Abstract
Rationale
Relapse is one of the main challenges facing the current treatment of cocaine addiction. Understanding its neurobiological mechanism is a critical step toward developing effective anti-relapse therapies.
Objectives
Emerging evidence indicates that glutamate-mediated activation of dopamine (DA) neurons in the ventral tegmental area (VTA) may be critically involved in cocaine-induced relapse to drug-seeking behavior. Activity of VTA DA neurons is modulated by multiple neurotransmitter systems including opioids, serotonin, dopamine, and acetylcholine. Recent studies demonstrated that activation of κ-opioid receptors (κORs) in the rat VTA directly inhibits the activity of a subpopulation of DA neurons projecting to the prefrontal cortex (PFC) and amygdala. Because we previously showed that blockade of DA receptors in the dorsal PFC inhibits cocaine-induced reinstatement of extinguished cocaine-seeking behavior suggesting a critical role of the VTA–PFC DA circuit in this process, we tested the hypothesis that activation of κORs in the VTA will block cocaine-induced reinstatement in rats.
Methods
Rats were trained to self-administer intravenous cocaine (0.125 mg/infusion) under a modified fixed-ratio five schedule. After extinction of the learned behavior, the effects of activation of VTA κORs on cocaine-induced reinstatement were studied.
Results
The κOR agonist U50 488 (0–5.6 μg/side) microinjected into the VTA dose-dependently decreased cocaine-induced reinstatement. The effects could not be explained by either a disruption of operant behavior or diffusion of the drug to the areas surrounding the VTA. Moreover, the effect was reversed by norbinaltorphimine.
Conclusions
The VTA DA neurons expressing functional κORs are critically involved in cocaine-induced reinstatement in rats.
Keywords: Cocaine, Reinstatement, κ-Opioid receptors, Dopamine, Ventral tegmental area, Prefrontal cortex, Amygdala
Introduction
A high rate of relapse to cocaine-seeking behavior is a hallmark of cocaine addiction. Given that relapse can occur even after a long period of abstinence, it is hypothesized that such a maladaptive behavior is due to persistent neuroplastic changes in the brain induced by chronic cocaine abuse. Indeed, dopamine (DA) neurons in the ventral tegmental area (VTA) have emerged as an important target for cocaine-induced neuroplasticity. For example, Bonci et al. (Chen et al. 2008) recently showed that cocaine-induced long-term potentiation (LTP) at excitatory synapses onto VTA DA neurons in rats lasts at least 3 months after a 14-day cocaine self-administration (SA). Although food and sucrose SA also induces LTP in DA neurons, the LTP disappears within 21 days suggesting that the long-lasting LTP is a unique effect of cocaine SA. Given the important role of VTA DA neurons in reward, motivation, and learning (Koob and Le Moal 1997; Schultz 2002; Robinson and Berridge 2003), it is hypothesized that such a long-lasting LTP may be an important mechanism involved in the vulnerability to relapse (Wolf 2003; Kauer 2004). Relapse can be triggered by several factors including drugs, drug-associated environmental stimuli, and stress (Stewart 2003; Kalivas et al. 2005; See 2005). There is evidence that the VTA may be critically involved in cocaine-induced relapse to drug-seeking behavior. For example, reversible inactivation of VTA DA neurons with a mixture of GABAa and GABAb agonists inhibits cocaine-induced reinstatement of extinguished cocaine-seeking behavior (McFarland and Kalivas 2001). We recently showed that blockade of ionotropic glutamate receptors (iGluRs) in the VTA also blocks cocaine-induced reinstatement (Sun et al. 2005). Together, these data suggest that glutamate-mediated activation of DA neurons in the VTA and subsequent increases in DA input to DA neuron-projected regions are critically involved in cocaine-induced reinstatement. Thus, development of long-lasting LTP at excitatory synapses onto VTA DA neurons could be a critical mechanism underlying the persistent nature of relapse.
Note that besides GABA and glutamate, several other neurotransmitters/modulators such as opioids, serotonin, dopamine, and acetylcholine also modulate the activity of VTA DA neurons (White 1996). For example, activation of μ opioid receptors (μORs) in the VTA increases the activity of DA neurons, and this effect is believed to be mediated by inhibition of local GABA release and consequent disinhibition of DA neurons (Johnson and North 1992). Thus, cocaine-induced reinstatement could be changed by manipulating these neuromodulators. Indeed, activation of VTA μORs by morphine reinstates cocaine-seeking behavior (Stewart 1984) presumably due to μOR-mediated disinhibition of VTA DA neurons. Recently, it has been shown that activation of κ-opioid receptors (κORs) in the VTA of rats inhibits the activity of a subpopulation of DA neurons projecting to the prefrontal cortex (PFC) and amygdala without affecting the GABAergic interneurons suggesting a direct effect on DA neurons (Margolis et al. 2006; Margolis et al. 2008). This idea is further supported by the fact that inhibition can be induced in the presence of tetrodotoxin, a Na+ channel blocker that blocks evoked activity from the local circuit. Consistent with these data, intra-VTA administration of U69 593, a selective κOR agonist, decreases the extracellular level of DA in the PFC but not the nucleus accumbens (NAcc), and such an effect is blocked by a specific κOR antagonist norbinaltorphimine (Margolis et al. 2006). Because we previously demonstrated that blockade of DA receptors in the dorsal PFC (dPFC) dose-dependently decreases cocaine-induced reinstatement (Sun and Rebec 2005) suggesting that the VTA–dPFC DA circuit is critically involved in this process, we predict that activation of VTA κORs in rats will also inhibit cocaine-induced reinstatement. To this end, this study aimed to investigate the effects of bilateral microinjection of U50 488, a selective κOR agonist, into the VTA of rats on cocaine-induced reinstatement of extinguished cocaine-seeking behavior.
Materials and methods
Subjects
Male Sprague–Dawley rats (320–380 g) purchased from Harlan Industries (Indianapolis, IN) were housed individually in plastic home cages in a temperature- and humidity-controlled colony room on a 12-h reverse light–dark cycle (lights off at 08:00). One week before operant training, rats were placed on a restricted diet to reach 85–90% free-feeding weight. After training, free access to food was available for 1 week before and after surgery. Food restriction was then reinstated to maintain 85–90% of free-feeding weight throughout the experiments. Water was always available except during the experimental sessions. The experiments were conducted during the dark cycle (between 09:00 and 18:00). The principles of laboratory animal care were followed, and all procedures followed the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Tennessee Health Science Center Animal Care and Use Committee.
Operant training
Rats were first trained to press a lever for food pellets with a fixed ratio 1 (FR 1) schedule in a standard operant chamber. Each lever press was reinforced by one 45 mg food pellet (Research Diet, New Brunswick, NJ) followed by a 5 s timeout during which lever presses had no programmed consequences. After rats earned 60 pellets within 30 min under FR1, the schedule increased to FR 3 and then to FR 5. The training continued until the rats obtained 60 pellets within 30 min under FR 5.
Surgery
Rats were anesthetized with intramuscular injection of a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). Details of catheterization of the jugular vein are described elsewhere (Caine et al. 1999; Sun and Rebec 2003). After catheterization, rats were fixed in a stereotaxic apparatus. Two guide cannulas (26 ga, Plastics One, Roanoke, VA) were bilaterally implanted 2 mm above the VTA with the guide cannulas angled 10° toward the midline (VTA: AP: −5.6 mm, ML: ±0.3 mm, and DV: −8.0 mm, relative to bregma, midline, and skull surface, respectively). For the anatomical control group, the cannulas were implanted 3.5 mm above the VTA with the same angle as above. Four stainless steel screws were implanted in the skull for support. The cannulas and screws were held in place with dental cement. An obturator (Plastics One, Roanoke, VA) was inserted into each guide cannula to prevent blockage. For food SA groups, the surgical procedure was the same except that rats were not catheterized. After surgery, all rats were allowed to recover for 1 week during which 0.1 ml of the antibiotic gentamicin (10 mg/ml, Biowhitaker, Walkersville, MD) was injected through the catheter daily, and the catheter was flushed twice a day with heparinized physiological saline (30 U/ml). Catheter patency was evaluated by injecting 0.1 ml Brevital (1%) through the catheter as necessary. Loss of muscle tone within 5 s after injection indicates a patent catheter.
Cocaine and food SA training
For cocaine SA training, rats were trained to press a lever to self-administer intravenous (i.v.) cocaine in a daily 2-h session contingent upon a modified FR5 (mFR5) schedule. The mFR5 schedule is the same as the FR5 schedule, except that the first reinforcement is delivered after the first response on the active lever. Cocaine (0.125 mg) was infused in a volume of 0.05 ml over a 1 s period accompanied by a compound conditioned stimulus (CS), which consisted of two flashing cue lights and a tone for 20 s; during the 20 s period (timeout), responding was recorded but had no programmed consequences. The active lever was randomly assigned to either the left or right lever and counterbalanced among rats. The session ended when 2 h passed, or 60 infusions were delivered, whichever occurred first. Rats were trained for 5–6 days/week until they reached the training criterion (the number of cocaine infusions varied by <20% in three consecutive training sessions). Note that rats were trained to self-administer food pellets before they were trained to self-administer cocaine. The main reason for this arrangement was that the pre-training facilitates the cocaine SA training. One common concern with i.v. drug SA is that the catheter may fail (leak or blockade) within several weeks after catheterization. Thus, the pre-training will shorten the time to establish stable SA behavior and minimize the catheter issue.
For food SA training, rats were trained under the mFR 5 schedule. After each food pellet, there was also a 20-s timeout during which both levers were retracted. This arrangement was aimed to simulate the procedure of cocaine SA in which no cocaine was available during the timeout period. The session ended when 30 min passed or 60 pellets were delivered, whichever occurred first. Rats were trained for 5 days/week, and the training continued until the response rate varied by <10% in three consecutive training sessions.
Extinction
Extinction training was conducted in 60 min daily sessions for both food and cocaine groups. During the session, responding was recorded but had no programmed consequences. Extinction training continued until the response rate (responses/h) fell below 20% of the rate during the last cocaine SA session for cocaine groups or below 10% of the rate during the last food SA session for food groups.
Reinstatement
For cocaine-induced reinstatement, rats received an intraperitoneal (i.p.) injection of 10 mg/kg cocaine immediately before the 1 h testing session. Responding on either lever had no consequences. For food-induced reinstatement, the 1 h session started with non-contingent delivery of five trains of food pellets every 15 s. Each train consisted of 3 food pellets spaced 4 s apart. Thereafter, the same five trains was delivered every 30 s, 60 s, and 120 s. Thus, a total of 60 pellets were delivered. Responding had no programmed consequences during the session. This reinstatement procedure aimed to generate levels of reinstatement similar to those seen during cocaine-induced reinstatement.
Microinjections and testing procedures
On test days, the obturator was removed from the guide cannula. The drug was bilaterally microinjected into the brain regions in a volume of 0.3 μl through a 33 ga injection cannula (Plastics One, Roanoke, VA), which extended 2 mm below the guide cannula. The volume was delivered over a 1 min period, and the injection cannula stayed in place for another minute to ensure drug diffusion. After the microinjection, the injection cannula was removed, and the obturator was inserted back into the guide cannula.
Two groups of rats were used to study the dose–response effects of U50 488 in the VTA on cocaine and food SA, respectively. Rats received four different doses of U50 488 (0, 1, 3.2, and 5.6 μg/side) before the testing session based on a within-subject design. Immediately after the microinjection, rats were placed in the chamber and the session started 5 min later. The testing order for the doses was randomized and counterbalanced among rats. The schedule of reinforcement during the testing session was the exact same as during the SA training session. Testing was typically conducted on Tuesdays and Fridays, and SA training continued between the tests until the response rate returned to the pre-test level. It usually took ∼2 days.
Similarly, a within-subject design was used to study the dose–response effects of U50 488 on cocaine-induced reinstatement in a separate group of rats. After the SA training, rats received extinction training until their responding reached the extinction criteria. The reinstatement session was conducted the next day. Before the test session, rats received bilateral microinjections of U50 488 and thereafter, an i.p. injection of 10 mg/kg cocaine. Immediately after the injections, rats were placed in the chamber, and the session started 5 min later. Similarly, the testing order for the doses was randomized and counterbalanced among rats to minimize potential test order effects. To determine whether the effects of U50 488 microinjected into the VTA were due to diffusion of the drug to the area dorsal to the VTA where the drug molecules are most likely to diffuse, the effects of U50 488 directly microinjected 1.5 mm above the VTA were also determined in a separate group of rats. For this experiment, only the drug vehicle and the minimum effective dose of U50 488 (3.2 μg/side) that inhibited cocaine-induced reinstatement were tested. To determine whether the effects were specific to reinstatement of cocaine-seeking behavior, the effects of the minimum effective dose on food-induced reinstatement of extinguished food-seeking behavior were also studied in a separate group of rats.
To determine whether the effect of U50 488 was mediated by κORs, we studied whether norbinaltorphimine (BNI), a selective κOR antagonist, can reverse the effect of U50 488 in a separate group of rats using a within-subject design. To this end, BNI of 3.0 μg/side was microinjected into the VTA the day before the reinstatement session. The next day, U50 488 of 3.2 μg/side was microinjected into the VTA immediately followed by i.p injection of 10 mg/kg of cocaine. The dose choice for BNI was based on our pilot study that 1 μg/side failed to reverse the effect of the dose 3.2 μg/side of U50 488. BNI was microinjected the day before the reinstatement session because of the well known fact that this drug has a slow onset and long duration of effect (Carey and Bergman 2001). In addition to the drug–drug interaction study, cocaine-induced reinstatement was also tested after microinjection of drug vehicle into the VTA in the same group of rats. The order of the two tests was counterbalanced among rats.
Histology
After the experiments, rats were anesthetized with ketamine (100 mg/kg, i.p.) and transcardially perfused with physiological saline followed by formalin (5%). The brains were removed, soaked in formalin for at least 24 h, and sliced at 100 μm thickness with a vibratome. The sections were mounted on gelatin-coated slides and stained with cresyl violet. The positions of the microinjection cannula were inspected under a light microscope.
Drugs
U50 488 (trans-(±)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]benzeneacetamide methanesulfonate) and norbinaltorphimine dihydrochloride (17,17′-(dicyclopropyl-methyl)-6,6′,7,7′-6,6′-imino-7,7′-binorphinan-3,4′,14,14′-tetrol dihydrochloride) were purchased from Sigma (St Louis, MO) and dissolved in physiological saline to prepare different concentrations (0–18.6 and 10 mg/ml, salt, respectively). The pH of the solutions was adjusted to ∼7.0 with sodium hydroxide. Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (Bethesda, MD) and was dissolved in physiological saline to prepare a solution with a concentration of 2.5 mg/ml (salt).
Statistics
Responses (lever presses) were recorded during the SA, extinction, and reinstatement sessions. Control data were obtained from the training sessions before the test sessions. A repeated one-way ANOVA was used for analyzing dose-dependent effects, and the effects of different doses were compared with Bonferroni's post tests. The significance level was set at 0.05.
Results
Histology
The positions of all microinjection sites are shown schematically in Fig. 1. All placements were within the VTA or the area dorsal to the VTA.
Fig. 1.
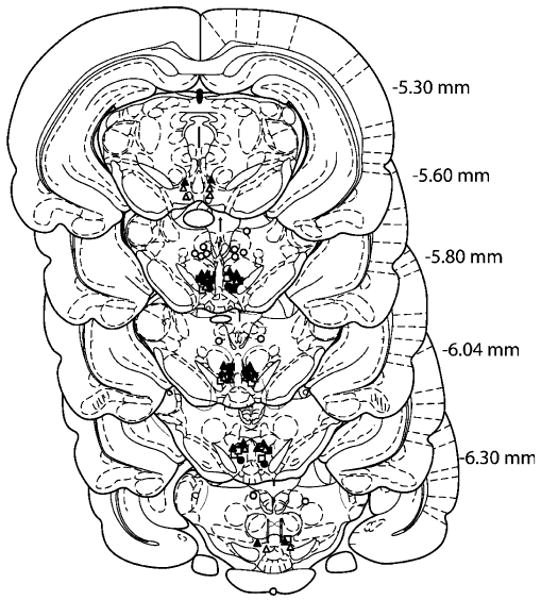
Schematic depiction of microinjection sites in the VTA and the area dorsal to the VTA. Coronal brain section images were adapted from the atlas of Paxinos and Watson (1998). Open triangles, circles, and filled circles represent the microinjection sites in cocaine SA (VTA), cocaine reinstatement (dorsal to the VTA), and cocaine reinstatement (VTA) groups, respectively. The filled triangles represent the microinjection sites in the drug–drug interaction group. Open and filled squares represent the microinjection sites in food reinstatement (VTA) and food SA (VTA) groups, respectively
Experiment 1. Effects of U50 488 in the VTA on cocaine SA
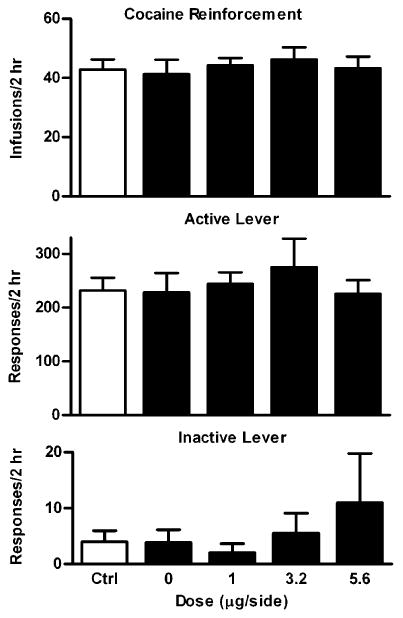
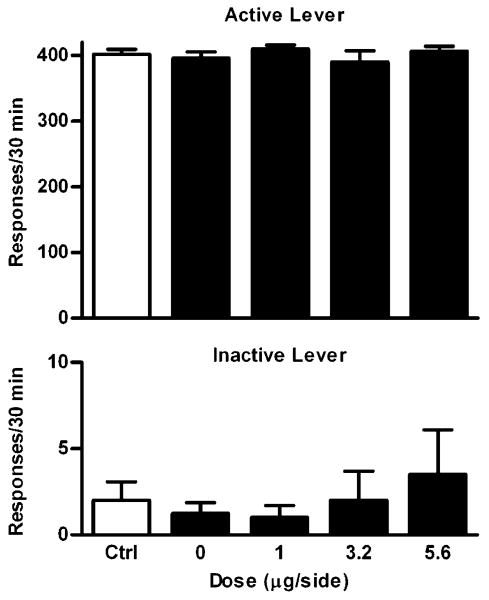
Rats (n=7) reached the SA training criteria on an average of 12.5 days (range, 10–15). The average number of infusions on the last SA session was 45±8. As shown in Fig. 2, a repeated one-way ANOVA revealed that U50 488 had no significant effects on the total number of infusions (F4,24=0.76, P=0.56), total responses on the active (F4,24= 1.45, P=0.25) or inactive lever (F4,24=0.87, P=0.49) at all the doses tested.
Fig. 2.
Dose–response effects of intra-VTA U50 488 on cocaine SA maintained under the mFR-5 schedule. Data are presented as mean ± SEM. Ctrl indicates the averaged data from the cocaine SA training sessions before the test sessions. At 0 μg/side, drug vehicle (saline) was given in a volume of 0.3 μl/side. Upper panel: Effects on the number of cocaine infusions. Middle panel: Effects on responding on the active lever. Lower panel: Effects on responding on the inactive lever. No significant effects were observed
Experiment 2. Effects of U50 488 in the VTA on cocaine-induced reinstatement of extinguished cocaine-seeking behavior
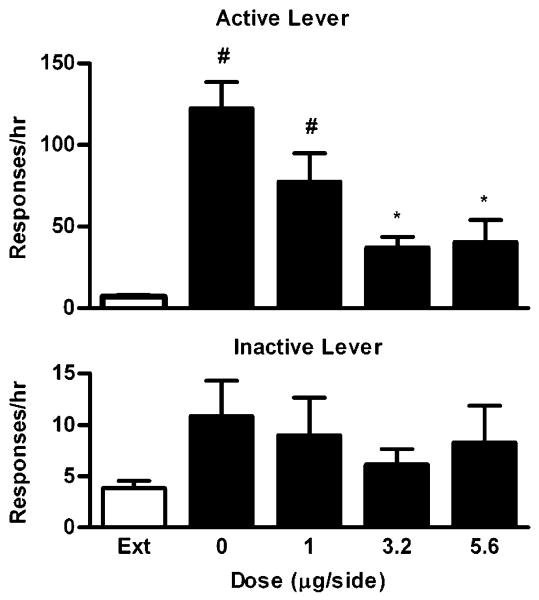
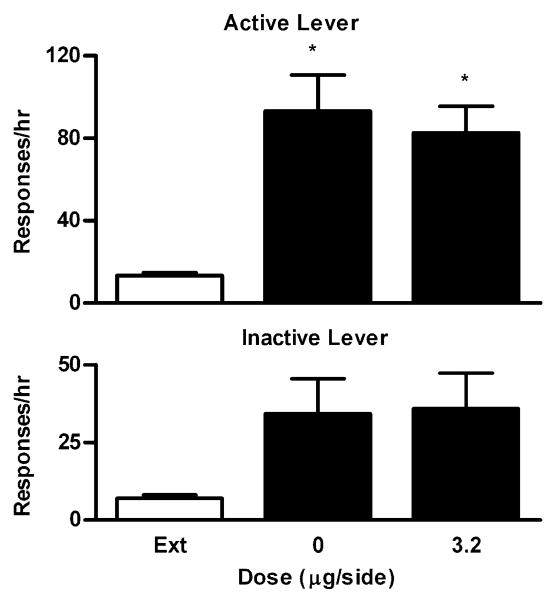
The effects of U50 488 on cocaine-induced reinstatement were studied in a group of rats (n=7) who were trained to self-administer cocaine just as in Experiment 1. The average number of infusions during the last SA session was 41 ± 10. Then rats went through extinction training until they met the criteria (range, 5–11 days). Reinstatement testing began the next day. As shown in Fig. 3, a repeated, one-way ANOVA revealed that U50 488 dose-dependently decreased responding on the active lever (F4,24=13.44, P< 0.0001), an indicator of reinstated cocaine-seeking behavior. Specifically, U50 488 significantly decreased cocaine-seeking behavior at the doses of 3.2 or 5.6 μg/side (Bonferroni's tests, P<0.001) but not at 1 μg/side (Bonferroni's tests, P>0.05) compared with vehicle (0 μg/side). At 3.2 or 5.6 μg/side, the levels of responding on the active lever were no longer different from the extinction level (Bonferroni's tests, P> 0.05). In contrast, U50 488 had no significant effects on responding on the inactive lever (F4,24=1.03, P=0.41).
Fig. 3.
Dose–response effects of intra-VTA U50 488 on cocaine-induced reinstatement of extinguished cocaine-seeking behavior. Data are presented as mean ± SEM. Ext indicates the averaged data from the extinction sessions before the reinstatement sessions. Upper panel: Effects on responding on the active lever. Lower panel: Effects on responding on the inactive lever. * and # indicate significant differences from 0 μg/side and Ext, respectively
Experiment 3. Effects of BNI on U50 488-induced inhibition of cocaine-induced reinstatement
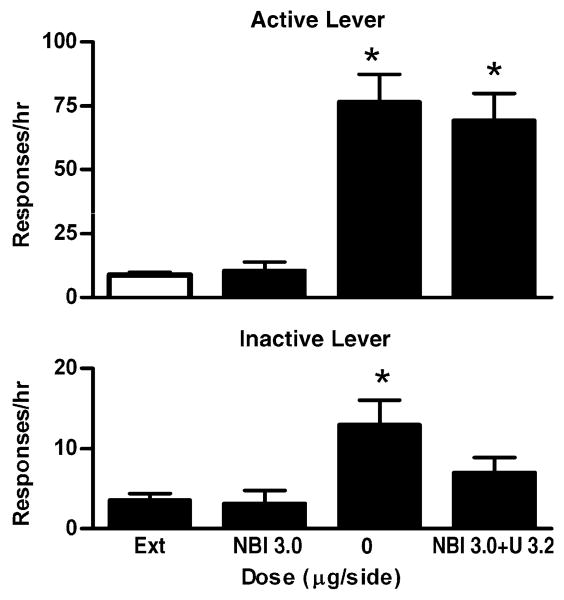
To determine whether the inhibitory effects of U50 488 on cocaine-induced reinstatement were mediated by κORs in the VTA, we tested whether the specific κOR antagonist BNI can reverse such effects in a separate group of rat (n=7). As shown in Fig. 4, cocaine significantly reinstated extinguished drug-seeking behavior when drug vehicle was microinjected into the VTA (F3,18=24.94.19, P<0.05, Bonferroni's tests, P<0.001). BNI alone (3.0 μg/side) microinjected into the VTA did not affect drug-seeking behavior under the extinction condition. In addition, after microinjection of BNI into the VTA, the minimum effective dose of U50 488 (3.2 μg/side) that inhibited cocaine-induced reinstatement no longer had such effects (compared with drug vehicle, Bonferroni's tests, P>0.05). There was a moderate increase in responding on the inactive lever induced by cocaine priming after drug vehicle microinjected into the VTA (compared with the extinction level, Bonferroni's tests P<0.05). Such an increase was not observed after BNI and U50 488 microinjected into the VTA.
Fig. 4.
Effects of BNI on U50 488-mediated inhibition of cocaine-induced reinstatement of extinguished cocaine-seeking behavior. Data are presented as mean ± SEM. Ext indicates the averaged data from the extinction sessions before the reinstatement sessions. BNI indicates the extinction session immediately after microinjection of BNI into the VTA. BNI3.0+U3.2 indicates the cocaine-induced reinstatement session after BNI and U50 488 were microinjected into the VTA (BNI was microinjected the day before microinjection of U50 488). Upper panel: Effects on responding on the active lever. Lower panel: Effects on responding on the inactive lever. * indicates significant differences from Ext
Experiment 4. Effects of U50 488 in the area dorsal to the VTA on cocaine-induced reinstatement
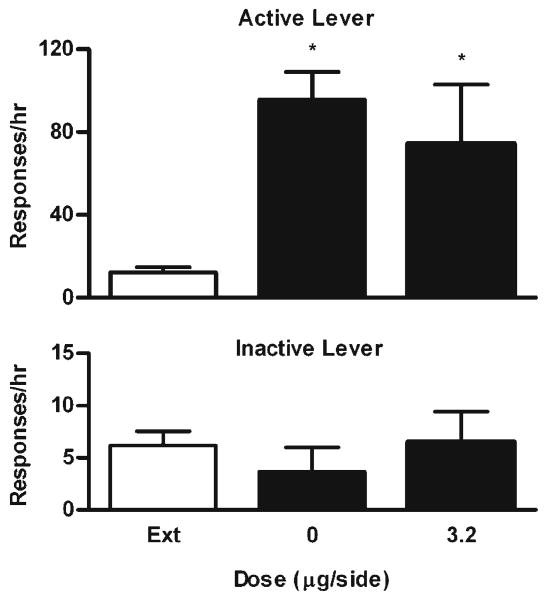
To determine whether the effects of U50 488 were due to diffusion of the drug into the neighboring regions, the effects of the drug microinjected into an area 1.5 mm dorsal to the VTA on cocaine-induced reinstatement were studied in a separate group of rats (n=6). The minimum effective dose of U50 488 was tested in this experiment. As shown in Fig. 5, cocaine significantly reinstated cocaine-seeking behavior compared with the extinction level (F2,10=7.19, P<0.05, Bonferroni's tests, P<0.01 and P<0.05). There were no significant differences in the effects between vehicle and 3.2 μg/side (Bonferroni's tests, P>0.05). In addition, U50 488 had no effects on responding on the inactive lever (F2,10=0.82, P=0.47).
Fig. 5.
Effects of U50 488 in the area dorsal to the VTA on cocaine-induced reinstatement of extinguished cocaine-seeking behavior. Data are presented as mean ± SEM. Ext indicates the averaged data from the extinction sessions before the reinstatement sessions. Upper panel: Effects on responding on the active lever. Lower panel: Effects on responding on the inactive lever. * indicates significant differences from Ext
Experiment 5. Effects of U50 488 in the VTA on food SA
To determine whether the effects of U50 488 were due to a general disruption of operant behavior, we studied the effects of the drug in the VTA on operant behavior maintained by food reinforcement. A group of rats (n=4) were trained to self-administer food pellets under the mFR5 schedule until they met the criteria (range, 6–11 days). The dose–response effects were studied as in Experiment 1. At all doses tested, rats earned 60 pellets, the maximum number allowed in the half hour sessions. As shown in Fig. 6, a repeated one-way ANOVA revealed that U50 488 had no significant effects on responding on the active (F4,12=1.77, P=0.20) or inactive levers (F4,12=0.59, P=0.67).
Fig. 6.
Dose–response effects of intra-VTA U50 488 on food SA maintained under the mFR5 schedule. Data are presented as mean ± SEM. Ctrl indicates the averaged data from the food SA training sessions before the test sessions. Upper panel: Effects on responding on the active lever. Lower panel: Effects on responding on the inactive lever. The drug had no effects on food SA
Experiment 6. Effects of U50 488 in the VTA on food-induced reinstatement of extinguished food-seeking behavior
To determine whether the effects of U50 488 were specific to cocaine-seeking behavior, food-induced reinstatement of food-seeking behavior was studied in a separate group of rats (n=7). Similarly, only the minimum effective dose of U50 488 was chosen for this experiment. As shown in Fig. 7, a repeated one-way ANOVA revealed a significant treatment effect (F2,12=14.8, P<0.001). Bonferroni's tests revealed that responding on the active lever was significantly higher at 3.2 and 0 μg/side (drug vehicle) than the extinction level (P<0.01 and P< 0.001, respectively). However, there were no significant differences between the two doses (Bonferroni's tests, P>0.05). Interestingly, there was a significant treatment effect on responding on the inactive lever (F2,12=4.67, P<0.05). However, the Bonferroni's post test did not reveal any significant differences between the pairs.
Fig. 7.
Effects of intra-VTA U50 488 on food-induced reinstatement of extinguished food-seeking behavior. Data are presented as mean ± SEM. Ext indicates the averaged data from the extinction sessions before the reinstatement sessions. Upper panel: Effects on responding on the active lever. Lower panel: Effects on responding on the inactive lever. * indicates significant differences from Ext
Discussion
Our data demonstrated that activation of VTA κORs dose-dependently inhibited cocaine-induced reinstatement of extinguished cocaine-seeking behavior in rats. The effects cannot be attributed to disruption of the ability of rats to execute the operant behavior required by the task because the same treatment did not change operant behavior during food or cocaine SA training. Neither can the effects be ascribed to diffusion of the drug to areas surrounding the VTA because an effective dose of U50 488 in the VTA, when microinjected into an area dorsal to the VTA where the drug molecules are most likely to diffuse, failed to inhibit cocaine-induced reinstatement. In addition, the effects of U50 488 in the VTA appeared to be specific to reinstatement of cocaine-seeking behavior because intra-VTA administration of U50 488 had no effects on reinstatement of food-seeking behavior.
Consistent with our results, systemic administration of a selective κOR agonist U69 593 blocks cocaine-induced reinstatement in rats (Schenk et al. 1999). Interestingly, this study also showed that U69 593 decreases cocaine SA maintained within a certain dose range. However, the effects disappear at high cocaine training doses. Thus, the effects of κOR agonists on cocaine SA depend on the training dose. Lack of effects of U50 488 on cocaine SA in the present study is consistent with the fact that our cocaine training dose is within the high dose range (0.25–1 mg/kg/infusion). Based on these observations, we predict that κOR antagonists will either have no effect or will enhance cocaine-induced reinstatement. Consistent with this prediction, intragastric administration of JDtic, a selective κOR antagonist, did not have effect on cocaine-induced reinstatement in rats (Beardsley et al. 2005). In fact, there is a trend toward increases after 30 mg/kg of JDtic compared with drug vehicle (∼100 vs ∼60 responses) although the difference was not statistically significant. Given the fact that the response rate is already high after cocaine priming, the apparent lack of enhancement of cocaine-induced reinstatement by JDtic could be due to a ceiling effect. Alternatively, there may not be much κOR-mediated inhibitory tone on DA neurons during cocaine-induced reinstatement. Thus, blockade of κORs in the VTAwould not facilitate activation of DA neurons or consequently, enhance cocaine-induced reinstatement. Consistent with this idea, the present study showed that BNI microinjected into the VTA alone did not increase cocaine-seeking behavior under the extinction condition, suggesting that there was no significant κOR-mediated inhibitory tone on DA neurons in the VTA.
Interestingly, systemic administration of κOR agonists in the absence of cocaine has been shown to reinstate extinguished cocaine-seeking behavior. For example, systemic administration of the selective κOR agonists spiradoline or enadoline reinstates extinguished cocaine-seeking behavior in squirrel monkeys (Valdez et al. 2007). Such effects, however, are not mediated by κORs but by the corticotropin-releasing factor (CRF) receptors because the effects are not reversed by selective κOR antagonists but by CRF1 antagonists. These data suggest that systemic administration of κOR agonists may engage different systems to regulate cocaine-seeking behavior dependent upon the presence or absence of cocaine.
There is evidence that different subregions of the VTA are differentially involved in the behavioral effects of cocaine. For example, rats readily self-administer cocaine into the caudal but not the rostral region of the VTA (Rodd et al. 2005). Overexpression of CREB (cAMP response element-binding protein) in the rostral region increases the rewarding effects of cocaine measured in the conditioned place preference procedure whereas overexpression of a dominant-negative form of CREB makes cocaine aversive (Olson et al. 2005). To the best of our knowledge, we are not aware of any studies that have investigated the differential roles of these two regions in cocaine-induced reinstatement. Most of our microinjection sites are within the caudal region (5.6–6.3 mm posterior to Bregma) (Olson et al. 2005; Rodd et al. 2005). Given the fact that the two subregions are very close to each other and the possibility that the drug may diffuse from the microinjection sites to the surrounding area, we cannot definitively draw the conclusion that the κORs in the caudal region are critical to the observed effects here. Further studies into this issue are warranted.
It is known that κOR agonists in the VTA induce aversive effects (Bals-Kubik et al. 1993). Thus, there is concern that such aversive effects could generally decrease motivated behavior and contribute to the inhibitory effect of U50 488 on cocaine-induced reinstatement. It should be noted that the aversive effects are typically observed in drug naïve animals (Shippenberg et al. 2007). Thus, it is unclear whether κOR agonists in the VTA produce aversive effects in rats with a history of cocaine SA. In addition, if the aversive effects are responsible, U50 488 should also inhibit food-induced reinstatement. This prediction is in contrast to our observation. The fact that U50 488 failed to change either cocaine or food SA further argues against this explanation.
DA neurons in the VTA project to several cortical and limbic regions such as the PFC, amygdala, and NAcc. In rats, κORs are functionally expressed on PFC- and amygdala- but not NAcc-projecting DA neurons in the VTA (Margolis et al. 2006; Margolis et al. 2008). Thus, the effects of U50 488 on cocaine-induced reinstatement could be due to selective inhibition of the VTA–PFC or VTA–amygdala DA circuits or both. Indeed, the PFC, in particular, the dPFC including anterior cingulate area 1 and dorsal part of the prelimbic cortex, plays a critical role in cocaine-induced reinstatement of extinguished cocaine-seeking behavior. For example, microinjections of cocaine directly into the PFC including portions of the dPFC reinstate cocaine-seeking behavior (Park et al. 2002). In addition, reversible inactivation of the dPFC inhibits cocaine-induced reinstatement (McFarland and Kalivas 2001; Capriles et al. 2003; McLaughlin and See 2003). There is evidence that DA signaling in the dPFC plays a critical role in cocaine-induced reinstatement. For example, microinjection of DA into the dPFC reinstates cocaine-seeking behavior (McFarland and Kalivas 2001). Conversely, we recently demonstrated that blockade of D1-like receptors in the dPFC specifically reduces cocaine-induced reinstatement without affecting food-induced reinstatement of extinguished food-seeking behavior (Sun and Rebec 2005). These data suggest that increased DA signaling in the dPFC through D1-like receptors may be specifically involved in cocaine-induced reinstatement. This idea is consistent with the evidence that chronic cocaine exposure preferentially increases D1-like receptor-mediated signaling in the dPFC (Bowers et al. 2004). For example, AGS3, a member of the activator-of-G-protein-signaling family, is upregulated in the rat PFC by repeated exposure to cocaine (Bowers et al. 2004). This protein disrupts the interaction between Giα and Gβγ subunits and thus, decreases Gi-mediated signal transduction. Because D2-like receptors are Gi-coupled receptors, the up-regulation of AGS3 may decrease D2-like receptor-mediated signaling. Thus, an increase in DA input to the PFC during cocaine-induced reinstatement will preferentially increase D1-like receptor-mediated signaling. These data combined with our current observations support the idea that the VTA–PFC DA circuit is critically involved in cocaine-induced reinstatement.
The amygdala plays a critical role in cue-induced reinstatement of extinguished cocaine-seeking behavior (Meil and See 1997; Grimm and See 2000; Kruzich and See 2001; Kantak et al. 2002; McLaughlin and See 2003; Yun and Fields 2003), and DA input to the amygdala appears to be important in this process (See et al. 2001). In contrast, the role of the amygdala in cocaine-induced reinstatement is less clear. On one hand, reversible inactivation of the basolateral or central nucleus of the amygdala (BLA or CeN) fails to block cocaine-induced reinstatement (McFarland and Kalivas 2001). On the other hand, inactivation of either rostral or caudal part of the BLA inhibits cocaine-induced reinstatement (Kantak et al. 2002). The reason for the difference is not entirely clear. There is, however, a key difference in the reinstatement procedures used in the two studies. The cocaine cues were presented during cocaine-induced reinstatement in the latter but not in the former study. It is likely that cocaine-induced reinstatement in the latter study is due to the combined contributions from cocaine and the cues. Thus, the effects on reinstatement in the latter study are probably due to the inhibition of the cue-related component. This could partially explain the difference between the two studies. However, a recent study showed that blockade of D1-like receptors in the central nucleus or the rostral region of the amygdala inhibits cocaine-induced reinstatement in the absence of the cues (Alleweireldt et al. 2006). At present time, it is unclear to us how to reconcile these data. Thus, the current study cannot rule out the role of the VTA–amygdala DA circuit in the effects of U50 488 on cocaine-induced reinstatement although the data are consistent with the idea that the VTA-dPFC DA circuit is critically involved in the process. Further investigations are needed to clarify this issue.
In summary, our results showed that activation of VTA κORs in rats dose-dependently decreased cocaine-induced reinstatement. These data indicate that the activation of the VTA-dPFC/amygdala DA circuits is critically involved in this process.
Acknowledgments
The project described was supported by Grant numbers DA021278 (WLS) and DA023215 (JDS) from the National Institute on Drug Abuse, and its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDA or NIH. All procedures followed the National Institute of Health Guidelines for the Care and Use of Laboratory Animals. There is no conflict of interest in relation to this article.
References
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 2006;31:363–374. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology. 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking.[see comment] Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. J Pharmacol Exp Ther. 1999;291:353–360. [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carey GJ, Bergman J. Enadoline discrimination in squirrel monkeys: effects of opioid agonists and antagonists. J Pharmacol Exp Ther. 2001;297:215–223. [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 2000;22:473–479. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci USA. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci. 2005;25:5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of cocaine within the posterior ventral tegmental area of Wistar rats: evidence for involvement of serotonin-3 receptors and dopamine neurons. J Pharmacol Exp Ther. 2005;313:134–145. doi: 10.1124/jpet.104.075952. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacologia. 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Reinstatement of heroin and cocaine self-administration behavior in the rat by intracerebral application of morphine in the ventral tegmental area. Pharmacol Biochem Behav. 1984;20:917–923. doi: 10.1016/0091-3057(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Stewart J. Stress and relapse to drug seeking: studies in laboratory animals shed light on mechanisms and sources of long-term vulnerability. Am J Addict. 2003;12:1–17. [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology. 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV. Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology. 2005;30:2073–2081. doi: 10.1038/sj.npp.1300744. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Ruedi-Bettschen D, Spealman RD. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- White FJ. Synaptic regulation of mesocorticolimbic dopamine neurons. Annu Rev Neurosci. 1996;19:405–436. doi: 10.1146/annurev.ne.19.030196.002201. [DOI] [PubMed] [Google Scholar]
- Wolf ME. LTP may trigger addiction. Mol Interv. 2003;3:248–252. doi: 10.1124/mi.3.5.248. [DOI] [PubMed] [Google Scholar]
- Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on discriminative stimulus task. Neuroscience. 2003;121:747–757. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]