Abstract
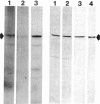
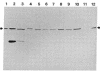
A cytosolic phosphoprotein that appears to function in membrane fusion during exocytosis of secretory products has previously been isolated from Paramecium tetraurelia. This phosphoprotein, parafusin, with Mr 63,000, is rapidly dephosphorylated via a Ca2+-dependent process when secretagogues induce exocytosis in competent cells. Dephosphorylation does not occur in exocytosis-incompetent cells. Polyclonal antibodies against purified parafusin have now been used to show that this protein is present in unicellular organisms and cells of metazoan groups of wide evolutionary divergence, such as yeast, insects, and mammals, including humans. These results suggest that parafusin was present early in the history of eukaryotes and that it is of functional importance in the general mechanism of exocytosis and membrane fusion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisson J., Lefort-Tran M., Pouphile M., Rossignol M., Satir B. Genetic analysis of membrane differentiation in Paramecium. Freeze-fracture study of the trichocyst cycle in wild-type and mutant strains. J Cell Biol. 1976 Apr;69(1):126–143. doi: 10.1083/jcb.69.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan D. M., Satir B. H. Protein phosphorylation/dephosphorylation and stimulus-secretion coupling in wild type and mutant Paramecium. J Biol Chem. 1982 Dec 10;257(23):13903–13906. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murtaugh T. J., Gilligan D. M., Satir B. H. Purification of and production of an antibody against a 63,000 Mr stimulus-sensitive phosphoprotein in Paramecium. J Biol Chem. 1987 Nov 15;262(32):15734–15739. [PubMed] [Google Scholar]
- Pollack S. Mutations affecting the trichocysts in Paramecium aurelia. I. Morphology and description of the mutants. J Protozool. 1974 May;21(2):352–362. doi: 10.1111/j.1550-7408.1974.tb03669.x. [DOI] [PubMed] [Google Scholar]
- Satir B. H., Busch G., Vuoso A., Murtaugh T. J. Aspects of signal transduction in stimulus exocytosis-coupling in Paramecium. J Cell Biochem. 1988 Apr;36(4):429–443. doi: 10.1002/jcb.240360411. [DOI] [PubMed] [Google Scholar]
- Srisomsap C., Richardson K. L., Jay J. C., Marchase R. B. Localization of the glucose phosphotransferase to a cytoplasmically accessible site on intracellular membranes. J Biol Chem. 1988 Nov 25;263(33):17792–17797. [PubMed] [Google Scholar]
- Zieseniss E., Plattner H. Synchronous exocytosis in Paramecium cells involves very rapid (less than or equal to 1 s), reversible dephosphorylation of a 65-kD phosphoprotein in exocytosis-competent strains. J Cell Biol. 1985 Dec;101(6):2028–2035. doi: 10.1083/jcb.101.6.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]