Abstract
In Sorghum bicolor, a group of phytoalexins are induced at the site of infection by Colletotrichum sublineolum, the anthracnose fungus. These compounds, classified as 3-deoxyanthocyanidins, have structural similarities to the precursors of phlobaphenes. Sorghum yellow seed1 (y1) encodes a MYB transcription factor that regulates phlobaphene biosynthesis. Using the candystripe1 transposon mutagenesis system in sorghum, we have isolated functional revertants as well as loss-of-function alleles of y1. These near-isogenic lines of sorghum show that, compared to functionally revertant alleles, loss of y1 lines do not accumulate phlobaphenes. Molecular characterization of two null y1 alleles shows a partial internal deletion in the y1 sequence. These null alleles, designated as y1-ww1 and y1-ww4, do not accumulate 3-deoxyanthocyanidins when challenged with the nonpathogenic fungus Cochliobolus heterostrophus. Further, as compared to the wild-type allele, both y1-ww1 and y1-ww4 show greater susceptibility to the pathogenic fungus C. sublineolum. In fungal-inoculated wild-type seedlings, y1 and its target flavonoid structural genes are coordinately expressed. However, in y1-ww1 and y1-ww4 seedlings where y1 is not expressed, steady-state transcripts of its target genes could not be detected. Cosegregation analysis showed that the functional y1 gene is genetically linked with resistance to C. sublineolum. Overall results demonstrate that the accumulation of sorghum 3-deoxyanthocyanidin phytoalexins and resistance to C. sublineolum in sorghum require a functional y1 gene.
PHYTOALEXINS are chemically diverse antimicrobial compounds that are induced in response to microbes. Examples include isoflavonoids and pterocarpans in legumes, sulfur-containing indole derivatives in cruciferous plants (Tsuji et al. 1992), sesquiterpenoids in solanaceous plants, and coumarins in umbelliferous plants (Knogge et al. 1987). In rice (Oryza sativa) and sorghum (Sorghum bicolor), flavonoid compounds have been shown to act as phytoalexins against Magnaporthe grisea and Colletotrichum spp., respectively (Snyder and Nicholson 1990; Kodama et al. 1992). In sorghum leaves, a suite of reddish-brown flavonoid compounds are induced in the epidermal cells at the site of attempted fungal ingress (Snyder and Nicholson 1990). These pigments belong to the 3-deoxyanthocyanidin class, which includes luteolinidin, 5-methoxy-luteolinidin, apigeninidin, caffeic acid ester of arabinosyl 5-O-apigeninidin,and 7-methoxyapigeninidin (Snyder and Nicholson 1990; Lo et al. 1996; Wharton and Nicholson 2000). Biosynthesis of these flavonoid compounds can be induced by inoculation of seedlings with the Cochliobolus heterostrophus fungus. Attempted penetration by this nonpathogenic fungus of sorghum leads to extremely rapid induction of 3-deoxyanthocyanidins (Lo and Nicholson 1998; Aguero et al. 2002). Traditionally, phytoalexin induction and biosynthesis have been studied in such incompatible systems for this reason. However, the profile of phytoalexins induced in this interaction can be directly compared to that of a compatible interaction (Rogers et al. 1996).
The contribution of flavonoid phytoalexins to resistance against Colletotrichum sublineolum in sorghum has been investigated by comparing the response of several sorghum cultivars that differentially produce 3-deoxyanthocyanidins (Wharton and Julian 1996; Tenkouano et al. 1998; Lo et al. 1999; Basavaraju et al. 2009). These studies, although performed on non-isogenic lines, indicated that phytoalexin production in the resistant cultivars was not only more rapid but also more intense than in the susceptible lines. In addition, phytoalexin accumulation was associated with the distortion of fungal hyphae and restriction of fungal proliferation in the resistant cultivars. On the other hand, extensive fungal colonization of the tissue was reported on the leaf blades of susceptible sorghum plants (Nicholson et al. 1987; Tenkouano et al. 1998; Lo et al. 1999). Although sorghum 3-deoxyanthocyanidins have been implicated in anthracnose leaf blight (ALB) resistance, the genetic and molecular mechanisms controlling their biosynthesis are unclear.
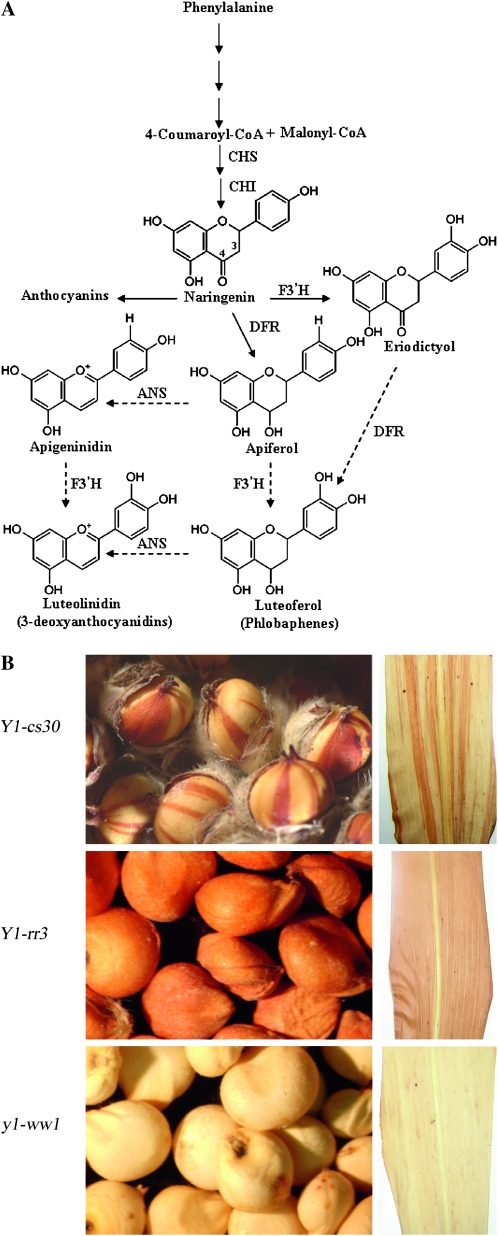
One of the approaches for elucidating the role of phytoalexins in disease resistance has been the use of phytoalexin-deficient (pad) mutants in Arabidopsis thaliana (Glazebrook and Ausubel 1994; Glazebrook et al. 1997; Hammerschmidt 1999; Thomma et al. 1999). In this work, isolation and characterization of loss-of-function mutants has been performed to dissect the sorghum phytoalexin biosynthetic pathway. The 3-deoxyanthocyanidins have structural similarity with flavan-4-ols, which are precursors of the red phlobaphenes (Figure 1) (Kambal and Bate-Smith 1976; Styles and Ceska 1977). A parallel pathway has been suggested for the biosynthesis of 3-deoxyanthocyanidins (Figure 1) (Lo and Nicholson 1998; Boddu et al. 2004, 2005). However, direct genetic evidence for the regulation of this branch of the pathway has been lacking because of the unavailability of near isogenic lines. The mutants analyzed in this study are in the yellow seed1 (y1) gene, which encodes an R2R3 MYB transcription factor. We have previously shown that y1 regulates phlobaphene accumulation and that a Y1-candystripe (Y1-cs) allele carrying an active transposon in y1 produces a variegated seed pericarp phenotype (Chopra et al. 1999). The Y1-cs allele also showed partial deficiency in the biosynthesis of 3-deoxyanthocyanidins, providing the first clue to the dual role of this transcription factor (Chopra et al. 2002). We have now developed near-isogenic mutant and functional y1 lines and demonstrated an overlap between phlobaphene and 3-deoxyanthocyanidin biosynthesis. The potential contribution of Y1-regulated 3-deoxyanthocyanidins in protecting sorghum plants against anthracnose disease was demonstrated through this pathway.
Figure 1.—
Flavonoid biosynthetic pathway of anthocyanins, phlobaphenes, and 3-deoxyanthocyanidins in sorghum. Phenylalanine undergoes a series of enzymatic steps to give rise to 4-coumaroyl-CoA. CHS then catalyzes the stepwise condensation of one molecule of coumaroyl-CoA and three molecules of malonyl-CoA as the first step in the flavonoid biosynthetic pathway to form naringenin chalcone. This then undergoes stereo-specific isomerization by CHI to form naringenin, the common precursor of anthocyanins, phlobaphenes, and 3-deoxyanthocyanidins. The fate of naringenin is determined by the genetic constitution of the plant and the environmental conditions. CHS, chalcone synthase; CHI, chalcone isomerase; DFR, dihydroflavonol reductase; F3′ H, flavonoid 3′ hydroxylase. (B) Seed pericarp and mature leaf phenotypes of sorghum genetic stocks carrying Y1-cs30, Y1-rr3, and y1-ww1 alleles.
MATERIALS AND METHODS
Sorghum and fungal stocks:
We have used the maize genetics nomenclature to name and describe sorghum [Sorghum bicolor (L.) Moench] y1 alleles. The locus or gene is designated as y1, whereas alleles are shown as dominant (functional) or recessive (nonfunctional) followed by a two-letter suffix representing pericarp and glume pigmentation. For example, Y1-rr specifies a red pericarp and red glumes and y1-ww indicates a white pericarp and white glumes. The Y1-rr3, y1-ww1, and y1-ww4 alleles used in this study originated from a common progenitor line (CS8110419) that carries a mutable Y1-cs30 (candystripe) allele (Chopra et al. 1999). The Y1-rr3, y1-ww1, and y1-ww4 alleles resulted from spontaneous excision of the candystripe1 (cs1) transposon. The functionally revertant allele, Y1-rr, arises with a frequency of ∼10%, and the structure of one such allele has been described previously (Chopra et al. 1999; Boddu et al. 2004). The nonfunctional y1-ww alleles were obtained with a frequency of 0.1%, and the molecular structure of two y1-ww alleles is described here.
Fungal cultures of C. heterostrophus [anamorph Bipolaris maydis (Nisikado and Miyake) Shoemaker] and C. sublineolum (P. Henn, Kabat, and Bubak) were maintained on potato dextrose agar or oatmeal agar under constant illumination at 26° for 7–10 days. The conidial suspensions were prepared in a Tween/water mixture as described previously (Lo and Nicholson 1998; Lo et al. 1999). Conidial suspensions were filtrated through cheesecloth and diluted to a concentration of 106 spores·ml−1.
Plant growth and fungal inoculation conditions:
To obtain seedlings with uniformly etiolated mesocotyls, seeds were surface sterilized for 1 hr with 10% commercial bleach, washed in running tap water, and then imbibed in sterile water for 12 hr. Seeds were planted in germination paper rolls and incubated in the dark for 5–7 days at 26° in a growth chamber. To inoculate, the conidial suspension was sprayed onto etiolated seedlings followed by incubation in the dark at 26° in a growth chamber with 100% relative humidity. For controls, etiolated seedlings were sprayed with mock inoculum containing a Tween/water mixture only. Triplicate samples of mesocotyls were collected at 0, 3, 6, 9, 12, and 24 hr from both inoculated and control genotypes. At the time of tissue collection, seedlings were first photographed using a dissection scope SMZ1000 (Nikon) connected with a digital camera DXM1200F (Nikon). Mesocotyls were excised 5 mm above the point of attachment to the seed, flash frozen in liquid nitrogen, and stored at −80° for biochemical and gene expression analyses.
Fungal spores were harvested from cultures of C. sublineolum maintained on oatmeal agar and rinsed in water. To observe the development of disease symptoms, leaves of 6-week-old greenhouse grown plants were spray inoculated with a suspension of 106 conidia·ml−1 and 0.1% Tween 20 as a wetting agent. The plants were allowed to stand for 1 hr before respraying. Once surface moisture had evaporated from the leaves, the plants were placed in a mist chamber at 29° for 24 hr and transferred to a growth chamber maintained at a temperature of 29° and a light intensity of 20 μmol·m−2·s−1. High relative humidity was maintained by spraying water in the chamber twice a day. Disease progression and symptom development was recorded on 4 and 11 days post inoculation (dpi). Lesion density was measured at 4 dpi, and a percentage of the lesion area was recorded at 11 dpi. At 11 dpi, leaves were excised at the base and scanned. A total of 54 plants were used from each genotype and the lesion area on the image was measured using Imagej software (ImageJ 1.36b; Wayne Rasband, National Institutes of Health, Bethesda, MD). To determine the effect of genotype on conidiation, the fourth leaf was immobilized on trays and drop inoculated with 105 spores (10 drops/leaf, 5 plants/genotype), and the number of conidia produced 8 dpi was determined (Gao et al. 2007). Inoculations of plants were carried out independently a minimum of three times with similar results. An analysis of variance was computed for all these traits, considering the three inbred lines as source of variation. Mean comparisons were made using Fisher's protected LSD (Steel et al. 1996). Statistical analyses were computed with SAS version 9.1 (SAS Institute).
Cosegregation analysis was performed using a testcross population from (Y1-rr3×BT×623) × BT×623. BT×623 is an anthracnose-susceptible line of sorghum (Perumal et al. 2009). Parents, F1 plants, and 50 testcross progeny plants were genotyped using the y1 gene fragment F-3 corresponding to intron II as shown previously (Boddu et al. 2005). Disease phenotypes were rated resistant or susceptible on the basis of the corresponding disease-response rating of the two parental lines (1, Y1-rr3—resistant; 5, BT×623—susceptible).
Plasmids and probes:
Positions of y1 gene primers and probes used for gel blot hybridizations are shown in Figure 2C. The y1-cDNA probe was prepared by PCR amplification of exons I and II and 70 bp of exon III using Y1-F1, 5′-ACACACTGCGAGCTGAGAG-3′ and Y1-R3, 5′-CGAGTTCCAGTAGTTCTTGATC-3′ as forward and reverse primers, respectively. Flavonoid structural gene probes consisted of DNA fragments obtained from the plasmid pZMC2 containing a maize c2 cDNA (Wienand et al. 1986), plasmid pZMA1 containing a maize dfr1 cDNA (Schwarz-Sommer et al. 1987), pChi1 containing maize chi1 cDNA (from Erich Grotewold Ohio State University), and pSbF3′H3 containing a sorghum f3′h3 gene (Boddu et al. 2004). Maize gene probes did not pose any problem with hybridizations because the maize genes used here are highly homologous to sorghum. For all probe preparations, the [α-32P]dCTP was used as a label during random prime labeling of DNA fragments using prime-a-gene kit (Promega, Madison, WI).
Figure 2.—
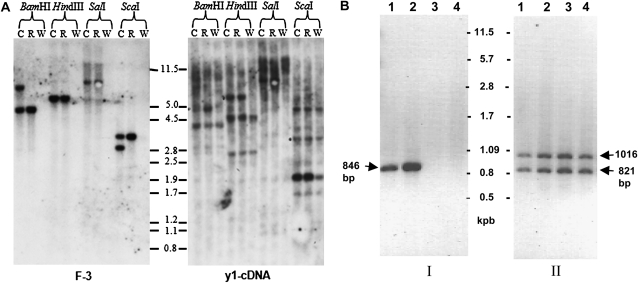
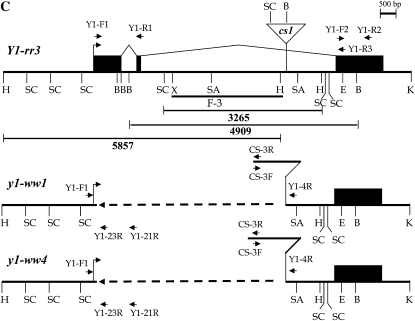
Molecular structure of the y1-ww1 and y1-ww4 alleles. (A) DNA gel blot analysis of Y1-rr3 (R), y1-ww1 (W), and their common progenitor Y1-cs30 (C lanes). A genomic DNA gel blot was hybridized with either an intron II-specific fragment of Y1-rr (F-3) or a y1-cDNA probe. Molecular weight markers in kilobase pairs are shown between the blots. (B) PCR reactions were carried out using genomic DNA as templates and two sets of primer pairs: I, YF-1 and YR-1; II, YF-2 and YR-2. The positions of these primers are shown in C. Lanes are the following: 1, Y1-cs30; 2, Y1-rr3; 3, y1-ww1; and 4, y1-ww4. (C) Line diagram of the structure of y1 in Y1-rr3, y1-ww1, and y1-ww4. A bent arrow represents the transcription start site. Solid boxes are exons joined by angled lines representing introns. The cs1 transposon inserted in intron II is shown as an inverted triangle. The open triangle in the maps of y1-ww1 and y1-ww4 represents the remnants of cs1. The dashed line in y1-ww1 and y1-ww4 indicates sequence deletion. The restriction enzyme sites shown are the following: B, BamHI; E, EcoRI; H, HindIII; K, KpnI; SA, SacI; SL, SalI; SC, ScaI; X, XhoI. The accession numbers corresponding to y1 and cs1 sequences are AY860968 and AF206660, respectively.
DNA and RNA gel blot analysis:
Plant genomic DNA was extracted from sorghum seedlings following the CTAB method (Saghai-Maroof et al. 1984). DNA was digested using restriction enzymes, reagents, and reaction conditions from Promega. DNA digests were fractionated on 0.8% agarose gels, and gels were blotted onto nylon membranes through standard protocol of capillary transfer (Sambrook and Russell 2001). DNA gel blots were hybridized using buffers and conditions described previously (Sekhon and Chopra 2009).
Total RNA was isolated from inoculated sorghum seedlings collected at various time points using TriReagent (Molecular Research Center, Cincinnati). For preparation of slot blots, 200 ng of the purified and denatured gene fragment or linear plasmid vector DNA was added per slot and transferred to nitrocellulose membrane using a slot blotter. RNA samples [24 hr post inoculation (hpi)] were reverse transcribed using [32P]dCTP as the radioactive label and first-strand cDNA was used as a probe. For RNA gel blots, 5 μg of total RNA per sample was fractionated on 1.2% denaturing gels containing 5% (v/v) formaldehyde. Gels were rinsed in DEPC-treated water and blotted onto nylon membranes (Osmonics, Minnetonka, MN) by standard capillary transfer. RNA blots were hybridized with 32P-labeled individual DNA probes for y1, chs1, chi1, f3′h3, and dfr1 genes. All blots were exposed to X-OMAT X-ray films (Kodak, Rochester, NY) for 3 days prior to developing. A boiling solution of 0.1% SDS was used for stripping the blot for subsequent hybridizations.
PCR analysis:
PCR analysis was performed on genomic DNA extracted from seedlings of Y1-rr3, Y1-cs30, y1-ww1, and y1-ww4. Forward primer Y1-F1, 5′-ACACACTGCGAGCTGAGAG-3′, and reverse primer Y1-R1, 5′-GACGTCGGCCCGAAGGTAGTTGATCC-3′, were used for amplification of a y1–DNA fragment corresponding to regions of exon I, intron I, and a part of exon II. In addition, Y1-F2 5′-CAAGAACTACTGGAATTCGCACCT-3′ and Y1-R2 5′-AGTACAGTACATGTGAAGAAG-3′ primers were used for amplification within exon III. PCR-based analyses of y1-ww1 and y1-ww4 alleles were done using several y1 gene and cs1 transposon-specific primers (not shown). Two y1 primers present on the flanking regions of the deletion gave a PCR product. These primers are Y1-F1 in exon I and Y1-4R (5′-GAGCTAATGAGACGTGTC-3′) in exon III. All genomic PCR reactions were performed using standard conditions with an annealing temperature of 58° and 30 cycles of amplification.
Spectrophotometry and thin-layer chromatography:
To detect the presence of 3-deoxyanthocyanidins, 100 mg of powdered tissues collected at 0, 24, and 36 hpi were incubated in 0.083% HCl in HPLC-grade methanol at 4° for 24 hr. The clear extracts were collected after centrifugation at 10,000 rpm for 10 min. Total anthocyanidin content was quantified spectrophotometrically at 480 nm using a UV mini-1240 spectrophotometer (Shimadzu Scientific Instruments, Columbia, MD), and the concentration was expressed as micromoles of 3-deoxyanthocyanidins using the molar extinction coefficient of luteolinidin (13,800 m−1·cm−1) (Nicholson et al. 1987; Yamaoka et al. 1990; Aguero et al. 2002).
For thin-layer chromatography (TLC), 100 mg of the ground tissues were extracted in HPLC-grade methanol and clarified by centrifugation at 14,000 rpm for 10 min. Equal aliquots of the clear extracts were loaded onto a 20 × 20 cellulose plate (Analtech, Newark, DE). TLC plates were developed in Forestal [CH3COOH:HCl:H2O (30:3:10, v/v)] as a mobile phase (Stafford 1965). Commercially available apigeninidin and luteolinidin (10 ng·μl−1) (Extrasynthese) were loaded as controls. These experiments were repeated at least three times using independent samples.
HPLC and liquid chromatography–mass spectrometry analysis:
For HPLC analysis of compounds accumulating in mesocotyls, 20 mg of tissue was placed in 1 ml of HPLC-grade methanol, and compounds were allowed to leach at 4° for 24 hr in the dark. Extracts were collected by centrifugation at 1000 rpm, filtered through 0.45-μm Acrodisc LC 13-mm syringe filters (Gelman Laboratory, Ann Arbor, MI), and evaporated down to 100 μl. To separate compounds, two reversed phase C18 columns connected in tandem (Supelco, Bellefonte, PA) were used, and a 50-μl sample was applied to the column fitted on a Waters 600 HPLC separator with a Waters 900 detector system. Spectral measurements were taken over a wavelength range of 230–550 nm, which is known to detect all flavonoid compounds (Grotewold et al. 1998). Detection of 3-deoxyanthocyanidins was carried out at 480 nm as described previously (Snyder and Nicholson 1990; Lo and Nicholson 1998). Pure luteolinidin and apigeninidin (Extrasynthese) were dissolved in HPLC-grade methanol and used as standards. To measure 3-deoxyanthocyanidins accumulating during C. sublineolum inoculation of greenhouse-grown plants, lesions were dissected and compounds were extracted as described above. Compounds were separated on a 1 × 150-mm BetaBasic C18 column with a solvent gradient of water containing 0.15% formic acid (solvent A) and methanol (solvent B) with a flow rate of 0.05 ml·min−1 delivered using a preinjection split. The program of solvent composition was established as the following: 0–5 min (99% solvent A; 1% solvent B) followed by a linear gradient to 50% solvent A, 50% solvent B at 12 min, another linear gradient to 100% of solvent B at 20 min, with a hold at 100% solvent B until 25 min. Liquid chromatography–mass spectrometry (LC–MS) analyses were carried out using a Quattro II mass spectrometer (Micromass, Beverly, MA) interfaced with a Shimadzu LC10ADvp pump. Electro-spray ionization was carried out in the positive ions mode for detection of 3-deoxyanthocyanidin compounds.
RESULTS
Null y1 alleles have internal deletions:
Genetic tests showed that two sorghum mutant stocks with the white pericarp and white glumes phenotype segregated as recessive alleles of y1 and were designated as y1-ww1 and y1-ww4. To characterize the cause of their nonfunctionality, gel blots were first hybridized with intron II probe F-3, and results are shown in Figure 2A. In the BamHI digest of Y1-cs30, the F-3 probe hybridized to two bands of ∼7.5 and 4.9 kb. The 7.5-kb band originates from digestion of BamHI sites at positions 2633 and 3911 within y1 and the cs1 transposon, respectively. The 4.9-kb band is generated due to BamHI sites at positions 2633 and 7542 in the y1 sequence. In the BamHI digest of Y1-rr3 DNA, the 7.5-kb band is not present because of the absence of the cs1 element. Interestingly, the F-3 probe did not hybridize to any genomic DNA fragments in the y1-ww1 BamHI digest. Similarly, bands of expected size were observed in HindIII, ScaI, and SalI digests of Y1-cs30 and Y1-rr3 DNA with fragment F-3. This probe did not detect any homologous sequences in y1-ww1 DNA digests, indicating that fragment F-3 may be either deleted or altered.
To test whether there is any modification in the y1-coding region in the y1-ww1 line, the DNA gel blot was hybridized with y1 cDNA (Figure 2A). In Y1-cs30 and Y1-rr3 BamHI digests, the probe hybridized to bands of approximate sizes of 5.0 and 4.0 kb, representing y1 and y2 genes, respectively. y2 is a pseudogene present at 8.5 kbp 3′ to y1 (Boddu et al. 2006). Interestingly, the cDNA probe hybridized only to the 4-kb BamHI band (y2 specific) in y1-ww1. In the HindIII digest, the 6.0 -kb y1-specific band is absent in y1-ww1. Similarly, in the ScaI digest, the 1.9-kb band belonging to y1 is also absent. This is indicated by the low intensity of this band in y1-ww1 as compared to Y1-rr3 and y1-cs30, both of which have y1- and y2-specific bands of 1.9 kb. SalI digests could not be resolved very well because of large fragment sizes. Similar gel blot hybridization results were obtained from characterization of the y1-ww4 allele (data not shown). These results established that the y1-ww1 and y1-ww4 alleles have deletions.
To further characterize the extent of the deletion within y1-ww1 and y1-ww4, PCR analysis was performed using two sets of primers (Figure 2B). Primers located in exons I and II gave rise to expected PCR products of 846 bp in both Y1-cs30 and Y1-rr3, while y1-ww1 and y1-ww4 did not show this amplification. However, primers corresponding to exon III detected y1 (1016 bp)-and y2 (821 bp)-specific PCR products in all four genotypes. These results together with DNA gel blot hybridization further confirmed that y1 either is deleted or significantly modified in y1-ww alleles. To precisely map the deletion within y1, PCR amplifications were performed using forward primers in the 5′ UTR, exon I, exon II, and reverse primers in the intron II or exon III regions. The primer combination Y1-F1 (5′ UTR) and Y1-4R (intron II) gave PCR products of ∼2.5 and 3.0 kb in y1-ww1 and y1-ww4 DNA, respectively. These PCR primers did not amplify any product from Y1-rr3 and Y1-cs30 DNAs. Furthermore, PCR-based investigations and sequencing of the deletion end points revealed that both y1-ww1 and y1-ww4 alleles have a common deletion point at the 5′ end of the y1 sequence and harbor a piece of cs1. As shown in Figure 2C, there is a leftover sequence of cs1 element. Interestingly, y1-ww1 carries 2291 bp of the leftover transposon whereas y1-ww4 carries 2818 bp. These leftover pieces correspond to the 3′ end of the cs1 present at its original position in intron II of y1. These molecular characterizations indicated that the two y1 nulls may have resulted from the improper excision of the cs1 transposon from y1. The mechanism leading to an interstitial deletion leaving a fractured transposon within the y1 sequence is not clear and currently under investigation.
Fungal-induced 3-deoxyanthocyanidins are not synthesized in y1-ww mesocotyls:
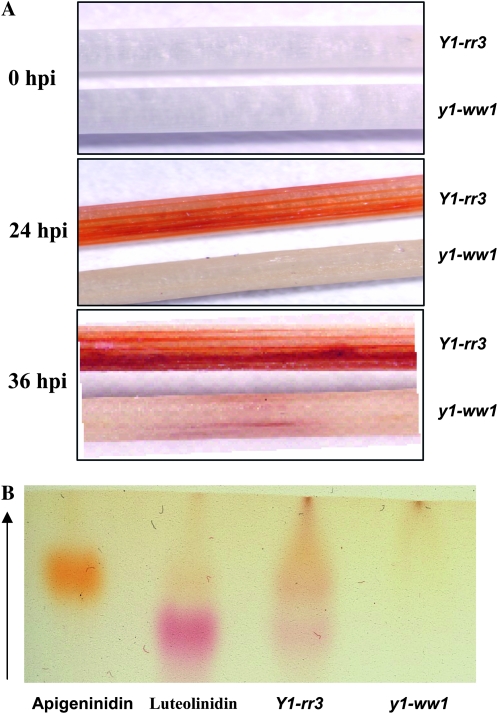
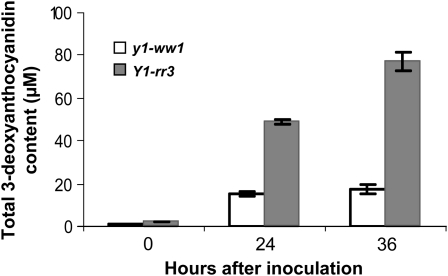
On the basis of the proposed role of y1 in the regulation of flavonoid biosynthesis, we tested the hypothesis that a functional y1 gene is required for the synthesis of 3-deoxyanthocyanidins. Etiolated mesocotyls inoculated with C. heterostrophus showed accumulation of reddish-brown pigments in Y1-rr3 (24 and 36 hpi), while these compounds were barely visible in y1-ww1 (Figure 3A) and y1-ww4 (not shown). Thin-layer chromatographic analysis of the Y1-rr3 extract showed the presence of two bands that comigrated with the standards for luteolinidin and apigeninidin. These bands were not detected in the y1-ww1 extract (Figure 3B). The acidified methanolic extracts of the inoculated Y1-rr3 tissues showed absorption spectra characteristic of 3-deoxyanthocyanidins (λmax 480 nm; data not shown). Total 3-deoxyanthocyanidin concentration produced at 0, 24, and 36 hpi indicated that, in response to fungal infection, sorghum seedlings carrying a functional y1 gene accumulated much higher levels of anthocyanidin compounds than the ones with a nonfunctional y1-ww1 allele (Figure 4). In addition, it revealed that the level of total anthocyanidins increased over time in seedlings carrying a functional y1 allele.
Figure 3.—
Comparative response of etiolated seedlings of Y1-rr3 and y1-ww1 after inoculation with C. heterostrophus. (A) Accumulation of reddish-brown phytoalexin compounds in Y1-rr3 and y1-ww1 mesocotyls at 0, 24, and 36 hpi with C. heterostrophus. The upper mesocotyl in each panel is from the Y1-rr3 allele while the lower one is from the y1-ww1 allele. (B) TLC analysis of methanolic extracts prepared from inoculated Y1-rr3 and y1-ww1 mesocotyls. Luteolinidin and apigeninidin were loaded as standards.
Figure 4.—
Spectrophotometry of induced compounds. Quantification of flavonoid compounds from methanolic extracts prepared from Y1-rr3 and y1-ww1 mesocotyls harvested at 0, 24, and 36 hpi. Absorbance was recorded at 480 nm. The error bars represent the standard error of three replicates.
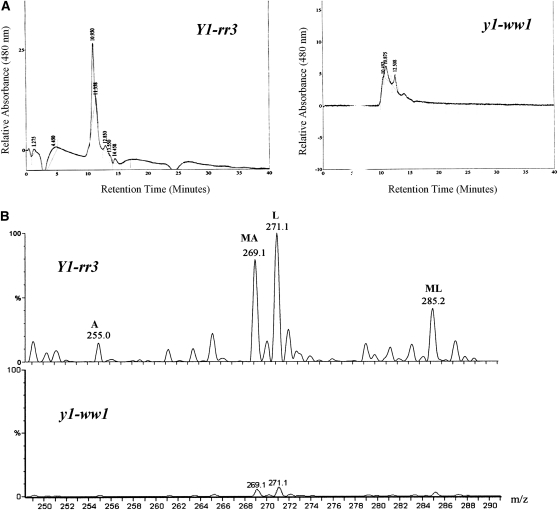
The identity of the compounds was confirmed by HPLC (Figure 5A). The retention times of primary standards of luteolinidin and apigeninidin were used to identify the induced compounds. We identified luteolinidin (retention time, ∼10 min), apigeninidin (retention time, ∼11 min), and 5-methoxyluteolinidin (retention time, ∼12.8 min) in Y1-rr3. y1-ww1 and y1-ww4 produced trace amounts of unknown compounds. Extracts of uninoculated mesocotyls did not show induction of any pigments or 3-deoxyflavonoid compounds (data not shown). LC–MS analysis of the Y1-rr3 extract showed three major and several minor ions (Figure 5B). Major peaks corresponded to luteolinidin [271 mass/charge ratio (m/z)], methoxy-apigeninidin (269 m/z), and methoxy-luteolinidin (285 m/z). One of the observed minor peaks was for a compound with a mass consistent with that of apigeninidin (255 m/z) while the other minor peaks could not be confirmed. In the y1-ww1 extract, minor peaks that had retention times of ∼10 and ∼12.8 min were observed. These peaks could be attributed to the presence of other flavonoid compounds such as pelargonidin produced in response to stress. This is in agreement with our spectrophotometric quantification of total anthocyanidins, which were detected in the y1-ww lines although at much lower levels (see Figure 4).
Figure 5.—
Characterization of the 3-deoxyanthocyanidins from inoculated mesocotyls of Y1-rr3 and y1-ww1 in response to C. heterostrophus. (A) HPLC chromatograms monitored at 480 nm. Pure luteolinidin and apigeninidin were eluted at ∼10 and 11 min, respectively. (B) LC-MS profile of the Y1-rr3 extract shows three major and a number of minor ions. The major ions at 269.1, 271.1, and 285.2 m/z correspond to methoxy-apigeninidin (MA), luteolinidin (L), and methoxy-luteolinidin (ML), respectively. One of the minor ions at 255 m/z corresponds to apigeninidin (A), while other minor ions have not been identified. The profile of the y1-ww1 extract shows two minor peaks with mass/charge ratios of 269.1 and 271.1.
y1 and flavonoid structural genes are coordinately induced after fungal ingress:
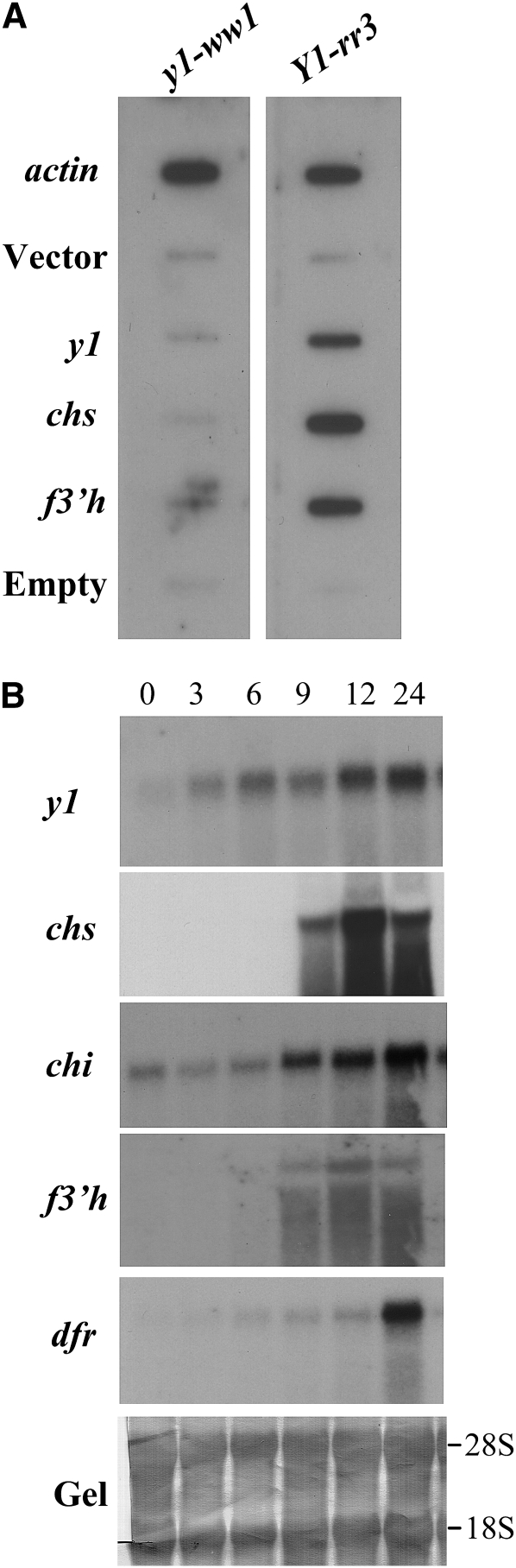
To test whether the expression of the flavonoid structural genes was dependant on y1 functionality, slot blot hybridization showed a significant level of y1 transcripts in Y1-rr3 while the signal observed in y1-ww1 was equivalent to the background level (Figure 6A). Interestingly, chs1 and f3′h3 expression were also induced after fungal inoculation in Y1-rr3 but not in y1-ww1. These results provided evidence that y1 and its putative target genes in the flavonoid pathway are induced after inoculation with C. heterostrophus and that the differential induction of flavonoid genes correlates with the allelic constitution at the y1 locus. Further, gel blots of total RNA isolated at 0, 3, 6, 9, 12, and 24 hpi were hybridized with y1 and flavonoid structural gene probes (Figure 6B). y1 transcripts were observed as early as 3 hpi and showed accumulation over time. Neither chs1 nor f3′h3 showed any detectable level of expression up to 6 hpi. Their transcripts started to accumulate by 9 hpi and reached a plateau at 12 hpi with a considerably higher level of chs1 induction than that of f3′h3. Interestingly, dfr1 exhibited much lower levels of induction at 6 and 12 hpi and then showed a sharp increase at 24 hpi. On the other hand, chi1 showed expression through all time points with upregulation at the later time points (9–24 hpi). These results indicated that the transcriptional machinery of the flavonoid pathway is activated in response to C. heterostrophus infection. In addition, our data established that the induction of y1 expression preceded that of these candidate target genes. Expression of y1 and the flavonoid structural genes studied here are coordinately regulated during sorghum–fungus interaction. In the y1-ww1 plants, there was neither induction of y1 nor the upregulation of the flavonoid structural genes with time (data not shown).
Figure 6.—
Gene expression analysis of y1 and the flavonoid structural genes. (A) Slot blot hybridization of radioactively labeled first-strand cDNA from RNA isolated 24 hpi. “Empty” means no DNA was added as a control for background hybridization. (B) Temporal expression of y1 and flavonoid structural genes in Y1-rr3 mesocotyls after inoculation with C. heterostrophus. Blots were probed with cDNA of y1, chalcone synthase (chs1), chalcone isomerase (chi1), flavonoid 3′ hydroxylase (f3′h3), and dihydroflavonol reductase (dfr1). Numbers at the top represent hours after inoculation with C. heterostrophus.
Y1-rr3 line shows resistance to anthracnose leaf blight:
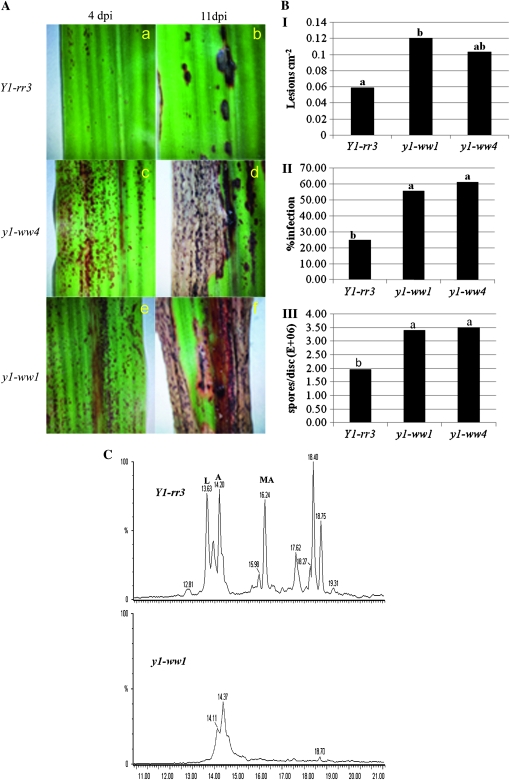
We examined fungal infection and disease progression in the Y1-rr3 and mutant y1-ww1 and y1-ww4 lines to determine the role of 3-deoxyanthocyanidins in disease resistance to C. sublineolum, the causal agent of sorghum ALB. By 4 dpi, all three lines produced pigmented lesions at the sites of primary infection (Snyder and Nicholson 1990) (Figure 7A). The lesions on the null lines progressed and eventually coalesced, resulting in necrosis of the infected leaves. At this stage, acervuli were observed in the lesions, and this phenotype has been documented (Lo et al. 1999). However, the lesions on the Y1-rr3 line were restricted and the rest of the leaf tissue remained healthy. The lesion density on Y1-rr3 was found to be 0.059 cm−2 while that on y1-ww1 and y1-ww4 was 0.120 cm−2 and 0.103 cm−2, respectively. Lesion density on y1-ww1 was greater than on Y1-rr3 while differences between Y1-rr3 and y1-ww4 were not significant (P = 0.0451), (Figure 7B, I). By 11 dpi, a significantly greater area of the leaf was covered in lesions in the y1-ww1 (55.78%) and y1-ww4 (61.21%) lines compared to the Y1-rr3 line (25.08%, P ≤ 0.01) (Figures 7B, II). Because the inhibition of fungal proliferation would result in reduced sporulation, the effect of genotype on fungal sporulation was determined 8 dpi. Analysis of variance showed that there was a highly significant difference between Y1-rr3 and the two null lines (P ≤ 0.01). The line Y1-rr3 had an average of 1.96 × 106 conidia/disc while y1-ww1 and y1-ww4 had 3.40 × 106 and 3.51 × 106conidia per disc, respectively (Figure 7B, III). This result shows that the presence of a functional y1 gene correlates with greater resistance to the fungus C. sublineolum in sorghum. Our result thus provides genetic evidence that a sorghum line that produces 3-deoxyanthocyanidins under the control of y1 is more resistant while two sibling lines that carry a transposon-induced deletion of the y1 gene are more susceptible to C. sublineolum.
Figure 7.—
Fungal infection and plant responses after infection with C. sublineolum. (A) Inoculated leaves of the three genotypes showing disease symptoms 4 dpi (a, c, and e) and 11 dpi (b, d, and f). Restriction of the disease is shown in the Y1-rr3 (b). Development of characteristic ALB symptoms with necrosis of the leaf as the lesions grow and coalesce is shown in the null y1-ww lines (d and f). (B) Quantification of lesion density at 4 dpi (I), percentage of infection area at 11 dpi (II), and sporulation at 8 dpi (III). (C) HPLC analysis of the induced 3-deoxyanthocyanidin compounds 72 hpi with C. sublineolum. (Top) A chromatogram of pigments extracted from lesions of inoculated Y1-rr3 leaves showing the accumulation of luteolinidin (L, retention time 13.63 min), apigeninidin (A, 14.2 min), and 7-methoxy-apigeninidin (MA, 16.2 min). (Bottom) A chromatogram of pigments extracted from inoculated plants of the y1-ww1 allele.
The pigments induced in the lesions at 72 hpi were extracted and analyzed. HPLC profiles of extracts from both null lines were similar and showed only two minor peaks corresponding to unknown compounds. The HPLC profile of the Y1-rr3 line, on the other hand, showed five major peaks with retention times of 13.63, 14.20, 16.24, 18.40, and 18.75 min (Figure 7C). Three of these peaks were identified, on the basis of their retention times, as luteolinidin (13.63 min), apigeninidin (14.2 min), and 7-methoxy-apigeninidin (16.2 min) while the other two have not been identified. Retention times of 3-deoxyanthocyanidins in this experiment are different from the ones observed in mesocotyl extracts (Figure 5A). These differences can be attributed to the use of two different HPLC systems (see materials and methods).
Cosegregation analysis of y1 and resistance to C. sublineolum:
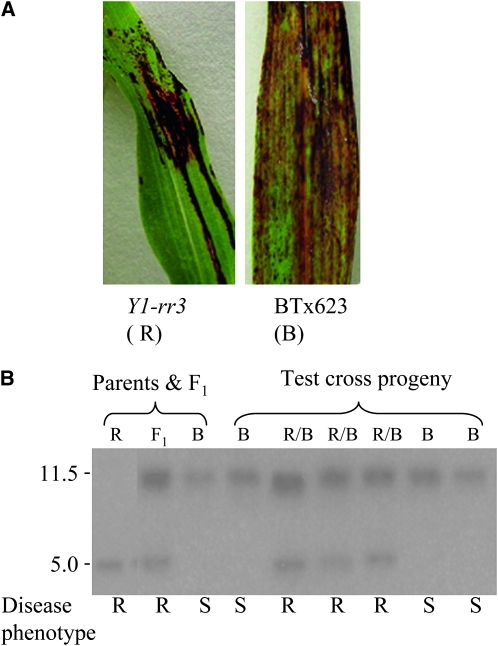
Genetic linkage between the functional y1 gene and resistance to C. sublineolum was tested in a testcross population derived from [(Y1-rr3 × BT×623) × BT×623]. The sorghum BT×623 line is susceptible to C. sublineolum (Figure 8A) (Lo et al. 1999) and carries a nonfunctional allele of y1 (Boddu et al. 2005). Parents, F1, and testcross progeny plants were grown in a greenhouse and genotyped at the seedling stage. DNA gel blots carrying BamHI-digested genomic DNA from testcross progeny plants were hybridized with the y1-intron II probe F-3. As shown previously, BT×623 carries a partially deleted y1 gene that gives rise to an 11.5-kb BamHI band hybridizing with the F3 probe, whereas a functional y1 gene present in Y1-rr3 shows an ∼5.0-kb restriction band (Chopra et al. 2002; Boddu et al. 2005). Results of the cosegregation analysis are shown in Figure 8B; the hybridization pattern of a set of representative samples shows that the heterozygous plants carry both 11.5- and 5.0-kb bands (“R/B” lanes) while the homozygous BT×623 segregants (“B” lanes) show the presence of the 11.5-kb band. Plants were inoculated with C. sublineolum (see materials and methods), and disease lesions were observed 7 dpi. Progeny plants were scored as resistant or susceptible, depending upon the phenotypes in comparison with disease lesions observed in the two parental lines (Figure 8A). Resistant and susceptible plants segregated, as expected, at a 1:1 ratio for a testcross (27 resistant/23 susceptible). Genotyping showed that all heterozygous (Y1-rr3/BT×623) plants were resistant while homozygous (BT×623) segregants were susceptible. This result further confirms that resistance to C. sublineolum is genetically linked to a functional y1 gene present in Y1-rr3.
Figure 8.—
Cosegregation analysis of disease phenotype and y1 gene in a testcross population of [(Y1-rr3 × BT×623) × BT×623]. (A) ALB phenotypes of C. sublineolum-infected leaves of Y1-rr3 and BT×623 observed at 7 dpi. (B) Genotype and disease phenotype of testcross progeny plants. “R/B” lanes indicate Y1-rr3/BT×623 heterozygotes, and “B” lanes are homozygous for BT×623. The disease phenotype corresponding to each plant is shown below the lane.
DISCUSSION
Sorghum responds to the invasion of both pathogenic and nonpathogenic fungi by the induction of 3-deoxyanthocyanidin phytoalexins (Nicholson et al. 1987; Lo et al. 1999). Although extensive studies have demonstrated the antimicrobial nature of these compounds and their elevated levels in resistant lines when exposed to pathogens, their biosynthetic pathway and its regulation remains obscure. Furthermore, present evidence for the role of 3-deoxyanthocyanidins in disease resistance is not conclusive since these studies were carried out using non-isogenic lines. The structural similarities between 3-deoxyanthocyanidins and flavan-4-ols, the precursors of the red phlobaphene pigments found in sorghum and maize, suggest that these compounds might be synthesized via a common or overlapping pathway (Grotewold et al. 1998; Chopra et al. 2002; Winefield et al. 2005). In a previous study, we showed that a mutable allele of an R2R3 MYB transcription factor encoded by Y1-cs is partially defective in the synthesis of 3-deoxyanthocyanidins (Chopra et al. 2002). In a recent study, we have found that biosynthesis of sorghum flavan-4-ols is under the regulatory control of y1 (Boddu et al. 2005, 2006). In this article, we have characterized a functionally revertant allele and two null alleles of y1. The molecular analysis and sequencing of the y1-ww1 and y1-ww4 alleles indicated that these have an internal deletion in the y1 sequence. This deletion removes most of the Myb domain and thus makes the Y1 protein nonfunctional. Moreover, expression analysis indicated that these y1-ww alleles are loss-of-function alleles and do not show any y1-specific transcript even during fungal infection. Biochemical analysis established that these y1 null alleles are deficient in flavan 4-ols and 3-deoxyflavonoid pigment accumulation, while the functional Y1-rr3 allele accumulates these compounds. Our disease assays revealed that, as compared to y1-ww null alleles, Y1-rr3 synthesizes the suite of 3-deoxyanthocyanidin compounds in response to infection with both C. heterostrophus and C. sublineolum. Furthermore, these data indicate that 3-deoxyanthocyanidins play a role in making the plant resistant to ALB.
Phytoalexin accumulation has been shown to be associated with de novo protein synthesis of enzymes required for their biosynthesis (Dixon et al. 1983; Cramer et al. 1985; Berenbaum and Zangerl 1992; Dixon and Paiva 1995). We have shown that genes coding for enzymes predicted to be involved in 3-deoxyanthocyanidin biosynthesis are induced and upregulated over time in response to infection with C. heterostrophus. The activation of biosynthetic genes in metabolic pathways is often coordinated by transcription factors. Chalcone synthase (CHS) and chalcone isomerase (CHI) are two early pathway enzymes that drive the flavonoid pathway toward the formation of naringenin, the common precursor of many flavonoid compounds including anthocyanins, flavones, and phlobaphenes (Dong et al. 2001; Winkel-Shirley 2001a,b). Gene expression analysis in the y1 mutant and wild-type genotypes further revealed that the transcription of these biosynthetic genes is y1 dependent. In addition, this investigation revealed that the accumulation of y1 transcripts preceded (3 hpi) that of the biosynthetic genes, which reached a peak several hours post inoculation. The coordinated induction of flavonoid structural genes reported here supports the idea of a common transcription factor. The cosegregation analysis in our testcross population shows that y1 is genetically linked to ALB resistance. To shunt naringenin toward 3-deoxyanthocyanidins, it is crucial to induce late-pathway enzymes such as DFR, ANS, and F3′H (see Figure 1). Similarly, induction of camalexin in Arabidopsis has been associated with transcriptional activation of both early pathway genes for the biosynthesis of tryptophan and late-pathway genes such as anthranilate synthase (Zhao and Last 1996; Schuhegger et al. 2007). In rice, the biosynthesis of sakurantein was shown to be correlated with the accumulation of naringenin and the induction of naringenin O-methyltransferase (Kodama et al. 1992; Rakwal et al. 2000, 2001).
In response to infection by the anthracnose fungus C. sublineolum, sorghum plants carrying a functional y1 allele accumulated known 3-deoxyanthocyanidins, resulting in a significant reduction in ALB disease symptoms. This is evinced by the localized red spots on the infected leaves. On the other hand, the y1-ww1 and y1-ww4 lines were deficient in the biosynthesis of 3-deoxyanthocyanidins and exhibited severe symptoms typical of anthracnose disease. The antifungal activity of these 3-deoxyanthocyanidins, and particularly of luteolinidin and methoxy luteolinidin, has been established (Nicholson et al. 1987). This investigation provides genetic evidence that a functional y1 confers increased resistance to ALB. The gene expression analysis supports our biochemical data, and both suggest that the functional y1 controls biosynthesis of sorghum 3-deoxyanthocyanidins. In addition to inducing the requisite pathway genes, y1 is predicted to contain promoter elements that are responsive to specific stresses such as pathogenesis or herbivory. Therefore, these favorable traits such as resistance to ALB can be introduced to related plant species more economically by transferring a single regulatory gene.
Acknowledgments
We are grateful to Thomas Peterson, Iowa State University, for his unconditional support during the early stages of this project. We thank Dan Jones, Michigan State University, for LC–MS analysis, and Germán Sandoya for help with statistical analyses. We very much appreciate the suggestions of anonymous reviewers. This work was supported by a U. S. Department of Agriculture-National Research Initiative award no. 2007-35318-17795, and research was performed under the Hatch project 4144. F.I. was supported by a predoctoral fellowship from the Egyptian Government and a dissertation award from the College of Agricultural Sciences, Pennsylvania State University.
This work is dedicated to the memory of Professor Ralph Nicholson, Purdue University, for his encouragement and enthusiasm in the study of the genetics and biochemistry of sorghum phytoalexins.
References
- Aguero, M. E., A. Gevens and R. L. Nicholson, 2002. Interaction of Cochliobolus heterostrophus with phytoalexin inclusions in Sorghum bicolor. Physiol. Mol. Plant Pathol. 61 267–271. [Google Scholar]
- Basavaraju, P., N. P. Shetty, H. S. Shetty, E. de Neergaard and H. J. L. Jørgensen, 2009. Infection biology and defence responses in sorghum against Colletotrichum sublineolum. J. Appl. Microbiol. 107 404–415. [DOI] [PubMed] [Google Scholar]
- Berenbaum, M. R., and A. R. Zangerl, 1992. Genetics of secondary metabolism and herbivore resistance in plants, pp. 415–438 in Herbivores: Their Interactions With Secondary Plant Metabolites, edited by G. A. Rosenthal and M. R. Berenbaum. Academic Press, San Diego.
- Boddu, J., C. Svabek, R. Sekhon, A. Gevens, R. L. Nicholson et al., 2004. Expression of a putative flavonoid 3′-hydroxylase in sorghum mesocotyls synthesizing 3-deoxyanthocyanidin phytoalexins. Physiol. Mol. Plant Pathol. 65 101–113. [Google Scholar]
- Boddu, J., C. Svabek, F. Ibraheem, A. D. Jones and S. Chopra, 2005. Characterization of a deletion allele of a sorghum Myb gene, yellow seed1, showing loss of 3-deoxyflavonoids. Plant Sci. 169 542–552. [Google Scholar]
- Boddu, J., C. H. Jiang, V. Sangar, T. Olson, T. Peterson et al., 2006. Comparative structural and functional characterization of sorghum and maize duplications containing orthologous Myb transcription regulators of 3-deoxyflavonoid biosynthesis. Plant Mol. Biol. 60 185–199. [DOI] [PubMed] [Google Scholar]
- Chopra, S., V. Brendel, J. B. Zhang, J. D. Axtell and T. Peterson, 1999. Molecular characterization of a mutable pigmentation phenotype and isolation of the first active transposable element from Sorghum bicolor. Proc. Natl. Acad. Sci. USA 96 15330–15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra, S., A. Gevens, C. Svabek, K. V. Wood, T. Peterson et al., 2002. Excision of the Candystripe1 transposon from a hyper-mutable Y1-cs allele shows that the sorghum Y1 gene controls the biosynthesis of both 3-deoxyanthocyanidin phytoalexins and phlobaphene pigments. Physiol. Mol. Plant Pathol. 60 321–330. [Google Scholar]
- Cramer, C. L., J. N. Bell, T. B. Ryder, J. A. Bailey, W. Schuch et al., 1985. Co-ordinated synthesis of phytoalexin biosynthetic enzymes in biologically-stressed cells of bean (Phaseolus vulgaris L.). EMBO J. 4 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, R. A., and N. L. Paiva, 1995. Stress-induced phenylpropanoid metabolism. Plant Cell 7 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, R. A., P. M. Dey and C. J. Lamb, 1983. Phytoalexins: enzymology and molecular biology. Adv. Enzymol. Relat. Areas Mol. Biol. 55 1–136. [DOI] [PubMed] [Google Scholar]
- Dong, X. Y., E. L. Braun and E. Grotewold, 2001. Functional conservation of plant secondary metabolic enzymes revealed by complementation of Arabidopsis flavonoid mutants with maize genes. Plant Physiol. 127 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, X. Q., W. B. Shim, C. Göbel, S. Kunze, I. Feussner et al., 2007. Disruption of a maize 9-lipoxygenase results in increased resistance to fungal pathogens and reduced levels of contamination with mycotoxin fumonisin. Mol. Plant Microbe Interact. 20 922–933. [DOI] [PubMed] [Google Scholar]
- Glazebrook, J., and F. M. Ausubel, 1994. Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc. Natl. Acad. Sci. USA 91 8955–8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook, J., M. Zook, F. Mert, I. Kagan, E. E. Rogers et al., 1997. Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics 146 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold, E., M. Chamberlin, M. Snook, B. Siame, L. Butler et al., 1998. Engineering secondary metabolism in maize cells by ectopic expression of transcription factors. Plant Cell 10 721–740. [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt, R., 1999. Phytoalexins: What have we learned after 60 years? Annu. Rev. Phytopathol. 37 285–306. [DOI] [PubMed] [Google Scholar]
- Kambal, A. E., and E. C. Bate-Smith, 1976. A genetic and biochemical study on pericarp pigments in a cross between two cultivars of grain sorghum, Sorghum bicolor. Heredity 37 413–416. [Google Scholar]
- Knogge, W., E. Kombrink, E. Schmelzer and K. Hahlbrock, 1987. Occurrence of phytoalexins and other putative defense-related substances in uninfected parsley plants. Planta 171 279–287. [DOI] [PubMed] [Google Scholar]
- Kodama, O., J. Miyakawa, T. Akatsuka and S. Kiyosawa, 1992. Sakuranetin, a flavanone phytoalexin from ultraviolet-irradiated rice leaves. Phytochemistry 31 3807–3809. [Google Scholar]
- Lo, S. C. C., and R. L. Nicholson, 1998. Reduction of light-induced anthocyanin accumulation in inoculated sorghum mesocotyls: implications for a compensatory role in the defense response. Plant Physiol. 116 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, S. C., I. Weiergang, C. Bonham, J. Hipskind, K. Wood et al., 1996. Phytoalexin accumulation in sorghum: identification of a methyl ether of luteolinidin. Physiol. Mol. Plant Pathol. 49 21–31. [Google Scholar]
- Lo, S. C. C., K. De Verdier and R. L. Nicholson, 1999. Accumulation of 3-deoxyanthocyanidin phytoalexins and resistance to Colletotrichum sublineolum in sorghum. Physiol. Mol. Plant Pathol. 55 263–273. [Google Scholar]
- Nicholson, R. L., S. S. Kollipara, J. R. Vincent, P. C. Lyons and G. Cadena-Gomez, 1987. Phytoalexin synthesis by the sorghum mesocotyl in response to infection by pathogenic and nonpathogenic fungi. Proc. Natl. Acad. Sci. USA 84 5520–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal, R., M. A. Menz, P. J. Mehta, S. Katilé, L. A. Gutierrez-Rojas et al., 2009. Molecular mapping of Cg1, a gene for resistance to anthracnose (Colletotrichum sublineolum) in sorghum. Euphytica 165 597–606. [Google Scholar]
- Rakwal, R., G. K. Agrawal, M. Yonekura and O. Kodama, 2000. Naringenin 7-O-methyltransferase involved in the biosynthesis of the flavanone phytoalexin sakuranetin from rice (Oryza sativa L.). Plant Sci. 155 213–221. [DOI] [PubMed] [Google Scholar]
- Rakwal, R., K. Shii, G. K. Agrawal and M. Yonekura, 2001. Protein phosphatase inhibitors activate defense responses in rice (Oryza sativa) leaves. Physiol. Plant. 111 151–157. [Google Scholar]
- Rogers, E. E., J. Glazebrook and F. N. Ausubel, 1996. Mode of action of the Arabidopsis thaliana phytoalexin camalexin and its role in Arabidopsis-pathogen interactions. Mol. Plant Microbe Interact. 9 748–757. [DOI] [PubMed] [Google Scholar]
- Saghai-Maroof, M. A., K. M. Soliman, R. A. Jorgensen and R. W. Allard, 1984. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 81 8014–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., and D. Russell, 2001. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Schuhegger, R., T. Rauhut and E. Glawischnig, 2007. Regulatory variability of camalexin biosynthesis. J. Plant Physiol. 164 636–644. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer, Z., N. Shepherd, E. Tacke, A. Gierl, W. Rohde et al., 1987. Influence of transposable elements on the structure and function of the A1 gene of Zea mays. EMBO J. 6 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon, R. S., and S. Chopra, 2009. Progressive loss of DNA methylation releases epigenetic gene silencing from a tandemly repeated maize Myb gene. Genetics 181 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, B. A., and R. L. Nicholson, 1990. Synthesis of phytoalexins in sorghum as a site-specific response to fungal ingress. Science 248 1637–1639. [DOI] [PubMed] [Google Scholar]
- Stafford, H. A., 1965. Flavonoids and related phenolic compounds produced in the first internode of Sorghum vulgare Pers. in darkness and in light. Plant Physiol. 40 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel, R. G. D., D. A. Dickey and J. H. Torrie, 1996. Principles and Procedures of Statistics: A Biometrical Approach. McGraw-Hill Higher Education, New York.
- Styles, E. D., and O. Ceska, 1977. Genetic control of flavonoid synthesis in maize. Can. J. Genet. and Cytol. 19 289–302. [Google Scholar]
- Tenkouano, A., F. R. Miller, R. A. Fredericksen and R. L. Nicholson, 1998. Ontogenetic characteristics and inheritance of resistance to leaf anthracnose in sorghum. Afr. Crop Sci. J. 6 249–258. [Google Scholar]
- Thomma, B., I. Nelissen, K. Eggermont and W. F. Broekaert, 1999. Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J. 19 163–171. [DOI] [PubMed] [Google Scholar]
- Tsuji, J., E. P. Jackson, D. A. Gage, R. Hammerschmidt and S. C. Somerville, 1992. Phytoalexin accumulation in Arabidopsis thaliana during the hypersensitive reaction to Pseudomonas synringae pv synringae. Plant Physiol. 98 1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton, P. S., and A. M. Julian, 1996. A cytological study of compatible and incompatible interactions between Sorghum bicolor and Colletotrichum sublineolum. New Phytol. 134 25–34. [Google Scholar]
- Wharton, P. S., and R. L. Nicholson, 2000. Temporal synthesis and radiolabelling of the sorghum 3-deoxyanthocyanidin phytoalexins and the anthocyanin, cyanidin 3-dimalonyl glucoside. New Phytol. 145 457–469. [DOI] [PubMed] [Google Scholar]
- Wienand, U., U. Weydemann, U. Niesbach-klösgen, P. A. Peterson and H. Saedler, 1986. Molecular cloning of the c2 locus of Zea mays, the gene coding for chalcone synthase. Mol. Gen. Genet. 203 202–207. [Google Scholar]
- Winefield, C. S., D. H. Lewis, E. E. Swinny, H. B. Zhang, H. S. Arathoon et al., 2005. Investigation of the biosynthesis of 3-deoxyanthocyanins in Sinningia cardinalis. Physiol. Plant. 124 419–430. [Google Scholar]
- Winkel-Shirley, B., 2001. a Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 126 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel-Shirley, B., 2001. b It takes a garden. How work on diverse plant species has contributed to an understanding of flavonoid metabolism. Plant Physiol. 127 1399–1404. [PMC free article] [PubMed] [Google Scholar]
- Yamaoka, N., P. C. Lyons, J. Hipskind and R. L. Nicholson, 1990. Elicitor of sorghum phytoalexin synthesis from Colletotrichum graminicola. Physiol. Mol. Plant Pathol. 37 255–270. [Google Scholar]
- Zhao, J. M., and R. L. Last, 1996. Coordinate regulation of the tryptophan biosynthetic pathway and indolic phytoalexin accumulation in Arabidopsis. Plant Cell 8 2235–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]