Abstract
Purpose
Radiation has many potential long-term effects on cancer survivors. Female cancer patients may suffer from decreased fertility depending on the site irradiated. Oncologists should be aware of these consequences and discuss options for fertility preservation prior to initiating therapy.
Design
A comprehensive review of the existing literature was conducted. Studies reporting the outcomes for female patients treated with cranio-spinal, abdominal, or pelvic radiation reporting fertility, pregnancy, or neonatal-related outcomes were reviewed.
Results
Cranio-spinal irradiation elicited significant hormonal changes in women that affected their ability to become pregnant later in life. Women treated with abdomino-pelvic radiation have an increased rate of uterine dysfunction leading to miscarriage, preterm labor, low birthweight, and placental abnormalities. Early menopause results from low-dose ovarian radiation. Ovarian transposition may decrease the rates of ovarian dysfunction.
Conclusions
There is a dose-dependent relationship between ovarian radiation therapy (RT) and premature menopause. Patients treated with RT must be aware of the impact of treatment on fertility and explore appropriate options.
Keywords: Fertility, Pregnancy, Neonatal, Radiation Therapy, Female
Introduction
Radiation therapy can disrupt the functioning of the hypothalamic-pituitary axis [1], directly cause ovarian failure [2–4], or cause damage that makes the uterus unable to accommodate the growth of a fetus to full term [5, 6]. These possible effects of radiation or other cancer treatments on fertility and pregnancy outcomes are of minimal concern to most patients, who are beyond their reproductive years. However, these issues have become increasingly important to the growing number of pediatric and young-adult cancer survivors. For example, an estimated 70% of pediatric patients and 90% of patients diagnosed with Wilms tumor will survive for 5 years [7]. A recent study by Schover et al. reported 76% of younger cancer survivors without children not only expressed a desire to have children, but were also concerned about reduction of fertility and possible treatment-related pregnancy complications and neonatal outcomes [8]. In this era of improved normal tissue-sparing with newer radiation techniques including intensity-modulated radiation therapy (IMRT) and proton radiotherapy, as well as improved fertility preservation methods in the field of reproductive medicine, it is critical that we evaluate the potential long-term effects of radiation therapy on fertility and neonatal outcomes, and understand the possible in counseling patients regarding fertility preservation and neonatal care. This paper aims to review the literature regarding the impact of radiotherapy on fertility, pregnancy, and neonatal outcomes among female patients. Additionally, this paper will review published reports on the efficacy of ovarian transposition as one means of preserving fertility. Although obviously important, the teratogenic effects of radiation on the fetus when given during pregnancy are outside the scope of this review.
How Does Radiation Therapy Affect Fertility and Pregnancy Outcomes?
Hormonal Dysfunction
Disruption of the hypothalamic-pituitary-ovarian axis is a well-established potential complication of cranial irradiation that can lead to amenorrhea and infertility. Radiation-induced damage is possible within the hypothalamus, pituitary gland, or both, and can lead to dysregulation of the hormonal milieu responsible for fine regulation of menstruation and fertility. This hormonal environment is predominantly balanced by the secretion of gonadotropin-releasing hormone (GnRH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, progesterone, and prolactin.
Post-Pubertal Studies
The effect of cranial irradiation on the development of endocrinopathies has been investigated in numerous retrospective series. In 1993, Constine and colleagues sought to investigate endocrine abnormalities among patients treated with cranial irradiation for primary brain tumors not involving the hypothalamic-pituitary axis. Of the 32 patients enrolled, 16 patients were female and 16 were male, with a mean age of 19 years at time of radiation. Doses delivered to the hypothalamus and pituitary ranged from 39.6 to 70.2 Gy, with a mean of 53.6 Gy. With a mean follow-up of 7 years, 70% of post-pubertal pre-menopausal females developed oligomenorrhea, and 50% showed low serum estradiol concentrations. In addition, 50% of post-pubertal pre-menopausal females developed mild hyperprolactinemia. Low to normal basal and GnRH-stimulated levels of FSH and LH suggest a primary hypothalamic, rather than pituitary, dysfunction [9].
Pai et al. subsequently addressed the latency period of radiation-induced endocrinopathies in patients with primary brain tumors treated with cranial irradiation. With a median age of 41.2 years and a median prescribed target dose of 68.4 cobalt gray equivalents, the 5-year and 10-year actuarial rates of hypogonadism were 29% and 36%, respectively, after correcting for hyperprolactinemia. After a median follow-up of 5.5 years, the 5-year and 10-year actuarial rates of hyperprolactinemia were 72% and 87%, respectively. The median time to develop hypogonadism and hyperprolactinemia was 4 years and 2.5 years, respectively [10]. There was no significant difference in the 5-year actuarial rates of hyperprolactinemia or hypogonadism by gender or by age (≤ 40 years vs. > 40 years). This study demonstrates that radiation-induced damage to the hypothalamus and pituitary gland occurs frequently after high-dose cranial radiotherapy, and can result in clinically evident endocrinopathies. These results also suggest that radiotherapy patients, including pre-menopausal females potentially interested in fertility, should be followed for several years, as there can be a latency period before development of these endocrinopathies [10].
Pre-pubertal Studies
Until recently, the effect of low-dose prophylactic cranial irradiation (PCI) for children with leukemia has not been well-documented [9, 10]. Bath et al. assessed hypothalamic-pituitary-ovarian function in 12 female long-term survivors of acute lymphoblastic leukemia (ALL) following PCI, treated to doses of 18–24 Gy, compared to healthy controls. Their median age at diagnosis and assessment was 4.7 and 20.8 years, respectively. Although all 12 patients treated with PCI achieved adult sexual development and menarche, they also demonstrated decreased LH secretion, attenuated LH surge, and shorter luteal phases compared with controls [1]. Additionally, patients with short luteal phases had significantly longer follow-up compared with patients with normal luteal phase lengths, suggesting a progressive effect. Greater LH surges have been correlated with higher rates of conception [11]; in contrast, shorter luteal phases have been associated with incipient ovarian failure and early pregnancy loss [12]. These findings suggest that pre-pubertal females who receive low-dose PCI may be at increased risk of incipient ovarian failure and early pregnancy loss. None of the patients in this study reported a history of pregnancy; however, the number of patients who had attempted pregnancy was not specified. Therefore, this study lacked sufficient pregnancy outcome data to confirm the finding of increased risk of ovarian failure/pregnancy loss. Though some studies have reported successful pregnancies after treatment for childhood ALL [13], a recent Scandinavian population-based cohort study for childhood ALL found that women who received PCI at doses of 18–24 Gy had a significantly lower first birth rate than those who received no radiation [14].
Lastly, some studies have suggested that cranial irradiation may induce precocious puberty [15–17], which has been attributed to cortical disinhibition of the hypothalamus [18]. Ogilvy-Stuart et al. demonstrated a correlation between cranial irradiation and early precocious puberty by evaluating 46 children treated with cranial irradiation to a median dose of 30 Gy for primary brain tumors. The study enrolled 30 boys and 16 girls with potential for early puberty <2 standard deviations below mean onset of puberty for respective genders. In both sexes, puberty began earlier among patients in the study compared to historical published controls (girls: 8.5 vs. 11.2 years; boys: 9.2 vs. 11.6 years) [15]. A significant linear association was demonstrated between younger age at irradiation and earlier onset of puberty [15]. These results suggest that cranial irradiation can result in precocious puberty in both sexes.
It is important to note that a major limitation in interpreting these studies is their out-dated pediatric techniques with respect to radiation treatment, planning target volume definition, and prescribed radiation doses. Pediatric radiation oncology practices have changed drastically in the last decade, including the increased availability of proton radiotherapy and treatment with decreasing radiation doses, especially in PCI. Thus, although many of these recently published studies of long-term data may reflect outcomes relevant for adult pediatric patients treated with similar doses and techniques, they may not accurately reflect the risk among pediatric patients treated more recently.
Ovarian Dysfunction
Ovarian Dysfunction due to Radiation Therapy
The nonrenewable pool of ovarian primordial follicles declines through atresia from 2 million at birth to 500,000 at menarche. By age 37–38, the total number of viable primordial follicles drops to 25,000, with associated accelerated loss and increased difficulty of spontaneous conceptions [19]. Ovarian follicular depletion is most commonly evaluated indirectly through the age at onset of menarche and serum hormonal concentrations, with FSH levels > 50 mIU/mL suggestive of menopause [20, 21].
Ionizing radiation can cause direct DNA damage to ovarian follicles, leading to follicular atrophy and decreased ovarian follicular reserve. This can hasten the natural decline of follicle numbers, leading to impaired ovarian hormone production, uterine dysfunction due to inadequate estrogen exposure, and early menopause. Although the radiosensitivity of the oocyte is thought to vary during the growth phase, primordial follicles are thought to be more radioresistant than maturing follicles [22]. Several factors have been identified as significant determinants of ovarian failure, including radiation dose, age at the time of radiation exposure, and extent of radiation treatment field [6, 23, 24].
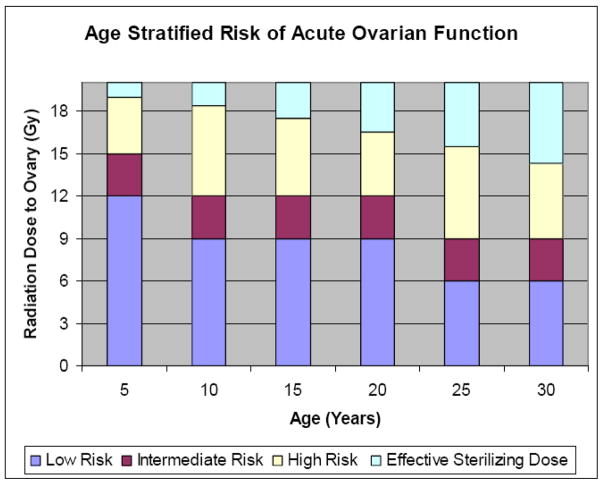
The human oocyte is generally extremely sensitive to radiation therapy. A recent mathematical model employed by Wallace et al. suggested that the dose required to destroy 50% of the immature oocytes (LD50) is less than 2 Gy [4]. These researchers employed the Faddy-Gosden model, incorporating decay as an instantaneous rate of temporal change based on the remaining population pool to estimate the radiosensitivity of the human oocyte. The authors solved the equation with data sets obtained from two cohorts of women with ovarian failure secondary to radiation therapy. Ovarian failure was defined as failure to undergo or complete pubertal development, or the onset of a premature menopause before age 40 [4]. A follow-up model by Wallace et al. sought to predict the age at which ovarian failure is likely after ovarian irradiation by accounting for age at treatment and radiation dose. The effective sterilizing dose (ESD), or dose of fractionated radiotherapy at which ovarian failure occurs immediately after treatment in 97.5% of patients, was found to decrease with increasing age at treatment. The estimated ESD at birth was 20.3 Gy; at 10 years, 18.4 Gy; at 20 years, 16.5 Gy; and at 30 years, 14.3 Gy. This model can be used to estimate the age at which premature ovarian failure occurs for individual patients from birth to 50 years at any given dose of radiotherapy. The wide individual variability in ovarian follicular reserve at time of treatment can explain differences in onset of premature ovarian failure between patients treated at similar ages. Clinical application of this model would allow physicians to counsel women on their reproductive potential following radiation therapy [25]. Figure 1 extrapolates data generated from this model and graphically depicts the risk of developing acute ovarian failure (AOF, defined as failure within 5 years of diagnosis), stratified by age and radiation dose to the ovary. This mathematical model has not yet been validated by any additional clinical studies.
Figure 1.
Risk of developing acute ovarian failure, defined as ovarian failure within 5 years stratified by age and radiation dose to the ovary (data extrapolated from reference 27).
Several studies have sought to investigate the degree of ovarian damage among long-term cancer survivors [26, 27]. Larsen et al. sought to assess ovarian function amongst a cohort of 100 female cancer survivors treated with chemotherapy and/or radiotherapy identified from the Childhood Cancer Registry. The median age of survivors was 5.4 years at time of diagnosis and 25.7 years at time of study entry. At study onset, 17 female survivors required hormone replacement therapy secondary to treatment-related ovarian failure and were found to have follicle-depleted or undetectable ovaries, elevated FSH and LH, and inhibin B below measurable levels. Seventy patients with spontaneous menstrual cycles had smaller ovarian volumes per ovary than controls (4.8 cm3 vs. 6.8 cm3, p<.001) and fewer antral follicles per ovary (7.5 vs. 11, p<.001). In addition, follicle number was inversely associated with ovarian irradiation, alkylating chemotherapy, older age at diagnosis, and longer follow-up. These results demonstrate that survivors with spontaneous menstrual cycles may have diminished menstrual reserves [26].
Subsequently, Chemaitilly et al. conducted a multicenter study of 3,390 female participants from the Childhood Cancer Survivor Study (CCSS) to determine the incidence of AOF and to identify potential risk factors. A total of 215 patients (6.3%) developed AOF. Older age at diagnosis (OR 1.8, p<0.0001) and treatment with abdominal or pelvic irradiation (OR 25.4, p<0.0001) were associated with AOF. Among survivors with AOF, 116 patients (54%) received at least 10 Gy to the ovaries. These results suggest that AOF can develop in a small subset of cancer survivors, especially those subjected to high doses of ovarian irradiation [27].
Along with the predictive model of ovarian failure created by Wallace et al. [25], these published data should assist physicians in counseling patients and their families at the time of diagnosis and before cancer therapy is initiated. Understanding which patients are at the highest risk of AOF will allow physicians to tailor radiation techniques to minimize ovarian dose, when possible, and to guide patients in discussions of fertility and fertility preservation options.
Precise dosimetric calculation of ovarian dose is difficult and depends on accurate algorithims of scattered radiation dose [28] and exact radiographic identification of the ovaries [25]. Use of higher-energy photons may reduce side scatter when ovaries are outside the primary field and external shielding can be considered to reduce external scatter from the linear accelerator. Again, it is important to note that with novel radiation techniques, including IMRT and proton radiotherapy, the ovaries may be spared from significant radiation, thus mitigating the potential adverse effects on fertility. However, there are still few published clinical or dosimetric data that directly address this issue. With the growing interest in IMRT and the increasing availability of proton radiotherapy, future studies will hopefully address the potential clinical benefit of these radiation techniques in fertility preservation.
Ovarian Failure Due to Chemotherapeutic Agents
Radiotherapy is frequently used in combination with chemotherapy. Although a thorough discussion of all chemotherapeutic effects on fertility and pregnancy is beyond the scope of this review, we will address some aspects of this topic due to its important effects on fertility. (For a more thorough review of this subject, please refer to Lee et al. [29]). The effect of chemotherapeutic drugs on ovarian function varies widely, depending on numerous factors including patient age, type and dose of chemotherapeutic agent, number of chemotherapy cycles, and differences in the definition of iatrogenic amenorrhea [30]. In general, however, studies of ovarian development in women treated with chemotherapy have demonstrated a minimal loss of primordial follicles, with the primary decrease in large maturing follicles [31, 32], suggesting an effect of chemotherapy on follicular development. Gonadotoxic chemotherapeutic agents, most notably alkylating agents (e.g., cyclophosphamide), may contribute to premature menopause [33, 34]. Although chemotherapeutic regimens could ideally be modified to minimize their effect on ovarian failure, the primary focus remains maximizing the probability of cure. Table 1 provides a brief summary of common chemotherapeutic agents and their associated risks of ovarian failure.
Table 1.
Chemotherapeutic agents stratified by associated risk of ovarian failure
| Chemotherapic Agents and Associated Risk of Ovarian Failure | |
|---|---|
| High Risk (Alkylating Agents) | Cyclophosphamide, Melphalan, Busulfan, Nitrogen Mustard, Chlorambucil, Procarbazine |
| Intermediate Risk | Cisplatin, Adriamycin |
| Low Risk | Methotrexate, 5-Fluorouracil, Vincristine, Bleomycin, Actinomycin D |
Uterine Dysfunction
Pelvic irradiation appears to put women at elevated risk for pregnancy-related complications, including spontaneous miscarriages, preterm labor and delivery, low birth weight, and placental abnormalities [7, 35, 36]. Table 2 reviews common adverse obstetrical and neonatal outcomes associated with radiation therapy. These findings have been attributed to reduced uterine volume, impaired uterine distensibility due to myometrial fibrosis, uterine vasculature damage, and endometrial injury [37–41]. The degree of uterine damage depends on the total radiation dose, site of irradiation, and patient age at time of treatment [5, 6, 37]. Studies suggest that the pre-pubertal uterus is more vulnerable than the adult uterus to the effects of pelvic irradiation, with doses of 14–30 Gy likely to cause uterine dysfunction [5, 6].
Table 2.
A glossary of terms defining adverse pregnancy and neonatal outcomes
| Definitions of Adverse Pregnancy and Neonatal Outcomes | |
|---|---|
| Threatened Labor | Labor after 37 weeks of gestation without delivery |
| Preterm Delivery | Delivery before gestational week 37 |
| Low Birth Weight | Birth Weight less than 2.500 grams |
| Small Gestational Age | Estimated fetal weight below the 10th percentile |
| Miscarriage (aka Spontaneous Abortion) | Spontaneous loss of pregnancy before 20 weeks gestation |
| Premature Low Birth Weight | Delivery before gestational week 37 weighing less than 2.500 grams |
| Malposition of the Fetus | Includes breech presentation, face presentation, brow presentation |
| Perinatal Infant Mortality | Fetal death after 20 weeks of gestation or before the 1st week of life |
| Placenta Accreta | Abnormal adherence of part or all of the placenta to the uterine wall |
| Placenta Percreta | Abnormal placentation where the placenta invades through the myometrium into the uterine serosa |
Larsen et al. evaluated the effect of radiotherapy on uterine volume in 100 childhood cancer survivors using transvaginal sonography. Based on uterine exposure to radiation, patients were divided into four groups. Median uterine volumes for control patients without irradiation (n=44) was 47 mL, compared with 40 mL for patients treated with radiation above the diaphragm (n=21), 34 mL for patients treated with radiation below the diaphragm (n=19), and 13 mL for patients treated with uterine irradiation (n=16). Among nulliparous patients, those who received uterine irradiation had significantly lower uterine volume than any other group (p<0.02). For the 13 nulliparous patients treated with direct uterine irradiation, smaller uterine volume was significantly associated with younger treatment age (p=0.02). In addition, there was a significant increase in mid-trimester abortions in patients who had higher uterine radiation exposure compared to those that did not (p=0.007). This study demonstrates that uterine irradiation in childhood may reduce adult uterine volume, which may lead to adverse pregnancy outcomes [37].
In addition to restricting uterine volume, radiation may also cause uterine vessel damage. Holm et al. evaluated the effect of total body irradiation (TBI) on uterine volume and uterine blood flow by ultrasound and Doppler. Twelve female patients diagnosed with childhood leukemia with a median age of 12.7 years were assessed 4.0 to 10.9 years after TBI. With a median follow-up of 21.5 years, the median uterine volume was 2.6 standard deviations below that of controls (range, −6.3 to −0.6, p=0.002). In addition, uterine blood flow was impaired, with systolic blood flow detectable in 6 of 9 patients and diastolic blood flow detectable in only 1 of 9 patients [38]. In contrast, healthy subjects demonstrate measurable diastolic blood flow in 35% of prepubertal and 100% of adult females [39]. Poor vascularization may result in diminished uterine response to cytotrophoblast invasion and decreased fetoplacental blood flow, which may impair fetal growth.
Additionally, uterine irradiation may injure the endometrium and prevent normal decidualization. This may increase the incidence of placental attachment disorders, including placental accreta or placental percreta. It has also been hypothesized that radiation therapy may lead to diffuse thinning of the myometrium, increasing the risk of uterine rupture. Case reports have described placenta percreta and uterine rupture in the setting of prior TBI [36] and pelvic radiation [40].
Adverse Pregnancy and Neonatal Outcomes After Radiotherapy
Several small series published in the 1980s demonstrated an increased risk of adverse pregnancy outcomes among women who had received abdominopelvic irradiation [41–44]. More recently, in 2000, Chiarelli and colleagues compared the risk of adverse pregnancy and neonatal outcomes in 340 female cancer survivors after abdominopelvic irradiation to the risk among patients treated with non-sterilizing agents and surgery. Compared with patients treated with surgery alone, survivors receiving abdominopelvic radiation with or without surgery were more likely to have low-birth-weight infants (OR: 3.64, 95% CI: 1.33–9.96), premature low-birthweight infants (OR: 3.29; 95% CI: 0.97–11.1), and perinatal infant mortality (OR 2.41, 95% CI: 0.50–11.5). Additionally, the likelihood of perinatal infant mortality and low birthweight were significantly related to radiation dose [35]. These findings were attributed to radiation-induced uterine damage.
Green et al. subsequently evaluated the risk of fetal loss among 1,915 female cancer survivors enrolled in the CCSS diagnosed between 1970 and 1986 [45]. All participants were sent questionnaires regarding pregnancy attempts, pregnancy, and pregnancy outcomes. Of 4,029 pregnancies reported, 2,349 occurred among patients previously treated with radiation therapy (58%). The relative risk (RR) of miscarriage was 1.40 in patients who received cranial irradiation, compared with those who received no radiation therapy (95% CI: 1.02–1.94). The RR of miscarriage was 2.22 (95% CI: 1.7–7.78) among those who received craniospinal irradiation compared with those who received no radiation, suggesting that spinal irradiation harms pregnancy outcome. Additionally, there was a trend toward increased risk of miscarriages among women whose ovaries were within or near the radiation field (RR 1.86, p=.14) or within 5 cm of the field edge (RR 1.64, p=0.06) compared with patients who did not receive radiation therapy. In contrast, risk of miscarriage was not elevated if the ovaries were shielded (RR 0.90, p=0.86). There was also a higher risk of low birthweight in infants born to patients treated with pelvic irradiation (RR 1.85, p=0.03) [45].
Signorello et al. carried out a similar study, focusing on the potential risk of preterm birth and restricted fetal growth among offspring of female cancer survivors enrolled in the CCSS from 1968 to 2002 [46]. With a total of 2,201 offspring of 1,264 female survivors, the authors found that offspring of patients treated with high-dose radiotherapy (> 5 Gy) to the uterus were at increased risk of preterm delivery (OR=3.5, 95% CI: 1.5–8.0), low birth weight (OR=6.8, 95% CI: 2.1–22.2), and small gestational age (OR=4.0, 95% CI: 1.6–9.8) compared to offspring of patients who did not receive radiotherapy. In addition, increased risk was apparent at lower uterine doses, starting at 50 cGy for preterm birth and at 250 cGy for low birthweight [46]. These studies all demonstrate an increased risk of adverse pregnancy and neonatal outcomes associated with prior history of abdominal irradiation.
The effect of low-dose flank irradiation on pregnancy and neonatal outcomes has also been evaluated in survivors of Wilms tumor [7, 41, 42]. Recently, Green et al. evaluated patients enrolled in four consecutive National Wilms Tumor Study Group (NWTSG) trials. Of 427 pregnancies reported, including 409 liveborn singletons, fetal malposition and early or threatened labor were significantly more frequent among irradiated women. Among those treated with doses > 25 Gy compared with no radiation, female subjects had a higher risk of early or threatened labor (OR 2.36, 95% CI: 0.93 – 6.02) and malposition (OR 6.26, 95% CI: 1.5 – 36.57). Green et al. also reported a significantly increased incidence of low birthweight and prematurity in the offspring of irradiated females. Incidence of fetal malposition, early or threatened labor, low birthweight, and prematurity were correlated with higher radiation doses [7]. In contrast, none of these complications was significantly more frequent in partners of men who received flank irradiation compared with those who were not [41, 42], which is consistent with prior reports.
Table 3 provides a summary of the largest published series evaluating the risk of adverse pregnancy and neonatal outcomes in female cancer survivors. These studies illustrate the importance of close obstetric monitoring of pregnant women who have received radiation to the pelvis or abdomen. These patients may be at higher risk for preterm or threatened labor, fetal malposition, impaired fetal growth, or placental attachment disorders and may benefit from obstetrical evaluation by maternal fetal medicine specialists.
Table 3.
Summary of the largest published series evaluating effect of radiotherapy on pregnancy and neonatal outcomes.
| Series | Study Population | Years of Study | # of Pregnanices | Irradiated Site | Adverse Pregnancy and Neonatal Outcomes | Comments | |
|---|---|---|---|---|---|---|---|
| Signorello et al (46) | Childhood Cancer Survivor Study | 1968–2002 | 2201 | Pelvic Irradiation | Preterm Delivery | OR 3.5, 95% Cl: 1.5–8.0, p=0.003 | Increased risks apparent at lower uterine RT doses, as low as 50 cGy. |
| Low Birth Weight | OR 6.8, 95% Cl: 2.1–22.2, p=.001 | ||||||
| Small Gestational Age | OR 4.0, 95% Cl: 1.6–9.8, p=.003 | ||||||
| Green et al. (45) | Childhood Cancer Survivor Study | 1970–1986 | 4029 | Cranial Irradiation | Miscarriage | RR 1.4, 95% Cl: 1.02–1.94, p=0.04 | A trend toward increased risk of miscarriage in patients treated with pelvic irradiation |
| Craniospinal Irradiation | Miscarriage | RR 3.63, 95% Cl: 1.70–7.78, p<.001 | |||||
| Pelvic Irradiation | Low Birth Weight | RR 1.84, 95% Cl: 1.07–3.18, p=0.03 | |||||
| Green et al. (7) | National Wilms Tumor Study Group | 1969–1999 | 427 | Flank Irradiation | Preterm Delivery/Threatened Labor | p=.03 | Increased risk with increasing RT doses; Trend toward increased risk of congenital malformations |
| Malposition of the Fetus | p=.007 | ||||||
| Prematurity | p=0.0005 | ||||||
| Low Birth Weight | p=.02 | ||||||
| Chiare et. al (35) | Ontario Cancer Registry | 1964–1688 | 594 | Abdominal-Pelvic Irradiation | Low Birth Weight | OR 3.64, 95% Cl 1.33–9.96 | Increased risk of perinatal mortality and low birth weight with increasing RT doses. |
| Premature Low Birth Weight | OR 3.29, 95% Cl: 0.97–11.1 | ||||||
| Perinatal Infant Mortality | OR 2.41, 95% Cl: 0.50–11.5 | ||||||
Can Ovarian Transposition Help Preserve Fertility?
Ovarian transposition, also known as oophoropexy, is a surgical procedure that moves the ovaries out of the radiation field. Since its initial proposal in the 1950’s as a means to preserve ovarian function among cervical cancer patients treated with definitive radiation therapy, [47], ovarian transposition has been considered for numerous other cancers, including Hodgkin’s lymphoma, pediatric sarcomas, and rectal cancer [48–51]. Traditionally, this procedure has been performed via laparotomy at time of staging for Hodgkin’s disease [52] or radical hysterectomy for cervical cancer [53]. More recently, it has been performed via laparoscopy [48–51]. Briefly, the ovary and fallopian tube are dissected from the uterus, mobilized out of the pelvis, and ligated to the peritoneum as highly and laterally as possible. The transposed ovaries may be sutured within the lateral paracolic gutter up the 12th rib, anterior to the psoas muscle above the pelvic brim, or within the far lateral pelvis [54]. The proper location at which to fix the transposed ovaries depends upon the planned radiation fields. For cervical cancer, the ovaries should be transposed high above the pelvic brim, since traditional pelvic field extends to the L4/L5 vertebral space. In contrast, for patients receiving pelvic lymph node irradiation or an inverted Y field for Hodgkin’s disease, the ovaries can be transposed medially.
Covens et al. performed dose calculations to estimate the radiation exposure to each transposed ovary in cervical cancer patients based on intracavitary radiation alone and on external-beam pelvic radiotherapy (45 Gy) with and without para-aortic nodal irradiation (45 Gy). They estimated the mean radiation dose to each ovary following lateral transposition (14.4 cm on the right, 14.3 cm on the left) for a course of intracavitary radiation as 1.26 Gy. The estimated doses for pelvic radiation without and with para-aortic lymph node irradiation were 1.35–1.90 Gy and 2.3–3.1 Gy, respectively [55].
Published reports evaluating the efficacy of ovarian transposition with respect to ovarian function preservation and fertility vary widely. [56]. Morice et al. sought to assess the effectiveness and complications of bilateral ovarian transposition before pelvic irradiation in cervical cancer patients. Of 104 patients, 59 were treated with vaginal brachytherapy (VB) alone to 60 Gy, and 25 other patients received pelvic radiation to 45 Gy with concurrent cisplatin, followed by VB boost of 15 Gy. Ovarian function was assessed by routine postoperative ultrasound and serial serum hormone levels after transposition. With a median follow-up of 31 months, ovarian function preservation rates were 100% for patients treated exclusively by surgery, 90% for those treated with VB, and 60% for patients with pelvic irradiation and VB. Complications of ovarian transposition include benign ovarian cysts (23%), chronic pelvic pain (3%), and ovarian metastases (1%) [53]. Other reported complications include vascular injury, fallopian tube infarction, and ovarian migration [49, 55, 57].
In addition, Morice and colleagues reported a separate study of 24 patients with pelvic malignancies treated with laparoscopic ovarian transposition. Bilateral laparoscopic ovarian transposition to the paracolic gutter was successfully performed in 22 patients (94%), and 19 patients (77%) received VB and/or pelvic external beam radiation. With a median follow-up of 31 months, ovarian preservation was achieved in 79% of subjects, and three pregnancies were achieved by 2 patients treated with VB alone and 1 patient treated with external beam to 25–35 Gy [58].
Kuohung et al. recently evaluated the efficacy of laparoscopic unilateral oophoropexy prior to craniospinal irradiation (CSI) for primary brain tumors among the pediatric population. They retrospectively compared the ovarian function of 15 patients treated with CSI who underwent unilateral oophoropexy to 11 patients treated with CSI alone. Mean age at diagnosis, length of follow-up, chemotherapy, and radiation treatment characteristics were similar between the two groups. However, there was a trend towards reduced ovarian dysfunction, defined as elevated FSH or persistent amenorrhea, in patients treated with oophoropexy compared to controls (13% vs. 45%, p=0.09). Fertility data were not available due to limited follow-up [59]. Table 4 provides an overview of the literature evaluating the efficacy of ovarian transposition in preserving ovarian function. Similar studies have reported ovarian function preservation and successful pregnancies following oophoropexy among patients with pelvic Hodgkin’s disease [49, 50, 60]. However, even with oophoropexy and placement of a lead central block to shield the uterus and ovaries, the ovaries can still receive 8–15% of the prescribed dose due to scatter and transmission through the shield [60]. With emerging normal tissue-sparing techniques of IMRT and proton radiotherapy, radiation dose to the ovaries may be significantly reduced.
Table 4.
Single institution retrospective series evaluating efficacy of ovarian transposition on ovarian preservation.
| Series | No of Pts | Pt Age | Median F/U (mnths) | Diagnosis | Site of Irradiation | Laterality of Oophoropexy | Rate of Ovarian Preservation |
|---|---|---|---|---|---|---|---|
| Kuohung et al. (59) | 15 | 5 – 14 | 71 | Medullo-blastoma | CSI | Unilateral | 87% (case) vs. 55% (control) (p=0.09) |
| Morice et al. (58) | 24 | 15–40 | 31 | Pelvic Malignancies | Pelvic | Bilateral | 79% |
| Morice et al. (53) | 104 | 21–42 | 31 | Cervical Cancer | Pelvic | Bilateral | 90% (VB) 60% (EBRT+VB) |
| Williams et al. (48) | 10 | 21–36 | Not Available | Hodgkin’s Disease (HD) | Pelvic | Not Specified | 50% overall; 83% pts with 0–2 cycles of chemotherapy |
| Classe et al. (49) | 4 | 22–37 | 21 | Hodgkin’s Disease | Inverted Y | Bilateral | 100% |
| Clough et al. (61) | 14 | 22–44 | 24 (mean) | Cervical Cancer (n=17), HD (n=2), Ependymoma (n=1) | Brachytherapy (n=9); Pelvic EBRT (n=2), EBRT+Brachy (n=3) | Unilateral | 86% overall; 100% among pts age <40 |
For women treated with pelvic irradiation, transposition of ovarian tissue outside the radiation field increases the chance of preserving ovarian function. The optimal approach to fertility preservation depends on the patient’s age, anticipated cancer treatment, time line prior to initiation of treatment, and whether the patients wants to get pregnant. Though outside the scope of this review, other methods of fertility preservation include embryo cryopreservation, oocyte preservation, ovarian tissue cryopreservation, and autotransplantation of the ovary to the upper extremity with creation of vascular anastomosis. A recent review by Lobo [61] nicely addresses these fertility preservation options and should be referred to for further discussion. A brief summary of these fertility preservation options is provided in Table 5.
Table 5.
Fertility Preservation Options for Female Cancer Patients Undergoing Cytotoxic Therapy
| Methods of Fertility Preservation | Definition | Status of Procedure | Considerations |
|---|---|---|---|
| Ovarian Transposition | Surgical repositioning of ovaries away from the radiation field | Standard | Outpatient surgical procedure, may need repositioning or IVF to conceive |
| Gonadal Shielding | Use of shielding to reduce the dose of radiation delivered to the reproductive organs | Standard | Evaluation by radiation oncologist to determine feasibility of shielding |
| Donor oocytes and gestational surrogacy | IVF using donor oocytes and/or implantation of the embryo in a surrogate carrier | Standard | Requires donor oocyte or surrogate carrier |
| Embryo cryopreservation | Harvesting eggs, IVF, and freezing embryos for later implantation | Standard | Outpatient surgical procedure, requires 10–14 days of ovarian stimulation, requires partner or sperm donor |
| Oocyte cryopreservation | Harvesting and freezing of unfertilized eggs | Investigational | Outpatient surgical procedures, requires 10–14 days of ovarian stimulation; expensive |
| Cryopreservation of ovarian tissue | Freezing of ovarian tissue and reimplantation after cancer treatment | Investigational | Outpatient surgical procedure, not appropriate if high risk of ovarian involvement |
| Ovarian suppression with GnRH analogs or antagonists | Use of hormonal agents to protect ovarian tissue during chemotherapy or RT | Investigational | More data with regards to chemotherapy; expensive |
Conclusion
Treatment-related effects on fertility, pregnancy, and neonatal outcomes are of great concern to the majority of young cancer survivors. Adverse effects on pregnancy can be caused by disruption of normal functioning of the hypothalamic-pituitary axis, uterine damage, or premature ovarian failure. Patients who undergo cranial irradiation are at risk for developing clinically detectable abnormalities of the gonadal axis. Women who have received pelvic irradiation appear to be at elevated risk for pregnancy-related complications, including spontaneous miscarriages, preterm labor and delivery, low birthweight, and placental abnormalities. These findings have been attributed to reduced uterine volume, impaired uterine distensibility, damaged uterine vasculature, and endometrial injury. Female cancer patients previously treated with pelvic or abdominal irradiation should receive close obstetric monitoring during pregnancy.
Whenever possible, direct irradiation to the ovaries should be avoided. Ovarian transposition should be considered in women of reproductive age before pelvic irradiation, though it should be performed soon before treatment initiation, as ovarian migration has been reported. However, even if the ovaries are outside of the radiation field, scatter dose can cause significant ovarian damage. Newer radiation techniques, including IMRT and proton radiotherapy, may mitigate these radiation-related treatment effects, but require further investigation. With emerging data regarding the timing to ovarian failure, patients can be educated and counseled regarding potential fertility and obstetrical issues. Due to the necessary complexity of their care, these patients will benefit from having a multidisciplinary team of caregivers including a radiation oncologist, pediatric oncologist, medical oncologist, a reproductive endocrinologist or gynecologist, and a maternal fetal medicine specialist. Only through a multidisciplinary approach will patients receive optimal care of their cancer and the best options for fertility preservation. Finally, and beyond the scope of this review, both established and emerging experimental techniques for fertility preservation may be options for these patients.
Footnotes
Conflicts of Interest Notification: None
Bibliography
- 1.Bath LE, et al. Hypothalamic-pituitary-ovarian dysfunction after prepubertal chemotherapy and cranial irradiation for acute leukaemia. Hum Reprod. 2001;16(9):1838–44. doi: 10.1093/humrep/16.9.1838. [DOI] [PubMed] [Google Scholar]
- 2.Bath LE, et al. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum --Reprod. 2003;18(11):2368–74. doi: 10.1093/humrep/deg473. [DOI] [PubMed] [Google Scholar]
- 3.Wallace WH, et al. Ovarian failure following abdominal irradiation in childhood: natural history and prognosis. Clin Oncol (R Coll --Radiol) 1989;1(2):75–9. doi: 10.1016/s0936-6555(89)80039-1. [DOI] [PubMed] [Google Scholar]
- 4.Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod. 2003;18(1):117–21. doi: 10.1093/humrep/deg016. [DOI] [PubMed] [Google Scholar]
- 5.Critchley HO, et al. Abdominal irradiation in childhood; the potential for pregnancy. Br J Obstet Gynaecol. 1992;99(5):392–4. doi: 10.1111/j.1471-0528.1992.tb13755.x. [DOI] [PubMed] [Google Scholar]
- 6.Bath LE, et al. Ovarian and uterine characteristics after total body irradiation in childhood and adolescence: response to sex steroid replacement. Br J Obstet Gynaecol. 1999;106(12):1265–72. doi: 10.1111/j.1471-0528.1999.tb08180.x. [DOI] [PubMed] [Google Scholar]
- 7.Green DM, et al. Pregnancy outcome after treatment for Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2002;20(10):2506–13. doi: 10.1200/JCO.2002.07.159. [DOI] [PubMed] [Google Scholar]
- 8.Schover LR, et al. Having children after cancer. A pilot survey of survivors’ attitudes and experiences. Cancer. 1999;86(4):697–709. doi: 10.1002/(sici)1097-0142(19990815)86:4<697::aid-cncr20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Constine LS, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328(2):87–94. doi: 10.1056/NEJM199301143280203. [DOI] [PubMed] [Google Scholar]
- 10.Pai HH, et al. Hypothalamic/pituitary function following high-dose conformal radiotherapy to the base of skull: demonstration of a dose-effect relationship using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2001;49(4):1079–92. doi: 10.1016/s0360-3016(00)01387-0. [DOI] [PubMed] [Google Scholar]
- 11.Baird DD, et al. Preimplantation urinary hormone profiles and the probability of conception in healthy women. Fertil Steril. 1999;71(1):40–9. doi: 10.1016/s0015-0282(98)00419-1. [DOI] [PubMed] [Google Scholar]
- 12.Soules MR, et al. Luteal phase deficiency: characterization of reproductive hormones over the menstrual cycle. J Clin Endocrinol Metab. 1989;69(4):804–12. doi: 10.1210/jcem-69-4-804. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson HS, Byrne J. Fertility and pregnancy after treatment for cancer during childhood or adolescence. Cancer. 1993;71(10 Suppl):3392–9. doi: 10.1002/1097-0142(19930515)71:10+<3392::aid-cncr2820711743>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 14.Nygaard R, et al. Reproduction following treatment for childhood leukemia: a population-based prospective cohort study of fertility and offspring. Med Pediatr Oncol. 1991;19(6):459–66. doi: 10.1002/mpo.2950190603. [DOI] [PubMed] [Google Scholar]
- 15.Ogilvy-Stuart AL, Clayton PE, Shalet SM. Cranial irradiation and early puberty. J Clin Endocrinol Metab. 1994;78(6):1282–6. doi: 10.1210/jcem.78.6.8200926. [DOI] [PubMed] [Google Scholar]
- 16.Oberfield SE, et al. Age at onset of puberty following high-dose central nervous system radiation therapy. Arch Pediatr Adolesc Med. 1996;150(6):589–92. doi: 10.1001/archpedi.1996.02170310023003. [DOI] [PubMed] [Google Scholar]
- 17.Byrne J, et al. Fertility in women treated with cranial radiotherapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;42(7):589–97. doi: 10.1002/pbc.20033. [DOI] [PubMed] [Google Scholar]
- 18.Gleeson HK, Shalet SM. The impact of cancer therapy on the endocrine system in survivors of childhood brain tumours. Endocr Relat Cancer. 2004;11(4):589–602. doi: 10.1677/erc.1.00779. [DOI] [PubMed] [Google Scholar]
- 19.Faddy MJ, et al. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342–6. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 20.Levy MJ, Stillman RJ. Reproductive potential in survivors of childhood malignancy. Pediatrician. 1991;18(1):61–70. [PubMed] [Google Scholar]
- 21.Levitt GA, Jenney ME. The reproductive system after childhood cancer. Br J Obstet Gynaecol. 1998;105(9):946–53. doi: 10.1111/j.1471-0528.1998.tb10256.x. [DOI] [PubMed] [Google Scholar]
- 22.Ogilvy-Stuart AL, Shalet SM. Effect of radiation on the human reproductive system. Environ Health Perspect. 1993;101(Suppl 2):109–16. doi: 10.1289/ehp.93101s2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace WH, et al. Ovarian failure following abdominal irradiation in childhood: the radiosensitivity of the human oocyte. Br J Radiol. 1989;62(743):995–8. doi: 10.1259/0007-1285-62-743-995. [DOI] [PubMed] [Google Scholar]
- 24.Lushbaugh CC, Casarett GW. The effects of gonadal irradiation in clinical radiation therapy: a review. Cancer. 1976;37(2 Suppl):1111–25. doi: 10.1002/1097-0142(197602)37:2+<1111::aid-cncr2820370821>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Wallace WH, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62(3):738–44. doi: 10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 26.Larsen EC, et al. Reduced ovarian function in long-term survivors of radiation-and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab. 2003;88(11):5307–14. doi: 10.1210/jc.2003-030352. [DOI] [PubMed] [Google Scholar]
- 27.Chemaitilly W, et al. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab. 2006;91(5):1723–8. doi: 10.1210/jc.2006-0020. [DOI] [PubMed] [Google Scholar]
- 28.Kase KR, SGK, Chen DTS. Radial Distribution of Scattered and Leakage Radiation Dose for Radiotherapeutic Equipment. IAEA-SM-249/25. 1982:111–124. [Google Scholar]
- 29.Lee SJ, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 30.Warne GL, et al. Cyclophosphamide-induced ovarian failure. N Engl J Med. 1973;289(22):1159–62. doi: 10.1056/NEJM197311292892202. [DOI] [PubMed] [Google Scholar]
- 31.Nicosia SV, Matus-Ridley M, Meadows AT. Gonadal effects of cancer therapy in girls. Cancer. 1985;55(10):2364–72. doi: 10.1002/1097-0142(19850515)55:10<2364::aid-cncr2820551011>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.Koyama H, et al. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer. 1977;39(4):1403–9. doi: 10.1002/1097-0142(197704)39:4<1403::aid-cncr2820390408>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Byrne J, et al. Early menopause in long-term survivors of cancer during adolescence. Am J Obstet Gynecol. 1992;166(3):788–93. doi: 10.1016/0002-9378(92)91335-8. [DOI] [PubMed] [Google Scholar]
- 34.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7(6):535–43. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 35.Chiarelli AM, Marrett LD, Darlington GA. Pregnancy outcomes in females after treatment for childhood cancer. Epidemiology. 2000;11(2):161–6. doi: 10.1097/00001648-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Norwitz ER, et al. Placenta percreta and uterine rupture associated with prior whole body radiation therapy. Obstet Gynecol. 2001;98(5 Pt 2):929–31. doi: 10.1016/s0029-7844(01)01435-1. [DOI] [PubMed] [Google Scholar]
- 37.Larsen EC, et al. Radiotherapy at a young age reduces uterine volume of childhood cancer survivors. Acta Obstet Gynecol Scand. 2004;83(1):96–102. doi: 10.1111/j.1600-0412.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 38.Holm K, et al. Ultrasound B-mode changes in the uterus and ovaries and Doppler changes in the uterus after total body irradiation and allogeneic bone marrow transplantation in childhood. Bone Marrow Transplant. 1999;23(3):259–63. doi: 10.1038/sj.bmt.1701569. [DOI] [PubMed] [Google Scholar]
- 39.Laursen EM, et al. Doppler assessment of flow velocity in the uterine artery during pubertal maturation. Ultrasound Obstet Gynecol. 1996;8(5):341–5. doi: 10.1046/j.1469-0705.1996.08050341.x. [DOI] [PubMed] [Google Scholar]
- 40.Pridjian G, Rich NE, Montag AG. Pregnancy hemoperitoneum and placenta percreta in a patient with previous pelvic irradiation and ovarian failure. Am J Obstet Gynecol. 1990;162(5):1205–6. doi: 10.1016/0002-9378(90)90018-3. [DOI] [PubMed] [Google Scholar]
- 41.Li FP, et al. Outcome of pregnancy in survivors of Wilms’ tumor. JAMA. 1987;257(2):216–9. [PubMed] [Google Scholar]
- 42.Hawkins MM, Smith RA. Pregnancy outcomes in childhood cancer survivors: probable effects of abdominal irradiation. Int J Cancer. 1989;43(3):399–402. doi: 10.1002/ijc.2910430309. [DOI] [PubMed] [Google Scholar]
- 43.Green DM, Fine WE, Li FP. Offspring of patients treated for unilateral Wilms’ tumor in childhood. Cancer. 1982;49(11):2285–8. doi: 10.1002/1097-0142(19820601)49:11<2285::aid-cncr2820491114>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 44.Byrne J, et al. Reproductive problems and birth defects in survivors of Wilms’ tumor and their relatives. Med Pediatr Oncol. 1988;16(4):233–40. doi: 10.1002/mpo.2950160403. [DOI] [PubMed] [Google Scholar]
- 45.Green DM, et al. Pregnancy outcome of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Am J Obstet Gynecol. 2002;187(4):1070–80. doi: 10.1067/mob.2002.126643. [DOI] [PubMed] [Google Scholar]
- 46.Signorello LB, et al. Female survivors of childhood cancer: preterm birth and low birth weight among their children. J Natl Cancer Inst. 2006;98(20):1453–61. doi: 10.1093/jnci/djj394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mc CM, Keaty EC, Thompson JD. Conservation of ovarian tissue in the treatment of carcinoma of the cervix with radical surgery. Am J Obstet Gynecol. 1958;75(3):590–600. doi: 10.1016/0002-9378(58)90614-8. discussion 600–5. [DOI] [PubMed] [Google Scholar]
- 48.Cowles RA, Gewanter RM, Kandel JJ. Ovarian repositioning in pediatric cancer patients: Flexible techniques accommodate pelvic radiation fields. Pediatr Blood Cancer. 2007;49(3):339–41. doi: 10.1002/pbc.20652. [DOI] [PubMed] [Google Scholar]
- 49.Williams RS, Littell RD, Mendenhall NP. Laparoscopic oophoropexy and ovarian function in the treatment of Hodgkin disease. Cancer. 1999;86(10):2138–42. [PubMed] [Google Scholar]
- 50.Classe JM, et al. Ovarian transposition by laparoscopy before radiotherapy in the treatment of Hodgkin’s disease. Cancer. 1998;83(7):1420–4. [PubMed] [Google Scholar]
- 51.Kurt M, et al. Successful spontaneous pregnancy in a patient with rectal carcinoma treated with pelvic radiotherapy and concurrent chemotherapy: the unique role of laparoscopic lateral ovary transposition. Eur J Gynaecol Oncol. 2007;28(5):408–10. [PubMed] [Google Scholar]
- 52.Carde P, et al. Clinical staging versus laparotomy and combined modality with MOPP versus ABVD in early-stage Hodgkin’s disease: the H6 twin randomized trials from the European Organization for Research and Treatment of Cancer Lymphoma Cooperative Group. J Clin Oncol. 1993;11(11):2258–72. doi: 10.1200/JCO.1993.11.11.2258. [DOI] [PubMed] [Google Scholar]
- 53.Morice P, et al. Ovarian transposition for patients with cervical carcinoma treated by radiosurgical combination. Fertil Steril. 2000;74(4):743–8. doi: 10.1016/s0015-0282(00)01500-4. [DOI] [PubMed] [Google Scholar]
- 54.Sella T, Mironov S, Hricak H. Imaging of transposed ovaries in patients with cervical carcinoma. AJR Am J Roentgenol. 2005;184(5):1602–10. doi: 10.2214/ajr.184.5.01841602. [DOI] [PubMed] [Google Scholar]
- 55.Covens AL, et al. Laparoscopic ovarian transposition. Eur J Gynaecol Oncol. 1996;17(3):177–82. [PubMed] [Google Scholar]
- 56.Howell SJ, Shalet SM. Fertility preservation and management of gonadal failure associated with lymphoma therapy. Curr Oncol Rep. 2002;4(5):443–52. doi: 10.1007/s11912-002-0039-6. [DOI] [PubMed] [Google Scholar]
- 57.Gabriel DA, et al. Oophoropexy and the management of Hodgkin’s disease. A reevaluation of the risks and benefits. Arch Surg. 1986;121(9):1083–5. doi: 10.1001/archsurg.1986.01400090115021. [DOI] [PubMed] [Google Scholar]
- 58.Morice P, et al. Laparoscopic ovarian transposition for pelvic malignancies: indications and functional outcomes. Fertil Steril. 1998;70(5):956–60. doi: 10.1016/s0015-0282(98)00284-2. [DOI] [PubMed] [Google Scholar]
- 59.Kuohung W, et al. Laparoscopic oophoropexy prior to radiation for pediatric brain tumor and subsequent ovarian function. Hum Reprod. 2008;23(1):117–21. doi: 10.1093/humrep/dem368. [DOI] [PubMed] [Google Scholar]
- 60.Le Floch O, Donaldson SS, Kaplan HS. Pregnancy following oophoropexy and total nodal irradiation in women with Hodgkin’s disease. Cancer. 1976;38(6):2263–8. doi: 10.1002/1097-0142(197612)38:6<2263::aid-cncr2820380612>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 61.Lobo RA. Potential options for preservation of fertility in women. N Engl J Med. 2005;353(1):64–73. doi: 10.1056/NEJMra043475. [DOI] [PubMed] [Google Scholar]
- 62.Clough KB, Goffinet F, Labib A, et al. Laparoscopic Unilateral Ovarian Transposition prior to Irradiation. Cancer. 1996;77(12):2638–45. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2638::AID-CNCR30>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]