Abstract
Traditionally, follicles have been grown in standard incubators with atmospheric oxygen concentration. However, preantral follicles exist in the avascular cortex of the ovary. This study examines the effectiveness of an oxygen delivery protocol that more closely mimics the in vivo environment of the ovary on oocyte viability, maturation, parthenogenetic activation, and fertilization from in vitro cultured rat preantral follicles. Of 54 oocytes cultured in the dynamic oxygen environment, 35 were viable while only 22 of 50 oocytes cultured within an ambient oxygen concentration remained viable (p < 0.05). Germinal vesicle breakdown was observed in 56% of oocytes from the dynamic oxygen group compared to 30% of oocytes from the ambient oxygen group (p < 0.05). Parthenogenetic activation was observed in a significant number of oocytes from the dynamic oxygen group, while none of the oocytes from the ambient oxygen group activated (p < 0.05). However, the proportions of oocytes from the dynamic oxygen group that remained viable underwent germinal vesicle breakdown, and activated were still significantly less than those from the in vivo control group (p < 0.05). Fertilization of the oocytes from the dynamic oxygen group was confirmed through a successful trial of intracytoplasmic sperm injection.
Introduction
Therapeutic treatments for cancer and other diseases often adversely affect the reproductive cells of a patient. The very therapy that kills the cancer cells also kills the germ cells within the reproductive organs. For girls and women, this is a particularly harsh consequence because mammalian females are born with all the eggs they will ever have.1 Should this original complement of eggs be destroyed, the result is sterility. For adult women, depletion of the supply of eggs results in menopause. Children whose eggs have been destroyed fail to undergo puberty. One step that can be taken to salvage some of the eggs is to remove and freeze the ovaries of girls and women before their undergoing potentially sterilizing therapies.2 This banked tissue can then be used for transplant back into the patient to restore hormone and egg production. However, the major drawback with this procedure is that re-implanting the ovarian tissue carries the very real risk of transferring cancer cells that were resident in the tissue, thus re-introducing the cancer as well.3 This possibility could be avoided entirely by using the preserved tissue as a source of follicles that would be grown to maturity in vitro. This process could then be used as a source of mature oocytes for routine in vitro fertilization (IVF) procedures. The in vitro approach has here to fore been limited in that there is currently no technology to produce fertilizable eggs from the immature follicles present in the frozen ovaries of most mammals, including humans.
The spherical follicle is the major anatomic and functional unit of the ovary. Follicles consist of an oocyte surrounded by epithelial-like granulosa cells. The granulosa cells are surrounded by a basement membrane. Outside the basement membrane is a mesenchymal layer of theca cells.1 In reproductive age mammals, the vast majority of the follicles exist as very small, nongrowing, primordial follicles in what has been called a resting pool of follicles.
An unknown mechanism results in the activation and growth of some follicles, while the majority of the follicle complement remains in the resting pool. When follicles are activated from the resting pool, they progress through morphologically distinct stages known in order as primary, secondary, preantral, antral, and Graafian, or preovulatory.1 At ovulation, an egg is released that undergoes mechanical transport to the fallopian tube, where it undergoes further maturation and if sperm are present, may undergo fertilization and further development as a zygote. In human IVF procedures, the recovered eggs are at the preovulatory stage and are matured and fertilized in culture or by intracytoplasmic sperm injection (ICSI). Some labs have begun to attempt to culture and mature retrieved eggs that are earlier than preovulatory in a process called in vitro maturation. This name is somewhat deceptive; however, in that these eggs are already from late antral follicles. In this study, we have grown follicles from very early secondary stages of development all the way up to ovulation, maturation, and fertilization—a much more complex and prolonged period than in vitro maturation of eggs.
Primordial follicles have a greater likelihood of surviving this cryopreservation due to their smaller size, slow metabolism, and the undifferentiated state of the cells that comprise them.4 Although primordial follicles derived from mice can be grown to maturity using static culture techniques under atmospheric oxygen conditions,5–7 to date there has been no successful complete in vitro follicle development from larger species, such as rats, pigs, and humans.8,9 In other words, intact rat, primate, or human preantral follicles have not yet been successfully cultured to maturation in vitro. Successful development of a technology in which preserved ovarian tissues can be utilized to restore fertility would be a major development in reproductive science.
Over the past several years, several groups have worked to develop a defined culture system for preantral follicular development. This work has identified a number of factors important for follicular growth in vitro. It has been shown both in vivo and in vitro that preantral follicles respond to follicle-stimulating hormone treatment with increased cell division, growth, and differentiation.10 Other factors that promote growth or differentiation of rat preantral follicles in short-term culture have been identified (Mullerian inhibitory substance,11 keratinocyte growth factor,12 growth and differentiation factor 9,13 and activin11). It has also been determined that transforming growth factor-β induces apoptosis,11 whereas cyclic guanosine monophosphate (cGMP) analogs inhibit apoptosis in preantral follicles.10 While these studies have added to our knowledge of early follicle development and greatly improved the media used for in vitro culture of isolated follicles, a number of obstacles still remain before follicles can be supported in the proper environment long enough to result in normal oocyte maturation.
We have recently performed studies to determine why rat follicles fail to progress beyond the preantral follicle stage in vitro. Our preliminary studies14 demonstrated that under conventional conditions, rat follicles frequently undergo flattening and rupture with loss of anatomic integrity that is important to the normal egg maturation process. When follicles were cultured in suspension culture systems, consisting of orbiting test tubes and rotating-wall vessels, the follicles did not rupture and exhibited more robust growth. Therefore, we believe that advances in bioreactor technologies hold great promise toward the development of a system that can support the complex needs of larger follicles that are required for proper development. In the short term, the rat model will allow for more complex studies of follicle development in vitro that are unnecessary for the maturation of the smaller mouse follicles. In the long term, it may represent the hope for young cancer patients to retain fertility. More generally, the problems associated with nurturing the development of larger follicles mirror many of the unmet challenges in tissue engineering as a whole.
Follicles have traditionally been grown in standard incubators with atmospheric oxygen concentrations. However, primordial and primary follicles exist in the avascular cortex of the ovary. Throughout this region, blood vessels are not observed in close proximity to these follicles.15 The oxygen available to these small follicles would have to diffuse from the peritoneal cavity or nearby large follicles that do have a blood supply. The partial pressure of oxygen in the peritoneal cavity has been measured at 40 mmHg, a far cry from the ∼140 mmHg obtained in media exposed to normal incubator conditions.16 Further, the partial pressure of oxygen is expected to be even further reduced by crossing the ovarian capsule. The oxygen gradient across the walls of microcapillaries is steep, reducing the intracapillary levels by half.17 The Po2 across the subrenal capsule drops from the peritoneal level of 40 to 14–19 mmHg.16 In contrast, an abundance of blood vessels are found in the region of the ovary that contains secondary and antral follicles.15 Differential regulation of follicle development in environments reflecting the in vivo Po2 has never been investigated, though it is likely that in vivo follicles experience a transition from relative hypoxia in early development to the high volume of blood flow and oxygen delivery for preovulatory follicles. The difficulty in achieving in vitro oocyte maturation may be due to the dysregulation of follicle development by exposure of early stage follicles to inappropriate oxygen concentrations.
In this study, we examined the effectiveness of a dynamic oxygen delivery protocol on oocyte viability and maturation. Ovarian follicles were cultured in suspension under two different oxygen conditions, a static 20% or a dynamic environment in which the oxygen was increased over the duration of the culture period. Along with an in vivo control group, the resulting oocytes were analyzed for survival, germinal vesicle breakdown, and polar body extrusion. Further, additional oocytes derived from these three groups were placed into strontium chloride to determine the efficiency of parthenogenetic activation. As a result of the activation study, oocytes from the dynamic oxygen group underwent ICSI to verify that they were competent to undergo fertilization.
Materials and Methods
Animals and ovarian dissection
All animal experiments were performed in accordance with National Institutes of Health guidelines and with institutional approval. Sprague-Dawley rats were obtained from Hilltop Lab Animals (Pittsburgh, PA) and housed under standard conditions. The animals were sacrificed by CO2 exposure and cervical dislocation. Ovaries were dissected and placed immediately in warmed culture medium, consisting of Leibovitz L-15 medium (Invitrogen, Carlsbad, CA). The follicles were then mechanically dissected from the ovary using a pair of syringes with 26-gauge needles. All follicles used in the experiments were measured in two dimensions, using an inverted microscope fitted with an ocular micrometer. Only intact follicles that were between 140 and 170 μm in diameter were used in culture.
Follicle culture
Culture media consisted of α-minimal essential medium (Gibco BRL–Invitrogen, Grand Island, NY) with additives of 8-bromo-cGMP (5 mM), ITS + (1% solution of insulin, 10 mg/L; transferrin, 5.5 mg/L; linoleic acid, 4.7 mg/L; and selenium, 5 mg/L), Pen/Strep (1%, penicillin 100 U/mL and streptomycin 100 μg/mL) (all from Sigma Chemical, St. Louis, MO), and recombinant follicle-stimulating hormone (0.7 IU/mL) (Serono Laboratories, Rockville, MD). Culture media was placed into 12 × 75 mm polypropylene culture test tubes (500 μL/tube) and cultured in 5% CO2 and 37°C humidified incubator. Two hundred microliters of culture media was exchanged every 3 days.
Suspension culture was attained by placing the 6 mL culture tubes in a circular rotator plate (Glas-Col, Terre Haute, IN), having a diameter of 30.5 cm, which was rotated around its horizontal axis at rate between 8 and 15 rpm. Therefore, as the plate rotates, the tubes slowly orbit the axis of the plate.
Oxygen protocols
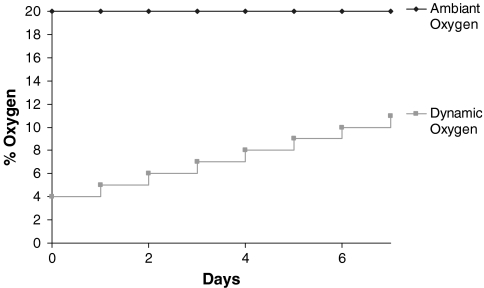
The incubators used for this study were both CO2 and O2 controlled. For the ambient oxygen group, the oxygen tension was cultured in the traditional static oxygen tension of 20% (Fig. 1). For the preliminary low oxygen experiment, the oxygen tension was set at 2% (15.2 mmHg). For the dynamic oxygen group, the oxygen tension in the incubator was initially set at 4% (30.4 mmHg), a concentration more similar to what a preantral follicle would experience in vivo.16 To mimic the in vivo transition from avascular to vascular oxygen levels, the oxygen tension was increased 1% every 24 h until the end of the 7-day culture period with a final oxygen tension of 11% (83.6 mmHg), a level within the normal range for arterial oxygen (Fig. 1).
FIG. 1.
Dynamic oxygen delivery protocol. The oxygen tension in the incubator was initially set at 4% (30.4 mmHg) and increased 1% every 24 h until the end of the 7-day culture period with a final oxygen tension of 11% (83.6 mmHg).
Histology
The morphology of preantral follicles cultured at different oxygen tensions was analyzed by hematoxylin and eosin staining. Preantral follicles were cultured in either 15% (115 mmHg) or 2% (15.2 mmHg) oxygen tension. After 7 days, the follicles were retrieved from culture and fixed in 4% paraformaldehyde. Follicles were then embedded in optimal cutting temperature compound for sectioning. Frozen sections were cut at 5 μm and stained with hematoxylin and eosin before being imaged using a Leica CMR microscope (Leica Microsystems, Wetzlar, Germany).
Ovulation induction and oocyte maturation
Follicles were removed from the culture media and placed into media containing α-minimal essential medium, Pen/Strep (1%, penicillin 100 U/mL and streptomycin 100 μg/mL), and hCG (1.0 IU/mL) for 12 h. Oocytes were then removed from their surrounding cumulus and placed into IVF-30 media (Vitrolife, Göteborg, Sweden). Oocytes were initially observed for viability. Oocytes with vacuoles in their cytoplasm or voids in their perivitelline space were considered nonviable. After 6 h in the IVF-30 media, oocytes were examined for germinal vesicle breakdown and the presence of an extruded polar body. Oocyte diameter was measured from inside the zona pellucida using SPOT version 3.2.4 software (Diagnostic Instruments, Sterling Heights, MI).
Superovulation protocol
To compare the quality of the oocytes from both culture conditions with an in vivo control, immature Sprague-Dawley rats were stimulated to superovulate using pregnant mare's serum gonadotropin (PMSG) subcutaneous injections. Rats were injected with PMSG (15 IU) at 10 a.m. The rats were then injected with human chorionic gonadotropin (30 IU) 48 h later. After 24 h, the rats were sacrificed, and the fallopian tubes dissected to retrieve the ovulated oocytes. The recovered oocytes were placed into the IVF media for 6 h and examined for germinal vesicle breakdown and the presence of an extruded polar body.
Parthenogenic activation
Follicles were removed from the culture media and placed into media containing α-minimal essential medium, Pen/Strep (1%, penicillin 100 U/mL and streptomycin 100 μg/mL), and hCG (1.0 IU/mL) for 12 h. Oocytes were then removed from their surrounding cumulus and placed into IVF-30 media (Vitrolife). To determine oocyte competency, oocytes from the dynamic oxygen group, the ambient oxygen group, and the in vivo control group were parthenogenically activated using strontium chloride–supplemented IVF-30 media. Only those oocytes that matured through germinal vesicle breakdown were put through the protocol. For mammalian oocytes, activation is triggered by intracellular calcium oscillations.18 These calcium oscillations occur as intracellular calcium is released across the egg.19 This process is necessary for the progression of the egg through the cell cycle.20 Studies on mouse21 and rat22 oocytes have shown that strontium can provoke the calcium oscillations necessary for activation. However, in the case of strontium-induced parthenogenetic activation, there is no consensus on an optimum protocol regarding strontium concentration used and time spent in strontium that would result in the most efficient activation rates and the formation of the greatest number of blastocysts.
Strontium chloride (Sigma Chemical) was dissolved into IVF-30 medium at a concentration of 0.625 mM. After the 6-h culture in IVF-30 media, oocytes from the dynamic oxygen group were placed into 50 μL drops of strontium-supplemented IVF-30 media. They were incubated for 8 h at 37°C in 5% CO2 in air. They were then washed in IVF media and cultured for 8 h in IVF media alone. Finally, they underwent a second 8-h culture in the strontium-supplemented IVF media before being placed back into IVF media for 24 h. Oocytes were then examined for the presence of two or more cells. For all incubation periods, the microdrops of IVF media were covered with mineral oil.
Oocyte DNA staining
Activated oocytes were stained with Hoechst 33342 to confirm the presence of a nucleus in each cell. At the end of the activation protocol, oocytes that displayed two or more cells were placed into a 1 μg/mL working solution of the Hoechst 33342 for 10 min followed by two phosphate-buffered saline washes, 5 min. Oocytes were then digitally imaged using a Leica DM IRB fluorescence microscope. Only those oocytes displaying positive staining in all cells were considered to be activated.
Intracytoplasmic sperm injection
ICSI was performed using a controlled volume microinjection system (Narishige, East Meadow, NY) attached to a Leica DM IRB microscope. Spermatozoa were collected from the cauda epididymidis of a mature male Sprague-Dawley rat. The spermatozoa were suspended in a 10% polyvinylpyrrolidone solution (Irvine Scientific, Santa Ana, CA). Spermatozoa were then injected into the oocytes in a drop of human tubal fluid (HTF) media supplemented with 10% v/v synthetic serum substitute (Irvine Scientific). After injection, the oocytes were transferred into IVF-medium and incubated at 37°C and 5% CO2 in air. The oocytes used for injections were retrieved from follicles cultured in the dynamic oxygen condition and cultured for an additional 6 h in the IVF media. Only those oocytes that matured through germinal vesicle breakdown were injected. The injected oocytes were observed over the next 24 h for the presence of two pronuclei (2pn) and subsequent progression to a two-cell embryo.
Statistical analysis
The percentages of oocytes that were viable, went through germinal vesicle breakdown, and extruded the first polar body were compared between oxygen treatments using the Fisher exact probability test. Significance was accepted at p < 0.05.
Results and Discussion
The overall goal of these studies was to develop a reliable method of attaining mature oocytes from in vitro cultured preantral ovarian follicles for the purpose of restoring fertility. The parameters used to compare the effects of the dynamic oxygen environment to those of the ambient control focused on oocyte viability and oocyte maturation. Oocyte maturation was evaluated by observing the progression of the cultured oocytes through a number of meiotic stages.
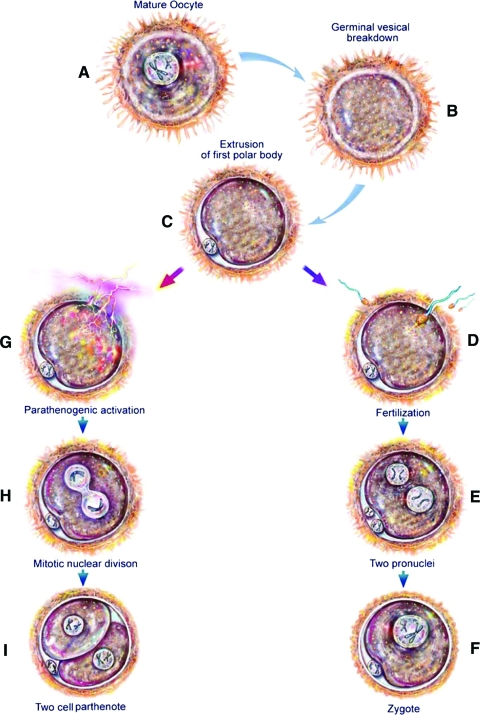
Oocytes that are still within a follicle are arrested in the prophase of the first meiotic division, the germinal vesicle stage (Fig. 2A). Mature oocytes can spontaneously resume meiosis in vitro if they are retrieved from a follicle and separated from the surrounding cumulus cells.23 This induces a process known as germinal vesicle breakdown in which the nucleus dissolves in preparation for the completion of meiosis (Fig. 2B). At the conclusion of meiosis I, the oocyte will extrude a polar body and enter meiosis II, at which point the oocyte will arrest in metaphase II until it is fertilized (Fig. 2C). For the purposes of this study, oocytes were observed for germinal vesicle breakdown and the presence of a polar body to evaluate the effectiveness of both the ambient oxygen and dynamic oxygen groups at producing mature oocytes.
FIG. 2.
Meiotic stages of the ovulated oocyte and its subsequent progression through either fertilization or parthenogenesis. (A) Mature ovulated oocyte. (B) Germinal vesicle breakdown, a process in which the nucleus dissolves in preparation for the completion of meiosis occurs. (C) After the first meiotic division, the first polar body is extruded. The oocyte will arrest at this stage until it is fertilized or stimulated to undergo parthenogenesis. (D) Fertilization occurs and triggers the second meiotic division. (E) The second polar body is extruded, and two pronuclei, one from the ovum and one from the sperm, are present. (F) The two pronuclei fuse into one nucleus, forming a zygote. (G) Parthenogenesis activation. (H) Activation triggers a mitotic nuclear division. (I) This is followed by cytokinesis and the formation of a two-cell parthenote. Color images available online at www.liebertonline.com/ten.
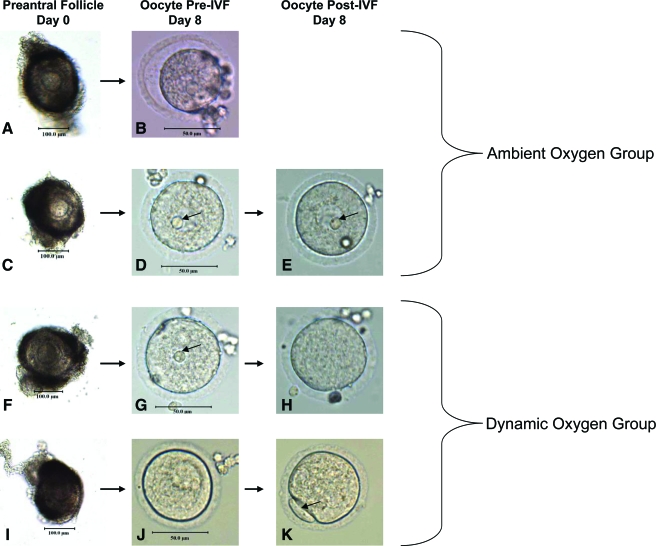
Figure 3 presents the different outcomes for the cultured oocytes. The first picture in each series (Fig. 3A, C, F, I) shows the preantral follicle before it was placed into the culture tube. The second picture in each series (Fig. 3B, D, G, J) displays the oocyte retrieved from the follicle shown in the first frame. These oocytes were yet to be cultured in the IVF media. The bottom 3 outcomes have an additional micrograph (Fig. 3E, H, K) showing the oocytes after a 6-h culture in the IVF media.
FIG. 3.
Micrographs of follicles and subsequent oocytes. (A, C, F, I) Follicles on day 0. (B) Nonviable oocyte on day 8 from ambient oxygen group. (D, G, J) Viable oocytes on day 8 before culture in IVF-30 media. (E) Oocyte after culture in IVF-30 media, no germinal vesicle breakdown from ambient oxygen group. (H) Oocyte after culture in IVF-30 media, germinal vesicle breakdown from dynamic oxygen group. (K) Oocyte after culture in IVF-30 media, germinal vesicle breakdown and extrusion of the first polar body from dynamic oxygen group. Color images available online at www.liebertonline.com/ten.
In vivo control
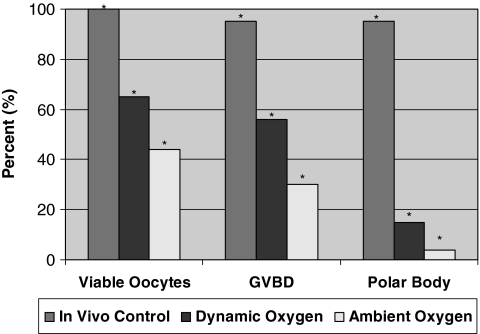
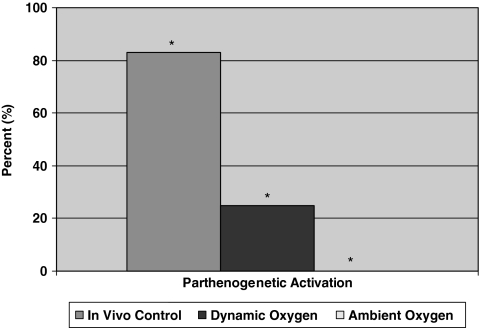
The superovulation protocol yielded a total of 19 oocytes that were observed for viability and maturation. Of the 19 oocytes recovered, all 19 were viable. After culturing the oocytes in the IVF medium, 18 (95%) went through germinal vesicle breakdown and extruded the first polar body (Fig. 4). The 18 oocytes were then subjected to the parthenogenetic activation protocol. Of the 18 oocytes, 15 (83%) were activated (Fig. 5).
FIG. 4.
Oocyte viability and maturation data (*p < 0.05).
FIG. 5.
Parthenogenesis data (*p < 0.05).
Ambient oxygen culture
In an attempt to develop a long-term culture system, immature ovarian follicles were cultured for 7 days in the suspension culture bioreactors under ambient oxygen tension. Figure 3A and B represents the outcome in which the oocyte had not survived the 7-day culture. Figure 3B shows a nonviable oocyte that had retreated from the zona pellucida leaving a sizable portion of the perivitelline space empty. Of the 50 oocytes cultured in the ambient oxygen environment, only 22 (44%) remained viable.
The 22 surviving oocytes from the viability study were placed into IVF-30 media to determine their efficiency at resuming meiosis. After 6 h in the IVF-30 media, germinal vesicle breakdown was observed in 15 of the oocytes. Figure 3G and H portrays a viable oocyte that went through germinal vesicle breakdown. The nucleus could clearly be seen in Figure 3G (arrow), but was no longer present after 6 h in IVF-30 media (Fig. 3H), signifying the resumption of meiotic maturation. This was in contrast to the outcome in Figure 3D and E in which the oocyte failed to undergo germinal vesicle breakdown as the nucleus was present (arrows) both before (Fig. 3D) and after (Fig. 3E) culture in the IVF-30 media. Ultimately, the ambient oxygen group produced two oocytes that extruded the first polar body. Figure 3J and K shows an oocyte that not only went through germinal vesicle breakdown, but also extruded the first polar body (arrow).
From this experiment, it was found that the majority of the oocytes retrieved from the ambient oxygen group neither survived the culture nor resumed meiosis. Compared to the in vivo control, there was a significant decrease in all three of the observed parameters, the proportion of oocytes that were viable, underwent germinal vesicle breakdown, and extruded the first polar body (Fig. 4). Although the yield of mature oocytes was low using this culture condition, we wanted to determine if the oocytes generated from the ambient oxygen environment could be parthenogenically activated. With the successful activation of these oocytes, we would take a first step toward the derivation of embryonic stem cell lines from in vitro matured preantral follicles.
In addition to being a source of fertilizable oocytes for the restoration of fertility, this study also examined whether in vitro matured oocytes could potentially be used to derive embryonic stem cell lines through parthenogenetic activation. Parthenogenesis is a form of asexual reproduction in some species in which the oocyte develops without ever being fertilized (Fig. 2G, H, I). A number of methods have been developed to induce the parthenogenetic activation of mammalian eggs. These eggs can divide mitotically progressing to the blastocyst stage, complete with an inner cell mass consisting of embryonic-like stem cells.
Embryonic stem cell lines have already been derived from the unfertilized oocytes of nonhuman primates.24,25 In the case of human eggs, it has been shown that parthenogenesis can be induced through several different methods.26,27 These activations have resulted in the acquisition of pluripotent stem cells from the resulting blastocyst.27 Although promising, the primate and human studies have been performed with in vivo matured oocytes. However, it has been found that preantral mouse follicles can be cultured to produce an oocyte capable of establishing an embryonic stem cell line from parthenogenesis.5 In this study, the issue of culturing larger follicles from more complex species for the purpose of producing oocytes capable of undergoing this activation was addressed.
To assess whether our culture method could produce the mature oocytes necessary to attain these stem cells, a number of oocytes from the ambient oxygen group were subjected to an activation protocol utilizing the chemical induction agent, strontium. A total of 22 oocytes from the ambient oxygen group were put through the parthenogenetic activation protocol. Of the 22 oocytes, none were activated.
It was determined that the functional capability of the oocytes cultured at higher oxygen tensions was significantly diminished compared to mature oocytes that activate in response to strontium exposure.28 This suggested that the parameters for this culture condition needed to be altered to develop a successful long-term culture technique that could produce mature oocytes with the same potential as those matured in vivo. We hypothesized that if the oxygen tension in the incubator were decreased to in vivo levels for developing preantral follicles, the result would be an increase in the proportion of mature functioning oocytes obtained from the culture.
Low oxygen culture
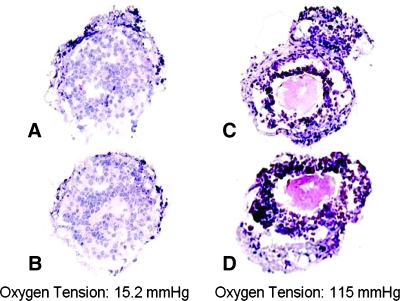
Our preliminary low oxygen experiments focused on the effect that this environment had on the morphology of the preantral follicles. Hematoxylin and eosin–stained sections were compared between follicles cultured for 7 days at 2% oxygen tension (Fig. 6A, B) and 15% oxygen tension (Fig. 6C, D). The low oxygen sections showed that the tissue structure was intact and the cells were not necrotic, which is a concern when culturing at such low levels of oxygen. Follicles grown in the high oxygen environment had ruptures in the basement membrane, compromising the three-dimensional integrity of the tissue along with a loss of cell junctions creating empty gaps around the oocyte. From these observations, it was found that not only could preantral follicles survive in a low oxygen environment, but also their three-dimensional structure more closely resembled in situ follicle structure. However, a static low oxygen tension is also not what developing follicles experience in vivo. We further hypothesized that for long-term cultures designed to support follicle maturation, a method of oxygen delivery that would increase the partial pressure of oxygen over the duration of the culture period should be implemented.
FIG. 6.
Hematoxylin and eosin–stained sections of follicles cultured for 7 days in (A, B) low oxygen tension (2%) or (C, D) high oxygen tension (15%).
Dynamic oxygen culture
Utilizing in vivo oxygen tensions to enhance tissue function during in vitro culture has been used in a number of related fields. It has been shown that maturing porcine oocytes in a low oxygen environment improves not only their ability to be parthenogenically activate but also the quality of the resulting blastocysts.29 It was also determined that culturing embryos at an oxygen tension characteristic of levels found in the oviduct and uterus improved both the establishment and maintenance of murine stem cells.30 It is becoming evident that the fields of follicular development, oocyte maturation, and embryo culture are benefiting from this trend to mimic the unique in vivo metabolic needs of these avascular tissues. In this spirit, several groups have examined the effects of lower oxygen tensions on follicle growth and development.31–33 It was found that by culturing preantral follicles under 5% oxygen, there was a significant increase in oocyte survival.31,33 Although these studies use oxygen tensions that are more suitable for early follicle development, the oxygen concentrations are still held constant for the duration of the culture periods. To better mimic the native oxygen environment, the oxygen concentrations for the dynamic oxygen protocol implemented in this study were based on the unique in vivo realities experienced by maturing follicles throughout their life cycle in the ovary.
For the purposes of our study, this dynamic oxygen protocol was then combined with the use of bioreactors that enable follicles to grow without flattening.14 As in the ambient oxygen culture, immature follicles were cultured for 7 days at which point the oocytes were retrieved and observed for viability, germinal vesicle breakdown, and polar body extrusion. Of the 54 oocytes cultured in the dynamic oxygen environment, 35 (65%) were viable. After 6 h in the IVF-30 media, germinal vesicle breakdown was observed in 30 (56%) oocytes from the dynamic oxygen group, with 8 of those oocytes extruding the first polar body.
Although these figures are still significantly less than the in vivo control values, significant increases were observed in the dynamic oxygen group for both the proportion of oocytes that remained viable and the proportion of oocytes that resumed meiosis compared to the ambient oxygen group (Fig. 4). The dynamic oxygen environment not only permitted the development of mature oocytes, but also significantly increased the number of oocytes that resumed meiosis by undergoing germinal vesicle breakdown, showing that both the quantity and quality of the oocytes were enhanced using this culture method. Further, it was determined that there was no significant difference (p > 0.05) in oocyte diameter between the in vivo control (70.4 ± 5.6 μm), the dynamic oxygen (68.5 ± 2.5 μm), and the ambient oxygen (68.8 ± 2.7 μm) groups. Next, we determined if the oocytes generated from the dynamic oxygen environment could be parthenogenically activated.
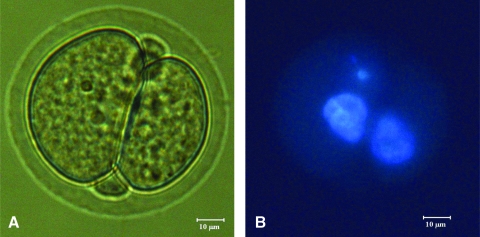
Utilizing strontium as a chemical-inducing agent, a total of 28 oocytes from the dynamic oxygen group were put through the parthenogenetic activation protocol. Of the 28 oocytes cultured in the dynamic oxygen environment, 7 were successfully activated. This is a significant increase when compared to the ambient oxygen group that failed to produce a single oocyte capable of activation (Fig. 5). Figure 7 shows a micrograph from an activated oocyte and its corresponding Hoechst-stained image. Figure 7A shows the activated oocyte at the two-cell stage, while Figure 7B is the fluorescent image of the oocyte with the nuclear-staining Hoechst dye confirming the presence of a nucleus in each of the two cells.
FIG. 7.
(A) Micrograph of activated oocyte (400 ×). (B) Hoechst-stained activated oocyte (400 ×). Color images available online at www.liebertonline.com/ten.
The derivation of embryonic stem cells from these parthenotes is beyond the scope of this paper. However, by activating these eggs, we confirmed that the oocytes retrieved from this improved culture environment have the potential to become a source for embryonic stem cells. Further, this indicates that culturing preantral follicles in a high oxygen environment will limit the functional potential of the oocytes.
Intracytoplasmic sperm injection
From the oocyte viability and maturation studies, it was found that the long-term culture of preantral ovarian follicles for the purpose of producing mature oocytes can become more efficient by altering the oxygen environment to mimic the native ovarian environment. However, to prove that this culture technique can ultimately be utilized as a method to restore fertility, the fertilization of a cultured oocyte must be demonstrated.
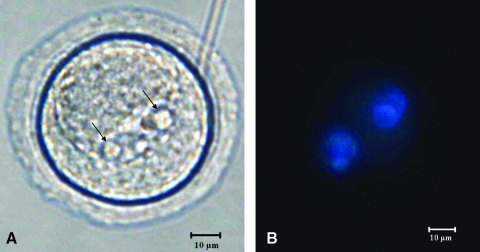
In this study, eight oocytes from the dynamic oxygen group were injected with sperm from a mature male Sprague-Dawley rat. Of these eight oocytes, 2pn were observed in two of the oocytes within 24 h. Figure 8A shows one of the 2pn oocytes, while Figure 8B is the fluorescent image of the oocyte with the nuclear-staining Hoechst dye confirming the presence of both pronuclei in the egg. None of these eggs progressed to the two-cell stage (Fig. 2F); however, the presence of both pronuclei in the egg confirms the fertilization (Fig. 2E).
FIG. 8.
(A) Micrograph of fertilized oocyte (400 ×). Arrows indicate the two pronuclei. (B) Hoechst-stained fertilized oocyte (400 ×). Color images available online at www.liebertonline.com/ten.
By fertilizing these oocytes, we have shown that dynamic oxygen culturing has the potential to restore fertility in females who undergo sterilizing therapy. As this project moves forward, we will look to improve the ICSI procedure and the media used to culture the embryos to support the embryo to later stages of development.
Conclusions
The dynamic oxygen environment produced a significantly higher yield of healthy oocytes than the static control. More importantly, the oocytes from the dynamic oxygen environment were significantly more efficient at progressing through germinal vesicle breakdown than high oxygen controls. In conclusion, these data demonstrate that both the yield and quality of the oocytes derived from in vitro cultured preantral follicles were improved by utilizing a dynamic oxygen protocol designed to mimic the in vivo environment of the ovary. Further, it was found that not only could immature rat preantral follicles be cultured to produce oocytes capable of parthenogenetic activation, but also the activation was only achieved when the oocyte had been cultured in the dynamic oxygen environment. Finally, the ex vivo ovulated oocytes could be fertilized by ICSI, and if we were able to demonstrate a similar approach with human follicles, then dynamic oxygen culturing would provide hope to young children who must sacrifice fertility for life.
Acknowledgments
We thank Dr. Jaspel Khillan for performing all ICSI injections and technical discussions of the procedures, Etta Volk for technical assistance in culturing injected oocytes and embryos, Randy McKenzie for his illustration work in Figure 2, and Nicki Zevola for assisting in the follicle dissections and imaging of the tissue. The authors thank Dr. Mohammad Ataai for acting as data monitor due to the authors being principals in O2Cyte LLP that controls intellectual property related to the oxygen protocol. Funding was provided by NIH/NIBIB 5 R21 EB004065-02 to A.J.R.
Disclosure Statement
No competing financial interests exist.
References
- 1.McGee E.A. Hsueh A.J.W. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 2.Wood C.E. Shaw J.M. Trounson A.O. Cryopreservation of ovarian tissue. Potential “reproductive insurance” for women at risk of early ovarian failure. Med J Aust. 1997;166:366. [PubMed] [Google Scholar]
- 3.Shaw J.M. Bowles J. Koopman P. Wood E.C. Trounson A.O. Fresh and cryopreserved ovarian tissue samples from donors with lymphoma transmit the cancer to graft recipients. Hum Reprod. 1996;11:1668. doi: 10.1093/oxfordjournals.humrep.a019467. [DOI] [PubMed] [Google Scholar]
- 4.Newton H. The cryopreservation of ovarian tissue as a strategy for preserving the fertility of cancer patients. Hum Reprod Update. 1998;4:237. doi: 10.1093/humupd/4.3.237. [DOI] [PubMed] [Google Scholar]
- 5.Lee S.T. Choi M.H. Lee E.J. Gong S.P. Jang M. Park S.H. Jee H. Kim D.Y. Han J.Y. Lim J.M. Establishment of autologous embryonic stem cells derived from preantral follicle culture and oocyte parthenogenesis. Fertil Steril. 2008;90:1910. doi: 10.1016/j.fertnstert.2007.01.099. [DOI] [PubMed] [Google Scholar]
- 6.Gosden R.G. Mullan J. Picton H.M. Yin H. Tan S.L. Current perspective on primordial follicle cryopreservation and culture for reproductive medicine. Hum Reprod Update. 2002;8:105. doi: 10.1093/humupd/8.2.105. [DOI] [PubMed] [Google Scholar]
- 7.Xu M. Kreeger P.K. Shea L.D. Woodruff T.K. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Telfer E.E. In vitro models for oocyte development. Theriogenology. 1998;49:451. doi: 10.1016/s0093-691x(97)00417-2. [DOI] [PubMed] [Google Scholar]
- 9.Hirshfield A.N. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 10.McGee E. Spears N. Minami S. Hsu S. Chun S. Billig H. Hsueh A.J.W. Preantral ovarian follicles in serum-free culture: suppression of apoptosis after activation of the cyclic guanosine 3’,5’-monophosphate pathway and stimulation of growth and differentiation by follicle stimulating hormone. Endocrinology. 1997;138:2417. doi: 10.1210/endo.138.6.5164. [DOI] [PubMed] [Google Scholar]
- 11.McGee E.A. Spears N. Smith R. Nachtigal M. Ingraham H. Hsueh A.J.W. Mullerian inhibitory substance (MIS) promotes growth but not differentiation of preantral ovarian follicles. Biol Reprod. 2001;64:293. doi: 10.1095/biolreprod64.1.293. [DOI] [PubMed] [Google Scholar]
- 12.McGee E.A. Chun S.Y. Lai S. He Y.E. Hsueh A.J.W. Keratinocyte growth factor promotes the survival, growth, and differentiation of preantral ovarian follicles Fertil Steril. 1999;71:732. doi: 10.1016/s0015-0282(98)00547-0. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi M. McGee E.A. Min G. Klein C. Rose U.M. van Duin M. Hsueh A.J.W. Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology. 1999;140:1236. doi: 10.1210/endo.140.3.6548. [DOI] [PubMed] [Google Scholar]
- 14.Rowghani N.M. Heise M.K. McKeel D. McGee E.A. Koepsel R.R. Russell A.J. Maintenance of morphology and growth of ovarian follicles in suspension culture. Tissue Eng. 2004;10:545. doi: 10.1089/107632704323061906. [DOI] [PubMed] [Google Scholar]
- 15.van Wezel I.L. Rodgers R.J. Morphological characterization of bovine primordial follicles and their environment in vivo. Biol Reprod. 1996;55:1003. doi: 10.1095/biolreprod55.5.1003. [DOI] [PubMed] [Google Scholar]
- 16.Tsai A.G. Frieseneneker B. Mazzoni M.C. Kerger H. Buerk B.G. Johnson P.C. Intaglietta M. Microvascular and tissue oxygen gradients in the rat mesentery (arteriolesyvessel wallyendotheliumyoxidative metabolism) Proc Natl Acad Sci USA. 1998;95:6590. doi: 10.1073/pnas.95.12.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chae Y.S. Kim W.S. Bae Y.H. Effect of cross-linked hemoglobin on functionality and viability of microencapsulated pancreatic islets. Tissue Eng. 2002;8:379. doi: 10.1089/107632702760184655. [DOI] [PubMed] [Google Scholar]
- 18.Swann K. Ozil J. Dynamics of the calcium signal that triggers mammalian egg activation. Int Rev Cytol. 1994;152:182. doi: 10.1016/s0074-7696(08)62557-7. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe L.F.Metz C.B.Monroy A.In biology of fertilization Proc Natl Acad Sci USA 889883.1991. 1946414 [Google Scholar]
- 20.Whitaker M. Patel R. Calcium and cell cycle control. Development. 1990;108:525. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]
- 21.Kline D. Kline J. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilised egg. J Biomed Chem. 1992;267:17624. [PubMed] [Google Scholar]
- 22.Kato M. Hirabayashi M. Aoto T. Ito K. Ueda M. Hochi S. Strontium-induced activation regimen for rat oocytes in somatic cell nuclear transplantation. J Reprod Dev. 2001;47:407. [Google Scholar]
- 23.Wassarman P.M. Oogenesis. In: Adashi E.Y., editor; Rock J.A., editor; Rosenwaks Z., editor. Reproductive Endocrinology, Surgery, and Technology. Philadelphia, PA: Lippincott–Raven; 1996. p. 2485. [Google Scholar]
- 24.Cibelli J.B. Grant K.A. Chapman K.B. Parthenogenetic stem cells in nonhuman primates. Science. 2002;295:819. doi: 10.1126/science.1065637. [DOI] [PubMed] [Google Scholar]
- 25.Vrana K.E. Hipp J.D. Goss A.M. McCool B. Riddle D. Walker S.J. Wettstein P. West M.D. Grant K.A. Cibella J.B. Nonhuman primate parthenogenetic stem cells. Proc Natl Acad Sci USA. 2003;100:11911. doi: 10.1073/pnas.2034195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers N.T. Hobson E. Pickering S. Lai F.A. Braude P. Swann K. Phospholipase Czeta causes Ca2+ oscillations and parthenogenetic activation of human oocytes. Reproduction. 2004;128:697. doi: 10.1530/rep.1.00484. [DOI] [PubMed] [Google Scholar]
- 27.Lin H. Lei J.Q. Wininger D. Nguyen M-T. Khanna R. Hartmann C. Yan W.-L. Huang S. Multilineage potential of homozygous stem cells derived from metaphase II oocytes. Stem Cells. 2003;21:152. doi: 10.1634/stemcells.21-2-152. [DOI] [PubMed] [Google Scholar]
- 28.Mizutani E. Jiang J.Y. Mizuno S. Tomioka I. Shinozawa T. Kobayashi J. Sasada H. Sato E. Determination of optimal conditions for parthenogenetic activation and subsequent development of rat oocytes in vitro. J Reprod Dev. 2004;50:139. doi: 10.1262/jrd.50.139. [DOI] [PubMed] [Google Scholar]
- 29.Iwamoto M. Onishi A. Fuchimoto D. Somfai T. Takeda K. Tagami T. Hanada H. Noguchi J. Kaneko H. Nagai T. Kikuchi K. Low oxygen tension during in vitro maturation of porcine follicular oocytes improves parthenogenetic activation and subsequent development to the blastocyst stage. Theriogenology. 2005;63:1277. doi: 10.1016/j.theriogenology.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Gibbons J. Hewitt E. Gardner D.K. Effects of oxygen tension on the establishment and lactate dehydrogenase activity of murine embryonic stem cells. Cloning Stem Cells Summer. 2006;8:117. doi: 10.1089/clo.2006.8.117. [DOI] [PubMed] [Google Scholar]
- 31.Cecconi S. Barboni B. Coccia M. Mattioli M. In vitro development of sheep preantral follicles. Biol Reprod. 1999;60:594. doi: 10.1095/biolreprod60.3.594. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y. Betzendahl I. Cortvrindt R. Smitz J. Eichenlaub-Ritter U. Effects of low O2 and ageing on spindles and chromosomes in mouse oocytes from pre-antral follicle culture. Hum Reprod. 2001;16:737. doi: 10.1093/humrep/16.4.737. [DOI] [PubMed] [Google Scholar]
- 33.Eppig J.J. Wigglesworth K. Factors affecting the developmental competence of mouse oocytes grown in vitro: oxygen concentration. Mol Reprod Dev. 1995;42:447. doi: 10.1002/mrd.1080420412. [DOI] [PubMed] [Google Scholar]