Abstract
The Drosophila gene CG14939 encodes a member of a highly conserved family of cyclins, the Y-type cyclins, which have not been functionally characterized in any organism. Here we report the generation and phenotypic characterization of a null mutant of CG14939, which we rename Cyclin Y (CycY). We show that the null mutant, CycYE8, is homozygous lethal with most mutant animals arresting during pupal development. The mutant exhibits delayed larval growth and major developmental defects during metamorphosis, including impaired gas bubble translocation, head eversion, leg elongation, and adult tissue growth. Heat-shock-induced expression of CycY at different times during development resulted in variable levels of rescue, the timing of which suggests a key function for zygotic CycY during the transition from third instar larvae to prepupae. CycY also plays an essential role during embryogenesis since zygotic null embryos from null mothers fail to hatch into first instar larvae. We provide evidence that the CycY protein (CycY) interacts with Eip63E, a cyclin-dependent kinase (Cdk) for which no cyclin partner had previously been identified. Like CycY, the Eip63E gene has essential functions during embryogenesis, larval development, and metamorphosis. Our data suggest that CycY/Eip63E form a cyclin/Cdk complex that is essential for several developmental processes.
CYCLINS are a family of highly conserved proteins that activate cyclin-dependent kinases (Cdks) to regulate the cell cycle, transcription, and other cellular processes. The founding members of the family, cyclins A and B, were first discovered as proteins that oscillated throughout the cell cycle, peaking in late G2 and M phases (Evans et al. 1983). These proteins were later shown to be required to activate the serine/threonine protein kinase, Cdc2 (also known as Cdk1), which is required for entry into M phase in most eukaryotes (Morgan 1997). Other cyclins with sequence similarity to cyclins A and B were subsequently identified and shown to be required at other points during the cell cycle (Murray 2004). The best characterized of these in metazoans include D-type cyclins, which partner with Cdk4 to control G1 phase events, and E-type cyclins, which partner with Cdk2 to control the transition from G1 to S phase. Several other members of the cyclin family do not show cell-cycle-dependent degradation or synthesis and some have been shown to play roles in cellular processes that are not directly related to cell cycle regulation. One group of cyclins, for example, regulates transcription by activating Cdks that can phosphorylate the carboxy-terminal tail of the large subunit of RNA polymerase II (Loyer et al. 2005). Several additional members of the cyclin family remain uncharacterized. Here we describe the initial characterization of one such novel cyclin encoded by the Drosophila gene CG14939, which we renamed Cyclin Y (CycY). Although this cyclin is highly conserved through evolution, no member of its family has been functionally characterized in any organism.
The defining feature of the cyclin family is a homologous region called the cyclin domain (Hadwiger et al. 1989; Hunt 1991), which includes the region responsible for interaction with a Cdk. Detailed studies on specific examples of Cdk/cyclin complexes have shown that the cyclin domain is essential and sufficient for interaction with and activation of the Cdk partner (Jeffrey et al. 1995; Morgan 1996). Thus, while specific Cdk partners have not been identified for every cyclin, all are thought to play the role of activating one or more Cdks. In addition to activating kinase activity, the cyclin may influence the substrate specificity or determine the subcellular localization of the active complex (Miller and Cross 2001).
Here we present data suggesting that one Cdk partner for Drosophila Cyclin Y is Ecdysone-induced protein 63E (Eip63E). The Eip63E gene encodes five highly related and apparently functionally redundant protein isoforms, all of which have homology to cyclin-dependent kinases (Sauer et al. 1996; Stowers et al. 2000). The proteins are most similar to the poorly characterized mammalian Cdks called PFTAIRE, so named because of the amino acid sequence in the conserved helix that binds to cyclins. Although a cyclin partner for Eip63E has not been identified, rescue experiments using mutant variants of the protein have suggested that its activity depends on cyclin binding (Stowers et al. 2000). In those experiments, mutation of a conserved glycine adjacent to the PFTAIRE (G243), which in other Cdks is required for cyclin binding, abolished the ability of an Eip63E transgene to rescue null Drosophila embryos to adulthood. Similarly, mutation of a conserved isoleucine (I249), which is also required for cyclin binding in other Cdks, diminished the ability of Eip63E to promote development. A directed yeast two-hybrid screen by Rascle et al. (2003) identified two potential regulators of Eip63E, Pif-1 and Pif-2, but neither of these proteins has any similarity to cyclins. In a high throughput yeast two-hybrid screen, however, one Eip63E-interacting protein that was identified was the CG14939 protein (Stanyon et al. 2004).
The name of Eip63E derives from the fact that one of the three transcription units of the Eip63E gene is induced in response to pulses of the steroid hormone ecdysone, which triggers crucial developmental transitions including metamorphosis. Phenotypic characterization of Eip63E loss-of-function mutants has shown that it has essential roles in several developmental processes (Stowers et al. 2000). The majority of zygotic null mutants die during larval development, while only a small percentage survive to pupation. The mutants that survive take 2–3 days longer to pupariate than their heterozygous siblings and are generally smaller than wild-type pupae. These phenotypes point to a role for Eip63E in larval development and metamorphosis and further suggest that this Cdk may be involved in growth control. Mutant eye clones, however, show no morphological or cell cycle defects, leading Stowers et al. (2000) to conclude that Eip63E does not regulate the cell cycle. Eip63E proteins have also been shown to be important for embryogenesis since zygotic null embryos from null mothers fail to hatch into first instar larvae. Interestingly, this maternal effect can be complemented by zygotic expression (Stowers et al. 2000). Thus, while it is clear that this ecdysone-inducible gene is important for metamorphosis and other developmental events, the molecular partners for Eip63E and the pathways in which it may function have yet to be discovered.
Here we report the generation of a null mutant allele of CycY and show that its phenotype is similar to that of Eip63E mutants. We show that CycY plays major essential roles during metamorphosis, especially during pupariation. We also show that maternally expressed CycY is essential for embryogenesis and that this requirement could be partially rescued by zygotic expression. Finally, we confirm that CycY and Eip63E specifically interact in Drosophila cells and show that the interaction depends on a conserved phosphorylation target on CycY, Ser389.
MATERIALS AND METHODS
Fly stocks:
The stocks carrying crol04418 (D'Avino and Thummel 1998), Eip63E81 (Stowers et al. 2000), Df(3L)GN50, also known as Eip63EGN50 (Stowers et al. 2000), P{Δ2-3} (Robertson et al. 1988), ovoD1 neoFRT40A (Chou and Perrimon 1996), Tubulin-Gal4, and Df(2L)Exel6030 were obtained from the Bloomington Stock Center (stock nos. 11374, 4513, 3687, 3664, 2121, 5138, and 7513, respectively). The XP insertion line d03228 (Thibault et al. 2004) was obtained from the Exelixis mutant collection at Harvard Medical School.
Plasmid cloning for P-element transformation:
pAS1 (A. Soans and R. L. Finley, unpublished results) is a modified pUAST (Brand and Perrimon 1993) vector encoding a myc-tag followed by 5′ and 3′ recombination tags (RTs) to facilitate cloning of open reading frames containing the same RTs from yeast two-hybrid vectors (Giot et al. 2003; Stanyon et al. 2004). A map of pAS1 is available at http://www.proteome.wayne.edu. pAS1–CycY was constructed by subcloning a fragment of the CycY cDNA beginning with the ATG and ending with the stop codon. The fragment was generated by PCR from the yeast two-hybrid clone using oligonucleotides (forward: 5′ TTGACTGTATCGCCGGAATTC; reverse: 5′ CCGGAATTAGCTTGGCTGCAG), which provided the 5′ and 3′ RTs at either end, respectively. The fragment was subcloned by gap repair in Escherichia coli (Parrish et al. 2004). The P{CycY} genomic clone was constructed by sequentially subcloning a 3.6-kb BamHI/NotI fragment and then a 4.0-kb AvrII/EcoRI fragment, each from BACR05B13 (BACPAC resources center), into pCaSpeR2 (Thummel et al. 1988). The whole insert is 7.3 kb, which includes the entire CycY gene and sequences 4032 bp upstream of the CycY start codon, and 1970 bp downstream of the stop codon, and includes none of the coding regions of crol or Pde1c. A P-element carrying the CycY cDNA expressed from a heat-shock promoter, pCaSpeR-hs-CycY, was constructed by subcloning the 1.5-kb HpaI/StuI fragment encoding myc–CycY from pAS1–CycY into the HpaI/StuI sites of pCaSpeR-hs (Thummel et al. 1988). P-element-mediated transformation was performed as previously described (Rubin and Spradling 1982).
Generation and molecular characterization of a CycY mutant allele:
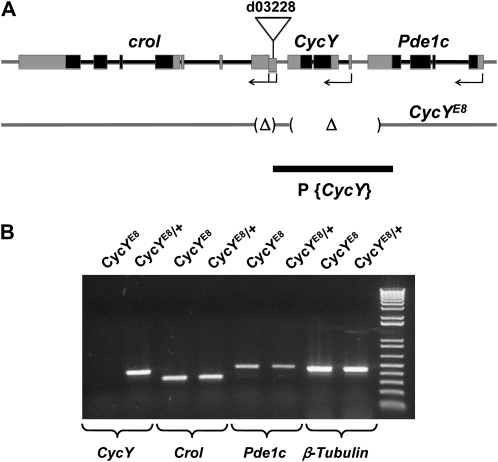
We used P-element imprecise excision to generate CycY mutant alleles. The starting P-element in d03228 is inserted 1958 bp downstream of the CycY stop codon and 5723 bp upstream of the crol start codon (Figure 1A). d03228 virgin females were mated with w*; CyO/Sp; Δ2-3, Sb/TM6 males, which provided P-transposase. F1 females (w*; d03228/CyO; Δ2-3, Sb/+) were then mated to w*; CyO/Sp; TM3, Ser/Sb males, and F2 progeny were screened for P-element excision by the reversion of eye color to white (Carney et al. 2004). One hundred white-eyed flies were collected and further balanced individually to make stocks. For each stock, genomic DNA was extracted from single flies and analyzed by PCR for the presence of CycY gene regions close to the d03228 insertion site. The excision that removes all CycY coding sequences was named E8 and was shown here to be a null allele of CycY (CycYE8). To make sure there is not a second site mutation on the same chromosome as CycYE8, the CycYE8 chromosome was cleaned up by homologous recombination with d03228 for seven generations. To determine the precise end point of the E8 deletion, genomic DNA was extracted from heterozygous CycYE8 adults and the region was amplified by PCR using primers 5′-GGGCCAAGCACAAATACAAACG-3′ and 5′-TGGTGAACGGCGAACAGAGC-3′. The PCR product, which is about 1 kb, was gel purified and sequenced from both ends. The deletion end points were determined by sequence alignment with wild-type genomic sequence. CycYE8 is missing 6119 bp of DNA from 734 bp downstream of the Pde1c stop codon to 1411 bp downstream of the CycY stop codon, and a second small region of 988 bp from 1955 bp downstream of the CycY stop codon to the first noncoding exon of crol transcripts RA-RC or the first intron of crol transcript RD. This removes the entire CycY transcript along with noncoding portions of the last exon of Pde1c and the first noncoding exon of crol (Figure 1A).
Figure 1.—
(A) The genomic region of CycY and the flanking genes crol and Pde1c. Exons of the CycY, crol, and Pde1c transcripts are indicated by boxes. Solid boxes represent coding regions and shaded boxes represent untranslated regions. Direction of transcription and approximate start sites are shown with arrows; Pde1c has five predicted transcripts that all start from the same position, CycY has one predicted transcript, and crol has three predicted transcripts (RA, RB, and RC) that start from the same position and one (RD) that starts further upstream as shown. The P-element in strain d03228 is inserted just upstream of exon 1 of crol transcripts RA-RC, and within exon 1 (offset box) of crol transcript RD. The two deleted regions in the CycYE8 allele, which was isolated by imprecise excision of the P-element in strain d03228, are indicated as Δ. The deletion removes the entire CycY gene, the first noncoding exon of crol, and a noncoding portion of the last exon of Pde1c. The genomic fragment used to create a transgene P{CycY} that complements CycYE8 is also depicted (solid bar). (B) Reverse-transcription PCR detecting expression of CycY, crol, Pde1c, or β-tubulin in homozygous CycYE8 or heterozygous CycYE8 (CycYE8/+) second instar larvae; the “+” chromosome is a CyO balancer with Act5C–GFP. A band of the expected size is detected for all genes in both genotypes, except for CycY in the homozygous CycYE8 larvae.
Lethal phase analysis:
Eggs were collected from w*; CycYE8/CyO, Act5C-GFP flies for 12 hr on apple juice plates with fresh yeast paste. After another 30 hr, the numbers of unhatched embryos and hatched first instar larvae were counted. Since homozygous CyO balancer is lethal during embryogenesis, an ∼75% hatching rate suggests no embryonic lethality. One hundred eighty w*; CycYE8/CyO, Act5C-GFP and 180 w*; CycYE8 (lacking GFP) first instar larvae were picked under a fluorescence dissection microscope and transferred into regular vials. The numbers of wandering third instar larvae, pupae, and adults were counted once a day for 15 days to score for a delay in puparium formation, progression through metamorphosis, and adult eclosion. To estimate the delay of puparium formation more accurately, we followed 180 first instar larvae for each genotype and calculated the average time to form pupa by counting the number of pupa newly formed after each 24-hr period and averaging over all individuals and days. Similarly, we analyzed the following animals: (1) w*; CycYE8/CyO, Act5C-GFP; P{CycY}, (2) w*; CycYE8; P{CycY}, (3) w*; CycYE8/CyO, Act5C-GFP and Df(2L)Exel6030/CyO, Act5C-GFP, (4) w*; CycYE8/Df(2L)Exel6030, (5) w*; CycYE8/CyO, Act5C-GFP; P{CycY} and w*; Df(2L)Exel6030/CyO, Act5C-GFP; P{CycY}, (6) w*; CycYE8/Df(2L)Exel6030; P{CycY}, (7) w*; Eip63E81/TM3, Ser Act5C-GFP and w*; Eip63EGN50/TM3, Ser Act5C-GFP, and (8) w*; Eip63E81/Eip63EGN50. All flies were incubated at 25° except where noted.
Phenotypic characterization:
Two hundred w*; CycYE8/CyO, Act5C-GFP and 200 w*; CycYE8 first instar larvae were collected (see above), transferred into individual fresh vials, and allowed to develop for 9 days. For each genotype, pupae at all developmental stages were collected, weighed, and imaged. The relative pupal length was measured on the basis of the image size. The average pupa weight and length were then calculated. To document the pupal phenotype, pupae at all developmental stages were carefully removed from the wall. For pharate adults, the pupal case was gently dissected. Images were taken with the Leica MZ 16FA stereomicroscope and Leica DFC 490 camera (kindly provided by Markus Friedrich). Similarly, we analyzed the following animals: (1) w*; CycYE8/CyO, Act5C-GFP; P{CycY}, (2) w*; CycYE8; P{CycY}, (3) w*; CycYE8/CyO, Act5C-GFP and Df(2L)Exel6030/CyO, Act5C-GFP, (4) w*; CycYE8/Df(2L)Exel6030, (5) w*; CycYE8/CyO, Act5C-GFP; P{CycY} and w*; Df(2L)Exel6030/CyO, Act5C-GFP; P{CycY}, (6) w*; CycYE8/Df(2L)Exel6030; P{CycY}, (7) w*; Eip63E81/TM3, Ser Act5C-GFP and w*; Eip63EGN50/TM3, Ser Act5C-GFP, and (8) w*; Eip63E81/Eip63EGN50.
Heat-shock induction and rescue efficiency:
Eggs from a self cross of CycYE8/CyO; hs-CycY/TM3, Ser flies were collected in glass vials with standard cornmeal Drosophila media for 24 hr and then heat shocked on each day as indicated in Figure 4. For each heat-shock induction, glass vials were incubated in a 37° water bath for 1 hr. Vials were kept at 25° otherwise. The numbers of flies with or without curly wings were counted separately. If CycYE8/CyO and CycYE8 flies have equal viability (full rescue), the number of CycYE8 flies should be half of that of CycYE8/CyO flies. The rescue efficiency was then determined by the number of CycYE8 adult flies divided by half of the number of CycYE8/CyO adult flies. The genotype of representative flies was confirmed by single-fly PCR.
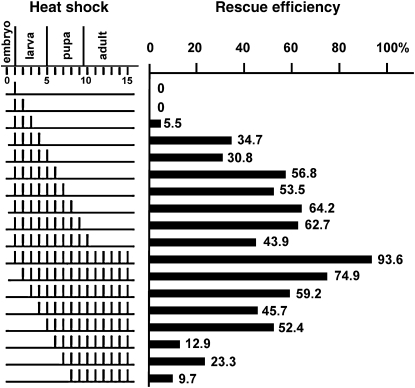
Figure 4.—
Temporal requirements for the expression of CycY. Embryos from a CycYE8/CyO; hs-CycY/TM3, Ser self-cross were collected for 24 hr and heat shocked for different regimes. Each row indicates a different heat-shock schedule. On the left side, each bar represents a single 1-hr heat shock at 37° on that particular day. The efficiencies of rescue to adulthood are shown on the right. The genotype of each adult was determined by the presence or absence of CyO and Ser balancer chromosomes (materials and methods). Representative adult genotypes were confirmed by single-fly PCR. For each condition, the total number of adults analyzed was between 200 and 300.
Generation of mosaic germline clones with homozygous CycYE8:
CycYE8 was recombined with FRT40A as previously described (Xu and Rubin 1993). Germline clones with homozygous CycYE8 were generated on the basis of the well-established approach of Chou and Perrimon (1996) with minor modification. w*; CycYE8 FRT40A/CyO females were crossed with hs-FLP/Y; ovoD1 FRT40A/CyO males for 3 days. Eggs were collected for 3 days and aged for 2 more days. Larvae, which were at either second or third instar stages, were heat shocked at 37° in a water bath for 2 hr. Females with straight wings (hs-FLP/w*; CycYE8 FRT40A/ovoD1 FRT40A) from the above cross were then mated to CycYE8/CyO, Act5C-GFP males to test for a maternal requirement for CycY. GFP positive (CycYE8 FRT40A/CyO, Act5C-GFP) and GFP negative (CycYE8 FRT40A/CycYE8) first instar larvae were picked and development was followed as described above.
Gene expression:
Gene expression was assayed by reverse-transcription PCR (Figure 1B) or quantitative real-time PCR (qPCR) (supporting information, Figure S5). Flies at the indicated developmental stages were collected and total RNA was extracted using the RiboPure kit (Ambion). The RNA samples were then treated with DNase from a DNA-free kit (Ambion) to remove contaminating genomic DNA. cDNAs were synthesized with a Transcriptor First Strand cDNA Synthesis kit (Roche) according to the manufacturer's protocol. qPCR reactions were performed using Brilliant SYBR Green QPCR Master Mix (Stratagene) in a 96-well plate. qPCR reactions were carried out in triplicate for each RNA sample. The primers used in this work are listed in Table S5. rp49 was used as the internal control and the RNA level of CycY was normalized to rp49 levels.
Co-affinity purification (co-AP) assays:
Co-AP assays were conducted by expressing pairs of N-terminally myc-tagged and NTAP-tagged proteins in Drosophila S2R+ cells, purification of the NTAP-tagged protein, and detection of associated myc-tagged proteins by immunoblotting. Myc-tagged proteins were expressed from pAS1. NTAP-tagged proteins were expressed from pDL4, a derivative of pUAST–NTAP (Veraksa et al. 2005) containing the NTAP tag followed by the same 5′ and 3′ RTs found in pAS1. Coding regions from the ATG to the stop codon of various Cdks or cyclins were amplified from yeast two-hybrid clones (Stanyon et al. 2004) with primers that added the 5′ and 3′ RTs and the products were subcloned into pAS1 or pDL4 by gap repair in E. coli. S2R+ cells were cotransfected with pairs of myc-tagged and NTAP-tagged plasmids along with pMT–Gal4 (Klueg et al. 2002), a Gal4 expression vector induced by CuSO4. Cells were grown in Schneider's Drosophila media (Invitrogen) supplemented with 10% FBS and 0.1 mg/ml gentamicin and were induced with 1 mm CuSO4. Cells were harvested 2 days after induction and lysed with 50 mm Tris-HCl pH 7.4, 180 mm NaCl, 5 mm EDTA, 1% NP-40, 10% glycerol, 50 mm NaF, 1 mm Na3VO4, 1× protease inhibitor cocktail (Roche). Clarified cell lysate was then incubated with rabbit IgG-conjugated agarose beads (Sigma) at 4° for 2 hr with shaking. After five washes with lysis buffer, co-purified proteins were eluted with 1× LDS sample loading buffer (Invitrogen), resolved by SDS–PAGE and detected by Western blotting using mouse anti-myc primary antibody (Santa Cruz) and goat anti-mouse HRP conjugated secondary antibody (BioRad) or goat anti-protein A peroxidase-conjugated antibody (Rockland Immunochemicals).
RESULTS AND DISCUSSION
CycY is a conserved uncharacterized cyclin:
Drosophila CG14939 has a single predicted transcript that encodes a protein with 406 residues (Figure 1A, Figure S1, B). Between amino acids 205 and 328 lies a cyclin domain, a conserved region that defines the cyclin family of proteins. The closest human homolog of CG14939 is a poorly characterized gene called Cyclin Y (CCNY). Genes in a number of other species have also been named Cyclin Y on the basis of their sequence similarity to human CCNY. CG14939 is more similar to the Y cyclins from other species than it is to any other Drosophila melanogaster gene (Figure S1 and Table S1), indicating that it belongs to this orthologous family of proteins. We therefore renamed CG14939 Cyclin Y (CycY). Outside of the cyclin domain the protein has virtually no sequence similarity to other cyclins. However, CycY has been highly conserved through evolution. Clear CycY orthologs are found in all metazoans with fully sequenced genomes, including bilaterians (e.g., insects, nematodes, vertebrates), cnidarians (e.g., the sea anenome, Nematostella vectensis), and the placozoan, Trichoplax adhaerens. Cyclin Y is also found in the choanoflagellate, Monosiga brevicollis, the closest known unicellular relative of metazoans, suggesting that the Y-type cyclins originated prior to the first multicellular species. Cyclin Y proteins from all of these species share substantial sequence similarity over most of their length, including regions outside of the cyclin domain (Figure S1, B). In contrast, plants, fungi, and other nonmetazoan species do not have proteins with extensive sequence similarity to CycY, though they do contain the CycY-specific cyclin domain; this cyclin domain is distinct from other cyclin domains and appears to be conserved throughout the eukaryotic kingdom (Figure S2). In metazoan species the level of CycY conservation is particularly high. For example, the Drosophila protein shares 52% identity with the human CCNY protein. This level of conservation is much higher than that observed for the cell cycle cyclins (e.g., cyclins A, B, D, and E), which share between 20 and 41% identity between human and Drosophila (Finley et al. 1996). This suggests that CycY has an important and potentially conserved function. Surprisingly, the function of Cyclin Y has not been studied and CycY mutants have not been reported for any model organism.
Generation of a CycY mutant:
To determine the function of Drosophila CycY, we set out to generate a loss-of-function mutant allele. We took advantage of the availability of a strain, d03228, bearing a P-element inserted 1958 bp downstream of the CycY stop codon and 5723 bp upstream of the start codon of the neighboring gene, crol (Figure 1A). This insertion itself has no visible effect on the function of any genes in this region since the homozygous d03228 adults are completely viable and normal. We used imprecise excision to generate a small deletion around the original P-element. The deletion, E8, completely removed the CycY coding region while leaving the coding regions of the neighboring genes intact (Figure 1A), as determined by PCR and sequencing (materials and methods). Expression of the neighboring genes, crol and Pde1c, was confirmed using RNA extracted from homozygous and heterozygous E8 second instar larvae (Figure 1B). In contrast, CycY transcription was undetectable in homozygous E8 larvae. Hereafter we refer to the E8 deletion as CycYE8.
Two additional lines of evidence indicate that CycY is the only gene affected in strain CycYE8. First, CycYE8 fully complemented crol04418, a lethal null allele of the neighboring gene (D'Avino and Thummel 1998); crol04418 also complemented the mutant phenotype of CycYE8 (see below). Thus, although CycYE8 lacks the first noncoding exon of crol, a crol transcript is expressed and appears to be fully functional. Second, as described in detail below, all of the abnormalities that we observed in homozygous CycYE8 mutants can be rescued either by a CycY genomic transgene (Figure 1A) or by ubiquitous expression of a CycY cDNA using heat-shock induction (see below). Combined these results indicate that the CycYE8 mutant strain is a null mutant for CycY.
CycY null mutants show delayed entry into pupariation and are pupal lethal:
Homozygous CycYE8 mutants or CycYE8 over a deficiency that removes CycY (Df(2L)Exel6030) produce no viable adults, indicating that CycY is an essential gene. To analyze the lethal phase, eggs from a self-cross of CycYE8/CyO flies were collected for 12 hr and aged for another 30 hr. Of 366 embryos examined, 89 (24.3%) remained unhatched while 277 (75.7%) hatched to first instar larvae. Since ∼25% of the embryos from this cross should be homozygous CyO, which is lethal during embryogenesis, one-third of the embryos that hatched should be homozygous CycYE8, indicating that zygotic expression of CycY is not essential for embryogenesis.
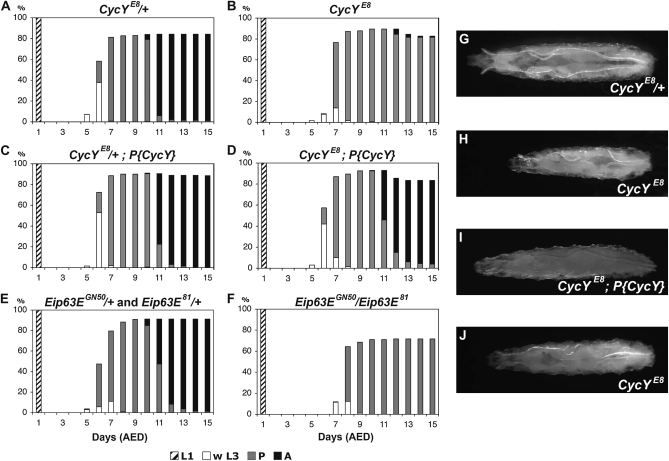
To evaluate whether CycY is required during larval and pupal development, we picked 180–200 first instar larvae of CycY null mutants (homozygous CycYE8 or CycYE8/Df(2L)Exel6030) or their siblings and followed their morphology and development for 15 days, after which no additional adults eclosed. CycY null mutants did not show obvious larval lethality since the majority (90 or 93%) of first instar larvae developed into pupae, which is a rate similar to their heterozygous siblings (84 or 94%, respectively) (Table S2). However, we did observe delayed growth during larval development. By the time third instar larvae in the heterozygous group started to wander, CycY null mutant larvae were still at the feeding stage and exhibited dramatically smaller body sizes (Figure 2, A, B, G, and H; Figure S3, A and B). The CycYE8 homozygotes eventually grew to sizes that were 80–90% of the heterozygotes before pupariation (Figure 2, G and J). The delay in larval growth could be rescued with a genomic CycY transgene (Figure 2, C, D, and I; Figure S3, C and D). The delay was also evident in the timing of pupariation. As shown in Figure 2, A and B, the first pupa of CycYE8 heterozygotes was observed at 6 days after egg deposition (AED), while the first pupa of CycYE8 homozygotes was observed at 7 days AED. On the basis of the number of pupae that formed each day in the two strains, we estimated that puparium formation of CycYE8 homozygous mutants was delayed for ∼13 hr relative to that of the heterozygous controls (materials and methods). The genomic CycY transgene shortened this delay to ∼5 hr. Similar results were obtained with the CycYE8/Df(2L)Exel6030 mutant (Figure S3).
Figure 2.—
Developmental timing of CycY and Eip63E mutants. (A–F) The development of 180 first instar larvae (L1) of each genotype was followed for 15 days. Genotypes include heterozygous CycYE8 (A and C) or homozygous CycYE8 (B and D). Larvae in C and D harbored a genomic CycY transgene on the third chromosome (P{CycY}). Larvae heterozygous for the Eip63E mutants, Eip63EGN50 or Eip63E81 (E), or transheterozygous Eip63EGN50/Eip63E81 (F) were also analyzed. The percentage of first instar larvae that developed into wandering third instar larvae (w L3), pupae (P), and adults (A) on each day after egg deposition (AED) is shown. (G–J) Typical third instar larvae of CycYE8/+ (G), CycYE8 (H), CycYE8; P{CycY} (I) at the same time point and CycYE8 after an additional day (J). In A, C, and G, the plus symbol (+) stands for an Act5C–GFP-marked CyO balancer chromosome; in E, + stands for an Act5C–GFP-marked TM3, Ser balancer chromosome.
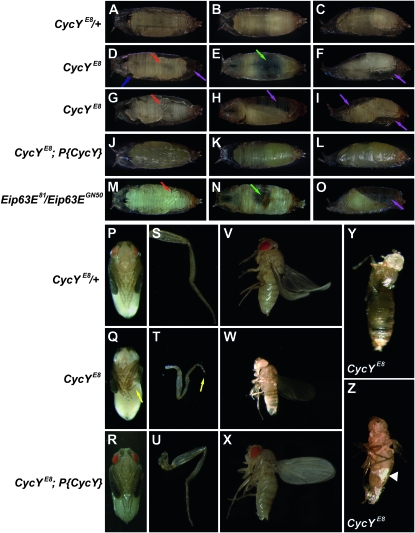
CycY null mutants were arrested predominately during pupal stages, but with variable expressivity. We scored the final developmental stages of animals from each genotype on the basis of the presence of defined morphological markers (Bainbridge and Bownes 1981). Two major lethal phases were observed. The early lethal phase was between pupal stages P3 and P5; for example, all 162 CycYE8 mutants that pupated developed to stage P3 but only 61% reached stage P5 (Table S2). In contrast, all of the 152 heterozygotes that pupated reached stage P5, and all but two eventually emerged as adults. The CycY null pupae that were arrested at stage P3 or P4 showed a variety of developmental defects, including defects in gas bubble translocation, head eversion, leg elongation, and adult tissue growth (Figure 3 and Table S3). Many mutant individuals stopped further development with the newly formed gas bubble still in the middle of the abdomen (Figure 3E). In others the gas bubble translocated to the posterior portion of the puparium as in wild type, but then failed to completely relocate to the anterior (Figure 3H), which may hinder head eversion (Chadfield and Sparrow 1985). Many of the mutant pupae showed different amounts of empty space inside the pupal case (Figure 3, D–I), which was probably due either to the failure of gas bubble translocation or to insufficient adult tissue growth. A defect in leg elongation was also prevalent. Some mutant individuals had partially elongated legs that were either shorter than normal and did not reach the bottom of the abdomen or were bent (e.g., Figure 3G). More severe cases showed no sign of leg elongation (Figure 3D). Wings also did not achieve full extension. The CycYE8/Df(2L)Exel6030 mutant had the same range of phenotypes as homozygous CycYE8 (Table S3 and Figure S4).
Figure 3.—
Metamorphosis defects in CycY and Eip63E mutants. (A–O) Representative early pupae from ventral, dorsal, and lateral views (left, middle and right columns, respectively). Genotypes include CycYE8/+ (A–C), homozygous CycYE8 (D–I), homozygous CycYE8 with the P{CycY} transgene (J–L), and Eip63E81/Eip63EGN50 (M–O). Defects are indicated by colored arrows. The CycYE8 homozygous mutant early pupae (second and third rows) and Eip63E null mutant early pupae (fifth row) show defects of leg elongation (red), head eversion (blue), gas bubble translocation (green), and adult tissue growth (purple). Early pupae of CycYE8 homozygotes with a genomic CycY transgene have no defects (fourth row). (P–R) Representative pharate adults of CycYE8/+ (P), homozygous CycYE8 (Q), and homozygous CycYE8 with the P{CycY} transgene (R). Homozygous CycYE8 mutant pharate adults have an obvious bent leg phenotype (yellow arrow in Q. CycYE8 homozygous mutant adult escapers either die soon after eclosion or survive for <2 days and have a much smaller body size (W) than heterozygous control adults (V) or CycYE8 mutants complemented with the P{CycY} transgene (X). Many of the adult escapers had malformed legs (T, yellow arrow), whereas legs were normal in heterozygous control adults (S), or CycYE8 mutants complemented with the P{CycY} transgene (U). In CycYE8 mutants arrested during eclosion (Y and Z), when the pupal case was manually removed (Z) a layer of white tissue (arrowhead) was evident. “+” stands for a CyO balancer chromosome with Act5C–GFP.
The late lethal phase of the CycY null was between stages P14 and P15, almost at the end of pupal development. For example, while 41% of the CycYE8 homozygous pupae reached stage P14, only 13% reached stage P15 (Table S2). The P14-arrested mutants exhibited the prominent malformed leg phenotype that was also observed during earlier pupal stages (Figure 3Q). In addition to the morphological defects, CycY null pupae were generally shorter and much lighter than wild-type pupae (Table 1).
TABLE 1.
CycY and Eip63E mutant pupae are smaller than wild-type pupae
| Average weighta |
Average lengthb |
|||
|---|---|---|---|---|
| Genotypec | mg (%) | n | % ± SD | n |
| CycYE8/+ | 1.12 (100) | 161 | 100 ± 4.0 | 31 |
| CycYE8/+ and Df(2L)Exel6030/+ | 1.18 (100) | 239 | 100 ± 4.8 | 44 |
| CycYE8 | 0.65 (58) | 137 | 90 ± 8.6 | 36 |
| CycYE8/Df(2L)Exel6030 | 0.75 (64) | 230 | 92 ± 6.4 | 44 |
| CycYE8/+; P{CycY} | 1.19 (100) | 265 | 100 ± 4.9 | 72 |
| CycYE8; P{CycY} | 1.13 (95) | 239 | 102 ± 5.0 | 72 |
| CycYE8/+; P{CycY} and Df(2L)Exel6030/+; P{CycY} | 1.21 (100) | 246 | 100 ± 4.4 | 47 |
| CycYE8/Df(2L)Exel6030; P{CycY} | 1.14 (94) | 279 | 101 ± 4.2 | 48 |
| Eip63EGN50/+ and Eip63E81/+ | 1.25 (100) | 104 | 100 ± 3.7 | 30 |
| Eip63EGN50/Eip63E81 | 0.75 (60) | 142 | 90 ± 5.3 | 40 |
Percentage of average weight is calculated relative to heterozygous siblings (100%).
Percentage of average length is calculated relative to heterozygous siblings (100%).
The plus symbol (+) stands for an Act5C-GFP-marked balancer chromosome; either CyO with CycYE8 and Df(2L)Exel6030 or TM3, Ser with Eip63EGN50 and Eip63E81.
Among the small fraction of CycYE8 pupae that reached stage P15, 8 out of 23 (35%) arrested during the process of eclosion. The remainder eclosed into adults, but the majority (13 out of 15) died very quickly with their wings still folded. Most of these adults displayed short bent legs (Figure 3T). Only two animals successfully eclosed into adults that looked normal, though they were smaller than newly emerged heterozygous control adults (Figure 3, V and W) and they survived for <2 days. When the mutants that were arrested during eclosion were manually dissected from the pupal case, a layer of white tissue could be seen, which seemed to adhere adult structures to the inside wall of the pupal case (Figure 3, Y and Z). All of the CycY null mutant defects described above could be rescued by introduction of a CycY genomic transgene (Figure 3, Table 1, Table S2, Table S3, and Figure S4).
The expression of CycY is essential during the transition from third instar larvae to prepupae:
The null mutant phenotype of CycY suggested an important function during metamorphosis. To determine the developmental time point at which CycY expression is required, we generated transgenic flies that expressed myc-tagged CycY from a heat-shock promoter. A series of different heat-shock regimes was performed to compare their ability to rescue the lethality of homozygous CycYE8 (Figure 4). Heat shock on the first 3 days after egg laying failed to rescue the viability of homozygous CycYE8 mutants. However, when heat shock was extended for 1 or 2 more days, which included late third instar larvae, the rescue ability was dramatically increased to 30–35%. If CycY was also provided during early pupal stages, the rescue ability increased further to 50–60%. If CycY expression was withheld until 4 days after egg laying, a 50% rescue rate could still be achieved. However, if heat shock was delayed for 1 more day, the rescue ability decreased to only 13% (Figure 4). Combined, these data suggest that the most important period for zygotic CycY expression is from the late larvae to the early stages of pupal development, consistent with the first major lethal phase of the CycYE8 mutant.
To see whether CycY is expressed at the developmental times when it appears to be needed, we used quantitative real-time PCR to determine the CycY mRNA levels. We found that the relative abundance of CycY mRNA fluctuated over a narrow range during development (Figure S5). The highest mRNA level was observed in 0- to 1-hr embryos, most likely due to maternal deposition. CycY message levels then decreased from later embryogenesis through the first and second instar larval stages but increased again in third instar larvae and peaked at pupal stages. The transcription variation of CycY is thus consistent with its essential requirement for pupariation.
CycY shows a maternal effect that can be partially rescued by zygotic expression:
The mutant phenotypes described above were based on zygotic null mutants, which showed normal embryogenesis and slow but otherwise normal larval development. To test whether maternally expressed CycY contributes to early development, we generated maternal null mutants using the ovoD1 dominant female sterile technique (Chou et al. 1993). Hs-FLP/w*; CycYE8 FRT40A/ovoD1 FRT40A females were heat shocked for 2 hr during larval development to express FLP recombinase and promote homologous recombination between the CycYE8 FRT40A and ovoD1 FRT40A chromosomes. Since ovoD1 is dominant female sterile, mothers will lay eggs only if homozygous CycYE8 FRT40A germline cells are generated and CycY is not essential for oogenesis. Mothers that received heat-shock treatment during larval development were crossed with w1118 males and the number and development of the eggs laid were monitored. We observed that heat-shock treated CycYE8 FRT40A/ovoD1 FRT40A females could lay similar numbers of eggs as heat-shock treated FRT40A/ovoD1 FRT40A females, indicating that CycY is not essential for at least some of the major processes of oogenesis. However, ∼40% of the eggs from CycYE8 mothers had fused dorsal appendages (data not shown), suggesting that CycY may play a role in axis specification.
To test for a maternal contribution to embryogenesis, females with homozygous CycYE8 germline cells were generated using the ovoD1 dominant female sterile technique (Chou et al. 1993), and were crossed with CycYE8/CyO, Act5C-GFP males. Zygotic null progeny were identified by absence of the GFP balancer. Interestingly, the majority (99.6%) of zygotic null embryos from null mothers failed to hatch, suggesting that maternal expression of CycY is essential for embryogenesis. Surprisingly, when females with homozygous CycYE8 germline cells were crossed with w1118 males, 7.3% of the embryos hatched into first instar larvae and 73% of these larvae developed into normal adults. Taken together, these data suggest that maternally provided CycY plays an important role during embryogenesis, but that this role can be accomplished at least to a limited extent by zygotic expression.
Eip63E is a potential binding partner of CycY:
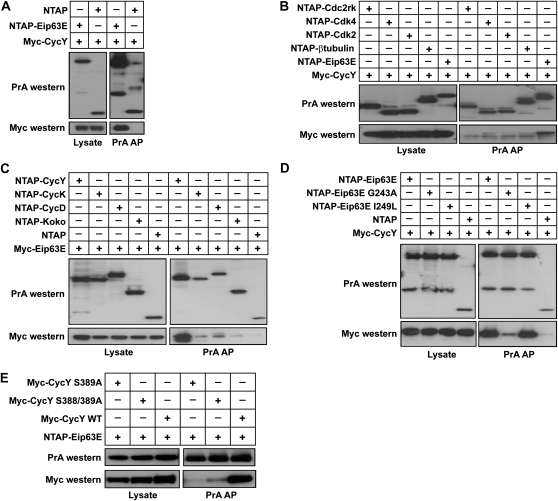
Cyclin proteins generally serve as regulatory subunits for Cdks. In a previous high throughput yeast two-hybrid screen (Stanyon et al. 2004) we identified an interaction between CycY and Eip63E, a Cdk with no known cyclin partner (Rascle et al. 2003; Stowers et al. 2000). To test specificity, we conducted additional two-hybrid assays using additional Cdks and cyclins (File S1 and Table S4). We found that CycY interacted only weakly or not at all with other Cdks, including Cdk1, Cdk2, Cdk4, Cdk5, Cdk7, Cdc2rk, and CG7597. Likewise, Eip63E interacted with CycY and CycC, a protein known to be promiscuous in two-hybrid assays, but only weakly or not at all with CycA, CycB, CycB3, CycD, CycE, CycG, CycH, CycJ, CycK, CycT, Koko, and CG16903. To further confirm and test the specificity of the Eip63E–CycY interaction, we expressed tagged versions of Cdks and cyclins in cultured Drosophila cells and tested interaction by co-AP followed by immunoblotting (materials and methods). In the co-AP assay, CycY interacted strongly with Eip63E but only weakly or not at all with Cdk2, Cdk4, or Cdc2rk (Figure 5, A and B). Eip63E, on the other hand, interacted much more strongly with CycY than with other cyclins tested, including CycK, CycD, and CG31232 (Koko) (Figure 5C). As expected, Glycine 243 (G243) of Eip63E, which is essential for its function in vivo (Stowers et al. 2000), is required for binding to CycY (Figure 5D). In further support of the interaction between these proteins, a recent study demonstrated an interaction between the human homolog of Eip63E, PFTK1, and human CycY using yeast two-hybrid and co-AP assays from human cells (Jiang et al. 2009). Taken together, our data and the studies with the human orthologs support the notion that CycY and Eip63E constitute a conserved cyclin–Cdk pair.
Figure 5.—
CycY preferentially interacts with Eip63E in Drosophila cells. Cells were cotransfected with the indicated constructs and lysed for co-affinity purification (co-AP) using IgG beads. Co-purified proteins were further detected by Western blot using anti-Myc or anti-protein A (PrA) antibody. (A) CycY interacts with Eip63E. (B) CycY interacts much more strongly with Eip63E than with Cdk2, Cdk4, or Cdc2rk. (C) Eip63E interacts much more strongly with CycY than with CycD, CycK, or CG31232 (Koko). (D) Eip63E G243A mutant interacts poorly with CycY. (E) CycY S389A mutants display decreased affinity for Eip63E.
A recent large-scale phosphoproteome study in Drosophila embryos identified several phosphorylated peptides from the CycY protein (Zhai et al. 2008). A number of the phosphorylation sites are in highly conserved serine residues, suggesting that they may affect CycY function (Figure S1, B). One of these residues, S389, has also been found to be phosphorylated in human CycY, both in nuclear and cytoplasmic fractions (Beausoleil et al. 2004; Olsen et al. 2006). Position Ser389 in the Drosophila protein is conserved in every species that we examined (Figure S1, B). Moreover in one of the two preceding positions of every CycY there is another serine (S388 in Drosophila), which was also identified as a phosphorylated residue in the human protein. As a first test of the potential importance of these residues we generated a Drosophila CycY S389A mutant and S388A/S389A double mutant and tested their Cdk-binding ability. The Ser389A mutant had a dramatically decreased ability to bind Eip63E (Figure 5E). The double mutant did not further diminish Cdk binding, indicating that S388 does not contribute to the interaction. While these results point to a role for S389 in Cdk interaction, we were unable to show that phosphorylation is important, since a S389E mutant also failed to interact with the Cdk (data not shown).
CycY and Eip63E have similar mutant phenotypes:
If Eip63E and CycY form a functional Cdk/cyclin complex in vivo, we might expect their mutant phenotypes to be similar. Previous studies have shown that Eip63E is important for embryogenesis, larval development, and morphogenesis (Stowers et al. 2000). Those studies demonstrated that the majority of Eip63E null mutants die during larval development, while a small percentage survive to pupal stages with an occasional adult escaper. Stowers et al. (2000) also showed that puparium formation in Eip63E mutants is delayed by 2–3 days, pupae are small, and the rare adult escapers have a bent-leg phenotype and short life spans. All of these phenotypes are similar to those we observed for CycYE8. To further compare the Eip63E and CycY loss-of-function phenotypes, we performed a detailed side-by-side phenotypic characterization. We used a transheterozygous null mutant, Eip63E81/Eip63EGN50 (Stowers et al. 2000) and compared its phenotype with that of CycYE8. We found that CycY and Eip63E null mutants showed similar developmental defects, though the Eip63E null mutant phenotype was generally more severe. Both mutants displayed a major lethal phase during metamorphosis (Figure 2, A, B, E, and F). While CycY mutants showed lethality during early or late pupal stages, the majority of Eip63E mutants died at earlier pupal stages (Table S2). Both mutants also showed similar metamorphosis defects, including gas bubble translocation defects, failed head eversion, and leg elongation defects (Figure 3; Table S3). In addition, pupae of both mutants were similarly small in weight and length (Table 1). Finally, both mutants exhibited delayed puparium formation, for 13 hr in the case of CycY, and 37 hr for Eip63E (Figure 2). We also note that Stowers et al. (2000) showed that Eip63E has a zygotically rescuable maternal contribution to embryogenesis, similar to our observation for CycY. The striking similarity between the mutant phenotypes of Eip63E and CycY, combined with the specific physical interaction between the proteins in yeast two-hybrid and co-AP assays, supports the idea that CycY and Eip63E may function together in vivo. We cannot exclude the possibility, however, that one or both proteins have additional partners. For example, one potential explanation for the earlier lethality and more severe phenotype of Eip63E mutants relative to the CycY null is that Eip63E may have functions independent of CycY and these may involve other cyclin partners. Alternatively, the subtle differences in CycY and Eip63E mutant phenotypes may be due to differences in the levels of perdurance of their maternal components. Further in vivo analysis of the interaction will be needed to distinguish these possibilities.
Conclusions:
Cyclin Y is a highly conserved protein that has not been characterized in any model organism. Only minimal information is available for the human ortholog, CCNY. The gene is broadly expressed in human tissues, with particularly high levels in testis (Jiang et al. 2009; Li et al. 2009). Localization studies with GFP fusions in cell lines have shown that one isoform of human CycY, which has also been called Cyclin X, is nuclear while another isoform may be anchored to the cell membrane via a conserved myristoylation signal (Jiang et al. 2009). Recently, CCNY was identified as a potential susceptibility factor for inflammatory bowel disease (IBD), a complicated genetic disorder affecting the intestinal mucosa. A single nucleotide polymorphism (SNP) located in an intron of CCNY was found to be strongly associated with the two IBD subphenotypes, Crohn's disease and ulcerative colitis (Franke et al. 2008; Weersma et al. 2009), though it is not yet clear whether CCNY plays a direct role in these diseases. Another study found that human CycY is among a number of proteins that are significantly upregulated in metastatic colorectal cancer cells (Ying-Tao et al. 2005), though again it is not clear whether this cyclin contributes to the phenotype of these cells. The establishment of a CycY-deficient animal model could provide a system for studying conserved functions of Cyclin Y and for understanding its potential role in human diseases.
Here we described the first mutant allele for a Y-type cyclin, a null for Drosophila CycY. We showed that CycY is an essential gene that is required for a broad range of developmental processes, including normal oogenesis, embryogenesis, larval and pupal development. The most obvious defects in the null were visualized during pupal development, and included defects in gas bubble translocation, head eversion, leg elongation, and adult tissue growth. Similar phenotypes have been described for a number of genes involved in the response to the steroid hormone ecdysone, including E74, EcR, BR-C, and crol, (Kiss et al. 1988; Fletcher and Thummel 1995; Bender et al. 1997; D'Avino and Thummel 1998). CycY may also be involved in the ecdysone response. Consistent with this possibility, we provide several lines of evidence, suggesting that at least one of the Cdk partners for CycY is the ecdysone-inducible protein, Eip63E. CycY and Eip63E preferentially interact in yeast two-hybrid assays and in co-AP assays from cultured Drosophila cells. The human orthologs of these proteins have also been shown to interact and to colocalize in human cell lines (Jiang et al. 2009). Finally, the mutations in Eip63E and CycY show a similar range of phenotypes. Our findings in Drosophila should provide a model system for further biochemical and genetic studies on the function of this conserved Cdk/cyclin pair.
Acknowledgments
We thank Victoria Meller and Stephen Guest for comments on the manuscript, Huamei Zhang and Aleric Soans for reagents and technical assistance, and Govindaraja Atikukke and members of the Finley lab for helpful discussions. We also thank Markus Friedrich for use of equipment and Alexey Veraksa and Spyros Artavanis-Tsakonas for reagents. This work was supported in part by National Institutes of Health grant R01HG001536.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.114017/DC1.
References
- Bainbridge, S. P., and M. Bownes, 1981. Staging the metamorphosis of Drosophila melanogaster. J. Embryol. Exp. Morphol. 66 57–80. [PubMed] [Google Scholar]
- Beausoleil, S. A., M. Jedrychowski, D. Schwartz, J. E. Elias, J. Villen et al., 2004. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc. Natl. Acad. Sci. USA 101 12130–12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, M., F. B. Imam, W. S. Talbot, B. Ganetzky and D. S. Hogness, 1997. Drosophila ecdysone receptor mutations reveal functional differences among receptor isoforms. Cell 91 777–788. [DOI] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 401–415. [DOI] [PubMed] [Google Scholar]
- Carney, G. E., A. Robertson, M. B. Davis and M. Bender, 2004. Creation of EcR isoform-specific mutations in Drosophila melanogaster via local P element transposition, imprecise P element excision, and male recombination. Mol. Genet. Genomics 271 282–290. [DOI] [PubMed] [Google Scholar]
- Chadfield, C. G., and J. C. Sparrow, 1985. Pupation in Drosophila melanogaster and the effect of the lethalcryptocephal mutation. Dev. Genet. 5 103–114. [Google Scholar]
- Chou, T. B., and N. Perrimon, 1996. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, T. B., E. Noll and N. Perrimon, 1993. Autosomal P[ovoD1] dominant female-sterile insertions in Drosophila and their use in generating germ-line chimeras. Development 119 1359–1369. [DOI] [PubMed] [Google Scholar]
- D'Avino, P. P., and C. S. Thummel, 1998. crooked legs encodes a family of zinc finger proteins required for leg morphogenesis and ecdysone-regulated gene expression during Drosophila metamorphosis. Development 125 1733–1745. [DOI] [PubMed] [Google Scholar]
- Evans, T., E. T. Rosenthal, J. Youngblom, D. Distel and T. Hunt, 1983. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33 389–396. [DOI] [PubMed] [Google Scholar]
- Finley, Jr., R. L., B. J. Thomas, S. L. Zipursky and R. Brent, 1996. Isolation of Drosophila cyclin D, a protein expressed in the morphogenetic furrow before entry into S phase. Proc. Natl. Acad. Sci. USA 93 3011–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, J. C., and C. S. Thummel, 1995. The ecdysone-inducible Broad-complex and E74 early genes interact to regulate target gene transcription and Drosophila metamorphosis. Genetics 141 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke, A., T. Balschun, T. H. Karlsen, J. Hedderich, S. May et al., 2008. Replication of signals from recent studies of Crohn's disease identifies previously unknown disease loci for ulcerative colitis. Nat. Genet. 40 713–715. [DOI] [PubMed] [Google Scholar]
- Giot, L., J. S. Bader, C. Brouwer, A. Chaudhuri, B. Kuang et al., 2003. A protein interaction map of Drosophila melanogaster. Science 302 1727–1736. [DOI] [PubMed] [Google Scholar]
- Hadwiger, J. A., C. Wittenberg, M. A. de Barros Lopes, H. E. Richardson and S. I. Reed, 1989. A family of cyclin homologs that control G1 phase in yeast. Proc. Natl. Acad. Sci. USA 86 6255–6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt, T., 1991. Cyclins and their partners: from a simple idea to complicated reality. Semin. Cell Biol. 2 213–222. [PubMed] [Google Scholar]
- Jeffrey, P. D., A. A. Russo, K. Polyak, E. Gibbs, J. Hurwitz et al., 1995. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature 376 313–320. [DOI] [PubMed] [Google Scholar]
- Jiang, M., Y. Gao, T. Yang, X. Zhu and J. Chen, 2009. Cyclin Y, a novel membrane-associated cyclin, interacts with PFTK1. FEBS Lett. 583 2171–2178. [DOI] [PubMed] [Google Scholar]
- Kiss, I., A. H. Beaton, J. Tardiff, D. Fristrom and J. W. Fristrom, 1988. Interactions and developmental effects of mutations in the broad-complex of Drosophila melanogaster. Genetics 118 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klueg, K. M., D. Alvarado, M. A. Muskavitch and J. B. Duffy, 2002. Creation of a GAL4/UAS-coupled inducible gene expression system for use in Drosophila cultured cell lines. Genesis 34 119–122. [DOI] [PubMed] [Google Scholar]
- Li, X., X. Wang, G. Liu, R. Li and L. Yu, 2009. Identification and characterization of cyclin X which activates transcriptional activities of c-Myc. Mol. Biol. Rep. 36 97–103. [DOI] [PubMed] [Google Scholar]
- Loyer, P., J. H. Trembley, R. Katona, V. J. Kidd and J. M. Lahti, 2005. Role of CDK/cyclin complexes in transcription and RNA splicing. Cell Signal 17 1033–1051. [DOI] [PubMed] [Google Scholar]
- Miller, M. E., and F. R. Cross, 2001. Cyclin specificity: How many wheels do you need on a unicycle? J. Cell Sci. 114 1811–1820. [DOI] [PubMed] [Google Scholar]
- Morgan, D. O., 1996. The dynamics of cyclin dependent kinase structure. Curr. Opin. Cell Biol. 8 767–772. [DOI] [PubMed] [Google Scholar]
- Morgan, D. O., 1997. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 13 261–291. [DOI] [PubMed] [Google Scholar]
- Murray, A. W., 2004. Recycling the cell cycle: cyclins revisited. Cell 116 221–234. [DOI] [PubMed] [Google Scholar]
- Olsen, J. V., B. Blagoev, F. Gnad, B. Macek, C. Kumar et al., 2006. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127 635–648. [DOI] [PubMed] [Google Scholar]
- Parrish, J. R., T. Limjindaporn, J. A. Hines, J. Liu, G. Liu et al., 2004. High-throughput cloning of Campylobacter jejuni ORfs by in vivo recombination in Escherichia coli. J. Proteome Res. 3 582–586. [DOI] [PubMed] [Google Scholar]
- Rascle, A., R. S. Stowers, D. Garza, J. A. Lepesant and D. S. Hogness, 2003. L63, the Drosophila PFTAIRE, interacts with two novel proteins unrelated to cyclins. Mech. Dev. 120 617–628. [DOI] [PubMed] [Google Scholar]
- Robertson, H. M., C. R. Preston, R. W. Phillis, D. M. Johnson-Schlitz, W. K. Benz et al., 1988. A stable genomic source of P-element transposase in Drosophila melanogaster. Genetics 118 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, G. M., and A. C. Spradling, 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218 348–353. [DOI] [PubMed] [Google Scholar]
- Sauer, K., K. Weigmann, S. Sigrist and C. F. Lehner, 1996. Novel members of the cdc2-related kinase family in Drosophila: cdk4/6, cdk5, PFTAIRE, and PITSLRE kinase. Mol. Biol. Cell 7 1759–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanyon, C. A., G. Liu, B. A. Mangiola, N. Patel, L. Giot et al., 2004. A Drosophila protein-interaction map centered on cell-cycle regulators. Genome Biol. 5 R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers, R. S., D. Garza, A. Rascle and D. S. Hogness, 2000. The L63 gene is necessary for the ecdysone-induced 63E late puff and encodes CDK proteins required for Drosophila development. Dev. Biol. 221 23–40. [DOI] [PubMed] [Google Scholar]
- Thibault, S. T., M. A. Singer, W. Y. Miyazaki, B. Milash, N. A. Dompe et al., 2004. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat. Genet. 36 283–287. [DOI] [PubMed] [Google Scholar]
- Thummel, C. S., A. M. Boulet and H. D. Lipshitz, 1988. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene 74 445–456. [DOI] [PubMed] [Google Scholar]
- Veraksa, A., A. Bauer and S. Artavanis-Tsakonas, 2005. Analyzing protein complexes in Drosophila with tandem affinity purification-mass spectrometry. Dev Dyn 232 827–834. [DOI] [PubMed] [Google Scholar]
- Weersma, R. K., P. C. Stokkers, I. Cleynen, S. C. Wolfkamp, L. Henckaerts et al., 2009. Confirmation of multiple Crohn's disease susceptibility loci in a large Dutch-Belgian cohort. Am. J. Gastroenterol. 104 630–638. [DOI] [PubMed] [Google Scholar]
- Xu, T., and G. M. Rubin, 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117 1223–1237. [DOI] [PubMed] [Google Scholar]
- Ying-Tao, Z., G. Yi-Ping, S. Lu-Sheng and W. Yi-Li, 2005. Proteomic analysis of differentially expressed proteins between metastatic and nonmetastatic human colorectal carcinoma cell lines. Eur. J. Gastroenterol. Hepatol. 17 725–732. [DOI] [PubMed] [Google Scholar]
- Zhai, B., J. Villen, S. A. Beausoleil, J. Mintseris and S. P. Gygi, 2008. Phosphoproteome analysis of Drosophila melanogaster embryos. J. Proteome Res. 7 1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]