+/c-kit+ adipose-derived murine stromal vascular cells into spontaneously beating cardiac myocytes. J Mol Cell Cardiol. 2007;43:362–370. doi: 10.1016/j.yjmcc.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gangenahalli GU. Singh VK. Verma YK. Gupta P. Sharma RK. Chandra R. Luthra PM. Hematopoietic stem cell antigen CD34: role in adhesion or homing. Stem Cells Dev. 2006;15:305–313. doi: 10.1089/scd.2006.15.305. [DOI] [PubMed] [Google Scholar]
- 27.Sauter B. Foedinger D. Sterniczky B. Wolff K. Rappersberger K. Immunoelectron microscopic characterization of human dermal lymphatic microvascular endothelial cells. Differential expression of CD31, CD34, and type IV collagen with lymphatic endothelial cells vs blood capillary endothelial cells in normal human skin, lymphangioma, and hemangioma in situ. J Histochem Cytochem. 1998;46:165–176. doi: 10.1177/002215549804600205. [DOI] [PubMed] [Google Scholar]
- 28.Muller AM. Hermanns MI. Skrzynski C. Nesslinger M. Muller KM. Kirkpatrick CJ. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol. 2002;72:221–229. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- 29.Fiedler U. Christian S. Koidl S. Kerjaschki D. Emmett MS. Bates DO. Christofori G. Augustin HG. The sialomucin CD34 is a marker of lymphatic endothelial cells in human tumors. Am J Pathol. 2006;168:1045–1053. doi: 10.2353/ajpath.2006.050554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pusztaszeri MP. Seelentag W. Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54:385–395. doi: 10.1369/jhc.4A6514.2005. [DOI] [PubMed] [Google Scholar]
- 31.Hu Y. Zhang Z. Torsney E. Afzal AR. Davison F. Metzler B. Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sengenes C. Lolmede K. Zakaroff-Girard A. Busse R. Bouloumie A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol. 2005;205:114–122. doi: 10.1002/jcp.20381. [DOI] [PubMed] [Google Scholar]
- 33.Ratajczak MZ. Machalinski B. Wojakowski W. Ratajczak J. Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- 34.Izadpanah R. Trygg C. Patel B. Kriedt C. Dufour J. Gimble JM. Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiyama E. Hiyama K. Telomere and telomerase in stem cells. Br J Cancer. 2007;96:1020–1024. doi: 10.1038/sj.bjc.6603671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang SK. Putnam LA. Ylostalo J. Popescu IR. Dufour J. Belousov A. Bunnell BA. Neurogenesis of Rhesus adipose stromal cells. J Cell Sci. 2004;117:4289–4299. doi: 10.1242/jcs.01264. [DOI] [PubMed] [Google Scholar]
- 37.Jun ES. Lee TH. Cho HH. Suh SY. Jung JS. Expression of telomerase extends longevity and enhances differentiation in human adipose tissue-derived stromal cells. Cell Physiol Biochem. 2004;14:261–268. doi: 10.1159/000080335. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez AM. Pisani D. Dechesne CA. Turc-Carel C. Kurzenne JY. Wdziekonski B. Villageois A. Bagnis C. Breittmayer JP. Groux H. Ailhaud G. Dani C. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madonna R. Willerson JT. Geng YJ. Myocardin a enhances telomerase activities in adipose tissue mesenchymal cells and embryonic stem cells undergoing cardiovascular myogenic differentiation. Stem Cells. 2008;26:202–211. doi: 10.1634/stemcells.2007-0490. [DOI] [PubMed] [Google Scholar]
- 40.Katz AJ. Tholpady A. Tholpady SS. Shang H. Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Y. Jahagirdar BN. Reinhardt RL. Schwartz RE. Keene CD. Ortiz-Gonzalez XR. Reyes M. Lenvik T. Lund T. Blackstad M. Du J. Aldrich S. Lisberg A. Low WC. Largaespada DA. Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 42.Anjos-Afonso F. Bonnet D. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2007;109:1298–1306. doi: 10.1182/blood-2006-06-030551. [DOI] [PubMed] [Google Scholar]
- 43.Kolf CM. Cho E. Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gronthos S. Franklin DM. Leddy HA. Robey PG. Storms RW. Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 45.Bianco P. Riminucci M. Gronthos S. Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 46.Crandall DL. Hausman GJ. Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997;4:211–232. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]