Abstract
Here we investigated the incidence of cortical spreading depolarizations (spreading depression and peri-infarct depolarization) after traumatic brain injury (TBI) and their relationship to systemic physiologic values during neurointensive care. Subdural electrode strips were placed on peri-contusional cortex in 32 patients who underwent surgical treatment for TBI. Prospective electrocorticography was performed during neurointensive care with retrospective analysis of hourly nursing chart data. Recordings were 84 hr (median) per patient and 2,503 hr in total. In 17 patients (53%), 280 spreading depolarizations (spreading depressions and peri-infarct depolarizations) were observed. Depolarizations occurred in a bimodal pattern with peak incidence on days 1 and 7. The probability of a depolarization occurring increased significantly as a function of declining mean arterial pressure (MAP; R2 = 0.78; p < 0.001) and cerebral perfusion pressure (R2 = 0.85; p < 0.01), and increasing core temperature (R2 = 0.44; p < 0.05). Depolarization probability was 7% for MAP values of >100 mm Hg but 33% for MAP of ≤70 mm Hg. Temperatures of ≤38.4°C were associated with a 21% depolarization risk, compared to 63% for >38.4°C. Intracranial pressures were higher in patients with depolarizations (18.3 ± 9.3 vs. 13.5 ± 6.7 mm Hg; p < 0.001). We conclude that depolarization phenomena are a common cortical pathology in TBI. Their association with lower perfusion levels and higher temperatures suggests that the labile balance of energy supply and demand is an important determinant of their occurrence. Monitoring of depolarizations might serve as a functional measure to guide therapeutic efforts and their blockade may provide an additional line of defense against the effects of secondary insults.
Key words: electroencephalography, hyperthermia, intracranial hypertension, spreading cortical depression, vascular hypotension
Introduction
Electrophysiologic studies of traumatic brain injury (TBI) have focused mainly on the occurrence of early seizures and mechanisms contributing to hyperexcitability of cortical networks. Clinically, continuous EEG monitoring to detect sub-clinical seizures, which occur in 22% of comatose TBI patients (Vespa et al., 1999), is increasingly used and prophylactic administration of anti-convulsants is widely practised in severe TBI (Hirsch, 2004). In recent years, however, it has been discovered that a novel electrophysiologic dysfunction—in the form of spreading mass tissue depolarizations—also occurs in many patients with severe TBI (Strong et al., 2002; Fabricius et al., 2006) during acute neurocritical care.
Spreading depolarizations are short-circuiting waves that depolarize neurons and astrocytes en masse and interrupt local cortical function (as evidenced by suppressed spontaneous cortical activity) for periods of minutes to hours (Somjen, 2001; Strong and Dardis, 2005; Dreier et al., 2006). Propagating through the cerebral cortex at a rate of ∼1–5 mm/min, spreading depolarizations cause ion fluxes 5—10-fold greater than those produced by seizures (Hablitz and Heinemann, 1989). Extracellular Ca2+, for instance, decreases from ∼1.3 to <0.1 mM, while K+ increases from 3 to 60 mM. In the ischemic penumbra, spreading depolarizations (synonym: peri-infarct depolarization) cause vasoconstriction (Shin et al., 2006; Strong et al., 2007) and lesion expansion (Back et al., 1996; Busch et al., 1996). Even in normally perfused cortex, the effects of depolarizations (synonym: spreading depression) include tissue acidification, vasogenic edema (Gursoy-Ozdemir et al., 2004), and depletion of extracellular glucose despite vasodilation (Hashemi et al., 2008).
Experimentally, spreading depolarizations occur spontaneously in response to intracerebral hemorrhage (Mun-Bryce et al., 2001), brain contusion (Trabold et al., 2006), and particularly focal ischemia (Nedergaard and Astrup, 1986; Hartings et al., 2003a). Therefore, in human TBI, depolarizations might arise as a direct consequence of the primary injury, which often consists of a mixture of these pathologies. However, primary TBI injury is often compounded by secondary brain insults that occur both early (pre-hospital) and late (in-hospital) after trauma. By promoting metabolic crisis and focal cortical pathology, it is possible that secondary insults might further contribute to increased depolarization activity.
Secondary insults are a powerful independent predictor of TBI outcome and their prevention is the focus of medical management of TBI (Chesnut et al., 1993a; Jones et al., 1994; Gopinath et al., 1994). For instance, hypotension can occur in up to 32% of severe TBI patients during intensive care, increasing poor outcomes from 17% to 66% (Chesnut et al., 1993b), and raised intracranial pressure (ICP) further contributes to deficits in cerebral perfusion, particularly on days 5–6 post-trauma (Cortbus et al., 1994). Hyperthermia also increases morbidity and mortality (Jones et al., 1994; Jiang et al., 2002) and occurs commonly in severe TBI (Albrecht et al., 1998; Geffroy et al., 2004). Importantly, a link between cortical spreading depolarizations and these systemic variables of temperature and cerebral perfusion would suggest a measurable, local, functional, and pathologic consequence of secondary insults, with implications for monitoring and medical management of the TBI patient.
In an ongoing study to determine the relationship of spreading depolarizations to clinical outcome, we have performed electrocorticographic (ECoG) monitoring of depolarization activity in a population of patients who underwent neurosurgical treatment for TBI. Here, data from the initial group of patients monitored were analyzed to determine the incidence and time course of depolarizations and their relationship to late secondary insults during neurointensive care.
Methods
Patient recruitment and clinical care
Thirty-five patients were prospectively enrolled in the Co-Operative Study of Brain Injury Depolarizations, an international collaboration on the clinical study of spreading depression and related phenomena, at two participating centers, King's College Hospital (London, UK) and Virginia Commonwealth University (Richmond, VA). Inclusion criteria were TBI diagnosis, clinical decision for craniotomy for lesion evacuation and/or decompression, and age of ≥18 years. Patients with fixed, dilated pupils or posterior fossa injuries were excluded. Research protocols were approved by institutional review boards and surrogate informed consent was obtained for all patients.
For electrocorticographic (ECoG) recordings, an electrode strip was placed on the surface of the cortex during neurosurgery after hematoma evacuation and hemostasis (Strong et al., 2002; Fabricius et al., 2006). The lead wire of the strip was externalized through a burr hole in the skull (if the bone flap was replaced) and the closed dura and scalp. When possible, the strip was placed along a single gyrus radiating outward from an accessible injured region such that the closest electrode contacts were placed on viable, but often edematous or contused cortex with a low load of subarachnoid blood.
After surgery, patients were transferred to the intensive care unit where continuous ECoG recordings were initiated. Throughout recordings, patients were ventilated and pharmacologically immobilized as required. Sedation was maintained with propofol or midazolam, and analgesia was provided with morphine or fentanyl. Phenytoin was administered for seizure prophylaxis in all patients except one. ICP was monitored, if clinically indicated, by a ventricular drainage catheter or an ICP transducer (Codman, Raynham, MA). ICP of >20 mm Hg was typically treated by intravenous vecuronium, ventricular drainage, mannitol, hypertonic saline, mild hyperventilation, and in a few patients by axial cooling. The target level for cerebral perfusion pressure (CPP) of >60 mm Hg was achieved by ICP control, intravenous fluids, and vasopressors. ECoG recordings were terminated and electrode strips were removed at the bedside by gentle traction when invasive neuromonitoring was no longer clinically required or after a maximum of 5 days. No hemorrhagic or infectious complications of the electrode strip were encountered. Clinical outcome was assessed at 6 months according to the Glasgow Outcome Scale–Extended.
Electrocorticography recordings and analysis
ECoG recordings were made from a linear subdural strip consisting of six electrodes, with 10 mm spacing between electrodes and 4.2 mm2 of exposed platinum per contact (Wyler, Ad-Tech Medical, Racine, WI). Data were acquired continuously in four active channels A–D from electrodes 2–6, which were connected in sequential bipolar fashion to two AC-coupled Dual Bioamp amplifiers (ADInstruments, New South Wales, Australia). Ground was provided by electrode 1 or a Ag/AgCl ground plate electrode placed on the back of the patient's shoulder. Data were recorded and reviewed with 200 Hz sampling by a Powerlab 16/SP analog-to-digital converter and Chart 5 software (ADInstruments). Hallmarks of spreading depolarizations are the amplitude depression of spontaneous activity in the 0.5—100-Hz band, reflecting the depolarization block of synaptic activity, and a slow potential change (SPC) of 1—5-mV peak-to-peak amplitude in the near-DC (<0.1 Hz) frequency band. Amplifiers were set to a high-pass cutoff of 0.02 Hz to enable detection of SPCs, which reflect in part the mass intracellular flux of cations during tissue depolarization.
Data were analyzed for spreading depolarizations, including both peri-infarct depolarizations and spreading depressions, according to methods described previously (Fabricius et al., 2006). Records were first marked for periods of interruption and poor quality to identify valid recording epochs. Spreading depressions were then identified by (1) the simultaneous occurrence of an SPC and depression of higher frequency activity (0.5–100 Hz) in individual channels, and (2) the sequential occurrence of SPCs and depressions on adjacent channels, evidencing the spread of depolarization across the cortex (Fig. 1). Stereotyped and propagating SPCs were acceptable evidence of a “peri-infarct depolarization” in instances where no spontaneous activity existed due to electrodes being positioned over penumbral tissue, or due to prolonged depression from a previous depolarization (Fabricius et al., 2006). To simplify presentation of results, peri-infarct depolarizations are considered together with spreading depressions throughout the manuscript as “spreading depolarization” phenomena.
FIG. 1.
Representative electrocorticographic recording of spreading depolarizations. Recordings from channels A–C show a series of three spreading depression-type depolarizations separated by 33- and 30-min intervals. Top three traces show recordings with high frequency activity filtered (low pass 0.05 Hz) to show slow potential changes that ranged 3.0 (Ch. A) to 0.8 (Ch. C) mV in amplitude. Phase reversals between adjacent channels (arrows) identify the electrode common to those channels as the active site of depolarization. The second three traces show the same recordings with low frequency activity filtered (high pass 0.5 Hz) to show depressions of spontaneous activity. Tracings of Ch. A before and during depressions (indicated by *) are shown at magnified time scale below. The time delays between depolarizations on adjacent electrodes, evidenced by slow potential changes (arrows, top traces) and depression onsets (dashed lines, bottom traces), indicate the rate of propagation across the cortex is 4 mm/min. Total recording duration shown is 1 hr 45 min.
Data analysis
Hourly MAP, ICP, CPP, and core temperature data for the duration of ECoG monitoring were extracted from nursing charts for assessment of values in relation to the timing of depolarizations. Data were analyzed by custom programs written in MATLAB 7 (Mathworks, Inc., Natick, MA). For each depolarization, a time stamp was assigned as the time of occurrence of the first SPC. Physiologic values associated with that event were determined as those recorded within the closest temporal proximity to the depolarization, with a maximum acceptable interval of 1 hr for MAP, ICP, and CPP, and a longer interval of 2 hr for temperature to accommodate more sparse nursing documentation. Due to missing data, there were no MAP values meeting this criterion for six of 280 depolarizations and no CPP/ICP values for 22 of 239 depolarizations. For temperature data, there were 11 missing values and one patient with 75 depolarizations was excluded as an outlier (see Fig. 6 below), leaving 194 (280 minus 86) depolarizations for temperature analysis. The median time difference of 15 min (1st, 3rd quartiles: 8, 22) between depolarizations and their associated MAP, ICP, and CPP data is consistent with that expected based on use of hourly nursing assessments. The median time difference for temperature data was only slightly longer (22; 1st, 3rd quartiles: 10, 38).
FIG. 6.
Hyperthermia is associated with temporal clusters of depolarizations. (A,B) Hourly core temperatures are shown through 3 and 5 days of electrocorticography monitoring in two patients. Raster plots shows times of depolarizations. (C) Head computed tomography scan (post-trauma day 5) of the patient excluded from analysis of temperature data. This patient had diffuse bilateral contusions and intracerebral hemorrhage at admission and was the only patient in the study to undergo bilateral decompressive craniectomies. In the first 2 days after surgery, 75 depolarizations occurred, more than twice the total of any other patient. Although the patient was cooled with the Arctic Sun 2000 (Medivance, Inc., Louisville, KY) and maintained at 35–36°C during this time, the rate of depolarizations was not altered. The patient subsequently died 19 days after trauma.
Statistical analysis
Statistical analyses were performed using Minitab 15 (Minitab, Inc., State College, PA) and GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). Mann-Whitney (M-W) tests were used to compare means and frequency counts were assessed using Chi-Square tests. Data are reported as mean ± standard deviation.
Results
Depolarization incidence
Three patients enrolled were excluded from the analysis due to noisy and uninterpretable ECoG recordings. Demographic and clinical characteristics for the remaining 32 patients are presented in Table 1. Most patients sustained severe TBI (GCS 3—8) resulting from the usual causes, and three patients sustained penetrating injuries. Surgery occurred within 24 hr of trauma in 28 patients (78%) and ECoG recordings commenced within 24 hr in 22 patients (69%). The median duration of recordings was 84 hr, and the total duration of ECoG analyzed for all 32 patients was 2,503 hr.
Table 1.
Summary of Study Population
| n = 32 | |
|---|---|
| Sex, male | 25 (78%) |
| Age | |
| Mean ± SD | 42 ± 17 |
| Range | 18–74 |
| Mechanism of Injury | |
| Motor vehicle accident | 11 (34%) |
| Fall | 14 (44%) |
| Assault | 5 (16%) |
| Gunshot | 2 (6%) |
| Marshall CT Classification | |
| Diffuse injury III | 2 (6%) |
| Diffuse injury IV | 2 (6%) |
| Evacuated mass lesion | 22 (69%) |
| Non-evacuated mass lesion | 6 (19%) |
| Pupil reactivity after resuscitation | |
| Both reacting | 21 (66%) |
| Only one reacting | 4 (13%) |
| Neither reacting | 7 (22%) |
| GCS on admission | n = 29a |
| 3–8 | 26 |
| 9–15 | 3 |
| GOS-E at 6 months | |
| Dead | 10 (31%) |
| Vegetative state | 0 (0%) |
| Severe disability | 4 (13%) |
| Moderate disability | 9 (28%) |
| Good recovery | 9 (28%) |
| Time of surgery post-injury (hr) | |
| Mean ± SD | 23.6 ± 33.1 |
| Range | 1.6–113.5 |
| ECoG recording duration (hr) | |
| Mean ± SD | 78.2 ± 33.9 |
| Range | 13–123 |
| Depolarization rate (per 24 hr) | n = 17b |
| Mean ± SD | 6.2 ± 5.5 |
| Range | 0.6–17.7 |
Sample size, n < 32, due to data not recorded, unobtainable, or untestable.
Only those patients with depolarizations.
In 17 of the 32 patients (53%), a total of 280 spreading depolarizations were observed. Of these, 42 (15%) met the criteria for peri-infarct depolarization and 238 (85%) were spreading depressions. Figure 1 shows ECoG tracings of a representative series of depolarizations identified by SPCs and suppression of spontaneous activity. Three patients exhibited spike-and-wave seizures in addition to depolarizations and are described in detail as patients 6, 7, and 9 in Fabricius et al. (2008). Patients with depolarizations had a mean of 6.2 events per 24 hr recording time. Of three patients with penetrating injuries, two had depolarizations at 2 and 17 events per 24 hr. At 6 months, favorable outcomes (GOS-E 5–8) were achieved in 47% of patients with depolarizations, compared to 66% of patients with no depolarization activity (power of 18% for α = 0.05 is insufficient for statistical testing).
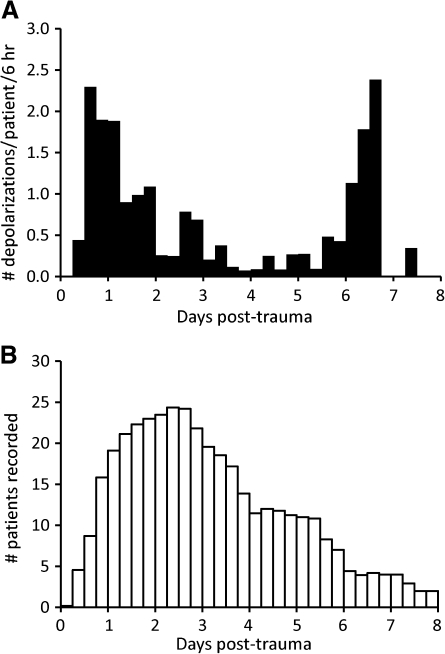
Figure 2A shows the temporal distribution of depolarizations after trauma, normalized to the number of patients recorded per time bin (Fig. 2B). There was an early peak incidence on days 1—2, which declined to minimal activity on days 4–5. A delayed secondary peak then occurred on days 6-7. Depolarization counts on days 1–7 differed significantly from expected counts, which were calculated based on actual recording durations on each day and the assumption of a uniform rate of depolarizations (χ2, p < 0.001). Because the delayed secondary peak was unexpected, data were examined more closely to assess the impact of delayed surgeries. Each of the five patients studied on day 7 had depolarizations, including two patients with recordings beginning 3 days post-trauma or earlier. One had a clearly accelerating pattern of depolarizations (i.e., one on day 4, three on each day 5 and 6, and 13 on day 7). The other three patients had delayed surgeries with recordings commencing 4.7–6.3 days post-trauma. An additional patient studied at 12.4–14.7 days post-trauma had 24 depolarizations (see Fig. 5 below).
FIG. 2.
Time course of depolarizations following trauma. (A) The total number of depolarizations accumulated for each 6-hr time bin was divided by the number of patients recorded in the corresponding interval. This eliminated bias from the differing number of patients recorded at various times to yield mean rates that can be compared across time. All patients, including those without depolarizations, were included in the analysis. (B) Number of patients recorded in each 6 hr bin is shown. Non-integer values result from the start or termination of recordings within a time bin (e.g., 4-hr recording = 0.67 patients).
FIG. 5.
Epoch of low cerebral perfusion is associated with a temporal cluster of depolarizations. Hourly cerebral perfusion and mean arterial pressure values are shown through 2 days of electrocorticography in a 45-year-old man who underwent neurosurgery 12 days after suffering a fall while intoxicated. Raster plot shows times of depolarizations. Early in monitoring, there is a 12-hr epoch in which mean arterial pressure drops ∼20 mm Hg and a series of repetitive depolarizations occurs. After blood pressure recovers, depolarization activity wanes and becomes sporadic. Thus, it is the instability in blood pressure that is temporally correlated with changing depolarization activity, but elevated intracranial pressure (16–20 mm Hg) causes cerebral perfusion to fall to critical levels during the episode of low blood pressure.
Time course of ischemic insults
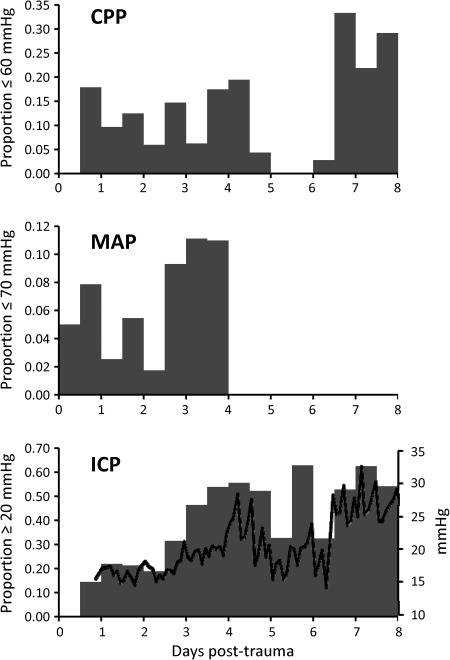
The bimodal temporal distribution of depolarizations following trauma is similar to the time course of CPP deficits reported by Cortbus et al. (1994), which occurred in both early (24 hr) and delayed (5–6 days) clusters. The time course of CPP deficits (≤60 mm Hg) in our patients was similar, with a relatively steady rate through the first 5 days and a delayed peak incidence on days 7–8 (Fig. 3). Analysis of MAP values of ≤70 mm Hg and ICP values of ≥20 mm Hg shows that early CPP deficits were attributable mainly to hypotension and late deficits to elevated ICP. Mean ICP values sharply elevated to >25 mm Hg on day 7, coincident with the delayed phase of depolarizations (Fig. 3, bottom panel).
FIG. 3.
Time course of ischemic insults. For each 12-hr time bin post-trauma, the number of hourly cerebral perfusion pressure, mean arterial pressure, or intracranial pressure values beyond the respective threshold was divided by the total number of values assessed in that bin. Cerebral perfusion and intracranial pressure analyses were based on 15 patients who exhibited depolarizations and had intracranial pressure monitoring. Analysis of mean arterial pressures was based on all 17 patients with depolarizations. The solid black line in the lower panel shows mean intracranial pressures in 2-hr increments.
Mean arterial pressure
To determine whether cortical depolarizations were associated with blood pressure, MAP values during depolarizations were compared with all MAP values throughout ECoG monitoring in the 17 patients with depolarizations. MAP values during depolarizations (n = 274) were significantly lower (86.9 ± 11.9 vs. 91.2 ± 13.2; M-W, p < 0.001), indicating that depolarizations did not occur randomly with respect to blood pressure. Rather, depolarizations occurred disproportionately often in moderate and low ranges of MAP (χ2, p < 0.001; Table 2). For instance, while 71.1% of all recorded MAP values were in the range of 71–100 mm Hg, a disproportionate 83.9% of depolarizations occurred in this range. In contrast, 23.5% of MAP values were >100 mm Hg, but only 8.4% of depolarizations occurred in this range.
Table 2.
Physiologic Values and Depolarization Probabilities
| |
Patients with depolarizations |
No Depolar. |
||||
|---|---|---|---|---|---|---|
| Depolarizations count (%) | ÷ | All values count (%) | = | Probabilities % | All values count (%) | |
| MAP | ||||||
| ≤70 | 21 (7.7) | 63 (4.8) | 33.3 | 39 (2.8) | ||
| 71–100 | 230 (83.9) | 944 (71.7) | 24.4 | 1172 (85.4) | ||
| >100 | 23 (8.4) | 309 (23.5) | 7.4 | 161 (11.7) | ||
| CPP | ||||||
| ≤60 | 42 (19.4) | 143 (12.9) | 29.4 | 127 (9.5) | ||
| 61–90 | 169 (77.9) | 842 (76.2) | 20.1 | 1088 (81.1) | ||
| >90 | 6 (2.8) | 120 (10.9) | 5.0 | 126 (9.4) | ||
| Temperature | ||||||
| ≤36 | 29 (14.9) | 145 (19.2) | 20.0 | 154 (15.0) | ||
| 36.1–38.4 | 114 (58.8) | 530 (70.1) | 21.5 | 789 (76.7) | ||
| >38.4 | 51 (26.3) | 81 (10.7) | 63.0 | 86 (8.4) | ||
Patients with depolarizations. Left column: Raw counts and percentages of depolarizations occurring with physiologic values in specified ranges. Middle: Raw counts and percentages of all physiologic values recorded throughout the duration of ECoG monitoring. Right: Probabilities of depolarization occurrence for each physiologic value range were computed by dividing counts in the left column by those in the middle.
No depolarizations. Raw counts and percentages of all physiologic values recorded throughout the duration of ECoG monitoring in patients not exhibiting depolarizations.
To determine the influence of MAP on the probability of a depolarization occurring, we divided the total number of depolarizations associated with a particular MAP range by the total number of values recorded in that range throughout monitoring. Figure 4A shows that depolarization probability increased significantly as a function of declining MAP (R2 =0.75; p < 0.001). For instance, for every hourly MAP value of >100 mm Hg, there was only a 7% probability of a depolarization occurring. For the 71–100 mm Hg range, the probability increased to 24%, and then to 33% for MAP of ≤70 mm Hg (Table 2). The incidence of hypotension was similar between patients with and without depolarizations (Table 2).
FIG. 4.
Depolarization probabilities increase with decreasing mean arterial and cerebral perfusion pressures. Depolarization probabilities were computed as the likelihood of observing a single event for every hourly mean arterial pressure (A) or cerebral perfusion pressure (B) value observed in a given range. Total number of depolarizations associated with a particular range of values was divided by the total number of values recorded in that range throughout monitoring (×100 = %). Analysis included only those patients exhibiting depolarizations. (A) Data points are based on analysis of data in 5-mm Hg bins. Because it is reasonable to assume that depolarization probability is dependent on arterial pressure, we performed non-linear regression using a forward selection procedure with a polynomial model. A quartery term significantly improved prediction (p < 0.01), while a quintic term did not (p > 0.05), yielding the best fit equation, y = 26.1 − 1.17 · x + 0.02 · x2–1.44 · 10−4 · x3 + 0.39 · 10−6 · x4. The equation fits with an adjusted R2 = 94.7 (ANOVA, p < 0.0001). Dashed lines indicate the 95% confidence bands of the equation. B) Data points are based on analysis of cerebral perfusion pressure data in 10-mm Hg bins. The regression equation is y = 0.64 – 5.66 · 10−3 · x and dashed lines shown 95% confidence bands.
Cerebral perfusion pressure
ICP and CPP were monitored in 30 patients, including 15 with depolarizations. As for MAP, CPP values during depolarizations (n = 217) were significantly lower than those obtained throughout monitoring in these 15 patients (70.4 ± 11.3 vs. 74.0 ± 13.1; M-W, p < 0.001). Similarly, the distribution of CPP values during depolarizations were significantly shifted toward lower ranges (χ2, p < 0.001; Table 2). For instance, while 10.9% of all CPP values were >90 mm Hg, only 2.8% of depolarizations occurred in this range. A greater proportion of depolarizations occurred when CPP was ≤60 mm Hg. Depolarization probabilities are shown as a function of CPP in Figure 4B. Linear regression provided a goodness-of-fit with R2 = 0.85 and a significant negative slope (p < 0.01). The incidence of CPP deficits (≤60 mm Hg) was similar between patients with and without depolarizations (Table 2).
Intracranial pressure
In patients with depolarizations, ICP values during depolarizations (n = 217) did not differ from those obtained throughout ECoG monitoring (18.1 ± 7.8 vs. 18.3 ± 9.3; M-W, p = 0.44). However, ICPs were significantly higher in patients with depolarizations (18.3 ± 9.3) compared to those without (13.5 ± 6.7; M-W, p < 0.001). Neither lower CPP nor MAP values similarly distinguished these groups. In fact, MAP values were significantly higher in patients with depolarizations (91.2 ± 13.2 vs. 88.1 ± 10.7; M-W, p < 0.001), while CPP was the same (74.0 ± 13.1 vs. 74.7 ± 12.1; M-W, p = 0.37). Taken together, these data suggest that elevated ICP increases the general risk for a patient to develop depolarizations, but that MAP changes determine the timing and frequency of depolarization occurrence. For example, the patient illustrated in Figure 5 experienced a series of repetitive depolarizations that was tightly time-locked to a 12-hr epoch of low MAP. ICP was persistently elevated at 16–20 mm Hg, causing CPP to fall to critical levels during the low MAP episode.
Temperature
Temporal clusters of depolarizations were also associated with periods of elevated core temperature (Fig. 6A,B). This effect was robust, except for a single patient with extensive intracranial lesions who had 75 depolarizations while being cooled at 35–36°C (Fig. 6C); this patient was excluded from the analysis as an outlier. For the remaining 16 patients, temperatures during depolarizations (n = 194) were shifted significantly toward higher ranges (χ2, p < 0.001; Table 2). Although only 11% of all temperatures throughout monitoring were >38.4°C, 26% of depolarizations occurred in this range. Depolarization probabilities, examined in incremental ranges of 0.4°C, were also positively correlated with temperature (R2 = 0.44, p < 0.05). For every hourly temperature ≤36.0°C, there was a 20% probability that a depolarization would occur. The probability was similar for the 36.1–38.4°C range (22%), but increased to 63% for temperatures ≥38.4°C. The incidence of hyperthermia (>38.4°C) was similar between patients with and without depolarizations (Table 2).
Figure 7 shows depolarization probability as a function of both temperature and MAP. Multiple linear regression revealed a significant dependence of depolarization probability on both variables (p's < 0.05), which combine to account for 42% of the variance (analysis of variance [ANOVA], p < 0.01). The effects of temperature and blood pressure compounded each other: depolarizations were most likely to occur at low MAP and high temperature, and least likely at high MAP and low temperature.
FIG. 7.
Depolarizations as a function of mean arterial pressure and core temperature. Plot shows depolarizaton probability as a function of hourly temperature and mean arterial pressure values occurring simultaneously in specified ranges. Analysis is based on 16 patients and 194 depolarizations. The numbers of depolarizations occurring when both temperature and pressures values were in the respective ranges of each category was divided by the total number of paired values observed in the respective category throughout electrocorticographic monitoring. Quotients were then multiplied by 100. Counts of <5 in the denominator were excluded, resulting in four categories with no data.
Discussion
We examined the incidence and timing of spreading depolarizations, including both spreading depression and its variant peri-infarct depolarizations, in a prospective ECoG study of 32 patients who underwent neurosurgery following TBI. We found that depolarizations occurred in 53% of patients, which is lower than has been reported following aneurysmal subarachnoid hemorrhage (72%)(Dreier et al., 2006) and malignant ischemic stroke (100%)(Dohmen et al., 2008) in humans. This finding is consistent with the greater incidence of depolarizations in experimental stroke (Nedergaard and Astrup, 1986; Hartings et al., 2003a) compared to trauma (Trabold et al., 2006).
Depolarization activity peaked early within the first 36 hr after trauma followed by a decline to minimal activity over 48–144 hr. This is not surprising considering a period of hypoperfusion exists during the first 24 hr after TBI (Robertson et al., 1992; Martin et al., 1997; Kelly et al., 1997). The early peak incidence of depolarizations may thus reflect ischemic conditions, particularly in the peri-contusion areas from which ECoG recordings were made (Furuya et al., 2003; Coles et al., 2004). The waning of depolarizations may be attributable to a later hyperemic phase that peaks at 48–72 hr (Martin et al., 1997).
An unexpected finding was that depolarizations recurred in a delayed secondary phase on days 6–7. Whether this activity continues is unknown because few patients were studied after this time point. This delayed peak partly reflects a subset of patients who underwent surgery after delayed deterioration. In addition, one patient studied from day 3 had an increase in frequency of depolarizations on day 7, suggesting that the secondary phase reflects the pathology underlying delayed deterioration rather than the surgical procedures themselves. This interpretation is supported by experimental studies of focal cerebral ischemia, in which a distinct secondary phase of cortical depolarizations develops with delayed increases in ICP (Hartings et al., 2003a; Umegaki et al., 2005). Here we observed a similar rise in average ICP from 15–20 to >25 mm Hg on day 7–8 (Fig. 3) in association with the secondary phase of depolarizations. While speculative, these changes may be causally related in a recursive manner. Spreading depression increases blood-brain barrier permeability and induces both vasogenic (Gursoy-Ozdemir et al., 2004) and cytotoxic (Kempski et al., 2000) edema. Conversely, rising ICP may induce depolarizations by exacerbating ischemia (Umegaki et al., 2005).
Clinical studies further support that depolarizations are an intrinsic component of delayed deterioration. In aneurysmal subarachnoid hemorrhage, delayed clusters of depolarizations develop between days 5–10 and are associated with the onset of delayed ischemic neurological deficits and sometimes the development of new infarcts (Dreier et al., 2006). Furthermore, the timing and bimodal nature of depolarization activity in our study is nearly identical to that recently reported for early seizures after TBI. With continuous EEG, Vespa et al. (2007) identified two peaks in nonconvulsive seizure incidence at early (29 hr) and delayed (140 hr) time points. This similarity is not likely to be coincidental. We have found that seizures occur almost exclusively in the sub-population of acutely brain-injured patients who exhibit depolarizations, and that both types of activity occur concomitantly with interacting and overlapping temporal patterns (Fabricius et al., 2008).
In patients with depolarizations, hypotension and cerebral perfusion deficits occurred at rates of 4.8% and 12.9%, respectively, and were associated with only a minority of all depolarizations observed (7.7% and 19.4%, respectively). Therefore, secondary ischemic insults, as defined by these systemic/global measures, are neither a necessary condition nor principal cause for depolarizations. This conclusion is further supported by the fact that 15 of 32 patients had no depolarizations, despite having similar rates of secondary ischemic insults as those patients with depolarizations. However, when ischemic insults do occur, they have a strong association with increased depolarization frequency. For instance, the likelihood of depolarization was three-fold greater for MAP ≤70 mm Hg when compared with the 70–100 mm Hg range. The additional decrease in risk of depolarizations when MAP exceeds a threshold of ∼100 mm Hg is also noteworthy. The recent IMPACT study showed that outcomes were optimized for patients with higher MAP values at hospital admission (i.e., 85–110 mm Hg) (Butcher et al., 2007). Our data support the suggestion that 70 mm Hg may be a conservative lower limit for MAP maintenance during neurointensive care.
ICP values were greater in patients with depolarizations (18.3 vs. 13.5), but were not further elevated specifically at the times depolarizations occurred. These results suggest that persistent ICP elevation increases a patient's risk for developing depolarizations by increasing the MAP threshold for reduction of CPP below critical levels (Fig. 5). That is, fluctuations in MAP appear to be an acute determinant of the specific timing and frequency of depolarizations within a patient's course, if ICP is sufficiently high. It is noteworthy that ICPs are also higher in TBI patients with nonconvulsive seizures compared to those without seizures (17.6 vs. 12.2 mm Hg, respectively)(Vespa et al., 2007). The present results suggest that nonconvulsive seizures, like depolarizations, may also be sensitive to cerebral perfusion, as shown experimentally (Hartings et al., 2003b).
A final risk factor associated with depolarization occurrence was hyperthermia. Hourly core temperatures of >38.4°C were associated with a 63% chance of depolarization, compared to only 21% for temperatures of ≤38.4°C. These results are consistent with findings that induced hyperthermia increases the frequency of depolarizations after focal cerebral ischemia in the rat (Chen et al., 1993). Furthermore, we found that blood pressure and temperature combine as risk factors to affect depolarization activity at all ranges for both variables. That is, low MAP increases depolarization probability even when temperature is low, but low MAP and high temperature together result in the greatest risk of depolarization (Fig. 7). Low perfusion and high temperature may act in concert by decreasing energy supply and increasing demand, respectively, such that the margin between the two is reduced beyond the threshold for maintenance of membrane polarization.
Although the effects of depolarizations on peri-injury cortex and clinical outcome are unknown, it is possible that depolarizations are a mechanism of lesion expansion that in part mediate the effects of secondary insults on TBI outcome. In rats, TBI compounded with a hypotensive episode (40 mm Hg) provoked additional depolarization activity and expanded contusion volume compared with TBI alone (Trabold et al., 2006). While the effects of hypotension and depolarization could not be differentiated, it is proven that peri-infarct depolarizations cause expansion of core infarction into the ischemic penumbra in animals (Busch et al., 1996; Back et al., 1996). Anecdotal evidence suggests a similar association of depolarizations with lesion development in human ischemic stroke (Dohmen et al., 2008), aneurysmal subarachnoid hemorrhage (Dreier et al., 2006), and TBI (Hartings et al., 2008), and the present data show a trend toward worse outcomes in patients exhibiting depolarizations (47% vs. 66% favorable outcome). Power analysis based on these initial data from 32 TBI patients suggests a substantially larger sample size is required to determine whether this difference in outcomes is significant.
The present study has several shortcomings. First, the temporal accuracy of assessments relative to continous ECoG data was limited by the use of hourly nursing chart data, and would be improved with continuous recordings of temperature, MAP, ICP, and other variables in the future. It should be noted, however, that the ECoG methods introduce an inherent limitation on the accuracy of these assessments. Depolarizations detected along the electrode strip may originate locally, but may also propagate from a distant, unmonitored site. Considering that depolarizations may propagate over cortical distances of at least several centimeters at a speed of 1–5 mm/min, uncertainty in their location of origin introduces an inherent uncertainty in their true time of onset, which may be up to 30 min or more from the time they are recorded at the electrode strip. Second, CPP is only a theoretical number. Future studies with direct measures of cerebral blood flow, ptiO2, or microdialysis in the recording area are required to draw any firm conclusions about local metabolism relating to depolarizations. Third, as an unfunded study, detailed data were not collected on therapeutic and injury variables such as specific head CT pathologies, therapeutic intensity level, and medications. Each of these factors is a possible candidate to influence depolarization activity and should be examined in combination with the factors identified here in a future prospective study with multivariate analysis.
Conclusions
We found that spreading depolarizations occur in 53% of neurosurgical TBI patients and can persist through at least 1 week post-trauma. While secondary brain insults were neither a necessary nor sufficient condition for depolarizations, their occurrence was strongly associated with increased depolarization occurrence. Higher ICPs were characteristic of patients with depolarizations, and the specific timing and frequency of depolarization events were related to epochs of lower MAP/CPP values and/or higher temperatures. These data suggest that cerebral perfusion and the balance of energy supply-demand are important factors in the elicitation of spreading depolarization waves in human TBI. ECoG monitoring of spreading depolarizations thus provides a continuous functional measure of peri-injury cortex that is sensitive to critical systemic variables targeted in TBI management. As such, ECoG may prove a valuable tool to guide therapy, with depolarizations possibly serving as an indication for active maintenance of normothermia and a guide for management of ICP and CPP tailored to individual patients. Although such a strategy of raising CPP to minimize ischemic insults and prevent depolarizations may have limitations because of side effects (Robertson et al., 1999), pharmacologic blockade of spreading depolarizations might provide an additional alternative line of defense against the effects of secondary brain insults.
Acknowledgments
We thank Charlotte Gilman, Marinella Gugliotta, and Amedeo Merenda for their valuable contributions. This work was supported in part by NINDS PO1 NS12587-27 (to R.B.), the German Research Foundation (DFG) SFB Tr3 D10 and DR 323/3-1 (to J.D.), and the Novo Nordisk Foundation (to M.F.).
Author Disclosure Statement
No competing financial interests exist.
References
- Albrecht II R.F. Wass C.T. Lanier W.L. Occurrence of potentially detrimental temperature alterations in hospitalized patients at risk for brain injury. Mayo Clin. Proc. 1998;73:629–635. doi: 10.1016/S0025-6196(11)64885-4. [DOI] [PubMed] [Google Scholar]
- Back T. Ginsberg M.D. Dietrich W.D. Watson B.D. Induction of spreading depression in the ischemic hemisphere following experimental middle cerebral artery occlusion: effect on infarct morphology. J. Cereb. Blood Flow Metab. 1996;16:202–213. doi: 10.1097/00004647-199603000-00004. [DOI] [PubMed] [Google Scholar]
- Busch E. Gyngell M.L. Eis M. Hoehn-Berlage M. Hossman K.A. Potassium-induced cortical spreading depressions during focal cerebral ischemia in rats: contribution to lesion growth assessed by diffusion-weighted NMR and biochemical imaging. J. Cereb. Blood Flow Metab. 1996;16:1090–1099. doi: 10.1097/00004647-199611000-00002. [DOI] [PubMed] [Google Scholar]
- Butcher I. Maas A.I. Lu J. Marmarou A. Murray G.D. Mushkudiani N.A. McHugh G.S. Steyerberg E.W. Prognostic value of admission blood pressure in traumatic brain injury: results from the IMPACT study. J. Neurotrauma. 2007;24:294–302. doi: 10.1089/neu.2006.0032. [DOI] [PubMed] [Google Scholar]
- Chen Q. Chopp M. Bodzin G. Chen H. Temperature modulation of cerebral depolarization during focal cerebral ischemia in rats: correlation with ischemic injury. J. Cereb. Blood Flow Metab. 1993;13:389–394. doi: 10.1038/jcbfm.1993.52. [DOI] [PubMed] [Google Scholar]
- Chesnut R.M. Marshall L.F. Klauber M.R. Blunt B.A. Baldwin N. Eisenberg H.M. Jane J.A. Marmarou A. Foulkes M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma. 1993a;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Chesnut R.M. Marshall S.B. Piek J. Blunt B.A. Klauber M.R. Marshall L.F. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir. 1993b;59:121–125. doi: 10.1007/978-3-7091-9302-0_21. [DOI] [PubMed] [Google Scholar]
- Coles J.P. Fryer T.D. Smielewski P. Chatfield D.A. Steiner L.A. Johnston A.J. Downey S.P. Williams G.B. Aigbirhio F. Hutchinson P.J. Rice K. Carpenter T.A. Clark J.C. Pickard J.D. Menon D.K. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J. Cereb. Blood Flow Metab. 2004;24:202–211. doi: 10.1097/01.WCB.0000103022.98348.24. [DOI] [PubMed] [Google Scholar]
- Cortbus F. Jones P.A. Miller J.D. Piper I.R. Tocher J.L. Cause, distribution, and significance of episodes of reduced cerebral perfusion pressure following head injury. Acta Neurochir. (Wien) 1994;130:117–124. doi: 10.1007/BF01405511. [DOI] [PubMed] [Google Scholar]
- Dohmen C. Sakowitz O.W. Fabricius M. Bosche B. Reithmeier T. Ernestus R.I. Brinker G. Dreier J.P. Woitzik J. Strong A.J. Graf R. Co-Operative Study of Brain Injury Depolarisations (COSBID) Spreading depolarizations occur in human ischemic stroke with high incidence. Ann. Neurol. 2008;63:720–728. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- Dreier J.P. Woitzik J. Fabricius M. Bhatia R. Major S. Drenckhahn C. Lehmann T.N. Sarrafzadeh A. Willumsen L. Hartings J.A. Sakowitz O.W. Seemann J.H. Thieme A. Lauritzen M. Strong A.J. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- Fabricius M. Fuhr S. Bhatia R. Boutelle M. Hashemi P. Strong A.J. Lauritzen M. Cortical spreading depression and peri-infarct depolarization in acutely injury human cerebral cortex. Brain. 2006;129:778–790. doi: 10.1093/brain/awh716. [DOI] [PubMed] [Google Scholar]
- Fabricius M. Fuhr S. Willumsen L. Dreier J.P. Bhatia R. Boutelle M.G. Hartings J.A. Bullock R. Strong A.J. Lauritzen M. Association of seizures with cortical spreading depression and peri-infarct depolarizations in the acutely injured human brain. Clin. Neurophysiol. 2008;119:1973–1984. doi: 10.1016/j.clinph.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya Y. Hlatky R. Valadka A.B. Diaz P. Robertson C.S. Comparison of cerebral blood flow in computed tomographic hypodense areas of the brain in head-injured patients. Neurosurgery. 2003;52:340–345. doi: 10.1227/01.neu.0000043931.83041.aa. [DOI] [PubMed] [Google Scholar]
- Geffroy A. Bronchard R. Merckx P. Seince P.F. Faillot T. Albaladejo P. Marty J. Severe traumatic head injury in adults: which patients are at risk of early hyperthermia? Intensive Care Med. 2004;30:785–790. doi: 10.1007/s00134-004-2280-y. [DOI] [PubMed] [Google Scholar]
- Gopinath S.P. Robertson C.S. Contant C.F. Hayes C. Feldman Z. Narayan R.K. Grossman R.G. Jugular venous desaturation and outcome after head injury. J. Neurol. Neurosurg. Psychiatry. 1994;57:717–723. doi: 10.1136/jnnp.57.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursoy-Ozdemir Y. Qiu J. Matsuoka N. Bolay H. Bermpohl D. Jin H. Wang X. Rosenberg G.A. Lo E.H. Moskowitz M.A. Cortical spreading depression activates and upregulates MMP-9. J. Clin. Invest. 2004;113:1447–1455. doi: 10.1172/JCI21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz J.J. Heinemann U. Alterations in the microenvironment during spreading depression associated with epileptiform activity in the immature neocortex. Dev. Brain Res. 1989;46:243–252. doi: 10.1016/0165-3806(89)90288-5. [DOI] [PubMed] [Google Scholar]
- Hartings J.A. Gugliotta M. Gilman C. Strong A.J. Tortella F.C. Bullock M.R. Repetitive cortical spreading depolarizations in a case of severe brain trauma. Neurol. Res. 2008;30:876–882. doi: 10.1179/174313208X309739. [DOI] [PubMed] [Google Scholar]
- Hartings J.A. Rolli M.L. Lu X.C. Tortella F.C. Delayed secondary phase of peri-infarct depolarizations after focal cerebral ischemia: relation to infarct growth and neuroprotection. J. Neurosci. 2003a;23:11602–11610. doi: 10.1523/JNEUROSCI.23-37-11602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartings J.A. Williams A.J. Tortella F.C. Occurrence of nonconvulsive seizures, periodic epileptiform discharges, and intermittent rhythmic delta activity in rat focal ischemia. Exp. Neurol. 2003b;179:139–149. doi: 10.1016/s0014-4886(02)00013-4. [DOI] [PubMed] [Google Scholar]
- Hashemi P. Bhatia R. Nakamura H. Dreier J.P. Graf R. Strong A.J. Boutelle M.G. Persisting depletion of brain glucose following cortical spreading depression, despite apparent hyperaemia: evidence for risk of an adverse effect of Leão's spreading depression. J. Cereb. Blood Flow Metab. 2009;29:166–175. doi: 10.1038/jcbfm.2008.108. [DOI] [PubMed] [Google Scholar]
- Hirsch L.J. Continuous EEG monitoring in the intensive care unit: an overview. J. Clin. Neurophysiol. 2004;21:332–340. [PubMed] [Google Scholar]
- Jiang J.Y. Gao G.Y. Li W.P. Yu M.K. Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J. Neurotrauma. 2002;19:869–874. doi: 10.1089/08977150260190456. [DOI] [PubMed] [Google Scholar]
- Jones P.A. Andrews P.J.D. Midgley S. Anderson S.I. Piper I.R. Tocher J.L. Housley A.M. Corrie J.A. Slattery J. Dearden N.M. Measuring the burden of secondary insults in head-injured patients during intensive care. J. Neurosurg. Anesthesiol. 1994;6:4–14. [PubMed] [Google Scholar]
- Kelly D.F. Martin N.A. Kordestani R. Counelis G. Hovda D.A. Bergsneider M. McBride D.Q. Shalmon E. Herman D. Becker D.P. Cerebral blood flow as a predictor of outcome following traumatic brain injury. J. Neurosurg. 1997;86:633–641. doi: 10.3171/jns.1997.86.4.0633. [DOI] [PubMed] [Google Scholar]
- Kempski O. Otsuka T. Seiwert T. Heimann A. Spreading depression induces permanent cell swelling under penumbra conditions. Acta Neurochir. 2000;76:251–255. doi: 10.1007/978-3-7091-6346-7_51. [DOI] [PubMed] [Google Scholar]
- Martin N.A. Patwardhan R.V. Alexander M.J. Africk C.Z. Lee J.H. Shalmon E. Hovda D.A. Becker D.P. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J. Neurosurg. 1997;87:9–19. doi: 10.3171/jns.1997.87.1.0009. [DOI] [PubMed] [Google Scholar]
- Mun-Bryce S. Wilkerson A.C. Papuashvili N. Okada Y.C. Recurring episodes of spreading depression are spontaneously elicited by an intracerebral hemorrhage in the swine. Brain Res. 2001;888:248–255. doi: 10.1016/s0006-8993(00)03068-7. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Astrup J. Infarct rim: effect of hyperglycemia on direct current potential and [14C]2-deoxyglucose phosphorylation. J. Cereb. Blood Flow Metab. 1986;6:607–615. doi: 10.1038/jcbfm.1986.108. [DOI] [PubMed] [Google Scholar]
- Robertson C.S. Contant C.F. Gokaslan Z.L. Narayan R.K. Grossman R.G. Cerebral blood flow, arteriovenous oxygen difference, and outcome in head injured patients. J. Neurol. Neurosurg. Psychiatry. 1992;55:594–603. doi: 10.1136/jnnp.55.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C.S. Valadka A.B. Hannay J.H. Contant C.F. Gopinath S.P. Cormio M. Uzura M. Grossman R.G. Prevention of secondary ischemic insults after severe head injury. Crit. Care Med. 1999;27:2086–2095. doi: 10.1097/00003246-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Shin H.K. Dunn A.K. Jones P.B. Boas D.A. Moskowitz M.A. Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J. Cereb. Blood Flow Metab. 2006;26:1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- Somjen G.G. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol. Rev. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- Strong A.J. Fabricius M. Boutelle M.G. Hibbins S.J. Hopwood S.E. Jones R. Parkin M.C. Lauritzen M. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002;33:2738–2743. doi: 10.1161/01.str.0000043073.69602.09. [DOI] [PubMed] [Google Scholar]
- Strong A.J. Dardis R. Depolarisation phenomena in traumatic and ischaemic brain injury. Adv. Tech. Stand. Neurosurg. 2005;30:3–49. doi: 10.1007/3-211-27208-9_1. [DOI] [PubMed] [Google Scholar]
- Strong A.J. Anderson P.J. Watts H.R. Virley D.J. Lloyd A. Irving E.A. Nagafuji T. Ninomiya M. Nakamura H. Dunn A.K. Graf R. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain. 2007;130:995–1008. doi: 10.1093/brain/awl392. [DOI] [PubMed] [Google Scholar]
- Trabold R. Schueler O.G. Eriskat J. Plesnila N. Baethmann A.J. Back T. Arterial hypotension triggers perifocal depolarizations and aggravates secondary damage in focal brain injury. Brain Res. 2006;1071:237–244. doi: 10.1016/j.brainres.2005.11.095. [DOI] [PubMed] [Google Scholar]
- Umegaki M. Sanada Y. Waezeggers Y. Rosner G. Yoshimine T. Heiss W.D. Graf R. Peri-infarct depolarizations reveal penumbra-like conditions in striatum. J. Neurosci. 2005;25:1387–1394. doi: 10.1523/JNEUROSCI.4182-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa P.M. Nuwer M.R. Nenov V. Ronne-Engstrom E. Hovda D.A. Bergsneider M. Kelly D.F. Martin N.A. Becker D.P. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J. Neurosurg. 1999;91:750–760. doi: 10.3171/jns.1999.91.5.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa P.M. Miller C. McArthur D. Eliseo M. Etchepare M. Hirt D. Glenn T.C. Martin N. Hovda D. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit. Care Med. 2007;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]