Abstract
While various problems with bone healing remain, the greatest clinical change is the absence of an effective approach to manage large segmental defects in limbs and craniofacial bones caused by trauma or cancer. Thus, nontraditional forms of medicine, such as gene therapy, have been investigated as a potential solution. The use of osteogenic genes has shown great potential in bone regeneration and fracture healing. Several methods for gene delivery to the fracture site have been described. The majority of them include a cellular component as the carrying vector, an approach known as cell-mediated gene therapy. Yet, the complexity involved with cell isolation and culture emphasizes the advantages of direct gene delivery as an alternative strategy. Here we review the various approaches of direct gene delivery for bone repair, the choice of animal models, and the various outcome measures required to evaluate the efficiency and safety of each technique. Special emphasis is given to noninvasive, quantitative, in vivo monitoring of gene expression and biodistribution in live animals. Research efforts should aim at inducing a transient, localized osteogenic gene expression within a fracture site to generate an effective therapeutic approach that would eventually lead to clinical use.
Introduction
Bone tissue has regenerative capabilities that enable the self-repair of fractures and tissue loss. The vast majority of fractures heal on their own, or recover after standard orthopedic procedures.1 In extreme situations, however, in which trauma or tumor surgery results in a critical-size bone defect (one spanning > 2 cm), complete regeneration cannot occur.2 Critical-size defects have become increasingly common nowadays due to the nature of modern warfare. Traumatic orthopedic injuries constitute the vast majority of injuries incurred on the battle field: 70% involve the musculoskeletal system; 26% of these are fractures and 82% of fractures are open fractures.3–6 Nontraditional approaches to bone healing, such as gene therapy,7 need to be considered as possible solutions in cases of critical-size bone loss, which have not been addressed by drug treatment or surgical methods.8 In the present paper, we review the various gene therapy approaches to bone repair reported during the last two decades. Used as therapeutic drugs for regenerative orthopedic medicine, genes hold several advantages over recombinant osteogenic proteins, including the low cost of gene production, sustained secretion of therapeutic proteins by transfected cells, and the ability for repeated administration of the transgene to achieve complete repair.
Gene therapies for bone repair can be divided into cell-mediated and acellular strategies. Much has been written about cell-mediated or stem cell–mediated gene therapy approaches and their potential for bone regeneration. Nevertheless, cell isolation and culture, integral components of most of these approaches, still pose hurdles in the transition of cell-mediated gene therapy to the clinical arena. Direct gene therapy can be regarded as a more straightforward approach to fracture repair. In this review we focus on acellular gene therapy for bone repair, highlighting potential gene delivery strategies, animal models, and outcome measures that are in use for evaluating the effectiveness of gene-based treatments.
Direct Gene Therapy Approaches for Bone Repair
Therapeutic DNA can be delivered to cells in vivo by means of viral or nonviral vectors. Localization of the vector within the fracture site can be assured either by physical placement at the target site or by gene release from a three-dimensional (3D) biomaterial implanted in the defect.
Physical placement methods include direct injection of the transgene into the fracture site. However, for the gene to penetrate cells in situ, it must be delivered by a virus or forced into cells' nuclei by an electric pulse or ultrasonic wave. Several studies by Evans and colleagues have shown the feasibility of inducing fracture repair in a rodent model by using adenoviral vectors that encode for a bone morphogenetic protein (BMP).9–15 Others have used an adenovirus encoding for vascular endothelial growth factor in a similar manner.16 The ease of adenovirus production, the minimally invasive method of administration, and the transiency of the gene expression that is induced make the adenoviral approach an attractive one for bone repair. Yet, it appears that complete fracture repair has not been achieved using this approach and that the timing of vector injection into the defect is critical.10 Moreover, clinical trials have shown that adenoviruses carry a risk of evoking the immune response, and this risk must be overcome before the method is clinically applicable.17
Potentially nonimmunogenic alternatives for direct gene delivery may be found in in vivo electroporation or sonoporation. Both methods are used to permeabilize cell membranes and translocate naked DNA into the nucleus by mechanisms as yet not fully understood. Using these methods, an osteogenic gene is directly injected into a fracture and an electric pulse or ultrasonic wave is applied to the site either trans- or percutaneously. So far electroporation and sonoporation have only been shown to form bone in ectopic sites when applied after delivery of genes from the BMP family.18–24 Given that the literature shows increased data on the use of ultrasound in fracture repair,25 it is conceivable that the sonoporation approach will gain much interest. Currently, in vivo electroporation is primarily used to induce cell death in cancer pretrials26 and clinical trials.27,28 For use in regenerative medicine, a careful pulse application would have to be designed to avoid tissue damage. This would be a valuable step, because we previously determined that an electroporation approach induces more bone formation than a sonoporation approach.18 Future studies should be performed to investigate the use of both electroporation and sonoporation in orthotopic fracture models. In addition, protocols or systems for sonoporation should be developed to improve this method's ability to achieve clinically relevant bone formation.
One major disadvantage of injecting an osteogenic gene into a fracture site is the difficulty encountered in localizing transfection to a specific limited region. Adverse effects associated with the spread of transfection include heterotopic ossification of adjacent muscle tissue and fusion of one bone to an adjacent bone, such as fusion of the tibia to the fibula. Moreover, if the fracture is located near a joint compartment, treatment may induce ossification of cartilaginous and ligamentous tissues, leading to joint dysfunction. Rubinsky and colleagues proposed a method to monitor the electroporation process in real time and thus control the extent of gene delivery—named “electrical impedance tomography.”29 This method is based on measuring the passive electrical properties, mainly conductivity, of electroporated tissues; it still needs to be validated for use in a fracture model.
An alternative to localizing transgene expression within the fracture site is entrapment of DNA in a biomaterial that is implanted into the defect. Using this technique, only cells surrounding or penetrating the biomaterial would be transfected. Indeed, the first method of localized gene therapy for bone healing involved a gene-activated matrix (GAM), in which naked plasmid DNA was physically entrapped in a polymer matrix sponge.30,31 Unfortunately, this approach suffered from a very low transduction efficiency. In recent studies investigators have utilized DNA, condensed using chemical vectors such as polyethyleneimine,32 liposomes,33 and calcium-phosphate precipitates,34 to increase the GAM's transfection efficiency and induce bone regeneration in fracture and dental models in vivo. Here as well, the nonviral approach is considered safer and may be attractive for clinical use. Yet, most biomaterials used to produce GAMs lack the mechanical properties usually required to sustain loads present in a long-bone fracture site. In this respect, structural bone grafts could provide an alternative biomaterial or scaffold with the desired mechanical properties.
Structural bone grafting is commonly used in orthopedic reconstructive surgeries such as spinal fusion, revision of failed total hip arthroplasty, or repair of osteolytic cavitary defects.35–37 Both experimental and clinical studies have shown that processed allografts fail to remodel and incorporate with host bone.38–40 Despite this, processed allografts remain the standard choice for structural bone grafting simply because of problems associated with autologous bone grafts—specifically, limitations in size and availability, chronic pain at the donor site, and complications of harvesting procedures.41,42 Unfortunately, the limited bone formation and remodeling associated with structural allografts are directly related to these allografts' 25–35% failure rate within 3 years due to infection, fracture, and nonunion.43,44 For allografts that survive longer, the failure rate at 10 years has been documented to be as high as 60%.45–47 Fractures occurring at this late stage are the result of an accumulation of microcracks that cannot be repaired by necrotic bone.
To improve these dismal results, we have set out to turn allografts into a gene delivery device. We demonstrated that recombinant adeno-associated virus (rAAV) can be freeze-dried onto any implantable surface without losing its infectivity.48 By coating allografts with rAAVs that express angiogenic, osteogenic, and osteoclastogenic genes, we were able to produce vascular ingrowth, new bone and marrow formation, and osteoclastic remodeling of necrotic cortical allografts.48,49
Experimental Animal Models for the Study of Fracture Repair
Animal models of bone fractures are useful for studying the basic science of bone healing as well as for the development of therapeutic modalities. Simple subcutaneous models are used to characterize the biological properties of different gene therapies, whereas more complex fracture models are used to simulate healing in the clinical setting. Dog, rabbit, and cat models have been used to examine repair of critical-size bone defects using various types of bone grafts.50–57 Large animal models are extremely valuable because they offer the best simulation of the clinical situation. However, these models have two major shortcomings. First, large animal models are significantly more costly—in time, money, and labor—than small animal models. As a consequence, fracture models in rodents have been the subjects of numerous studies. Second, large animal models produce few answers to questions about bone repair on molecular and cellular levels. With respect to these questions, murine models of human disease have provided remarkable information during the last decade.
Direct gene delivery for fracture repair has been demonstrated in long and flat bones of rodents. The rat femur is the foremost model used in the investigation of fracture repair.9,10,13,30 This rat model requires the use of an external fixation device to stabilize the edges of the fracture, allowing experimental animals to regain function of their hind limbs. Yet, the use of transgenic and knockout mouse models permits the direct evaluation of a single gene on a disease process; however, the femur fracture model is technically challenging when applied to a mouse. To address this problem, our laboratory focused its efforts on generating the first mouse model of fracture healing in the femur.58 Our model involves the use of an internal fixation rod that stabilizes the femur and allows placement of a biomaterial—allograft in our studies—at the site of the fracture. An additional murine model to be considered for acellular gene therapy is the radial fracture model, which we have described extensively.59–63 A nonhealing fracture is created by generating a 2.5-mm defect in the midportion of a mouse radius. The advantage of this model is that it does not require a fixation device because the ulna bone serves this purpose.
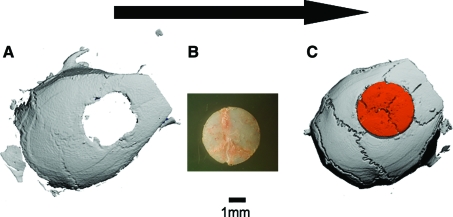
Major bone loss in craniofacial bones due to trauma, cancer, or birth defects poses another great challenge for surgical reconstruction. In experimental studies the most utilized model is a 5-mm disc-shaped defect generated in the calvaria.32,64,65 Figure 1 illustrates the salient features of this model. A calvarial defect is generated in a host mouse; the injury can be left as a nonhealing critical-size defect or repaired by gene therapy. Critical-size defects have also been created in rodents' mandibles and maxillas.66,67 However, there have been very few attempts to regenerate such defects using direct gene therapy,67 probably because of the technical challenges involved with creating a defect in a highly vascularized region covered by masticatory muscles. In this region minimal tissue damage can cause major morbidity due to loss of masticatory function.
FIG. 1.
The murine calvarial model of a critical-size bone defect implated with a recombinant adeno-associated virus (rAAV)–coated allograft. A three-dimensional reconstructed micro-computed tomography image of a murine calvarial defect is shown to illustrate the injury made in the host bone (A), which can be left untreated as a critical-size defect or be repaired a 5-mm-diameter allograft from another mouse (B). A three-dimensional reconstructed micro-computed tomography image of a calvarial defect that was implanted with a donor allograft coated with 109 rAAV-Luc particles (C). Color images available online at www.liebertonline.com/ten.
Outcome Measures for the Evaluation of Direct Gene Therapy for Fracture Healing
Bone healing is a complex process influenced by multiple variables. A single outcome measurement is not sufficient to assess bone healing and reconstitution. To address this, standard radiological, micro-computed tomography (μCT), histomorphometric, biomechanical, and molecular analyses have been developed.
X-ray–based imaging is commonly used in the clinical setting to evaluate fracture repair. Two-dimensional X-ray images have also been widely used to evaluate bone regeneration achieved using gene therapy techniques; however, these images are of low resolution and cannot be used for a quantitative analysis of bone formation. To provide high-resolution 3D images, the development of μCT systems was accelerated. State-of-the-art μCT imaging systems can generate 3D images at a resolution of 1 μm or less, and can be used to provide quantitative analyses of several parameters of bone formation, some of which are volume, mineral density, trabecular thickness, and connectivity-density. Advanced μCT scanners can even provide this information in live animals at decreased resolution. Further, new algorithms can make use of μCT data to predict the mechanical properties of bone formation68 and even to perform virtual biomechanical testing of scanned bones.69
To analyze the cellular and biochemical components of regenerated bone fractures, tissue must be excised from host animals and sectioned. Histological analyses are performed to determine what types of cells are present within the defect site, such as osteoblasts, osteoclasts, endothelial cells, and mesenchymal stem cells. Moreover, immunohistochemical analyses enable the detection of multiple proteins in the osteogenic matrix, such as collagens, osteocalcin (OC), and osteopontin. In gene therapy studies, one can use these methods to define what cells have been transfected at the target site by costaining for the transgene and specific cell types.18
Biomechanical testing is an important tool used to measure outcomes of bone regeneration. Conventional systems consist of a variety of ex vivo testing protocols for use on different types of bones; usually a controlled force is applied to the bone until the bone fails. New testing systems can be used to perform micromechanical analyses of small bone samples, thus allowing the use of rodent fracture models.70 Nanoscale mechanical testing systems, such as the nanoindenter, can be used to perform highly sensitive mechanical tests on millimeter-sized bone samples, providing the investigator with a quantitative analysis of the intrinsic material properties of regenerated bone tissue.62,63
Although μCT, histological analysis, and biomechanical testing provide excellent outcome measures of bone healing, they are mainly ex vivo assessments that restrict findings to cross-sectional data. To overcome this significant limitation, we have developed several alternatives that allow in vivo analyses of bone formation and fracture repair in the same experimental animal over a prolonged period of time. These alternatives include the use of μCT-based biomechanical analysis, mentioned earlier, and bioluminescence imaging (BLI) methods.71 Our approach relies on a transgenic mouse that expresses the firefly luciferase gene (Luc) driven by the OC promoter. In this OC-Luc murine model, transgene expression is restricted to osteoblasts that are actively laying down bone matrix.72 The BLI signal from these OC-Luc mice represents osteogenesis, and the intensity of the signal can be readily quantified in longitudinal studies.
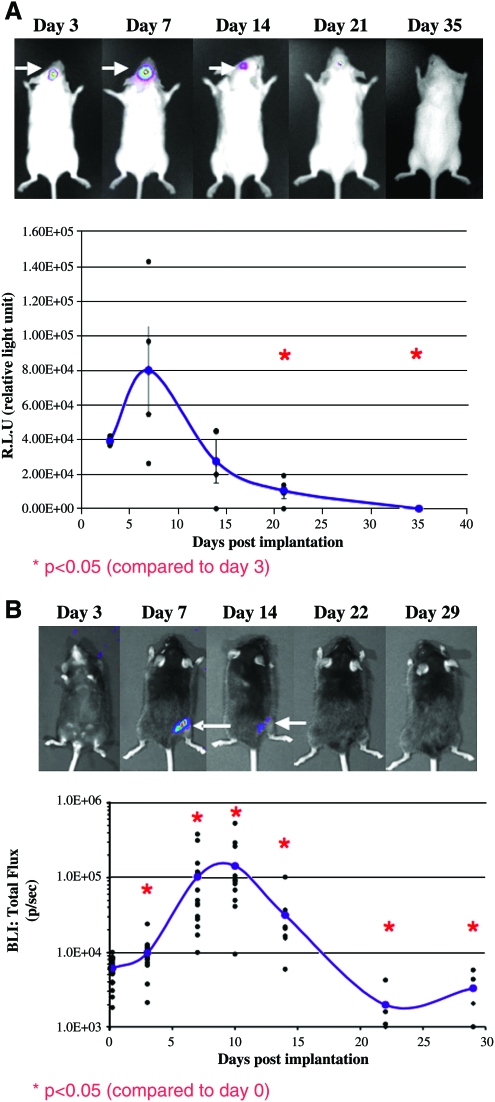
Another important obstacle that BLI overcomes is the lack of data on temporo-spatial transgene expression during gene therapy. To date, in most studies of bone gene therapy, investigators have used the invasive detection of reporter genes such as β-galactosidase (LacZ) in histological sections.20,21,49,67,73 However, this method requires the sacrifice of numerous animals and only provides nonquantitative 2D information on transgene expression. BLI, on the other hand, can provide real-time quantitative data on the expression and biodistribution of the Luc transgene, and thus can be used to evaluate the safety of the gene delivery method under investigation. For example, we used BLI in animals given rAAV-Luc–coated allografts to determine the initiation, peak, and limits of Luc transgene expression after implantation. Previously we had demonstrated that 5 × 109 particles/mm2 is the threshold dose of rAAV coating necessary to achieve peak transduction (∼4% cells proximal to the allograft), which cannot be significantly increased with higher titers.48,49,74 We therefore used this dose of rAAV-Luc to coat calvarial allografts that were later transplanted into FVB/N mice, or femoral allografts that were later transplanted into C57B/6 mice (Fig. 2). The same mice were subjected to BLI, while in an anesthetized state, at various time points during a 35-day period. A gross assessment of the BLI signal from both mouse groups revealed the following details: (i) in vivo transduction could only be observed at the surgical site; (ii) peak transduction occurred 1 week after transplantation; and (iii) transgene expression waned to undetectable levels by 3 weeks after transplantation. Quantification of the BLI signal from the surgical site confirmed that significant transgene expression was first detectable on day 3 posttransplantation.74 In both models we observed that in vivo transduction increased over the next 4 days, as a 3-fold and 10-fold increase in the BLI signal was observed from the transplanted rAAV-Luc–coated calvariae and femora, respectively. However, the BLI signal decreased thereafter, dropping to background levels by 3 weeks. Collectively, these results demonstrate that rAAV-coated allografts mediate local transient gene expression and minimize potential concerns about targeting inappropriate tissues and unregulated gene expression. Moreover, these kinetic characteristics are consistent with those from our previous studies, in which we used rAAV-LacZ–coated allografts and assessed transduction efficiency using in situ X-gal staining.48,49
FIG. 2.
Kinetic characteristics of rAAV-coated calvarial allograft transduction in vivo. Wild-type FVB/N (A) mice and C57B/6 mice (B) received rAAV-Luc–coated calvarial or femoral allografts, respectively. Longitudinal, noninvasive, bioluminescence imaging (BLI) was used to monitor luciferase expression over time in the same mice, while being under general anesthesia. A representative mouse from each group is shown to demonstrate luciferase expression at the different time points (arrows indicate the luciferase signal). Transgene expression decreased to undetectable levels by 3–4 weeks posttransplantation. Graphs show the differences in luciferase signal compared to day 3 (A) or day 0 (B) postimplantation (p < 0.05, two-tail t-test). Color images available online at www.liebertonline.com/ten.
From these kinetic findings we can infer an empirical advantage for bone repair as follows. Extensive molecular characterization studies of fracture healing have demonstrated that the host response over the 1st week is an inflammatory phase dedicated to innate immune protection and catabolism of damaged tissue.1,75 Thus, the lack of anabolic transgene expression during this period is not considered a shortcoming, as progenitor cells targeted by the trophic factors have yet to arrive at the repair site. Moreover, the peak transgene expression at 1 week is viewed as ideal to jumpstart the reparative phase of fracture healing, which is known to commence at this time.1,75 Finally, the extinguished gene expression by 3 weeks is viewed to have two empirical advantages. First, it alleviates concerns about unregulated long-term gene expression, which could lead to unwanted side effects such as heterotopic ossification and fusion of adjacent joints. Second, it allows for the final remodeling phase of fracture healing, which occurs naturally.
It is important to note that during the recent years other reporter genes have been developed that can allow long-term, real-time monitoring of gene expression. These include, among others, the human sodium iodide symporter (hNIS) and herpes simplex virus type 1 thymidine kinase (HSV-TK-1).76 The expression of these genes is monitored by nuclear imaging systems such as single-photon emission CT and positron emission tomography, which are routinely used in the clinic. The advantages of these systems lies in the ability to monitor gene expression in large animals and even in humans, detecting signals from deep tissues, due to the high sensitivity of the radiolabeled probes. However, the effect of radioactivity on bone formation is yet to be determined.
Discussion
In summary, several direct gene therapy approaches to the repair of nonhealing fractures or bone defects have been described in the literature. The use of viral vectors, primarily adenovirus encoding for BMP genes, has been shown to be effective in fracture healing; however, concerns remain regarding these vectors' safety. Other methods include electroporation and sonoporation, which are promising with regard to safety but whose efficacy in bone fracture repair still remains to be demonstrated. Collectively, viral and physical placement methods of gene delivery are attended by a difficulty in localizing the transgene to the fracture site. To overcome this hurdle, GAM systems have been developed that can be implanted in the defect site and restrict gene transfection to local cells. Because the biomaterials forming GAMs are mechanically inferior, however, we developed a method of localized gene delivery using AAV-coated allograft–mediated transduction, which has been shown to be highly effective in a murine femoral fracture model.
In this review we highlighted the importance of quantitative outcome measures to evaluate the transgene pattern of expression and biodistribution in vivo. Based on our BLI results we postulated that the kinetic characteristics of gene expression and biodistribution in the rAAV-coated allograft system are limited by several factors in vivo. The first is the time it takes for the virus to rehydrate, which is concomitant with hematoma formation around the allograft. As such, a soluble virus that is competent to infect target cells cannot be present until after an innate immune boundary is established, which effectively limits the migration of the vector to proximal tissues. Based on the well-established knowledge that the rate-limiting step of rAAV transduction is second-strand synthesis,77 which is known to occur on the order of days,77 it is not surprising that it takes a week to achieve peak transgene expression. It has also been formally established that rAAV is maintained as a replication-defective episome, which has only been shown to integrate into host chromosomes at an extremely low frequency in special cell types (i.e., polyploidy hepatocytes).78 Thus, given that the primary target cells in this gene therapy have been shown to be transitional inflammatory cells adjacent to the allograft and proliferating mesenchymal cells in the fracture callus,48,49 the most likely explanation for the loss of gene expression is that the vector is cleared via dilution and apoptosis of the target cells during tissue remodeling. Based on this mechanism of in vivo transduction, we conclude that the use of rAAV-coated allografts is a safe and effective means to achieve gene therapy for bone regeneration in humans, and that this method warrants further investigation in large animal models. Further, this freeze-dried rAAV–based method of in vivo gene delivery should achieve similar transduction efficiency, kinetic characteristics, and biodistribution when applied to any type of implantable biomaterial.
Acknowledgments
This work was supported by the following research grants from the National Institutes of Health: R21 DE017096, R01 DE019902 and P50 AR054041.
Disclosure Statement
No competing financial interests exist.
References
- 1.Einhorn T.A. The science of fracture healing. J Orthop Trauma. 2005;19:S4. doi: 10.1097/00005131-200511101-00002. [DOI] [PubMed] [Google Scholar]
- 2.Hollinger J.O. Kleinschmidt J.C. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg. 1990;1:60. doi: 10.1097/00001665-199001000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Covey D. Aaron R. Born C. Calhoun J. Einhorn T. Hayda R. Levin L. Mazurek M. Murray C. Powell E. Schwarz E. Wenke J. Orthopaedic war injuries: from combat casualty care to definitive treatment: a current review of clinical advances, basic science, and research opportunities. Instr Course Lect. 2008;57:65. [PubMed] [Google Scholar]
- 4.Covey D. Combat orthopaedics: a view from the trenches. J Am Acad Orthop Surg. 2006;14:S10. doi: 10.5435/00124635-200600001-00004. [DOI] [PubMed] [Google Scholar]
- 5.Owens B.D. Kragh J.F. Wenke J.C. Macaitis J. Wade C.E. Holcomb J.B. Combat wounds in operation Iraqi freedom and operation enduring freedom. J Trauma Inj Infect Crit Care. 2008;64:295. doi: 10.1097/TA.0b013e318163b875. [DOI] [PubMed] [Google Scholar]
- 6.Owens B.D. Kragh J.F. Macaitis J. Svoboda S.J. Wenke J.C. Characterization of extremity wounds in operation Iraqi freedom and operation enduring freedom. J Orthop Trauma. 2007;21:254. doi: 10.1097/BOT.0b013e31802f78fb. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman J.R. Ghivizzani S.C. Evans C.H. Gene transfer approaches to the healing of bone and cartilage. Mol Ther. 2002;6:141. doi: 10.1006/mthe.2000.0663. [DOI] [PubMed] [Google Scholar]
- 8.Awad H.A. Zhang X. Reynolds D.G. Guldberg R.E. O'Keefe R.J. Schwarz E.M. Recent advances in gene delivery for structural bone allografts. Tissue Eng. 2007;13:1973. doi: 10.1089/ten.2006.0107. [DOI] [PubMed] [Google Scholar]
- 9.Betz V.M. Betz O.B. Glatt V. Gerstenfeld L.C. Einhorn T.A. Bouxsein M.L. Vrahas M.S. Evans C.H. Healing of segmental bone defects by direct percutaneous gene delivery: effect of vector dose. Hum Gene Ther. 2007;18:907. doi: 10.1089/hum.2007.077. [DOI] [PubMed] [Google Scholar]
- 10.Betz O.B. Betz V.M. Nazarian A. Egermann M. Gerstenfeld L.C. Einhorn T.A. Vrahas M.S. Bouxsein M.L. Evans C.H. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther. 2007;14:1039. doi: 10.1038/sj.gt.3302956. [DOI] [PubMed] [Google Scholar]
- 11.Egermann M. Baltzer A.W. Adamaszek S. Evans C. Robbins P. Schneider E. Lill C.A. Direct adenoviral transfer of bone morphogenetic protein-2 cDNA enhances fracture healing in osteoporotic sheep. Hum Gene Ther. 2006;17:507. doi: 10.1089/hum.2006.17.507. [DOI] [PubMed] [Google Scholar]
- 12.Egermann M. Lill C.A. Griesbeck K. Evans C.H. Robbins P.D. Schneider E. Baltzer A.W. Effect of BMP-2 gene transfer on bone healing in sheep. Gene Ther. 2006;13:1290. doi: 10.1038/sj.gt.3302785. [DOI] [PubMed] [Google Scholar]
- 13.Betz O.B. Betz V.M. Nazarian A. Pilapil C.G. Vrahas M.S. Bouxsein M.L. Gerstenfeld L.C. Einhorn T.A. Evans C.H. Direct percutaneous gene delivery to enhance healing of segmental bone defects. J Bone Joint Surg Am. 2006;88A:355. doi: 10.2106/JBJS.E.00464. [DOI] [PubMed] [Google Scholar]
- 14.Southwood L.L. Frisbie D.D. Kawcak C.E. Ghivizzani S.C. Evans C.H. McIlwraith C.W. Evaluation of Ad-BMP-2 for enhancing fracture healing in an infected defect fracture rabbit model. J Orthop Res. 2004;22:66. doi: 10.1016/S0736-0266(03)00129-3. [DOI] [PubMed] [Google Scholar]
- 15.Baltzer A.W.A. Lattermann C. Whalen J.D. Wooley P. Weiss K. Grimm M. Ghivizzani S.C. Robbins P.D. Evans C.H. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7:734. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- 16.Tarkka T. Sipola A. Jämsä T. Soini Y. Ylä-Herttuala S. Tuukkanen J. Hautala T. Adenoviral VEGF-A gene transfer induces angiogenesis and promotes bone formation in healing osseous tissues. J Gene Med. 2003;5:560. doi: 10.1002/jgm.392. [DOI] [PubMed] [Google Scholar]
- 17.Raper S.E. Chirmule N. Lee F.S. Wivel N.A. Bagg A. Gao G.P. Wilson J.M. Batshaw M.L. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Sheyn D. Kimelman-Bleich N. Pelled G. Zilberman Y. Gazit D. Gazit Z. Ultrasound-based nonviral gene delivery induces bone formation in vivo. Gene Ther. 2008;15:257. doi: 10.1038/sj.gt.3303070. [DOI] [PubMed] [Google Scholar]
- 19.Osawa K. Okubo Y. Nakao K. Koyama N. Bessho K. Osteoinduction by microbubble-enhanced transcutaneous sonoporation of human bone morphogenetic protein-2. J Gene Med. 2009;11:633. doi: 10.1002/jgm.1331. [DOI] [PubMed] [Google Scholar]
- 20.Kishimoto K.N. Watanabe Y. Nakamura H. Kokubun S. Ectopic bone formation by electroporatic transfer of bone morphogenetic protein-4 gene. Bone. 2002;31:340. doi: 10.1016/s8756-3282(02)00825-6. [DOI] [PubMed] [Google Scholar]
- 21.Kawai M. Bessho K. Kaihara S. Sonobe J. Oda K. Iizuka T. Maruyama H. Ectopic bone formation by human bone morphogenetic protein-2 gene transfer to skeletal muscle using transcutaneous electroporation. Hum Gene Ther. 2003;14:1547. doi: 10.1089/104303403322495052. [DOI] [PubMed] [Google Scholar]
- 22.Kawai M. Bessho K. Maruyama H. Miyazaki J.I. Yamamoto T. Human BMP-2 gene transfer using transcutaneous in vivo electroporation induced both intramembranous and endochondral ossification. Anat Rec A Discov Mol Cell Evol Biol. 2005;287A:1264. doi: 10.1002/ar.a.20245. [DOI] [PubMed] [Google Scholar]
- 23.Kawai M. Bessho K. Maruyama H. Miyazaki J. Yamamoto T. Simultaneous gene transfer of bone morphogenetic protein (BMP)-2 and BMP-7 by in vivo electroporation induces rapid bone formation and BMP-4 expression. BMC Musculoskelet Disord. 2006;7 doi: 10.1186/1471-2474-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotajima S. Kishimoto K.N. Watanuki M. Hatori M. Kokubun S. Gene expression analysis of ectopic bone formation induced by electroporatic gene transfer of BMP4. Upsala J Med Sci. 2006;111:231. doi: 10.3109/2000-1967-044. [DOI] [PubMed] [Google Scholar]
- 25.Busse J.W. Kaur J. Mollon B. Bhandari M. Tornetta P. Schuenemann H.J. Guyatt G.H. Low intensity pulsed ultrasonography for fractures: systematic review of randomised controlled trials. Br Med J. 2009;338:b351. doi: 10.1136/bmj.b351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Sakere B. André F. Bernat C. Connault E. Opolon P. Davalos R. Rubinsky B. Mir L. Tumor ablation with irreversible electroporation. PLoS ONE. 2007;2:e1135. doi: 10.1371/journal.pone.0001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campana L.G. Mocellin S. Basso M. Puccetti O. De Salvo G.L. Chiarion-Sileni V. Vecchiato A. Corti L. Rossi C.R. Nitti D. Bleomycin-based electrochemotherapy: clinical outcome from a single institution's experience with 52 patients. Ann Surg Oncol. 2009;16:191. doi: 10.1245/s10434-008-0204-8. [DOI] [PubMed] [Google Scholar]
- 28.Daud A.I. DeConti R.C. Andrews S. Urbas P. Riker A.I. Sondak V.K. Munster P.N. Sullivan D.M. Ugen K.E. Messina J.L. Heller R. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26:5896. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granot Y. Rubinsky B. Methods of optimization of electrical impedance tomography for imaging tissue electroporation. Physiol Meas. 2007;28:1135. doi: 10.1088/0967-3334/28/10/001. [DOI] [PubMed] [Google Scholar]
- 30.Fang J.M. Zhu Y.Y. Smiley E. Bonadio J. Rouleau J.P. Goldstein S.A. McCauley L.K. Davidson B.L. Roessler B.J. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci USA. 1996;93:5753. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonadio J. Smiley E. Patil P. Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y.C. Simmons C. Kaigler D. Rice K.G. Mooney D.J. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12:418. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- 33.Lutz R. Park J. Felszeghy E. Wiltfang J. Nkenke E. Schlegel K.A. Bone regeneration after topical BMP-2-gene delivery in circumferential peri-implant bone defects. Clin Oral Implants Res. 2008;19:590. doi: 10.1111/j.1600-0501.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 34.Endo M. Kuroda S. Kondo H. Maruoka Y. Ohya K. Kasugai S. Bone regeneration by modified gene-activated matrix: effectiveness in segmental tibial defects in rats. Tissue Eng. 2006;12:489. doi: 10.1089/ten.2006.12.489. [DOI] [PubMed] [Google Scholar]
- 35.Brigman B.E. Hornicek F.J. Gebhardt M.C. Mankin H.J. Allografts about the knee in young patients with high-grade sarcoma. Clin Orthop Relat Res. 2004;421:232. doi: 10.1097/01.blo.0000127132.12576.05. [DOI] [PubMed] [Google Scholar]
- 36.Jaffe K.A. Morris S.G. Sorrell R.G. Gebhardt M.C. Mankin H.J. Massive bone allografts for traumatic skeletal defects. South Med J. 1991;84:975. doi: 10.1097/00007611-199108000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Mankin H.J. Gebhardt M.C. Tomford W.W. The use of frozen cadaveric allografts in the management of patients with bone-tumors of the extremities. Orthop Clin North Am. 1987;18:275. [PubMed] [Google Scholar]
- 38.Goldberg V. Stevenson S. The biology of bone grafts. Semin Arthroplasty. 1993;4:58. [PubMed] [Google Scholar]
- 39.Burchardt H. Glowczewskie F. Rudner C. Enneking W.F. Jones H. Freeze-dried allogeneic segmental cortical-bone grafts in dogs. J Bone Joint Surg Am. 1978;60:1082. [PubMed] [Google Scholar]
- 40.Chalmers J. Transplantation immunity in bone homografting. J Bone Joint Surg Br. 1959;41:160. doi: 10.1302/0301-620X.41B1.160. [DOI] [PubMed] [Google Scholar]
- 41.Summers B.N. Eisenstein S.M. Donor site pain from the Ilium—a complication of lumbar spine fusion. J Bone Joint Surg Br. 1989;71:677. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 42.Younger E. Chapman M. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Berrey B.H. Lord C.F. Gebhardt M.C. Mankin H.J. Fractures of allografts—frequency, treatment, and end-results. J Bone Joint Surg Am. 1990;72A:825. [PubMed] [Google Scholar]
- 44.Lord C.F. Gebhardt M.C. Tomford W.W. Mankin H.J. Infection in bone allografts—incidence, nature, and treatment. J Bone Joint Surg Am. 1988;70A:369. [PubMed] [Google Scholar]
- 45.Wheeler D.L. Enneking W.F. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005;435:36. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- 46.Enneking W.E. Campanacci D.A. Retrieved human allografts—a clinicopathological study. J Bone Joint Surg Am. 2001;83A:971. [PubMed] [Google Scholar]
- 47.Hornicek F.J. Gebhardt M.C. Tomford W.W. Sorger J.I. Zavatta M. Menzner J.P. Mankin H.J. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001;382:87. doi: 10.1097/00003086-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Ito H. Koefoed M. Tiyapatanaputi P. Gromov K. Goater J.J. Carmouche J. Zhang X. Rubery P.T. Rabinowitz J. Samulski R.J. Nakamura T. Soballe K. O'Keefe R.J. Boyce B.F. Schwarz E.M. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koefoed M. Ito H. Gromov K. Reynolds D.G. Awad H.A. Rubery P.T. Ulrich-Vinther M. Soballe K. Guldberg R.E. Lin A.S.P. O'Keefe R.J. Zhang X.P. Schwarz E.M. Biological effects of rAAV-caAlk2 coating on structural allograft healing. Mol Ther. 2005;12:212. doi: 10.1016/j.ymthe.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 50.Stevenson S. Li X.Q. Davy D.T. Klein L. Goldberg V.M. Critical biological determinants of incorporation of non-vascularized cortical bone grafts—quantification of a complex process and structure. J Bone Joint Surg Am. 1997;79A:1. [PubMed] [Google Scholar]
- 51.Lee F.Y.I. Hazan E.J. Gebhardt M.C. Mankin H.J. Experimental model for allograft incorporation and allograft fracture repair. J Orthop Res. 2000;18:303. doi: 10.1002/jor.1100180219. [DOI] [PubMed] [Google Scholar]
- 52.Henry W.B. Schachar N.S. Wadsworth P.L. Castronovo F.P. Mankin H.J. Feline model for the study of frozen osteoarticular hemijoint transplantation—qualitative and quantitative assessment of bone healing. Am J Vet Res. 1985;46:1714. [PubMed] [Google Scholar]
- 53.Aebi M. Regazzoni P. Schwarzenbach O. Segmental bone-grafting—comparison of different types of graft in dogs. Int Orthop. 1989;13:101. doi: 10.1007/BF00266370. [DOI] [PubMed] [Google Scholar]
- 54.Kohler P. Ehrnberg A. Kreicbergs A. Osteogenic enhancement of diaphyseal reconstruction—comparison of bone-grafts in the rabbit. Acta Orthop Scand. 1990;61:42. doi: 10.3109/17453679008993064. [DOI] [PubMed] [Google Scholar]
- 55.Schwarz N. Schlag G. Thurnher M. Eschberger J. Dinges H.P. Redl H. Fresh autogeneic, frozen allogeneic, and decalcified allogeneic bone-grafts in dogs. J Bone Joint Surg Br. 1991;73:787. doi: 10.1302/0301-620X.73B5.1894667. [DOI] [PubMed] [Google Scholar]
- 56.Burchardt H. Enneking W.F. Transplantation of bone. Surg Clin North Am. 1978;58:403. doi: 10.1016/s0039-6109(16)41492-1. [DOI] [PubMed] [Google Scholar]
- 57.Johnson A.L. Eurell J.A. Schaeffer D.J. Evaluation of canine cortical bone-graft remodeling. Vet Surg. 1992;21:293. doi: 10.1111/j.1532-950x.1992.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 58.Tiyapatanaputi P. Rubery P.T. Carmouche J. Schwarz E.M. O'Keefe R.J. Zhang X. A novel murine segmental femoral graft model. J Orthop Res. 2004;22:1254. doi: 10.1016/j.orthres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 59.Turgeman G. Pittman D.D. Muller R. Kurkalli B.G. Zhou S. Pelled G. Peyser A. Zilberman Y. Moutsatsos I.K. Gazit D. Engineered human mesenchymal stem cells: a novel platform for skeletal cell mediated gene therapy. J Gene Med. 2001;3:240. doi: 10.1002/1521-2254(200105/06)3:3<240::AID-JGM181>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 60.Moutsatsos I.K. Turgeman G. Zhou S. Kurkalli B.G. Pelled G. Tzur L. Kelley P. Stumm N. Mi S. Muller R. Zilberman Y. Gazit D. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol Ther. 2001;3:449. doi: 10.1006/mthe.2001.0291. [DOI] [PubMed] [Google Scholar]
- 61.Zilberman Y. Kallai I. Gafni Y. Pelled G. Kossodo S. Yared W. Gazit D. Fluorescence molecular tomography enables in vivo visualization and quantification of nonunion fracture repair induced by genetically engineered mesenchymal stem cells. J Orthop Res. 2008;26:522. doi: 10.1002/jor.20518. [DOI] [PubMed] [Google Scholar]
- 62.Pelled G. Tai K. Sheyn D. Zilberman Y. Kumbar S. Nair L.S. Laurencin C.T. Gazit D. Ortiz C. Structural and nanoindentation studies of stem cell-based tissue-engineered bone. J Biomech. 2007;40:399. doi: 10.1016/j.jbiomech.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 63.Tai K. Pelled G. Sheyn D. Bershteyn A. Han L. Kallai I. Zilberman Y. Ortiz C. Gazit D. Nanobiomechanics of repair bone regenerated by genetically modified mesenchymal stem cells. Tissue Eng A. 2008;14:1709. doi: 10.1089/ten.tea.2007.0241. [DOI] [PubMed] [Google Scholar]
- 64.Dazai S. Akita S. Hirano A. Rashid M.A. Naito S. Akino K. Fujii T. Leukemia inhibitory factor enhances bone formation in calvarial bone defect. J Craniofac Surg. 2000;11:513. doi: 10.1097/00001665-200011060-00002. [DOI] [PubMed] [Google Scholar]
- 65.Gafni Y. Pelled G. Zilberman Y. Turgeman G. Apparailly F. Yotvat H. Galun E. Gazit Z. Jorgensen C. Gazit D. Gene therapy platform for bone regeneration using an exogenously regulated, AAV-2-based gene expression system. Mol Ther. 2004;9:587. doi: 10.1016/j.ymthe.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 66.Steinhardt Y. Aslan H. Regev E. Zilberman Y. Kallai I. Gazit D. Gazit Z. Maxillofacial-derived stem cells regenerate critical mandibular bone defect. Tissue Eng A. 2008;14:1763. doi: 10.1089/ten.tea.2008.0007. [DOI] [PubMed] [Google Scholar]
- 67.Ashinoff R.L. Cetrulo C.L. Galiano R.D. Dobryansky M. Bhatt K.A. Ceradini D.J. Michaels J. McCarthy J.G. Gurtner G.C. Bone morphogenic protein-2 gene therapy for mandibular distraction osteogenesis. Ann Plast Surg. 2004;52:585. doi: 10.1097/01.sap.0000123023.28874.1e. [DOI] [PubMed] [Google Scholar]
- 68.Reynolds D.G. Hock C. Shaikh S. Jacobson J. Zhang X.P. Rubery P.T. Beck C.A. O'Keefe R.J. Lerner A.L. Schwarz E.M. Awad H.A. Micro-computed tomography prediction of biomechanical strength in murine structural bone grafts. J Biomech. 2007;40:3178. doi: 10.1016/j.jbiomech.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 69.van Lenthe G. Stauber M. Müller R. Specimen-specific beam models for fast and accurate prediction of human trabecular bone mechanical properties. Bone. 2006;39:1182. doi: 10.1016/j.bone.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 70.Xie C. Reynolds D. Awad H. Rubery P.T. Pelled G. Gazit D. Guldberg R.E. Schwarz E.M. O'Keefe R.J. Zhang X.P. Structural bone allograft combined with genetically engineered mesenchymal stem cells as a novel platform for bone tissue engineering. Tissue Eng. 2007;13:435. doi: 10.1089/ten.2006.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zilberman Y. Gafni Y. Pelled G. Gazit Z. Gazit D. Bioluminescent imaging in bone. Methods Mol Biol. 2008;455:261. doi: 10.1007/978-1-59745-104-8_18. [DOI] [PubMed] [Google Scholar]
- 72.Bar I. Zilberman Y. Zeira E. Galun E. Honigman A. Turgeman G. Clemens T. Gazit Z. Gazit D. Molecular imaging of the skeleton: quantitative real-time bioluminescence monitoring gene expression in bone repair and development. J Bone Miner Res. 2003;18:570. doi: 10.1359/jbmr.2003.18.3.570. [DOI] [PubMed] [Google Scholar]
- 73.Sonobe J. Okubo Y. Kaihara S. Miyatake S.I. Bessho K. Osteoinduction by bone morphogenetic protein 2-expressing adenoviral vector: application of biomaterial to mask the host immune response. Hum Gene Ther. 2004;15:659. doi: 10.1089/1043034041361208. [DOI] [PubMed] [Google Scholar]
- 74.Yazici C. Yanoso L. Xie C. Reynolds D.G. Samulski R.J. Samulski J. Yannariello-Brown J. Gertzman A.A. Zhang X. Awad H.A. Schwarz E.M. The effect of surface demineralization of cortical bone allograft on the properties of recombinant adeno-associated virus coatings. Biomaterials. 2008;29:3882. doi: 10.1016/j.biomaterials.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Einhorn T. The cell and molecular biology of fracture healing. Clin Orthop. 1998;355 (Suppl):S7. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 76.Serganova I. Ponomarev V. Blasberg R. Human reporter genes: potential use in clinical studies. Nucl Med Biol. 2007;34:791. doi: 10.1016/j.nucmedbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 77.Ferrari F.K. Samulski T. Shenk T. Samulski R.J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Russell D.W. AAV vectors, insertional mutagenesis, and cancer. Mol Ther. 2007;15:1740. doi: 10.1038/sj.mt.6300299. [DOI] [PubMed] [Google Scholar]