Abstract
Context:
Staphylococcus aureus is spread via direct contact with persons and indirect contact via environmental surfaces such as weight benches. Athletes participating in direct-contact sports have an increased risk of acquiring S aureus infections.
Objective:
To determine (1) potential environmental reservoirs of S aureus in football and wrestling locker rooms and weight rooms, (2) environmental bacterial status after employing more stringent cleaning methods, (3) differences in colonization rates between athletes and nonathletes, (4) exposed body locations where Staphylococcus was recovered more frequently, and (5) personal hygiene practices of athletes and nonathletes.
Design:
Cross-sectional study.
Setting:
Locker room and strengthening and conditioning facilities at a National Collegiate Athletic Association Division II university.
Patients or Other Participants:
Collegiate football players and wrestlers, with nonathlete campus residents serving as the control group.
Intervention(s):
Infection control methods, education of the custodial staff, and education of the athletes regarding the Centers for Disease Control and Prevention guidelines for infection prevention.
Main Outcome Measure(s):
Cultures were taken from the participants' noses, fingertips, knuckles, forearms, and shoes and from the environment.
Results:
Before the intervention, from the 108 environmental samples taken from the football locker room and weight room, 26 (24%) contained methicillin-susceptible S aureus (MSSA) and 33 (31%) contained methicillin-resistant S aureus (MRSA). From the 39 environmental samples taken from the wrestling locker room and pit areas, 1 (3%) contained MSSA and 4 (10%) contained MRSA. The MRSA rates were different between the 2 locations according to a χ2 test (P = .01). Seven MRSA isolates were recovered from football players and 1 from a wrestler; no MRSA isolates were recovered from the control group. The fingertip location of S aureus recovery from football players was significant when compared with both other locations in football players and fingertips in wrestlers and the control group (P < .05). Football players and wrestlers shared more personal items than the control group (P < .05). After the intervention, the football locker room and weight room samples were negative for S aureus.
Conclusions:
Intact strengthening and conditioning equipment, proper hygiene, and proper disinfection methods lowered both environmental and human S aureus recovery at 1 university.
Keywords: bacteria, infections, communicable diseases
Key Points.
Methicillin-resistant Staphylococcus aureus (MRSA) was recovered from more football players than wrestlers.
Recovery of MRSA from football players' fingertips was greater than from other locations, and football players and wrestlers shared more personal items than the control group.
Education of the custodial staff regarding appropriate disinfection procedures and of the athletes regarding infection-control measures reduced the amount of Staphylococcus in the athletic environment.
Staphylococcus aureus infections in athletic teams have steadily increased over time.1–4 Risk factors for athletes acquiring Staphylococcus infections include frequent antibiotic use; lack of personal hygiene; exposure to football turf1; direct skin contact5; taking steam baths6; and sharing bars of soap, soap dispensers,2 and towels.7 Borchardt et al8 linked weight-room fomites to Staphylococcus transmission. Previous authors2,3,5,6 have concentrated on Staphylococcus recovery from the external nares, axillae, or groin, but studies of other body parts serving as reservoirs for Staphylococcus are lacking.
The goals of this study were to determine the following: (1) potential environmental reservoirs of S aureus in football and wrestling locker rooms and weight rooms at 1 university, (2) environmental bacterial status after employing more stringent cleaning methods, (3) differences in colonization rates between athletes and nonathletes, (4) locations on the human body where S aureus would be recovered more frequently, and (5) personal hygiene practices of athletes and nonathletes.
METHODS
An athletic trainer at a National Collegiate Athletic Association Division II school with approximately 11 000 students noted an increase from 9 diagnosed S aureus infections the previous year to 19 infections during the studied year in a collegiate football team. The year the study was conducted, the football team played in 6 away games. The wrestling team had 9 diagnosed cases in both years. A commercially supplied disinfectant was used in all areas of the football locker and weight rooms, so the first step was to evaluate its effectiveness. The disinfectant, which was diluted by the custodial staff, was tested against a laboratory strain of methicillin-susceptible S aureus (MSSA; ATCC 25923) and did not inhibit the bacteria as stated by the manufacturer. The diluted disinfectant had been allowed to remain in the clear jug (which held approximately 3500 mL) until all of it was used, often longer than a month. Thus, determining environmental and human Staphylococcus reservoirs and providing proper disinfectant dilution and storage education was needed.
Participants
The research protocols were approved by the institutional review board, and all volunteers signed informed consent forms before the study began. Because Staphylococcus is acquired via direct contact, we investigated its recovery from fingertips, knuckles, and forearms in addition to traditional testing of the inside of the external nares. Furthermore, shoe soles can transfer bacteria to wrestling-mat surfaces and locker-room floors, with which athletes have contact during matches and other activities.
Based upon the number of infections reported and the frequent direct contact with others, we tested 70 football players (99% of the team players) and 25 wrestlers (100% of the team athletes). Participants testing positive were retested 2 months later, after new disinfection methods had been implemented. The control group consisted of 50 on-campus nonathletes: 23 men and 27 women. Although the athletes were all male, we did not control for sex in the nonathlete group because most of the men approached were already part of the athletic group or were in another sport. Recruitment for the control group occurred at 4 large dormitories and lunch areas on campus.
Each athlete answered a survey asking about team position, previous diagnosis with Staphylococcus, personal items players shared, and hand-washing habits. The survey administered to the control group asked about involvement with any person in collegiate athletics, previous participation in high school athletics, personal items shared, and hand-washing habits. After the season, the 70 football players took a second survey, so that we could see if they had learned anything about infection control during that time. We did not resurvey the wrestlers because their season had ended, and the coach had previously addressed infection-prevention methods with them.
Environmental Sampling
Sterile, cotton-tipped applicators were used to take 108 initial environmental samples from the football locker room and weight room and 39 samples from the wrestling locker room and pit areas. The wrestling team's lockers, weight room, and practice areas were located in a single building across campus from the football team. However, the football locker room was located in a different building from the football weight room and was not shared with other students. The football weight room was shared with the cross-country and track teams.
Samples were then plated onto microbiologic media and tested for the presence of methicillin-resistant S aureus (MRSA) as described below. Staphylococcus aureus was found on a swab taken from the blower unit, which was part of the heating and air-conditioning system, so we placed 5 environmental exposure plates throughout the football weight room, with lids removed, for 1 hour when no one was using the room. The exposure plates would grow Staphylococcus distributed throughout the room by the air-handling unit. Positive and negative controls (blind samples to the microbiologist) were taken and tested in addition to the samples listed above.
Infection-control methods in the weight room included replacing the torn vinyl on the weight benches and replacing the foam flooring with nonabsorbent flooring. In the football locker room, floor and bench carpeting was removed. Water bottles were bleached and no longer shared among football players during practice or games. Instead, an assistant squirted water into the players' mouths, or players drank from a hands-free hose system.
Another control method employed was educating the custodial staff on proper SaniMaster 4 (Ecolab, Incorporated, St Paul, MN) disinfectant concentrations, disinfecting frequency, and disinfecting techniques, such as mopping and spraying equipment, shower stalls, and floors. The working solution of disinfectant was also prepared more frequently to protect its efficacy. When tested, the disinfectant inhibited growth of the laboratory strain of S aureus.
After proper disinfection measures were implemented, we tested 32 samples from the football locker and weight rooms, including the flooring, the lockers, the door handles, and all benches, to ensure that the new methods were controlling bacterial levels. Because no new Staphylococcus infections had been diagnosed, we did not retest every item. Disinfected wrestling mats tested negative. We did not retest the weight locker rooms because (1) the season was over by that time and the rooms were not being disinfected any longer, (2) other groups were then using the locker rooms, and (3) the wrestling team's incidence of Staphylococcus infections was less than that of the football team.
Staphylococcus Testing
Using sterile swabs (product 4140; Copan Diagnostics, Inc, Corona, CA), we collected bacterial samples from the nares, knuckles, forearms, and shoes of 70 football players, 25 wrestlers, and 50 control students. To ensure complete and representative bacterial counts, each external naris was swabbed, and the fingertips (the entire fat pad from the first phalange) of at least 4 fingers of the dominant hand were directly pressed onto the agar surface of petri dishes selective for growing Staphylococcus. Three middle knuckles on the dominant hand were swabbed over their entire lengths and were tested. Areas of approximately 4 × 2 in (10 × 5 cm) on the supinated side of 1 forearm and 3 in (8 cm) on 1 shoe sole were also swabbed. We chose to test the middle knuckles because they are more likely to contact another person or athletic equipment than the other knuckles. Given that weights are frequently lifted onto or using the forearms and the forearms are exposed during practice, we wanted to determine the frequency of Staphylococcus recovery from those areas. The forearm and shoe the participant presented to us were swabbed, so most samples (but not necessarily all) were from the dominant side.
Swabs (and fingertips) were directly plated onto an oxacillin-resistance screening agar base (agar, product OXCM1008B; antibiotic supplement, product OXSR0195E; Oxoid Limited, Basingstoke, United Kingdom) because of its selectivity for Staphylococcus.9 Plates were incubated at 35°C for 24 to 48 hours. Mannitol fermentation yielded dark blue colonies resistant to oxacillin, allowing for differentiation between probable S aureus and other bacterial colonies, which appeared white. The number of blue colonies was recorded as a quantitative measure of an individual's MRSA colonization.
Isolates were tested for the production of the coagulase enzyme (product Z202; Hardy Diagnostics, Santa Maria, CA) via the tube method, which indicates S aureus and separates it from the commonly isolated Staphylococcus epidermidis. The MRSA was confirmed using the US Food and Drug Administration–approved penicillin binding protein 2a (PBP2a) latex agglutination test (product DR0900A; Oxoid Limited), which tests for the presence of the mecA gene. The mecA gene confers resistance to β-lactam antibiotics such as penicillin and methicillin.
Dark blue isolates testing positive for the PBP2a test were then subcultured onto Mueller Hinton plates that we made. A zone of inhibition (no bacterial growth) was measured to determine susceptibilities to commonly prescribed antibiotics for Staphylococcus infections: clindamycin, vancomycin, gentamicin, tetracycline, trimethoprim/sulfamethoxazole, rifampin, and nitrofurantoin.
Statistical Analysis
Statistical analysis was performed using a χ2 contingency test (P < .05) to determine recovery rate significance of environmental and human sampling, as well as the significance of sharing personal items.
RESULTS
Participants
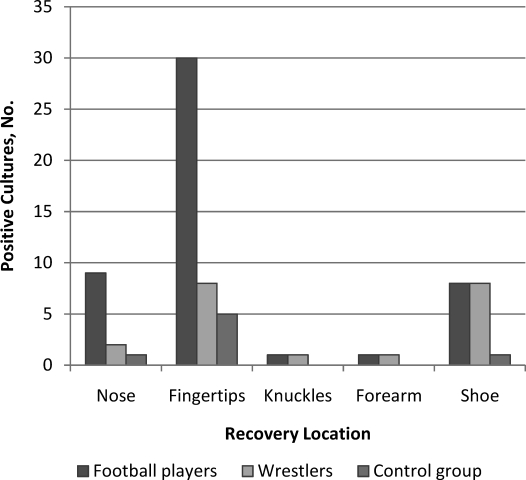
Two people (1 man, 1 woman; 4%) in the control group tested positive for S aureus, 1 each for the shoe and fingertip. Neither isolate was identified as MRSA. We used the same criteria public health agencies use: for an individual to be considered a carrier for Staphylococcus, a nasal sample must test positive. The locations from which Staphylococcus was recovered in the football, wrestling, and control groups are shown in Figure 1.
Figure 1.
Comparison of Staphylococcus aureus recovery from body locations of the football players, wrestlers, and control group. Recovery locations in football players were different from those of wrestlers and the control group, and recovery from the fingertip location in football players was greater than that from other locations in football players (P < .05).
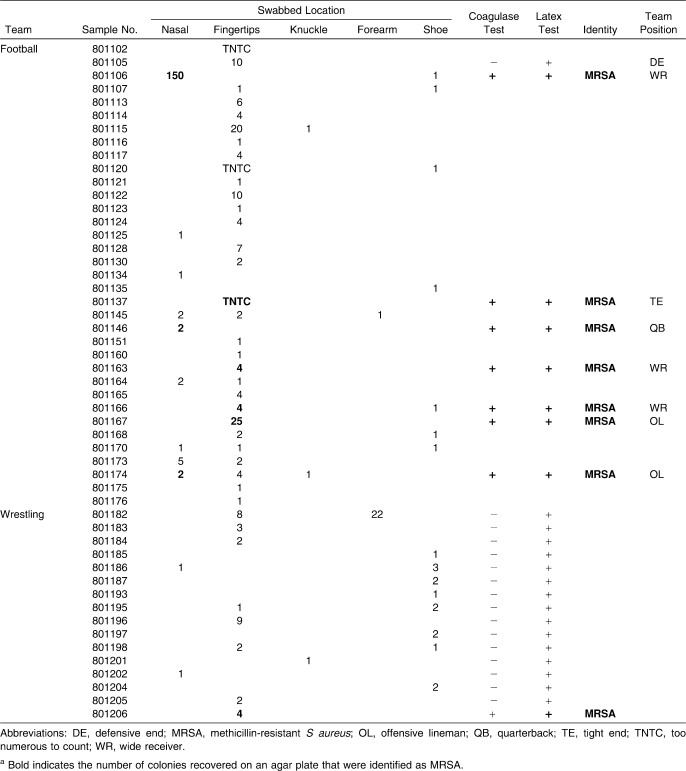
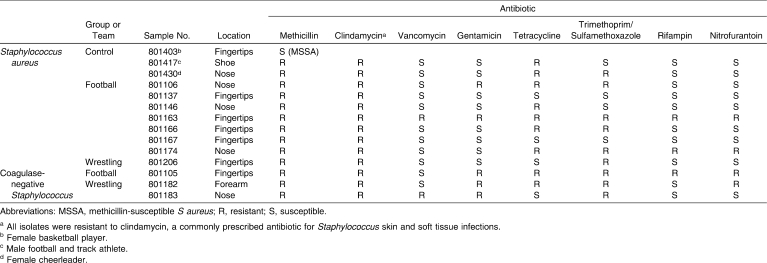
Of the 70 football players, 33 individuals (47%) tested positive for Staphylococcus at 1 location, and 15 players (21%) tested positive at multiple locations. Staphylococcus aureus was recovered from the nares in 9, from the fingertips in 30, from the knuckles in 2, from the forearm in 1, and from the shoes in 7 (Table 1). Seven athletes (10%) harbored MRSA: 3 in the nares and 4 on the fingertips. Based on the antibiotic susceptibilities shown in Table 2, we recovered 2 strains: 1 susceptible to tetracycline, sulfamethoxazole/trimethoprim, and rifampin, and 1 resistant to all 7 antibiotics. Of the 25 wrestlers, 16 (64%) tested positive for Staphylococcus: from the nares in 2, from the fingertips in 8, from the knuckle in 1, from the forearm in 1, and from the shoes in 8. Three people (12%) tested positive in 2 locations each. In 1 person (4%), MRSA was found on the fingertips and tested susceptible to tetracycline, rifampin, and nitrofurantoin.
Table 1.
Sampled Human Locations That Tested Positive for Staphylococcus aureusa
Table 2.
Antibiotic Susceptibilities of Recovered Methicillin-Resistant Staphylococcus Isolates
A χ2 contingency test revealed that the human locations (eg, nares, fingertips, shoes) of recovered Staphylococcus were significant in potential transmission among football players (P < .05) but not among wrestlers. The frequency of Staphylococcus recovery was not different between the teams, but the frequency was different between both teams and the control group (P < .05). Thus, Staphylococcus was recovered more frequently from the athletes than from the control group.
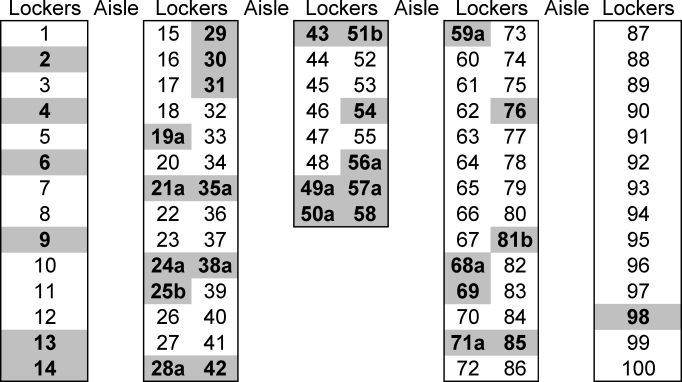
Geographically, the lockers of carriers, athletes with a diagnosed Staphylococcus infection, and athletes who were both carriers and had infections are shown in Figure 2. The center and center-left lockers appeared to be associated with more Staphylococcus than the lockers on the right side of the room, although statistical analysis did not show a difference.
Figure 2.
The football locker room layout showing geographic locations of carriers (ie, those with positive nasal swabs) in bold print. a Designates individuals with a reported infection; b designates individuals who both tested positive as carriers and were diagnosed with Staphylococcus infection. The locker numbers have been changed to protect human subjects.
In the survey of shared personal items, 12 of the control group (24%), 10 of the football players (14%), and 6 of the wrestlers (24%) indicated that they had not shared any of the listed items in the past year. The football players shared water bottles more frequently; the wrestlers shared more towels, soap, and deodorant; and the control group shared more clothes (Figure 3). A χ2 contingency tested revealed a difference between the athletes and the control group for sharing water bottles, towels, soap, deodorant, and clothes (P < .05). No differences were seen in the sharing of sweatbands, sponges, flip-flops, or backscratchers. All football players and wrestlers received education on covering wounds, not sharing personal items, and proper hand washing, based on the recommendations of the Centers for Disease Control and Prevention.10 We did not find any association between carrier status or infection and reported hand-washing habits.
Figure 3.
Personal items shared within the groups of football players, wrestlers, and control. Sharing differed between the athletes and the control group (P < .05).
Environment
Of the 108 environmental samples taken from the football locker and weight rooms, 26 (24%) were positive for MSSA and 33 (31%) were positive for MRSA. Of the 39 environmental samples taken from the wrestling locker room and pit areas, 1 (3%) was positive for MSSA and 4 (10%) were positive for MRSA. The locations from which MRSA was initially isolated are shown in Table 3. A χ2 contingency test showed a difference in MRSA rates between the teams (P = .01). Initial MRSA-positive environmental results were seen on the torn vinyl of weight benches and the chalk trays in the weight room, the hand dryer used for equipment, and several locker handles in the locker room. The carpet on the locker room floor and the carpet covering both teams' locker-room benches also tested positive for Staphylococcus in several locations, as did the foam flooring. The water bottles used during practices and games also tested positive. The environmental exposure plates were all negative, suggesting that Staphylococcus was not being blown through the room by the air-handling unit. The clothes washers and dryers and clean clothes also tested negative, indicating that laundering was not a source of bacteria.
Table 3.
Football and Wrestling Environmental Locations That Initially Tested Positive for Methicillin-Resistant Staphylococcus aureus
Once the proper disinfectant concentration was prepared and the frequency of disinfection was increased from once per day to 3 times per day (before and after practices and scheduled weight training), football areas previously testing positive retested negative. The shoe and ankle braces were not tested (these items were no longer stored in the lockers), and the water bottles were not retested because they were no longer being handled by players.
DISCUSSION
Participants' Sampling
Transmission of S aureus is primarily through direct contact; other means of transmission are thought to be via sharing equipment,11 dust,12 and aerosols.13 The Centers for Disease Control and Prevention10 estimates that 25% to 30% of the general population harbors S aureus and 1% harbors MRSA. Our results showed a 6% S aureus rate in our control group, a 47% rate in football players, and a 64% rate in wrestlers. In addition, the locations from which the bacteria were recovered in football players were significant, but these locations were not significant in wrestlers. Perhaps the high rate in the wrestlers can be attributed to the types of physical activity required of and the types of uniforms worn by the athletes. Wrestlers have direct upper and lower body contact because the uniforms are basically tank tops with shorts, whereas football players have short-sleeved tops and pants that extend to the knee. This difference in uniforms could explain why location was not significant in wrestlers: the majority of their body is subject to direct body contact. The S aureus rate was higher in wrestlers, but the MRSA rate was 0% in the control group, 4% in wrestlers, and 10% in football players. One possible explanation is that the wrestling coach had been educating his players for the past several years regarding proper hygiene, whereas the football team had not received education before this investigation.
Because nares, fingertips, and shoes were the main locations from which we recovered Staphylococcus, education appears to be a key to preventing infections. With the nares and fingertips harboring S aureus more often than other sites, the potential for Staphylococcus epidemics exists. Our overall culturing results were consistent with those of Beam and Buckley,11 Kazakova et al,4 Kirkland and Adams,14 and Romano et al.15
One group2 identified linemen and cornerbacks as having the highest risk among football players of acquiring Staphylococcus. In our study, 2 wide receivers, 1 tight end, 1 quarterback, and 2 offensive linemen harbored Staphylococcus. However, we were unable to determine if the bacteria recovered from the fingertips were resident or transient flora because of the frequent contact with other surfaces, and our study did not span several years.
All S aureus isolates recovered from athletes in our study were resistant to clindamycin, which is often the drug of choice for soft tissue infections. Trimethoprim/sulfamethoxazole is another drug of choice for soft tissue infections,16 but half of the isolates we recovered were resistant to it. If the bacteria are resistant to the administered antibiotic, the result may be septicemia; therefore, the clinician should be careful to select an antibiotic to which the infection is susceptible.
Environmental Sampling
Overall, MRSA was recovered from the locker rooms and weight rooms of both the football and wrestling teams at 1 university. Education in the proper dilution concentration of the disinfectant and increased frequency of use, in combination with carpet removal and the replacement of torn vinyl weight benches, lowered the Staphylococcus recovery rate to below detectable levels. This intervention helped to alleviate a continual source of potential infection by Staphylococcus.
The survey regarding personal hygiene habits provided an indication of potential ways Staphylococcus was being transmitted among players. Football players responded favorably to the infection-prevention education. A follow-up survey containing the same questions yielded 22 individuals reporting not sharing items, as compared with the initial 10. However, just because an individual reported that he was not sharing items does not mean he truly modified his behavior over the long term. The same 70 individuals filled out both the preintervention and postintervention surveys, so a direct comparison could be made.
Limitations
Limitations of this study include results from only a single university and the number of students willing to participate in a control group. In addition, the survey answers can only be taken at face value and do not indicate if an individual truly has modified sharing behaviors.
In conclusion, persons involved with athletics need to be aware of potential sources of Staphylococcus, how to prevent infections, and means of disinfection. In our study, we were able to educate athletes and implement appropriate disinfection practices to lower environmental Staphylococcus to below detectable limits. We were also able to see a correlation between body site of Staphylococcus recovery and Staphylococcus rates, thus providing a limited insight into transmission epidemiology, which we hope will be investigated and further elucidated in the future.
Acknowledgments
We thank Microbiology Laboratory technician William Kirby for laboratory support for this project; the Human Subjects Committee; David Glover, MD, the team physician; and the athletic teams and nonathletes for their participation.
REFERENCES
- 1.Romero D. V., Treston J., O'Sullivan A. L. Hand-to-hand: preventing MRSA. Nurse Pract. 2006;31(5):16–23. doi: 10.1097/00006205-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen D. M., Mascola L., Bancroft E. Recurring methicillin-resistant Staphylococcus aureus infections in a football team. Emerg Infect Dis. 2005;11(4):526–532. doi: 10.3201/eid1104.041094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen P. R. Cutaneous community-acquired methicillin-resistant Staphylococcus aureus infection in participants of athletic activities. South Med J. 2005;98(6):596–602. doi: 10.1097/01.SMJ.0000163302.72469.28. [DOI] [PubMed] [Google Scholar]
- 4.Kazakova S. V., Hageman J. C., Matava M., et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352(5):468–475. doi: 10.1056/NEJMoa042859. [DOI] [PubMed] [Google Scholar]
- 5.Grundmann H., Aires-de-Sousa M., Boyce J., Tiemersma E. Emergence and resurgence of methicillin-resistant Staphylococcus aureus as a public health threat. Lancet. 2006;368(9538):874–885. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 6.Gosbell I. B. Epidemiology, clinical features and management of infections due to community methicillin-resistant Staphylococcus aureus (cMRSA) Intern Med J. 2005;35(suppl 2):S120–S135. doi: 10.1111/j.1444-0903.2005.00985.x. [DOI] [PubMed] [Google Scholar]
- 7.Wilkoff L. J., Westbrook L., Dixon G. J. Factors affecting the persistence of Staphylococcus aureus on fabrics. Appl Microbiol. 1969;17(2):268–274. doi: 10.1128/am.17.2.268-274.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borchardt S. M., Yoder Y. S., Dworkin M. S. Is recurrent emergence of community-associated methicillin-resistant Staphylococcus aureus among participants in competitive sports limited to participants? Clin Infect Dis. 2005;40(6):906–907. doi: 10.1086/428354. [DOI] [PubMed] [Google Scholar]
- 9.Simor A. E., Goodfellow J., Louie L., Louie M. Evaluation of new medium, oxacillin resistance screening agar base, for the detection of methicillin-resistant Staphylococcus aureus from clinical specimens. J Clin Microbiol. 2001;39(9):3422. doi: 10.1128/JCM.39.9.3422.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Statement on questions and answers about methicillin-resistant Staphylococcus aureus in schools. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/Features/MRSAinSchools.html. Accessed October 13, 2008.
- 11.Beam J. W., Buckley B. Community-acquired methicillin resistant Staphylococcus aureus: prevalence and risk factors. J Athl Train. 2006;41(3):337–340. [PMC free article] [PubMed] [Google Scholar]
- 12.Leonas K. K., Jinkins R. S. The relationship of selected fabric characteristics and the barrier effectiveness of surgical gown fabrics. Am J Infect Control. 1997;25(1):16–23. doi: 10.1016/s0196-6553(97)90048-1. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh Y. L., Merry J. The adherence of Staphylococcus aureus, Staphylococcus epidermidis, and Escherichia coli on cotton, polyester and their blends. J Appl Bacteriol. 1986;60(6):535–544. doi: 10.1111/j.1365-2672.1986.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 14.Kirkland E. B., Adams B. B. Methicillin-resistant Staphylococcus aureus and athletes. J Am Acad Dermatol. 2008;59(3):494–502. doi: 10.1016/j.jaad.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Romano R., Lu D., Holtom P. Outbreak of community-acquired methicillin-resistant Staphylococcus aureus skin infections among a collegiate football team. J Athl Train. 2006;41(2):141–145. [PMC free article] [PubMed] [Google Scholar]
- 16.Patel M., Waites K. B., Moser S. A., Cloud G. A., Hoesley C. J. Prevalence of inducible clindamycin resistance among community- and hospital-associated Staphylococcus aureus isolates. J Clin Microbiol. 2006;44(4):2481–2484. doi: 10.1128/JCM.02582-05. [DOI] [PMC free article] [PubMed] [Google Scholar]