Abstract
Background
Mesenchymal stem cells hold promise for cardiovascular regenerative therapy. Derivation of these cells from the adipose tissue might be easier compared to bone marrow. However, the in vivo fate and function of adipose stromal cells (ASC) in the infarcted heart has never been compared directly to bone marrow derived mesenchymal cells (MSC).
Methods
ASC and MSC were isolated from transgenic FVB mice with β-actin promoter driving firefly luciferase and green fluorescent protein (Fluc-GFP) double fusion reporter gene, and were characterized using flow cytometry, microscopy, bioluminescence imaging (BLI) and luminometry. FVB mice (n=8/group) underwent myocardial infarction followed by intramyocardial injection of 5×105 ASC, MSC, fibroblasts (Fibro, positive control), or saline (negative control). Cell survival was measured using BLI for 6 weeks and cardiac function was monitored by echocardiography and pressure-volume (PV) analysis. Ventricular morphology was assessed using histology.
Results
ASC and MSC were CD34−, CD45−, c-Kit−, CD90+, Sca-1+, shared similar morphology, and had a population doubling time of ∼2 days. Cells expressed Fluc reporter genes in a number-dependent fashion, as confirmed by luminometry. After cardiac transplantation, both cell types showed drastic donor cell death within 4−5 weeks. Furthermore, transplantation of either cell type was not capable of preserving ventricular function and dimensions, as confirmed by PV-loops and histology.
Conclusion
This is the first study comparing the in vivo behavior of both cell types in the infarcted heart. ASC and MSC do not tolerate well in the cardiac environment, resulting in acute donor cell death and a subsequent loss of cardiac function similar to control groups.
Keywords: Myocardial infarction, molecular imaging, bone marrow stem cells, mesenchymal stem cells, adipose stromal cells
INTRODUCTION
Almost 80 million Americans suffer from cardiovascular disease (CVD), and with an average of one death every 36 seconds, CVD is the number one killer of Americans. Despite a wide range of therapeutic options to prevent progression of heart failure, end-stage disease can only be treated by heart transplantation which is, in turn, hampered by a lack of suitable donor organs (1). Bone marrow mononuclear stem cells (BMSC) have raised hope as a new therapeutic modality as they were believed to differentiate into cardiomyocytes when transplanted into the infarcted murine myocardium (2). Although this observation is subject to controversy (3), BMSC-mediated cardiac repair has recently been introduced into clinical medicine (4). A small stromal subset of BMSC, called mesenchymal stem cells (MSC), is capable of proliferation in vitro (5) and therefore gains popularity as a candidate to replace the infarcted myocardium. MSC have been proposed to improve cardiac function after myocardial infarction in both animals (6) and humans (7), and might even have an immunomodulating effect (8). However, the process of bone marrow harvesting can be painful and is limited in the quantity of aspirate.
Recently, stromal cells have been isolated from the adipose tissue (9), which would be an ideal source regarding procurement procedure (e.g., elective abdominoplasty) and yield. These adipose stromal cells (ASC) largely express the same surface markers as MSC (10) and have shown to preserve cardiac function after infarction (11). Although the in vitro properties of ASC and MSC have been compared before (12-14), there are no reports evaluating the cellular behavior and functional effects of either cell type when transplanted into the ischemic myocardium. Here, we present the first report using a molecular imaging technique to unveil and compare the in vivo behaviors and functional effects of ASC and MSC following transplantation into the infarcted heart.
METHODS
Animals
All animal study protocols were approved by the Stanford Animal Research Committee. The donor group consisted of male L2G mice (n=4, 8 weeks old), which were bred on FVB background and ubiquitously express green fluorescent protein (GFP) and firefly luciferase (Fluc) reporter genes driven by a β-actin promoter as previously described (15). Recipient animals (n=37) consisted of syngeneic, female FVB mice (8 weeks old, Jackson Laboratories, Bar Harbor, ME). Animals were randomized into 4 recipient groups (n=8/ group): (1) adipose tissue-derived stromal cells (ASC), (2) bone-marrow-derived mesenchymal cells (MSC), (3) fibroblasts (Fibro) as cellular control group, and (4) phosphate buffered saline (PBS) as non-cellular control group.
Cell culture of Fibro, ASC, and MSC
Donor mice were sacrificed by cervical dislocation after ample anesthesia with isoflurane, and were placed in 70% ethanol for 5 minutes. (A) For the isolation of Fibro, skin biopsies were taken from the tail and ears, minced and incubated overnight in collagenase type II (400 U/mL, Gibco-Invitrogen, Carlsbad, CA), dissolved in DMEM (Gibco, NY) supplemented with 20% heat-inactivated fetal bovine serum (FBS, Hyclone, Logan, UT), 1% antibiotics/antimycotic solution (Penicillin/Streptomycin, Gibco-Invitrogen, Carlsbad, CA) at 37°C and 5% CO2 in air as described (16). The next day, cells were dislodged from digested tissue by repeated pipetting and were passed through 70 μm sterile netting into sterile 15-ml centrifuge tubes. The samples were centrifuged for 5 minutes at 1200 rounds per minute (rpm), and the cell pellet was resuspended in DMEM/20% FBS/1%Penicillin-Streptomycin to be plated in a 25 cm2 tissue flask at 37°C/5%CO2. (B) For the isolation of ASC, the adipose tissue was isolated from the inguinal and abdominal region as described before (17). In brief, the adipose tissue was washed in PBS and digested using 5 mL 0.075% collagenase (type I, Gibco-Invitrogen, Carlsbad, CA) in PBS for 30 minutes, followed by deactivation by DMEM/20% FBS/1%Penicillin-Streptomycin. After centrifuging for 5 minutes at 1200 rpm, the cell pellet was resuspended and incubated for 10 minutes in ACK lysing buffer to eliminate red blood cells. The suspension was centrifuged, resuspended in DMEM/ 20% FBS/1%Penicillin-Streptomycin, filtered through a 70 μm mesh, and plated in a 25 cm2 tissue flask at 37°C/5%CO2 to grow ASC. (C) For the isolation of MSC, the long bones were explanted, washed and flushed with PBS using a 25-Gauge needle to collect bone marrow. After passing through a 70 μm strainer, the isolate was centrifuged at 1200 rpm for 5 minutes, washed and resuspended into DMEM/20%FBS/1%Penicillin/Streptomycin medium to grow MSC as described (5).
Flow cytometry
At passage 8−10, the cells were labeled using specific FITC-conjugated antibodies against CD34, CD45, C-kit, Sca-1, CD90, and CD106 and processed through a FACSCalibur system (BD, San Jose, CA) according to the manufacturer's protocol. Results were compared to appropriate isotype controls.
In vitro firefly luciferase (Fluc) assays
Cells were dislodged from culture flasks to be resuspended in PBS. Cell suspensions were divided into a 6-well plate in known concentrations. After administration of D-Luciferin (Xenogen, Alameda, CA, 4.5ug/mL), peak signal (photons/second/square centimeter/steridian or p/s/cm2/sr) was measured using a charged coupled device (CCD) camera (IVS200, Xenogen, Alameda, CA). Same amounts of dislodged cells were lysed using 200 μL of 10× Passive Lysis Buffer (Promega, Madison, WI) and centrifuged at maximum speed for 2 minutes at 4°C. For every sample, 20 μL of supernatant was added to 100 μL of Luciferase Assay Reagent (LAR-II, Promega, Madison, WI) and luminosity in relative light units (RLU) was measured on a 20/20n luminometer (Turner Biosystems, Sunnyvale, CA). All samples were conducted in triplets.
Surgical model
Female FVB mice (8 weeks old) were intubated with a 20-gauge angiocath (Ethicon Endo-Surgery, Inc. Cincinnati, OH) and were placed under general anesthesia with isoflurane (2%). Myocardial infarction (MI) was created by ligation of the mid-left anterior descending (LAD) artery with 8−0 Ethilon suture through a left anterolateral thoracotomy. After approximately 10 minutes, the infarct region was injected with 5×105 cells or PBS respective of group randomization using a Hamilton syringe with a 29-gauge needle. The chest was closed in 4 layers with 5−0 vicryl suture. All surgical procedures were performed in a blinded fashion by one micro-surgeon (G.H.) with several years of experience with this model.
Echocardiography
Echocardiography studies were performed 2, 4 and 6 weeks postoperatively. Three independent two-dimensional transversal-targeted M-mode traces were obtained at the level of the papillary muscles using a 14.7-MHz transducer on a Sequoia C512 Echocardiography system (Siemens, Malvern, PA). Using the enclosed software, left ventricular end-diastolic and end-systolic posterior and anterior dimensions were measured by a blinded member of our group (A.Y.S.) and processed to calculate left ventricular fractional shortening (LVFS).
In vivo optical bioluminescence imaging (BLI)
BLI was performed using the IVIS200 (Xenogen, Alameda, CA) system. Recipient mice were anesthetized with isoflurane, shaved and placed in the imaging chamber. After acquisition of a baseline image, mice were intraperitoneally injected with D-Luciferin (400 mg/kg body weight; Xenogen, CA). Mice were imaged on postoperative day 2, 4, 7, 10, and at week 2, 4, 5, and 6. Peak signal (p/s/cm2/sr) from a fixed region of interest (ROI) was evaluated using Living Image 2.50.1 software (Xenogen, CA).
Invasive hemodynamics
Invasive hemodynamic measurements were conducted by closed-chest pressure-volume (PV) loop analysis prior to sacrifice at week 6. The animal was placed under general anesthesia as described above. After midline neck incision, a 1.4 F conductance catheter (Millar Instruments, Houston, TX) was retrogradely advanced through the right carotid artery into the left ventricle. The measurements of segmental conductance were recorded which allowed extrapolation of the left ventricular volume, which was coupled with pressure. These data were analyzed in a blinded fashion using PVAN 3.4 Software (Millar Instruments, Houston, TX) and Chart/Scope Software (AD Instruments, Colorado Springs, CO).
Postmortem histology
Hearts (n=3/group at week 6) were flushed with saline and placed in 2% paraformaldehyde for 2 hours at room temperature followed by 12−24 hours in 30% sucrose at 4°C. The tissue was embedded in Optical Cutting Temperature (OCT) Compound (Tissue-Tek. Sakura Finetek USA Inc., Torrance, CA) and snap frozen on dry ice. Five-micron sections were cut in both the proximal and apical regions of the infarct zone. Slides were stained for H&E. Stained tissue was examined by Leica DMRB fluorescent microscope.
Ex vivo TaqMan PCR
Animals were sacrificed and hearts were explanted, followed by mincing and homogenization in 2 mL DNAzol (Invitrogen, Carlsbad, CA, USA). DNA was isolated according to the manufacturer's protocol. The DNA was quantified on a ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) and 500 ng DNA was processed for TaqMan PCR using primers specific for the Sry locus selectively found on the Y chromosome in the male donor cells. RT-PCR reactions were conducted in iCylcer IQ Real-Time Detection Systems (Bio-Rad, Hercules, CA, USA). Lower cycle numbers represent higher donor cell counts (18). Samples were conducted in triplets.
Effects of gender mismatch, myocardial ischemia, and green fluorescent protein (GFP) on cell survival
To investigate the effect of our gender mismatch model, we injected 5×105 male and female cells into the hindlimbs of male FVB mice (n=5). To assess the effects of myocardial milieus (ischemia vs. non-ischemia) on cell survival, 5×105 MSC were injected into non-infarcted hearts from female FVB recipients (n=3). To evaluate whether GFP could have an effect on transplanted cell survival, 5×105 GFP+-Fluc+ cells were injected into the hindlimbs of female FVB mice that previously received intramyocardial GFP-Fluc plasmid injection 3 months prior (n=3). As control, 5×105 GFP+-Fluc+ were similarly injected in non-manipulated FVB mice (n=3). In these experiments, cell survival was measured using in vivo optical bioluminescence imaging as described above.
Statistics
Statistics were calculated using SPSS 15.0 (SPSS Inc., Chicago, IL). Descriptive statistics included mean and standard error. Comparison between groups was performed using a one-way between groups ANOVA, or, when compared over time, one-way repeated measures ANOVA, both with Bonferroni correction. A logarithmic transformation of values was performed when needed to ensure normal distribution within each group and significance was assumed when P<0.05.
RESULTS
Characterization of ASC, MSC, and Fibro
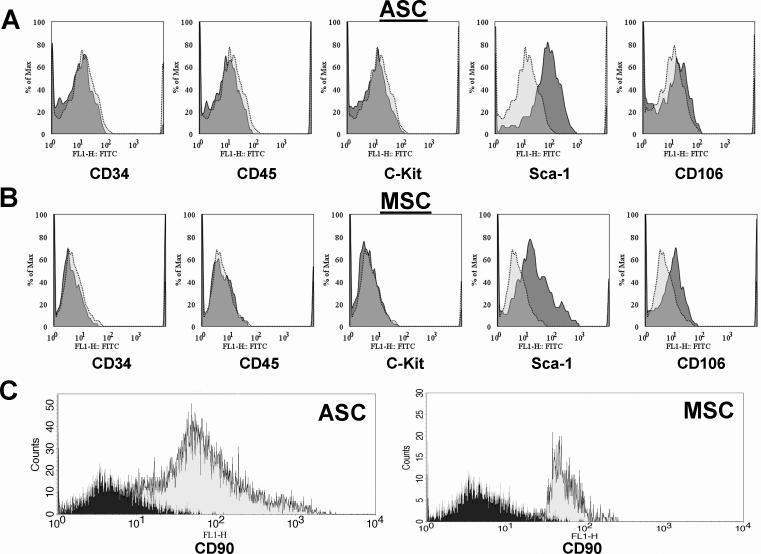
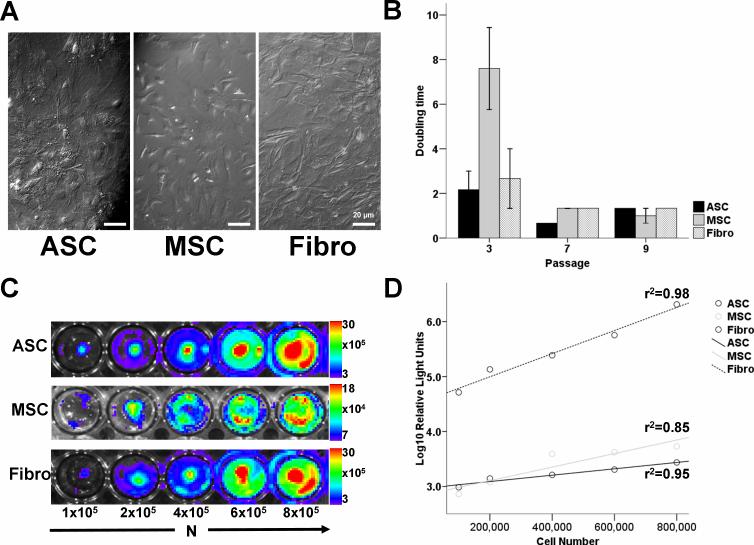
After culturing for approximately 8 passages, hematopoietic cells were eliminated from both ASC and MSC cultures. This was confirmed by flow cytometry showing absent CD34, CD45, and C-kit markers. Moreover, both populations were Sca-1+, CD90+, and CD106+ (MSC) or CD106− (ASC) (Figure 1), consistent with prior literature comparing expression patterns of ASC and MSC (13). On microscopy, both cell types showed spindle-shaped morphology (Figure 2a). Following isolation, MSC have slower population doubling time compared to ASC and Fibro before eventually growing like ASC and Fibro with an average population doubling time of approximately 2 days at passage 7 (Figure 2b). All populations were furthermore tested for the expression of the reporter gene firefly luciferase (Fluc) (Figure 2c). In all groups, cell number and Fluc signal correlated robustly with r2 values of 0.95 (ASC), 0.80 (MSC), and 0.97 (Fibro). Moreover, the assay of Fluc enzyme activity by luminometry also showed good correlation with cell number (ASC: r2=0.95, MSC: r2=0.85, Fibro: r2=0.98, Figure 2d). Importantly, Fluc enzyme activity correlated well with the previous BLI findings (ASC: r2=0.86, MSC: r2=0.75, Fibro: r2=0.95). Taken together, these data suggest that BLI is a reliable tool for measuring viable cell numbers and can be used instead of luminometry. Moreover, we found a robust correlation between in vivo BLI signals and ex vivo TaqMan PCR cycle counts of Sry expression, indicating that cardiac BLI signal is representative of the presence of male donor cells in the female hearts (Supplemental Figure 1).
Figure 1. In vitro assessment of cell surface marker expression.
(a) After 8−10 passages, flow cytometry showed that the ASC population was depleted from hematopoietic cells. Moreover, these cells tested negative for CD106, but positive for the stem cell antigen (Sca-1). (b) MSC also showed negative hematopoietic expression, but differed from their adipose tissue-derived counterparts regarding a positive expression for CD106. Dotted, light grey areas represent isotype controls. (c) Both ASC and MSC expressed the mesenchymal-specific marker CD90 (Black areas represent isotype controls).
Figure 2. In vitro characterization of morphology, expansion time, and reporter gene expression.
(a) When kept into culture, all cell types shared a spindle-shaped morphology (Bright-field Hoffman modulated contrast image, bar represents 20μm). (b) After an initial period of relatively slower in vitro growth of MSC, all cell types had mutual doubling times of approximately 2 days at passage 9. Bars represent mean ± SEM. (c) In vitro optical bioluminescence imaging (BLI) signal from firefly luciferase (Fluc) expression of increasing numbers of cells in 24-well plates show a correlative increase in signal intensity. Scale bars represent peak signal in photons/s/cm2/sr. (d) Lysates from the cell populations shown in figure 2c also showed robust correlation of cell number with Fluc enzyme on conventional luminometry.
Kinetics of cell survival by longitudinal bioluminescence imaging
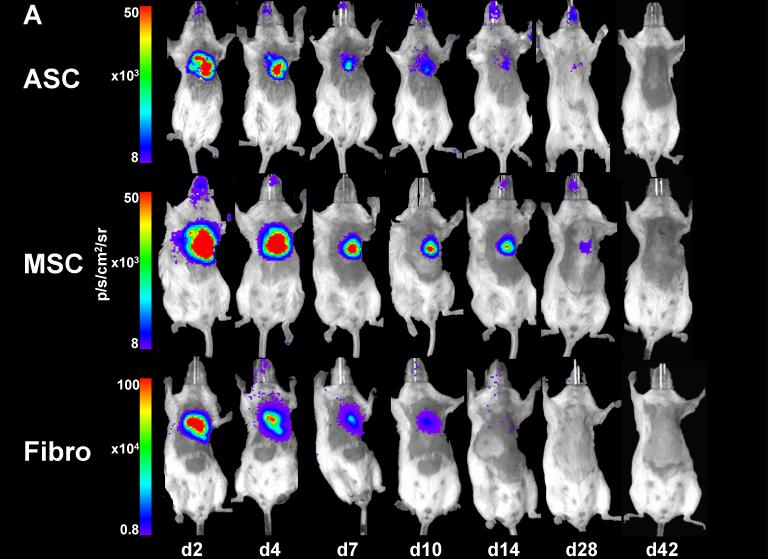
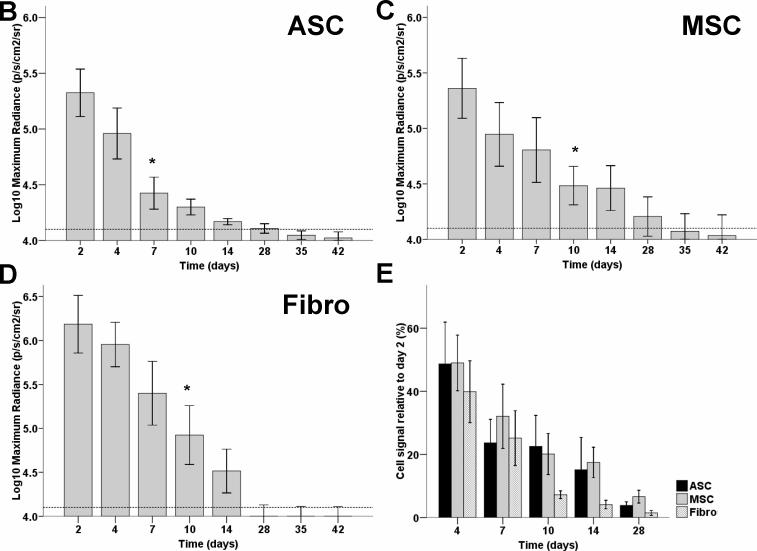
Previously, several groups have used RT-PCR or histological techniques and observed radical cell death after MSC transplantation into the ischemic myocardium (18, 19). However, these techniques do not allow for longitudinal imaging of cellular kinetics. Our BLI data showed that 2 days after intramyocardial transplantation, all cell types exhibited robust signals from the cardiac region, thereby confirming successful transplantation (20). However, in the following days, all three cell types experienced significant donor cell death (Figure 3a). Quantitative analysis shows decreased signals at day 7−10 as compared to day 2, which reached background levels by week 4−5 (Figure 3b-d). When normalized to the signal of day 2, there were no significant differences between ASC, MSC, and the Fibro control group (Figure 3e, P=NS, repeated measurements ANOVA).
Figure 3. Longitudinal in vivo optical bioluminescence imaging (BLI) of intramyocardially transplanted cells in living mice.
(a) Representative figures of animals within each group show that, after an initial strong cardiac signal 2 days after transplantation, robust cell death resulted in a remarkable decrease in signal over 4 weeks. Scale bars represent BLI signal in photons/s/cm2/sr. (b-d) Graphic representation of decreasing signals in the (b) ASC, (c) MSC, and (d) Fibro groups which are indicative of cell death. Background signals (dotted line) were reached between week 3 (Fibro) to week 5 (MSC) (n>7 in all groups, * indicates P<0.05 compared to the signal on day 2, ANOVA). (e) Normalized graph of reporter gene signals from transplanted cells showing that, when compared over time and controlled for initial differences in signal between cell types, there were no significant differences between ASC, MSC, and cellular control groups (Bars represent mean ± SEM, p=NS, repeated measurements ANOVA).
Influence of gender mismatch, myocardial milieus, and GFP expression on in vivo transplanted cell survival
To enable ex vivo validation of our in vivo BLI study, we performed a gender mismatch model with male donors and female recipients followed by TaqMan PCR of male Sry gene. From studies with organ transplant patients, it has been noticed that male patients receiving female grafts have decreased graft survival and require more immunosuppressant drugs (21). Although this effect was generally less apparent in male-female transplants (21), we set out to investigate the role of gender mismatch by transplanting equal numbers of male and female ASC into hindlimbs of male mice. As shown in Supplemental Figure 2, there were no significant differences in cell survival between the cell types from both genders. After two weeks, BLI signals were 6.3×104±0.8×104 p/s/cm2/sr for male ASC and 5.7×104±1.3 ×104 p/s/cm2/sr for female ASC (P=NS). In our study, it is also possible that GFP can elicit an immune response (22), which may have led to a decreased survival in our cardiac experiments. In order to differentiate between the effects of ischemic versus normal cardiac tissue on stem cell survival, MSC were also transplanted into non-infarcted hearts. While acute survival was observed in the non-ischemia group, the cells were still not capable of surviving for a prolonged period (Supplemental Figure 3). In order to explore the influence of GFP-expression on cell survival, we transplanted GFP+-Fluc+ into the hindlimbs of naïve FVB mice or FVB mice that were pre-sensitized by means of previous Fluc-GFP plasmid injection. By comparison of cell survival pattern, again there were no significant differences between BLI signals from both animals (Supplemental Figure 4). In summary, while both gene mismatch and GFP immunogenicity could have affected cell survival, our direct comparison studies suggest that they were not the main contributing factors for the loss of imaging signal, and thus cell survival, in our experiments.
Assessment of cardiac contractility by echocardiography
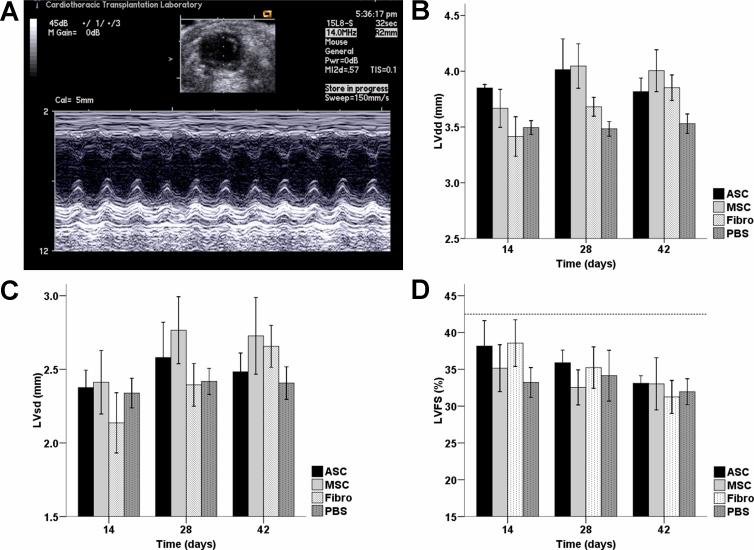
Previously, it has been observed that ASC (11) and MSC (23) preserved left ventricular dimensions and fractional shortening after infarction. However, there is no comparative functional data available between both cell types, after prolonged time in culture. In the current study, measurements of left ventricular dimensions revealed a gradual increase in diameters in both diastole and systole, suggesting negative remodeling in all groups without any significant benefits of cell transplantation when evaluated at weeks 2, 4, and 6 (Figure 4a-c). For the cell groups (ASC, MSC, Fibro), after an initial non-significant increase in left ventricular fractional shortening (LVFS) compared to the PBS group at week 2, the increasing ventricular dilatation resulted in a declining LVFS over time (Figure 4d). By week 6 after cell transplantation, there was a trend toward improved LVFS in the ASC (33.1±1.0%) and MSC (33.0±3.5%) groups compared to the Fibro (31.3±2.2%) and PBS (32.0±1.8%) control groups, of which the latter (Figure 4d, P =NS, repeated measurements ANOVA) had similar values compared to the literature (24). For complete echocardiography results, please refer to Supplemental Table 1.
Figure 4. In vivo measurements of cardiac function over time.
(a) Representative M-mode traced two-dimensional picture taken at the level of the papillary muscle whereby left ventricular diastolic and systolic diameters can be measured. (b,c) Cell transplantation after myocardial infarction was not capable of preventing an increase in left ventricular diastolic (LVdd, b) or systolic diameters (LVsd, c). (d) Left ventricular performance, as measured by left ventricular fractional shortening (LVFS), was preserved only in the short term after cell transplantation. The dotted line represents the mean pre-operative fractional shortening of all operated animals. Six weeks after transplantation, there was a comparable LVFS in the ASC and MSC groups. Although cardiac function was slightly better than control groups, there was no significant difference when controlled for the trend over time (Bars represent means ± SEM, n>7 in all groups, p=NS, repeated measurements ANOVA).
Hemodynamic measurements using pressure-volume (PV) loops
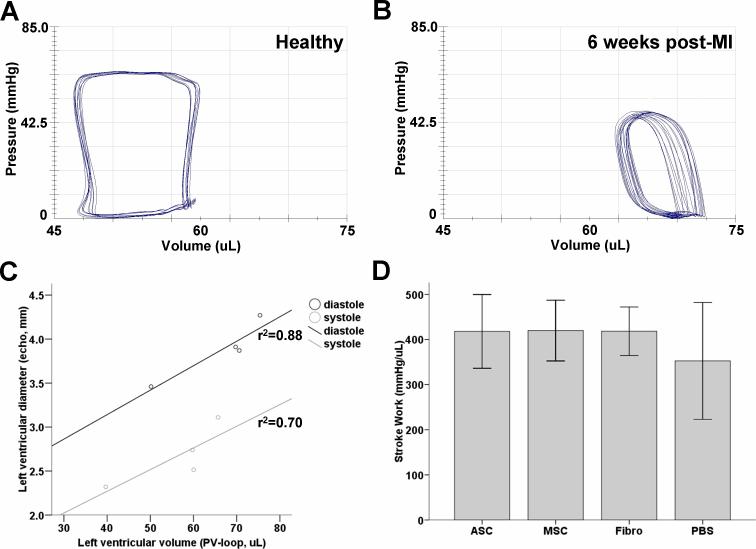
Recently, it has been shown that MSC were capable of preserving myocardial compliance, as measured by invasive hemodynamic measurements (25). Thus, to validate the echocardiography measurements of ventricular dimensions, invasive steady-state hemodynamic measurements of left ventricular diastolic and systolic volumes were conducted immediately after echocardiography at week 6. When plotted versus each other, mean diastolic and systolic volumes and diameters from each group correlated with r2 values of 0.88 and 0.70, respectively (Figure 5a-c). Stroke work and cardiac output, important parameters of ventricular performance (26), did not differ among cellular groups but was slightly better than the PBS control group (Figure 5d,e, P=NS, ANOVA). Ventricular contraction was not different between groups, but there was a trend towards an improved ventricular relaxation, as measured by the minimum ΔP/Δt, in the ASC and MSC groups (Figure 5f, g, P=NS, ANOVA). Furthermore, an increase in arterial elastance, suggestive of higher afterload caused either by arterial stiffening or increased peripheral resistance (27), was seen in the PBS group but this was not significantly different from the ASC and MSC group and the Fibro controls (Figure 5d, P=NS, ANOVA).
Figure 5. Invasive steady-state pressure-volume measurements of cardiac performance.
(a,b) Pressure-volume recordings of (a) a normal, non-operated animal, and (b) PBS control animal 6 weeks after infarction with typical right shift. (c) Mean values of left ventricular volumes, as measured by pressure-volume loops, were correlative with the earlier acquired left ventricular diameters, as measured by echocardiography (dots represent mean measured values from each group). (d, e) Although transplantation of ASC and MSC resulted in a similar trend of preservation of (d) stroke work and (e) cardiac output compared to the PBS group, there was no significant difference compared to the cellular control. (f) Although maximum dP/dt was not different among groups, there was a (g) trend towards better minimum dP/dt, which is indicative of better ventricular relaxation. (h) Similarly, there was a trend towards lower afterload, as shown by lower arterial elastance, in all cellular groups compared to the PBS group (Bars represent means ± SEM, n>6 in every group, p=NS, ANOVA).
Post-mortem histology
Immediately after PV-analysis, the animals were euthanized after which the hearts were explanted. Gross morphology showed that ventricular dilatation and fibrous scar formation had not been prevented by cell transplantation (Figure 6). As suggested by the BLI, echocardiography, and PV findings, transplantation of both ASC and MSC did not result in either repopulation (data not shown), or endogenous preservation of myocardial tissue.
Figure 6. Macroscopic pictures of representative explanted hearts show no prevention of cardiac remodeling after cell transplantation.
6 weeks after transplantation of (a) ASC, (b) MSC, and (c) Fibro there are no gross signs of tissue preservation compared to (d) PBS (H&E stained slides of representative hearts).
DISCUSSION
This study has evaluated for the first time the in vivo behavior and functional effects of both adipose tissue-derived stromal cells (ASC) and bone marrow-derived mesenchymal stem cells (MSC) after injection into the ischemic myocardium, as compared to a cellular (Fibro), and non-cellular (PBS) control group. The major findings are as follows: (1) molecular imaging using the Fluc reporter gene is a reliable tool for repetitively monitoring donor cell survival in vivo; (2) similar to the Fibro control group, ASC and MSC rapidly die off after injection into the infarcted heart; and (3) ASC and MSC were not capable of significantly preventing left ventricular remodeling and subsequent loss of cardiac function.
Until now, ASC and MSC studies have been largely based on in vitro studies (10) or investigations that monitored cell location and quantity by post-mortem histological analysis (28) or PCR techniques (18). To study the true in vivo behavior of stem cells, one needs to be able to repetitively image cell location and count in a non-invasive fashion. One such approach would be the labeling of stem cells with iron particles which would enable imaging by MRI (29). However, this technique does not provide insight into cell number since the same amount of iron particles are divided among daughter cells during cell proliferation or ingested by macrophages in case of cell death (30). By contrast, the current study demonstrates that molecular imaging of the Fluc reporter gene with the D-Luciferin reporter probe can provide repetitive, longitudinal in vivo imaging of donor cell survival in the infarcted heart of the same animal, thereby preventing sampling biases that can occur with the use of multiple animals that need to be sacrificed on different time points to perform conventional histological staining (31).
The clinical relevance of this study is significant. MSC have been suggested as potential treatments for a wide variety of diseases including graft versus host disease, osteogenesis imperfecta, rheumatoid arthritis, multiple sclerosis, and myocardial infarction (32). Since the ASC and MSC appear to be comparable cells with also similar in vivo behavior, it would be possible to yield stromal cells from fat instead of bone marrow. Not only would this provide a less restricting source regarding yield, it would also be a more patient friendly isolation procedure. In fact, it has even been proposed that human ASC are superior to MSC with regard to their paracrine and angiogenic potential in response to ischemia (33). Despite these reported advantages, an important finding from our study is that both MSC and ASC do not survive for longer term following transplantation into the infarcted heart. Based on our quantitative measurements of BLI signals, all cells have died by week 6. This is in concordance with earlier PCR findings from Muller-Ehmsen et al., who noticed robust cell death after transplantation with less than 2% of the initially transplanted MSC being present at 6 weeks after cardiac transplantation (18). Similarly, Nakamura and colleagues were only able to find 4.4% engraftment of MSC (19) 1 week after intramyocardial transplantation, while Amsalem et al. were unable to find any MSC 4 weeks after transplantation (23). Moreover, Mangi and colleagues observed robust cell death early after transplantation, which was mitigated by transfecting the MSC with the pro-survival gene Akt-1 (34). Thus, although prolonged survival of MSC and ASC has been proven possible in different models (35, 36), the environment in the heart proves hostile to these cells, resulting in decreased cell survival.
There have been several studies reporting preservation of cardiac function after myocardial infarction and subsequent ASC (11) and MSC (37) transplantation. On the contrary, in this study we did not observe any functional benefit from ASC or MSC after transplantation into the infarcted heart. In fact, after an initial preservation, we have observed decreasing fractional shortening in both ASC and MSC groups from week 2 to week 6. Although these differences could be a consequence of differences in the experimental animal and surgical models, the observed short-term effect of cell transplantation is in concordance with findings from a growing body of experimental studies. Ultra-sensitive small animal MRI has recently been used to show that MSC fail to repair the ischemic heart and do not have any beneficial effect on cardiac function after infarction (38). This non-beneficial effect corresponds to other studies, which show that non-transduced MSC do not ameliorate functional heart failure after transplantation (39). In addition, Dai and colleagues observed no long-term functional effect after an initial significant benefit 1 month after transplantation of MSC in infarcted rat hearts (28). In combination with the BLI data of the current study and as suggested in a recent review (40), the dismal cell survival of ASC and MSC might underlie this short term effect. This poor survival pattern makes robust repopulation impossible and furthermore limits the protective paracrine action of the cells. This paracrine effect has been shown to be of crucial importance in MSC function (34, 41). By transfecting MSC with Akt-1, researchers have observed not only a better cell survival under hypoxic conditions, but also a robust increase in paracrine factors which resulted in a preservation of morphology and function of the infarcted heart (34, 41), effects that were also shown using MSC transfected with the pro-survival gene Bcl-2 (39). Thus, further research is needed to identify the factors responsible for the acute donor cell death in the heart (both infarcted and non-infarcted). Improving stem cell survival may offer a sustained paracrine effect which could lead to protective effects on resident cardiomyocytes from apoptosis and a subsequent preservation of cardiac function.
Several limitations of this study can be raised. First, we used Fluc- and GFP-expressing cells in order to be able to investigate cellular fate in case of robust cell survival after 6 weeks. It has been suggested that GFP could impair actin-myosin interactions in muscle cells (42) which might have influenced cardiac function. However, the lack of functional benefit of transplantation of either cell type in this study is also in concordance with other studies using non-GFP-labeled MSC (28, 38). Second, we chose to use longer-term cultured cells because this generally increases purity and also because the ability to culture these cells underlies a crucial advantage which is important for its off-the-shelf clinical potential. However, it has been shown that higher passage MSC had decreased growth factor release under hypoxic conditions and that passage number of MSC was inversely correlated to the protective effect on infarcted hearts (43). Thus, it is possible that the lack of functional benefit is a consequence of dismal cell survival, diminished paracrine signaling, and high passage of the cells.
In conclusion, we have reported that the stromal population from the adipose tissue has close in vitro resemblance with its counterpart from the bone marrow. Importantly, using non-invasive molecular imaging techniques, this study has shown for the first time that these cells also have similar in vivo behavior in the infarcted heart. Finally, we did not observe a clear functional benefit after transplantation of both cell types. These results should be a stimulus for further research regarding improvement of cellular behavior to ultimately be able to restore cardiac function after myocardial infarction by transplanting long-term cultured, off-the-shelf adipose tissue-derived stromal cells.
Supplementary Material
Supplemental Figure 1: In vivo optical bioluminescence signals parallel ex vivo male donor cell Sry expression on TaqMan PCR. Facilitated by the male-to-female transplant model, in vivo BLI signals were controlled by TaqMan PCR-assessment of Sry expression of the male donor cells in the female hearts. (a,b) The hearts of 2 mice (MSC 1,2) with different BLI signals were explanted, the DNA was extracted, and TaqMan PCR was performed on 3 samples from each heart. (c) Higher BLI signal in MSC 1 correspond to lower PCR cycle counts, indicating higher Sry expression caused by increased donor cell count. (d) Correlation plot of BLI signals vs. TaqMan PCR cycle (r2=0.79).
Supplemental Figure 2: Effect of donor-recipient gender mismatch on in vivo cell survival. To assess whether gender mismatch had influenced cell survival, both 5×105 male and female ASC were transplanted into male mice. (a, b) Imaging signals from either cell type were comparable without any significant differences. Scale bars represent BLI signal in photons/s/cm2/sr (Bars represent means ± SEM, n=5, p=NS, ANOVA).
Supplemental Figure 3: Effect of ischemia on in vivo cell survival. In order to differentiate between the effects of ischemic versus normal cardiac tissue on stem cell survival, 5×105 MSC were transplanted into both infarcted and non-infarcted hearts. No significant differences in cell survival were observed in a 3-week period. Scale bar represents BLI signal in photons/s/cm2/sr (n=3/group, p=NS, ANOVA).
Supplemental Figure 4: Effect of donor GFP-expression on in vivo cell survival. Both (a) ASC (b) and MSC expressed GFP (in vitro fluorescence microscopy pictures), which might have influenced cell survival by possible immunogenicity. However, after transplantation of 5×105 GFP+-Fluc+ MSC into pre-sensitized and naïve FVB mice, (c) longitudinal BLI and (d) quantification of signal intensity revealed no significant differences between imaging signals from day 0 to day 28. Scale bars represent BLI signal in photons/s/cm2/sr (Bars represent means ± SEM, n=3/group, p=NS, ANOVA).
Supplemental table 1: Non-invasive echocardiography measurements. Values are reported in mean ± SEM. No symbols indicate P=NS (Repeated measurements ANOVA).
Acknowledgements
The authors thank V. Mariano for animal care and ms. P. Chu for assistance with histology.
Support: This study was supported in part by grants from the NIH HL074883 and Burroughs Wellcome Award (JCW). K.E.A. van der Bogt was supported by the American Heart Association (Medical Student Research Award), Fulbright committee, VSB fund, and the Michael van Vloten fund.
ABBREVIATIONS
- ASC
Adipose-derived stromal cells
- BLI
Bioluminescence imaging
- FBS
Fetal bovine serum
- Fibro
Fibroblasts
- Fluc
Firefly luciferase
- MSC
Mesenchymal stem cells
Footnotes
Conflicts of interest: None
REFERENCES
- 1.Rosamond W, Flegal K, Friday G, et al. Heart Disease and Stroke Statistics--2007 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006 doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 3.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 4.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 5.Schrepfer S, Deuse T, Lange C, et al. Simplified protocol to isolate, purify, and culture expand mesenchymal stem cells. Stem Cells Dev. 2007;16(1):105. doi: 10.1089/scd.2006.0041. [DOI] [PubMed] [Google Scholar]
- 6.Silva GV, Litovsky S, Assad JA, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 7.Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94(1):92. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 9.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 10.Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14(4−6):311. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 11.Li B, Zeng Q, Wang H, et al. Adipose tissue stromal cells transplantation in rats of acute myocardial infarction. Coron Artery Dis. 2007;18(3):221. doi: 10.1097/MCA.0b013e32801235da. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 13.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33(11):1402. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R880. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 15.Cao YA, Wagers AJ, Beilhack A, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci U S A. 2004;101(1):221. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289(1):E23. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- 17.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller-Ehmsen J, Krausgrill B, Burst V, et al. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006;41(5):876. doi: 10.1016/j.yjmcc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura Y, Wang X, Xu C, et al. Xenotransplantation of long-term-cultured swine bone marrow-derived mesenchymal stem cells. Stem Cells. 2007;25(3):612. doi: 10.1634/stemcells.2006-0168. [DOI] [PubMed] [Google Scholar]
- 20.Sheikh AY, Lin SA, Cao F, et al. Molecular Imaging of Bone Marrow Mononuclear Cell Homing and Engraftment in Ischemic Myocardium. Stem Cells. 2007 doi: 10.1634/stemcells.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeier M, Dohler B, Opelz G, Ritz E. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002;13(10):2570. doi: 10.1097/01.asn.0000030078.74889.69. [DOI] [PubMed] [Google Scholar]
- 22.Bubnic SJ, Nagy A, Keating A. Donor hematopoietic cells from transgenic mice that express GFP are immunogenic in immunocompetent recipients. Hematology. 2005;10(4):289. doi: 10.1080/10245330500093468. [DOI] [PubMed] [Google Scholar]
- 23.Amsalem Y, Mardor Y, Feinberg MS, et al. Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation. 2007;116(11 Suppl):I38. doi: 10.1161/CIRCULATIONAHA.106.680231. [DOI] [PubMed] [Google Scholar]
- 24.Rohde LE, Aikawa M, Cheng GC, et al. Echocardiography-derived left ventricular end-systolic regional wall stress and matrix remodeling after experimental myocardial infarction. J Am Coll Cardiol. 1999;33(3):835. doi: 10.1016/s0735-1097(98)00602-0. [DOI] [PubMed] [Google Scholar]
- 25.Berry MF, Engler AJ, Woo YJ, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290(6):H2196. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 26.Lips DJ, van der Nagel T, Steendijk P, et al. Left ventricular pressure-volume measurements in mice: comparison of closed-chest versus open-chest approach. Basic Res Cardiol. 2004;99(5):351. doi: 10.1007/s00395-004-0476-5. [DOI] [PubMed] [Google Scholar]
- 27.Shioura KM, Geenen DL, Goldspink PH. Assessment of cardiac function with the pressure-volume conductance system following myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2007;293(5):H2870. doi: 10.1152/ajpheart.00585.2007. [DOI] [PubMed] [Google Scholar]
- 28.Dai W, Hale SL, Martin BJ, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112(2):214. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 29.Kraitchman DL, Heldman AW, Atalar E, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107(18):2290. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Suzuki Y, Huang M, et al. Comparison of Reporter Gene and Iron Particle Labeling for Tracking Fate of Human Embryonic Stem Cells and Differentiated Endothelial Cells in Living Subjects. Stem Cells. 2008 doi: 10.1634/stemcells.2007-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang GY, Xie X, Wu JC. Overview of stem cells and imaging modalities for cardiovascular diseases. J Nucl Cardiol. 2006;13(4):554. doi: 10.1016/j.nuclcard.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Dazzi F, Horwood NJ. Potential of mesenchymal stem cell therapy. Curr Opin Oncol. 2007;19(6):650. doi: 10.1097/CCO.0b013e3282f0e116. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y, Kim H, Cho H, Bae Y, Suh K, Jung J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem. 2007;20(6):867. doi: 10.1159/000110447. [DOI] [PubMed] [Google Scholar]
- 34.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9(9):1195. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 35.Degano IR, Vilalta M, Bago JR, et al. Bioluminescence imaging of calvarial bone repair using bone marrow and adipose tissue-derived mesenchymal stem cells. Biomaterials. 2008;29(4):427. doi: 10.1016/j.biomaterials.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Shen LH, Li Y, Chen J, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27(1):6. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 37.Tang YL, Zhao Q, Qin X, et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80(1):229. doi: 10.1016/j.athoracsur.2005.02.072. [DOI] [PubMed] [Google Scholar]
- 38.Carr CA, Stuckey DJ, Tatton L, et al. Bone marrow-derived stromal cells home to and remain in the infarcted rat heart but fail to improve function: an in vivo cine-MRI study. Am J Physiol Heart Circ Physiol. 2008;295(2):H533. doi: 10.1152/ajpheart.00094.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Ma N, Ong LL, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25(8):2118. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 40.Wollert KC, Drexler H. Mesenchymal stem cells for myocardial infarction: promises and pitfalls. Circulation. 2005;112(2):151. doi: 10.1161/CIRCULATIONAHA.105.551895. [DOI] [PubMed] [Google Scholar]
- 41.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J. 2006;20(6):661. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 42.Agbulut O, Coirault C, Niederlander N, et al. GFP expression in muscle cells impairs actin-myosin interactions: implications for cell therapy. Nat Methods. 2006;3(5):331. doi: 10.1038/nmeth0506-331. [DOI] [PubMed] [Google Scholar]
- 43.Crisostomo PR, Wang M, Wairiuko GM, et al. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock. 2006;26(6):575. doi: 10.1097/01.shk.0000235087.45798.93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: In vivo optical bioluminescence signals parallel ex vivo male donor cell Sry expression on TaqMan PCR. Facilitated by the male-to-female transplant model, in vivo BLI signals were controlled by TaqMan PCR-assessment of Sry expression of the male donor cells in the female hearts. (a,b) The hearts of 2 mice (MSC 1,2) with different BLI signals were explanted, the DNA was extracted, and TaqMan PCR was performed on 3 samples from each heart. (c) Higher BLI signal in MSC 1 correspond to lower PCR cycle counts, indicating higher Sry expression caused by increased donor cell count. (d) Correlation plot of BLI signals vs. TaqMan PCR cycle (r2=0.79).
Supplemental Figure 2: Effect of donor-recipient gender mismatch on in vivo cell survival. To assess whether gender mismatch had influenced cell survival, both 5×105 male and female ASC were transplanted into male mice. (a, b) Imaging signals from either cell type were comparable without any significant differences. Scale bars represent BLI signal in photons/s/cm2/sr (Bars represent means ± SEM, n=5, p=NS, ANOVA).
Supplemental Figure 3: Effect of ischemia on in vivo cell survival. In order to differentiate between the effects of ischemic versus normal cardiac tissue on stem cell survival, 5×105 MSC were transplanted into both infarcted and non-infarcted hearts. No significant differences in cell survival were observed in a 3-week period. Scale bar represents BLI signal in photons/s/cm2/sr (n=3/group, p=NS, ANOVA).
Supplemental Figure 4: Effect of donor GFP-expression on in vivo cell survival. Both (a) ASC (b) and MSC expressed GFP (in vitro fluorescence microscopy pictures), which might have influenced cell survival by possible immunogenicity. However, after transplantation of 5×105 GFP+-Fluc+ MSC into pre-sensitized and naïve FVB mice, (c) longitudinal BLI and (d) quantification of signal intensity revealed no significant differences between imaging signals from day 0 to day 28. Scale bars represent BLI signal in photons/s/cm2/sr (Bars represent means ± SEM, n=3/group, p=NS, ANOVA).
Supplemental table 1: Non-invasive echocardiography measurements. Values are reported in mean ± SEM. No symbols indicate P=NS (Repeated measurements ANOVA).