Abstract
Context:
Prior authors have reported associations among increased risk of injury and factors of the female athlete triad, as defined before the 2007 American College of Sports Medicine position stand, in collegiate and adult club sport populations. Little is known about this relationship in an adolescent competitive sports population.
Objective:
To examine the relationship among disordered eating, menstrual dysfunction, and low bone mineral density (BMD) and musculoskeletal injury among girls in high school sports.
Design:
Prospective cohort study.
Setting:
The sample consisted of 163 female athletes competing in 8 interscholastic sports in southern California during the 2003–2004 school year. Each participant was followed throughout her respective sport season for occurrence of musculoskeletal injuries.
Main Outcome Measure(s):
Data collected included daily injury reports, the Eating Disorder Examination Questionnaire that assessed disordered eating attitudes and behaviors, a dual-energy x-ray absorptiometry scan that measured BMD and lean tissue mass, anthropometric measurements, and a questionnaire on menstrual history and demographic characteristics.
Results:
Sixty-one athletes (37.4%) incurred 90 musculoskeletal injuries. In our BMD z score model of ≤−1 SD, a history of oligomenorrhea/amenorrhea during the past year and low BMD (z score ≤−1 SD) were associated with the occurrence of musculoskeletal injury during the interscholastic sport season. In our BMD z score model of ≤−2 SDs, disordered eating (Eating Disorder Examination Questionnaire score ≥4.0), a history of oligomenorrhea/amenorrhea during the past year, and a low BMD (z score ≤−2 SDs) were associated with musculoskeletal injury occurrence.
Conclusions:
These findings indicate that disordered eating, oligomenorrhea/amenorrhea, and low BMD were associated with musculoskeletal injuries in these female high school athletes. Programs designed to identify and prevent disordered eating and menstrual dysfunction and to increase bone mass in athletes may help to reduce musculoskeletal injuries.
Keywords: adolescents, female athletes, menstrual status, musculoskeletal injuries, sports
Key Points.
In this sample of high school female athletes, disordered eating, oligomenorrhea/amenorrhea, and low bone mineral density were associated with musculoskeletal injury.
Bone mineral density levels below expected ranges for age were also associated with musculoskeletal injury.
Regular, close monitoring of adolescent female athletes during both seasonal and off-season training may be warranted, so that potential problems can be recognized and addressed promptly.
The participation by girls in interscholastic sports in the United States has increased from 1.84 million during the 1988–1989 school year to 3 million high school athletes during the 2007–2008 school year.1 This participation represents an increase of 65.9% for girls, compared with 27.6% for boys during the 2-decade span. Although many benefits can be gained from involvement in interscholastic sports, the increased activity among girls may also present greater opportunities for musculoskeletal injury. In fact, authors2–4 of recent prospective studies of high school populations have reported higher injury rates among female athletes than male athletes in similar sports. Negative consequences of sport-related musculoskeletal injuries among female athletes include reduced playing time and possible loss of an entire season of sport participation. Over the long term, injuries may lead to chronic musculoskeletal problems, restricting pain-free mobility and participation in fitness-enhancing activities later in life.5
Information regarding risk factors specific to musculoskeletal injuries among female high school athletes is limited. Growing evidence suggests that the 3 interrelated conditions of the female athlete triad (low energy availability, with or without disordered eating; menstrual dysfunction; and low bone mass) may place a female athlete at greater risk for musculoskeletal injury, primarily stress fracture.6 However, few researchers7–9 have reported the association among musculoskeletal injury and all triad components simultaneously in female athletes. Instead, most investigators have evaluated the association among musculoskeletal injury and 1 or 2 individual components.
Of the 3 components, menstrual dysfunction has exhibited the strongest relationship to injury, showing positive associations with increased risk of stress fractures in collegiate athletes,10,11 competitive club track-and-field athletes,7,12 adult recreational runners or athletes,10,13 and military recruit populations.14,15 Additionally, menstrual dysfunction has been associated with longer interruption of training due to musculoskeletal injuries.16
The association between low bone mass and musculoskeletal injury, primarily stress fracture, has also been reported in adult competitive cross-country runners,8 competitive club track-and-field athletes,7 adult athletes,13 and military recruits.17 Several authors have reported an association between disordered eating and musculoskeletal injury in female collegiate athletes9,18 and military recruits.19 However, evidence reviewed in the 2007 American College of Sports Medicine (ACSM) position stand on the female athlete triad6 indicated that low energy availability due to dietary restriction and excessive exercise, rather than disordered eating alone, likely leads to menstrual dysfunction and low bone mass. As such, research reported to date may underestimate the true association between energy availability and injury, as previous measures were limited to assessing only eating disorders or disordered eating.
In a recent study of girls' high school varsity sports, Nichols et al20 reported estimates of 18.2%, 23.5%, and 21.8% for disordered eating, menstrual irregularity, and low bone mass, respectively. Although only 1.2% of the athletes had the full triad simultaneously, 48.2% met criteria for at least 1 triad component, indicating that these conditions occur as early as adolescence for female athletes. Although the literature in collegiate and competitive female athletes suggests a relationship among 1 or more triad components and stress fracture or other musculoskeletal injury, whether relationships exist among 1 or more triad components as risk factors for musculoskeletal injury in adolescent athletes is presently unclear. In a study of 5461 adolescent girls, Loud et al21 did not show an association between stress fracture and disordered eating or menarcheal age. However, their findings were limited in that the cross-sectional design used did not allow them to determine if these risk factors preceded the stress fractures, and the lack of association between disordered eating and stress fracture may have been due to low statistical power. Further, they did not specify if stress fractures or sport activities occurred during an organized sport season. Thus, the purpose of our study was to prospectively examine the relationship among the 3 triad components, as defined before the revised 2007 ACSM position stand,6 and the risk of sport-related musculoskeletal injury among female athletes in an interscholastic multisport population.
METHODS
Participants
The sample consisted of female athletes representing 8 interscholastic sports from 6 high schools in southern California. Participants who had experienced menarche were 13 to 18 years old; those who had not begun to menstruate were required to be 15 to 18 years old to participate. Data from athletes who reported taking any medications known to affect bone mass were excluded from analysis. Subsequently, only 1 athlete was excluded because of chronic use of a corticosteroid inhaler for asthma. A total of 589 athletes (total possible athletes = 740, 79.6% response rate) completed questionnaires regarding eating behaviors and menstrual history and were measured, without shoes, for height and weight. One research assistant for every 6 to 8 athletes reviewed the Eating Disorder Examination Questionnaire (EDE-Q) response scale and questions deemed more difficult to interpret, defined terminology (eg, binge episode), and then remained in the room to assist athletes who requested further clarification as they completed the questionnaires individually. After stratification by eating behavior or attitude (normal versus elevated EDE-Q score) and menstrual status (eumenorrhea versus oligomenorrhea/amenorrhea), we randomly selected 170 of the 589 athletes to receive a bone mineral density (BMD) scan.20 The study was approved by the university's institutional review board. Written parental consent and athlete assent were provided by study participants. Passive consent was obtained to collect and analyze injury data from athletes who did not want to participate in other study measures.
Data Collection
Injury
Before each sport season, coaches and athletic trainers were trained in using the Athletic Health Care System Daily Injury Report (DIR) form.4,22 From the first official day of practice until the last regular or postseason competition, coaches or athletic trainers recorded each athlete's daily participation at practices and games or meets and absences and limitations to participation because of injury. An injury was defined as any reported muscle, joint, or bone problem or injury resulting from participation in a practice, game, or meet and requiring the athlete to be removed from a practice, game, or meet or to miss a subsequent one.4,22 Injuries that did not occur during practices or game or meet participation were excluded. A day lost to injury was any day in which the athlete was not able or permitted to participate in an unrestricted manner. For injured athletes, coaches or athletic trainers recorded the body part injured (eg, knee) and injury type (eg, tendinitis). The DIR required 5 minutes or less per day to complete. For analysis purposes, athletes who reported facial contusions or lacerations only were considered uninjured, because the mechanisms of the triad components were not expected to increase the risk of these injury types. Major time-loss injuries were those resulting in loss of participation for 22 or more days.3
Eating Attitudes and Behaviors
The current study was limited to assessing disordered eating. Near the beginning of the season, each athlete completed the EDE-Q to identify disordered eating attitudes and behaviors.23,24 The EDE-Q, a shorter version of the Eating Disorder Examination and designed for self-report,25 comprises 4 subscales (weight concern, shape concern, eating concern, and dietary restraint) along with a global score, which is the composite mean score of the 4 subscales. Scores ranging from 0 to 6 on a Likert scale correspond with the number of days over the past 4 weeks the respondent has experienced a specific attitude, feeling, or behavior. The EDE-Q also assesses pathogenic eating behaviors, including bulimia and use of vomiting, laxatives, diuretics, or diet pills to control body weight. The EDE-Q has high internal consistency and moderate to high concurrent and criterion validity.24–26 In addition to its psychometric properties, the EDE-Q was chosen over other widely used questionnaires because it addresses a particular time frame and specifically assesses the frequency of eating behaviors. We evaluated EDE-Q scores as continuous and dichotomous variables. Athletes were classified as having an elevated weight concern, shape concern, eating concern, or dietary restraint behavior if they had a mean score of 4.0 or higher for that particular behavior. Athletes were classified as having disordered eating if they had a mean score ≥4.0 on the weight concern, shape concern, eating concern, or dietary restraint subscales; had a mean global score ≥4.0; or reported engaging in any pathogenic behavior 2 or more times in the past 4 weeks. A cutoff score of 4, which indicates that a specific attitude or behavior was reported on more than half of the past 28 days, was used to define disordered eating because it has been shown to predict eating disorders.23 Also, scores ≥4.0 correspond with the 80th to 95th percentiles from reference norms for adolescent girls.23 Based on this scoring rubric, we divided athletes into 2 groups: elevated EDE-Q (disordered eating) or normal EDE-Q.
Menstrual Status
After completing the EDE-Q, the athletes completed a menstrual status and history questionnaire, which was derived from an athlete preparticipation medical history form developed to screen for the presence of female athlete triad components.27 The criteria for classifying athletes with menstrual irregularity were primary amenorrhea (no onset of menses by age 15 years), secondary amenorrhea (cessation of menstrual cycles ≥3 consecutive months in the past year), or oligomenorrhea (menstrual cycles occurring at intervals longer than 35 days after the onset of menses by age 15 years).6 For analysis purposes, girls meeting any of these criteria were combined into a single (oligomenorrheic/amenorrheic) group and compared with girls having normal menses (eumenorrheic). The athletes were also asked if they were taking birth control pills or hormones to regulate their menstrual cycle.
Bone Mineral Density
Between 2 and 4 weeks after the questionnaires were completed, areal BMD (g·cm−2) at the spine (L1–L4), proximal femur, and total body and body composition (percentages of fat and lean tissue mass) were assessed by dual-energy x-ray absorptiometry using a Lunar densitometer (model DPX-NT; GE/Lunar Corporation, Madison, WI). Quality assurance tests were performed each morning of testing. The coefficient of variation in BMD in our laboratory is 0.6% for the total hip, 1.2% for the spine (L1–L4), and 0.99% for total body.
We used recommendations from the new ACSM position stand,6 derived from World Health Organization28 and International Society for Clinical Densitometry29 criteria, to define low bone mass in premenopausal women and adolescents. Athletes were categorized as having low bone mass if their BMD z score at the spine or total body was 1 or 2 SDs below the age-matched, sex-specific reference data from the GE/Lunar Corporation pediatric database (z score ≤−1 or ≤−2, respectively). We examined both z score levels to determine the severity of low bone mass, because athletes would normally be expected to have BMD values approximately 10% higher than nonathletes.6 Therefore, a BMD z score between −1 and −2 warrants concern.
Statistical Analyses
Although athletes were allowed to complete the study questionnaires for each season in which they competed in a sport during the 2003–2004 school year, they were selected and received a bone scan for the season in which they initially participated in our study. An athlete was included only once in our study analysis. That is, only questionnaires completed and injuries incurred by the athlete during the sport season in which she received her bone scan were used. Independent t tests and the χ2 test were used to compare demographic, physical, and menstrual history characteristics of injured and uninjured athletes. Because body mass has been reported to influence scores on eating attitudes questionnaires,30 we used analysis of covariance to control for body mass index (BMI) when comparing the mean EDE-Q scores between injured and uninjured athletes. Analysis of covariance was also used to control for chronologic age, gynecologic age, lean tissue mass, and sport group type according to mechanical loading characteristics (nonimpact or endurance sport: swimming, cross-country, track and field; high- or variable-impact sport: soccer, softball, volleyball, tennis, lacrosse) when comparing the BMDs of injured and uninjured athletes, as these variables are associated with BMD.
Crude odds ratios and 95% confidence intervals were calculated for injury, comparing the proportion of individuals in a high-risk group versus the proportion of individuals in a baseline or referent group for each of the potential risk factors.31 Athletes who reported using birth control (n = 13) were controlled for in the univariate and multivariable analyses involving athletes' reports of recent menstrual history. For multivariable analyses, the measure of association was the adjusted odds ratios, which were generated from a multiple logistic regression analysis.32 Items in the logistic regression model analyses included disordered eating, menstrual status, BMD, and factors suspected to confound the relationships, including sport group type, lean tissue mass, and gynecologic age. Adjusted models were conducted for ≤−1 SD and ≤−2 SDs BMD group levels.
For all statistical analyses, the alpha level was set a priori at .05. All analyses were conducted with SPSS (version 14.0; SPSS Inc, Chicago, IL) and STATA (version 5.0; STATA Corporation, College Station, TX).
RESULTS
Final Sample
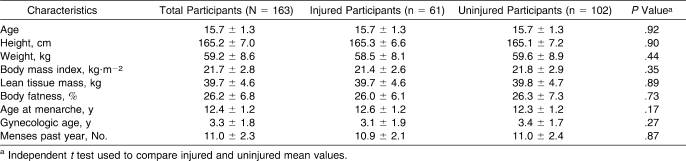
Of the 170 athletes, 7 athletes were excluded because 1 team did not provide injury information. Thus, the final sample size for study analysis was 163 athletes. The athletes' sports were track and field (n = 61), cross-country running (n = 33), soccer (n = 22), softball (n = 11), swimming (n = 9), volleyball (n = 11), tennis (n = 10), and lacrosse (n = 6). The mean characteristics for continuous variables (age, height, weight, BMI, lean tissue mass, total body fat percentage, menarcheal age, gynecologic age, and menses during the past year) are reported in Table 1 for the final sample (n = 163 athletes) and by injury status. Those athletes who incurred injuries were not different from the uninjured athletes on any of the continuous demographic or physical characteristics (P > .05). Overall, the menarcheal age of athletes reporting oligomenorrhea/amenorrhea during the past year was older than for athletes reporting normal menses (mean ± SD = 13.1 ± 1.4 versus 12.2 ± 1.1, P < .001). The sample included a racial and ethnic distribution of 55.2% (n = 90) Caucasian, 20.2% (n = 33) Latina, 14.1% (n = 23) African American, 6.1% (n = 10) Asian or Pacific Islander, and 4.3% (n = 7) other race or ethnicity. A χ2 test indicated that the racial and ethnic distribution did not vary by injury status (χ24 = 0.71, P = .95).
Table 1.
Selected Demographic and Physical Characteristics of Participants (Mean ± SD) by Musculoskeletal Injury Status
Injuries
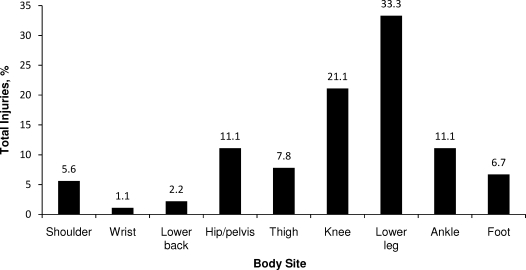
Of the 163 athletes studied for 1 season each, 61 (37.4%) incurred a total of 90 sport-related injuries. Although the difference was not significant (χ21 = 2.72, P = .13), athletes who participated in nonimpact or endurance sports sustained more musculoskeletal injuries (45.8%, n = 27) than those in high-impact or variable-impact, team, or anaerobic sports (32.7%, n = 34). The most common sites of injury were the lower leg (33.3%, n = 30) and knee (21.1%, n = 19; Figure 1). Overall, 41.7% (n = 5) of major time-loss injuries affected the lower leg. Overuse or chronic injuries accounted for 68.9% (n = 62) of the total and acute injuries for 31.1% (n = 28).
Figure 1.
Incidence of musculoskeletal injury (n = 90) by body site in 61 female high school athletes.
Disordered Eating and BMD Values by Injury Status
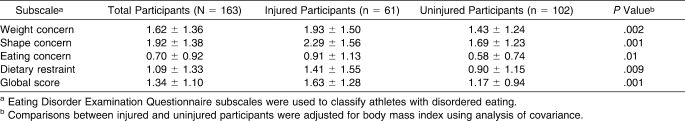
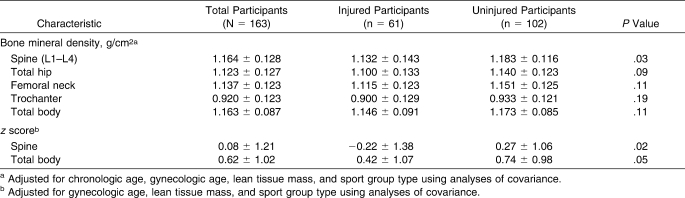
After we adjusted for BMI, injured athletes reported higher mean scores for all EDE-Q subscales and the global score than uninjured athletes (Table 2). Injured athletes had lower BMD at the spine (P = .03), and lower BMD z scores at the spine (P = .02) and for the total body (P = .05) than uninjured athletes after we adjusted for lean tissue mass, sport group type, and chronologic and gynecologic age (Table 3). Although injured athletes had lower BMD values at all other bone sites than uninjured athletes, these differences were not statistically different (P > .05).
Table 2.
Eating Disorder Examination Questionnaire Subscale Scores (Mean ± SD) by Musculoskeletal Injury Status
Table 3.
Bone Mineral Density (Mean ± SD) by Musculoskeletal Injury Status
Risk Factors for Injury
Disordered Eating Behaviors
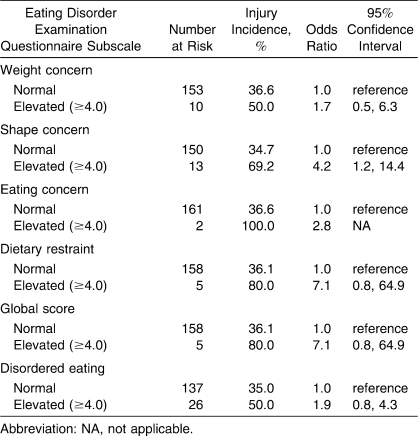
An elevated shape concern subscale score (≥4.0) was associated with a 4.2-fold increase in injury risk (Table 4). Athletes with an elevated dietary restraint subscale score or global score were 7 times more likely to incur a musculoskeletal injury than athletes who reported normal behavior scores, but the associations were not statistically significant (P = .08). No significant associations were found between groups for the other disordered eating scales.
Table 4.
Crude Odds Ratios for Musculoskeletal Injury by Self-Reported Eating Behaviors and Attitudes
Menstrual Status
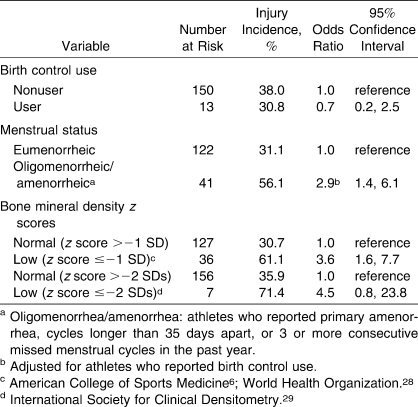
Athletes who reported oligomenorrhea/amenorrhea during the past year demonstrated an increase in injury risk that was almost 3-fold greater than that of athletes who reported eumenorrhea (P = .004; Table 5). No associations were found for other menstrual variables or birth control use.
Table 5.
Crude Odds Ratios for Musculoskeletal Injury by Self-Reported Menstrual Status and Low Bone Mineral Density Criteria
Bone Mineral Density
Athletes who had an overall BMD z score ≤−1.0 SD were 3.6 times more likely to incur an injury than athletes who had normal BMD values (P = .001; Table 5). Although athletes who had a BMD z score ≤−2 SDs were 4.5 times more likely to sustain an injury, the association was not significant (P = .08).
Risk Factors and Major Time-Loss Injury
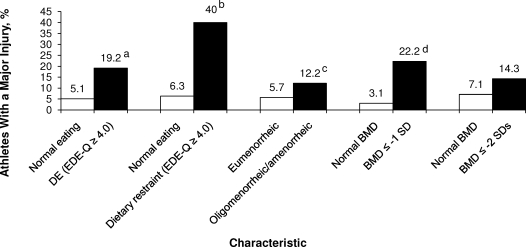
The incidence of injuries causing 22 or more days of lost participation by disordered eating, oligomenorrhea/amenorrhea, and low BMD status is illustrated in Figure 2. The incidence of major time-loss injury was higher for athletes who reported any disordered eating (P = .03) or dietary restraint behavior (P = .04) or had a BMD z score ≤−1.0 SD (P = .001).
Figure 2.
Relationship among disordered eating status, dietary restraint behavior status, menstrual status, BMD status, and major injuries (injuries that resulted in loss of practice, game, or meet participation >22 days). DE indicates disordered eating; EDE-Q, Eating Disorder Examination Questionnaire; BMD, bone mineral density. a Indicates P = .03; b P = .04; c P = .18; d P = .001.
Adjusted Final Risk Factor Models for Injury
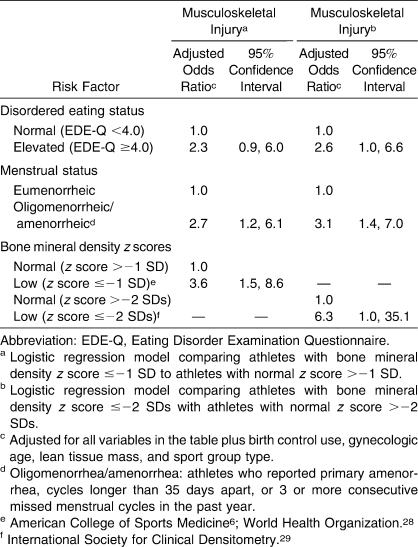
After adjusting for birth control use, lean tissue mass, sport group type, gynecologic age, and all 3 triad components (disordered eating, oligomenorrhea/amenorrhea, low BMD), the only significant components associated with sport-related injury in the final BMD z score ≤−1.0 SD logistic regression model were oligomenorrhea/amenorrhea and low bone density (Table 6). However, in the final BMD z score ≤−2.0 SDs logistic regression model, all 3 components were associated with injury.
Table 6.
Adjusted Odds Ratios for Musculoskeletal Injury by Disordered Eating Status, Menstrual Status, and Low Bone Mineral Density Level (Separate Models for z Scores of ≤−1 and ≤−2 SDs)
DISCUSSION
The purpose of our study was to determine if components of the female athlete triad, as defined before 2007,6 were associated with musculoskeletal injury in a sample of female high school athletes. Athletes who reported oligomenorrhea/amenorrhea during the past year or whose BMD was lower than expected for their age (based on reference norms) were at increased risk for a musculoskeletal injury. When we calculated a separate prediction model for athletes who had a BMD z score ≤−2 SDs, all triad components were associated with increased injury risk.
To our knowledge, we are the first to report associations among all triad components and musculoskeletal injury in girls' high school sports. The prospective design allowed us to determine the component status of each athlete before injuries occurred, thus reducing the likelihood of recall or measurement bias.31 The sample size was large enough to enable analysis of the triad factors and other important characteristics concurrently. The only other studies to prospectively examine the relationship of all 3 triad components with injury in the same study have been in collegiate, postcollegiate, or competitive club sport populations.7–9
Injury
During the season, 37.4% of the athletes had at least 1 sport-related musculoskeletal injury severe enough to result in their removal from, or nonparticipation in, a practice, game, or meet. Overall, although most injuries were considered chronic or overuse in nature, we did not report data regarding specific injury type (eg, sprain, tendinitis, stress fracture), which is a limitation of our study. We believed the coaches' and athletic trainers' reports regarding injured body locations were accurate because of the education they received about the sport injuries and how to report them on the DIR,4,22 but we were less sure about the accuracy of their reports regarding injury type without a clinician's diagnosis to confirm them. Thus, we did not report results regarding injury type because of the increased likelihood of misclassification. The greatest percentage of injuries affected the lower leg, a particularly important finding given that tibial stress fractures and other shin-related injuries may not be differentiated and may, thus, be misdiagnosed without additional clinical confirmation and diagnostic imaging.33
Menstrual Status and Injury
Our data confirm findings from studies of collegiate athletes, competitive club athletes and runners, recreational runners, and military recruits7,10–15 indicating that a history of menstrual disturbance is a risk factor for injury. Therefore, factors associated with oligomenorrhea/amenorrhea appear to reflect an increased risk for musculoskeletal injury in female athletes. In controlled laboratory studies,34,35 the direct negative effect of inadequate energy intake on hormones that regulate menstrual function and bone turnover has been documented. Additionally, a low energy state has also been reported to disrupt growth, thyroid, and stress hormones, thereby promoting a hormone profile indicative of a catabolic state.34–37 This same hormonal profile may also affect the health of other tissues, including muscle and connective tissue, which may explain the relationship between oligomenorrhea/amenorrhea and non–bone-related (eg, muscle strain) injuries.
Our findings indicated no association between oral contraceptive use and risk of injury. However, this finding may be a result of the small number of athletes who reported oral contraceptive use. Findings on the relationship between oral contraceptive use and risk of injury have been mixed in previous studies of female athletes and military recruits.15,18,38 However, these investigators examined only the association between oral contraceptive use and stress fracture occurrence, whereas not all injuries in our sample were bone related (ie, stress fractures). Thus, further studies are needed to examine the effects of oral contraceptive use on musculoskeletal injuries other than stress fractures in this population.
Unlike authors of previous prospective studies who have reported a relationship between older menarcheal age and stress fracture in competitive club track-and-field athletes7 and adult competitive cross-country runners,8 we did not find a relationship between menarcheal age and injury. Although our findings are in accordance with a cross-sectional study of stress fractures among adolescent females,21 they should be interpreted cautiously. The athletes reporting oligomenorrhea/amenorrhea during the past year had an older menarcheal age than athletes reporting normal menses. Therefore, because oligomenorrhea/amenorrhea was associated with injury in this study, older menarcheal age may still play a role in the increased likelihood of injury.
Low BMD and Injury
Low BMD was strongly associated with injury. This finding is consistent with the findings of several prospective and case-control studies whose authors7–9,13,39 have reported an association between low BMD and stress fracture or other musculoskeletal injury. When we examined risk of injury by BMD z scores, we found that athletes with low BMD were at a 4- and 6-fold increased risk of injury at −1 SD and −2 SDs, respectively. Our finding is similar to that reported by Kelsey et al,8 who observed that the rate of stress fractures increased with decreasing SD of whole body bone mass in young female cross-country runners. Important to note is that low BMD z scores at both −1 SD and −2 SDs were related to injury independent of oligomenorrhea/amenorrhea. It is possible that the effects of menstrual disturbance before the past year may have affected some athletes' BMD, as reported in studies of competitive club track-and-field athletes.7,12 Unfortunately, we did not assess the lifetime history of any menstrual disturbance in our athletes.
Our finding that injured athletes had lower mean lumbar spine BMD is consistent with findings of authors7,8,13 who have reported an association between lower lumbar spine BMD and increased stress fracture risk in competitive or adult runners and track-and-field athletes. Further, although non-statistically significant trends were observed, our injured athletes had lower mean total hip, femoral neck, trochanter, and total body BMD than uninjured athletes. Thus, these findings are relevant because they suggest that low bone mass, directly or indirectly, increases a female high school athlete's risk of bone or non–bone-related injury. Therefore, behaviors that promote optimal bone mineral accrual in high school athletes may also serve a protective role in minimizing the incidence of non–bone-related injury.
Disordered Eating and Injury
The adjusted mean scores for all disordered eating subscales and the global scale were higher among injured athletes than uninjured athletes. This finding supports results from several previous studies that showed more disordered eating among injured collegiate athletes,9,18 but the mean scores for injured athletes in our study fell within the normal range for eating behavior and attitudes. Of greater significance was a higher incidence of injury among athletes who had elevated EDE-Q scores (≥4.0) than among those who had normal scores when we compared athletes' BMD at the −2 SDs level. In our unadjusted risk estimates, an association was observed between injury and athletes who had elevated scores for the shape concern subscale. These findings indicate that unhealthy eating attitudes and behaviors were more apparent among the injured athletes. Further, although an association between elevated dietary restraint and any injury fell just short of statistical significance, a significant association was found between elevated dietary restraint and injuries lasting 22 days or more. This is important because authors12,40–43 have found that abnormal dietary restraint behaviors or restricted caloric intake may lead to amenorrhea, increased bone resorption, low BMD, or increased risk of stress fracture. These results lend support to the notion that inadequate energy intake relative to expenditure and possible macronutrient and micronutrient deficiencies may be largely related to the injured athletes' lower BMD. Our findings are notable in light of the new ACSM position stand,6 with its emphasis on low energy availability as the underlying cause of menstrual disturbance and poor bone health, and suggest that conditions of the female triad may concurrently increase a high school athlete's injury risk.
Severity of Injury
In earlier studies of athletes, researchers7,8,10–13,21 have predominantly examined the effects of triad components and likelihood of stress fracture. Thus, comparisons between our study and prior studies may be difficult, as we included all injuries and did not have clinical diagnoses for injuries suspected to be stress fractures. However, we were able to measure the severity of injury by time lost from participation. Disordered eating (particularly dietary restraint) and low BMD were associated with major injuries (injuries causing 22 days or more of lost participation). We observed only an increased trend between oligomenorrhea/amenorrhea and major injury, but Beckvid-Henrikkson et al16 reported that athletes with menstrual dysfunction had a longer interruption of training due to musculoskeletal injuries than those with regular cycles. Therefore, the sum of our findings may suggest that the athletes in our study with disordered eating behaviors may also have nutrient deficiencies or inadequate caloric intake relative to the energy demands of their sport, that is, low energy availability, which may adversely affect their bone, muscle, or connective tissues.44–46 Sustained low energy availability has been associated with menstrual dysfunction, hypoestrogenism, hypercortisolism, and low insulin-like growth factor levels, but it may also negatively affect the ability of these tissues to rebuild after injury and, consequently, prolong the athlete's recovery time.34,37,41,47–49 However, this possibility is speculative, and we are unaware of any reported direct associations between recovery from injury and metabolic or reproductive hormones. Future investigators should prospectively examine these relationships and the length of disability due to sport injury.
Limitations
Limitations of our study should be noted. First, we used self-reports instead of laboratory measures for menstrual status data. Interpreting menstrual data is difficult, especially in those athletes who report oligomenorrhea, which is not uncommon in the first few years after menarche.50 Yet our data were reliable20 and consistent with those of prior authors who used self-reported menstrual status in collegiate and elite athletes, and they provide additional evidence that menstrual irregularity is associated with musculoskeletal injury. Second, we collected our data before the revised ACSM position stand6 was published. Thus, because the EDE-Q measured only disordered eating attitudes and behaviors, another limitation of our study is that we did not collect data to determine energy availability, nor did we assess the athletes' calcium intake (low levels of calcium or dairy intake have been shown to be a risk factor for injury8,13). Third, we did not examine triad components separately by body site because of the small numbers of injuries for certain body sites. Additionally, we did not examine the relationship between triad components and injury by individual sport because several sports had small numbers of athletes.
Recommendations for Future Research
We suggest several recommendations for future research. First, further investigation into the relationship between sport injuries and triad components in high school athletes is needed, especially prospective studies with larger sample sizes of athletes representing all interscholastic sports. Second, future authors should examine associations among injuries by individual high school sports, especially in those athletes considered at greater risk of disordered eating behaviors and attitudes because of emphasis on leanness and weight control.6,18 Third, as recommended by the new ACSM position stand,6 we suggest that future investigators evaluate the association between energy availability, with and without eating disorders or disordered eating, and sport-related injuries, using objective measures that will capture the athlete's energy intake and exercise energy expenditure. Finally, it may be unlikely that the triad components alone result in injury. Studies are needed to determine if interactions among the triad components and other variables (eg, training history) increase an athlete's susceptibility to injury.
Conclusions
In this prospective study of 163 high school female athletes, disordered eating, oligomenorrhea/amenorrhea, and low BMD (especially a BMD z score of −2 SDs or lower) were associated with musculoskeletal injury. The association between oligomenorrhea/amenorrhea and injury provides additional support for menstrual dysfunction as an important risk factor for injury among female athletes. Preparticipation screening efforts to identify those with a history of menstrual irregularity, followed by proper medical management, are recommended. The findings we observed regarding a relationship between dietary restraint and injury also provide evidence for the value of screening for restrained eating and referring restrained eaters for medical or nutritional counseling.6,46 The BMD levels below the expected range for age were associated with musculoskeletal injury. Causes of low BMD are multifactorial, so athletes identified with menstrual dysfunction or disordered eating should be referred to their physicians for further screening because both factors have been found to be independently associated with low BMD. Given the potential for long-term health consequences resulting from this critical stage of growth and development, regular and close monitoring of adolescent female athletes during seasonal and off-season training appears warranted to recognize potential problems early and to facilitate appropriate medical intervention. Future researchers should conduct intervention studies designed to educate athletes, their parents, coaches, athletic trainers, and other sports health care professionals on the causes and adverse effects of the triad components. In addition to education, behavioral interventions to promote healthy eating and training practices are warranted at the high school level.
Acknowledgments
This research was supported in part by the National Athletic Trainers' Association Research & Education Foundation and the Graduate Division & Research Affairs, San Diego State University. We thank the high school athletic directors, coaches, athletic trainers, and athletes for their cooperation and participation in the study.
REFERENCES
- 1.National Federation of State High School Associations. High school sports participation increases again; boys, girls and overall participation reach all-time highs [press release] September 4, 2008. 2007–2008 National Federation of State High School Association Survey. http:///www.nfhs.org/web/2008/09/high_school_sports_participation. Accessed September 9, 2008.
- 2.Knowles S. B., Marshall S. W., Bowling J. M., et al. A prospective study of injury incidence among North Carolina high school athletes. Am J Epidemiol. 2006;164(12):1209–1221. doi: 10.1093/aje/kwj337. [DOI] [PubMed] [Google Scholar]
- 3.Powell J. W., Barber-Foss K. D. Sex-related injury patterns among selected high school sports. Am J Sports Med. 2000;28(3):385–391. doi: 10.1177/03635465000280031801. [DOI] [PubMed] [Google Scholar]
- 4.Rauh M. J., Koepsell T. D., Rivara F. P., Margherita A. J., Rice S. G. Epidemiology of musculoskeletal injuries among high school cross-country runners. Am J Epidemiol. 2006;163(2):151–159. doi: 10.1093/aje/kwj022. [DOI] [PubMed] [Google Scholar]
- 5.Garrick J. G., Requa R. K. Sports and fitness activities: the negative consequence. J Am Acad Orthop Surg. 2003;11(6):439–443. doi: 10.5435/00124635-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Nattiv A., Loucks A. B., Manore M. M., Sanborn C. F., Sundgot-Borgen J., Warren M. P. American College of Sports Medicine position stand: the female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867–1882. doi: 10.1249/mss.0b013e318149f111. [DOI] [PubMed] [Google Scholar]
- 7.Bennell K. L., Malcolm S. A., Thomas S. A., et al. Risk factors for stress fractures in track and field athletes: a twelve-month prospective study. Am J Sports Med. 1996;24(6):810–818. doi: 10.1177/036354659602400617. [DOI] [PubMed] [Google Scholar]
- 8.Kelsey J. L., Bachrach L. K., Proctor-Gray E., et al. Risk factors for stress fracture among young female cross-country runners. Med Sci Sports Exerc. 2007;39(9):1457–1463. doi: 10.1249/mss.0b013e318074e54b. [DOI] [PubMed] [Google Scholar]
- 9.Reinking M. F. Exercise-related leg pain in female collegiate athletes: the influence of intrinsic and extrinsic factors. Am J Sports Med. 2006;34(9):1500–1507. doi: 10.1177/0363546506287298. [DOI] [PubMed] [Google Scholar]
- 10.Barrow G. W., Saha S. Menstrual irregularity and stress fractures in collegiate female distance runners. Am J Sports Med. 1988;16(3):209–216. doi: 10.1177/036354658801600302. [DOI] [PubMed] [Google Scholar]
- 11.Nattiv A., Puffer J. C., Green G. Lifestyles and health risks of collegiate athletes: a multi-center study. Clin J Sport Med. 1997;7(4):262–272. doi: 10.1097/00042752-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Bennell K. L., Malcolm S. A., Thomas S. A., et al. Risk factors for stress fractures in female track and field athletes: a retrospective analysis. Clin J Sport Med. 1995;5(4):229–235. doi: 10.1097/00042752-199510000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Myburgh K. H., Hutchins J., Fataar A. B., Hough S. F., Noakes T. D. Low bone density is an etiologic factor for stress fractures in athletes. Ann Intern Med. 1990;113(10):754–759. doi: 10.7326/0003-4819-113-10-754. [DOI] [PubMed] [Google Scholar]
- 14.Rauh M. J., Macera C. A., Trone D. W., Shaffer R. A., Brodine S. K. Epidemiology of stress fracture and lower-extremity overuse injury in women recruits. Med Sci Sports Exerc. 2006;38(9):1571–1577. doi: 10.1249/01.mss.0000227543.51293.9d. [DOI] [PubMed] [Google Scholar]
- 15.Shaffer R. A., Rauh M. J., Brodine S. K., Trone D. W., Macera C. A. Predictors of stress fracture susceptibility in young female recruits. Am J Sport Med. 2006;34(1):108–115. doi: 10.1177/0363546505278703. [DOI] [PubMed] [Google Scholar]
- 16.Beckvid Henriksson G., Schnell C., Linden Hirschberg A. Women endurance runners with menstrual dysfunction have prolonged interruption of training due to injury. Gynecol Obstet Invest. 2000;49(1):41–46. doi: 10.1159/000010211. [DOI] [PubMed] [Google Scholar]
- 17.Beck T. J., Ruff C. B., Shaffer R. A., Betsinger K., Trone D. W., Brodine S. K. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone. 2000;27(3):437–444. doi: 10.1016/s8756-3282(00)00342-2. [DOI] [PubMed] [Google Scholar]
- 18.Beals K. A., Manore M. M. Disorders of the female athlete triad among collegiate athletes. Int J Sport Nutr Exerc Metab. 2002;12(3):281–293. doi: 10.1123/ijsnem.12.3.281. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong D. W., III, Rue J. P., Wilckens J. H., Frassica F. J. Stress fracture injury in young military men and women. Bone. 2004;35(3):806–816. doi: 10.1016/j.bone.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Nichols J. F., Rauh M. J., Lawson M., Ji M., Barkai H. S. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160(2):137–142. doi: 10.1001/archpedi.160.2.137. [DOI] [PubMed] [Google Scholar]
- 21.Loud K. J., Gordon C. M., Micheli L. J., Field A. E. Correlates of stress fractures among preadolescent and adolescent girls. Pediatrics. 2005;115(4):e399–e406. doi: 10.1542/peds.2004-1868. [DOI] [PubMed] [Google Scholar]
- 22.Rauh M. J., Margherita A. J., Rice S. G., Koepsell T. D., Rivara F. P. High school cross country running injuries: a longitudinal study. Clin J Sport Med. 2000;10(2):110–116. doi: 10.1097/00042752-200004000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Carter J. C., Stewart D. A., Fairburn C. G. Eating disorder examination questionnaire: norms for young adolescent girls. Behav Res Ther. 2001;39(5):625–632. doi: 10.1016/s0005-7967(00)00033-4. [DOI] [PubMed] [Google Scholar]
- 24.Fairburn C. G., Beglin S. J. Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord. 1994;16(4):363–370. [PubMed] [Google Scholar]
- 25.Cooper Z., Fairburn C. G. The Eating Disorder Examination: a semi-structured interview for the assessment of the specific psychopathology of eating disorders. Int J Eat Disord. 1987;6(1):1–8. [Google Scholar]
- 26.Mond J. M., Hay P. J., Rodgers B., Owen C., Beumont P. J. Validity of the Eating Disorder Examination Questionnaire (EDE-Q) in screening for eating disorders in community samples. Behav Res Ther. 2004;42(5):551–567. doi: 10.1016/S0005-7967(03)00161-X. [DOI] [PubMed] [Google Scholar]
- 27.Van de Loo D. A., Johnson M. D. The young female athlete. Clin Sports Med. 1995;14(3):687–707. [PubMed] [Google Scholar]
- 28.Kanis J. A., Melton L. J., III, Christiansen C., Johnston C. C., Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 29.Writing Group for the International Society for Clinical Densitometry Position Development Conference. Diagnosis of osteoporosis in men, premenopausal women, and children. J Clin Densitom. 2004;7(1):17–26. doi: 10.1385/jcd:7:1:17. [DOI] [PubMed] [Google Scholar]
- 30.Wilfley D. E., Schwartz M. B., Spurrell E. B., Fairburn C. G. Using the eating disorder examination to identify the specific psychopathology of binge eating disorder. Int J Eat Disord. 2000;27(3):259–269. doi: 10.1002/(sici)1098-108x(200004)27:3<259::aid-eat2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 31.Koepsell T. D. Epidemiologic Methods: Studying the Occurrence of Illness. New York, NY: Oxford Press; 2003. Weiss NS. [Google Scholar]
- 32.Rothman K. J., Greenland S. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. 2nd ed. [Google Scholar]
- 33.Bruckner P., Bennell K., Matheson G. Stress Fractures. Victoria, Australia: Blackwell Science; 1999. pp. 147–161. [Google Scholar]
- 34.Loucks A. B., Mortola J. F., Girton L., Yen S. S. Alterations in the hypothalamic-pituitary-ovarian and the hypothalamic-pituitary-adrenal axes in athletic women. J Clin Endocrinol Metab. 1989;68(2):402–411. doi: 10.1210/jcem-68-2-402. [DOI] [PubMed] [Google Scholar]
- 35.Loucks A. B., Heath E. M. Dietary restriction reduces luteinizing hormone (LH) pulse frequency during waking hours and increases LH pulse amplitude during sleep in young menstruating women. J Clin Endocrinol Metab. 1994;78(4):910–915. doi: 10.1210/jcem.78.4.8157720. [DOI] [PubMed] [Google Scholar]
- 36.Christo K., Prabhakaran R., Lamperllo B., et al. Bone metabolism in adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects. Pediatrics. 2008;121(6):1127–1136. doi: 10.1542/peds.2007-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindholm C., Hirschberg A. L., Carlstrom K., von Schoultz B. Altered adrenal steroid metabolism underlying hypercortisolism in female endurance athletes. Fertil Steril. 1995;63(6):1190–1194. [PubMed] [Google Scholar]
- 38.Cobb K. L., Backrach L. K., Sowers M., et al. The effect of oral contraceptives on bone mass and stress fractures in female runners. Med Sci Sports Exerc. 2007;39(9):1464–1473. doi: 10.1249/mss.0b013e318074e532. [DOI] [PubMed] [Google Scholar]
- 39.Magnusson H. I., Westlin N. E., Nyqvist F., Gardsell P., Seeman E., Karlsson M. K. Abnormally decreased regional bone density in athletes with medial tibial stress syndrome. Am J Sports Med. 2001;29(6):712–715. doi: 10.1177/03635465010290060701. [DOI] [PubMed] [Google Scholar]
- 40.Loucks A. B. Energy availability, not body fatness, regulates reproduction in women. Exerc Sport Sci Rev. 2003;31(3):144–148. doi: 10.1097/00003677-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Barrack M. T., Rauh M. J., Barkai H. S., Nichols J. F. Dietary restraint and low bone mass in female adolescent endurance runners. Am J Clin Nutr. 2008;87(1):36–43. doi: 10.1093/ajcn/87.1.36. [DOI] [PubMed] [Google Scholar]
- 42.Ihle R., Loucks A. B. Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res. 2004;19(8):1231–1240. doi: 10.1359/JBMR.040410. [DOI] [PubMed] [Google Scholar]
- 43.Guest N. S., Barr S. I. Cognitive dietary restraint is associated with stress fractures in women runners. Int J Sport Nutr Exerc Metab. 2005;15(2):147–159. doi: 10.1123/ijsnem.15.2.147. [DOI] [PubMed] [Google Scholar]
- 44.Manore M. M. Dietary recommendations and athletic menstrual dysfunction. Sports Med. 2002;32(14):887–901. doi: 10.2165/00007256-200232140-00002. [DOI] [PubMed] [Google Scholar]
- 45.Loucks A. B. Low energy availability in the marathon and other endurance sports. Sports Med. 2007;37(4–5):348–352. doi: 10.2165/00007256-200737040-00019. [DOI] [PubMed] [Google Scholar]
- 46.Bonci C. M., Bonci L. J., Granger L. R., et al. National Athletic Trainers' Association position statement: preventing, detecting, and managing disordered eating in athletes. J Athl Train. 2008;43(1):80–108. doi: 10.4085/1062-6050-43.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindholm C., Hirschberg A. L., Carlstrom K., von Schoultz B. Hormone anabolic/catabolic balance in female endurance athletes. Gynecol Obstet Invest. 1993;36(3):176–180. doi: 10.1159/000292621. [DOI] [PubMed] [Google Scholar]
- 48.Williams N. I., Helmreich D. L., Parfitt D. B., Caston-Balderrama A. L., Cameron J. L. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab. 2001;86(11):5184–5193. doi: 10.1210/jcem.86.11.8024. [DOI] [PubMed] [Google Scholar]
- 49.Steinacker J. M., Lormes W., Reissnecker S., Liu Y. New aspects of the hormone and cytokine response to training. Eur J Appl Physiol. 2004;91(4):382–391. doi: 10.1007/s00421-003-0960-x. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization multicenter study on menstrual and ovulatory patterns in adolescent girls, II: longitudinal study of menstrual patterns in the early postmenarcheal period, duration of bleeding episodes and menstrual cycles. World Health Organization Task Force on Adolescent Reproductive Health. J Adolesc Health Care. 1986;7(4):236–244. [PubMed] [Google Scholar]