Abstract
Despite progress in the treatment of glioblastoma, more than 95% of patients suffering from this disease still die within two years. Recent findings support the belief that cancer stem-like cells are responsible for tumor formation and ongoing growth. Here a method combining lectin microarray and LC-MS/MS was used to discover the cell surface glycoprotein markers of a glioblastoma-derived stem-like cell line. Lectin microarray analysis of cell surface glycans showed that two galactose-specific lectins Trichosanthes kirilowii agglutinin (TKA) and Peanut agglutinin (PNA) could distinguish the stem-like glioblastoma neurosphere culture from a traditional adherent glioblastoma cell line. Agarose-bound TKA and PNA were used to capture the glycoproteins from the two cell cultures, which were analyzed by LC-MS/MS. The glycoproteins were quantified by spectral counting, resulting in the identification of 12 and 11 potential glycoprotein markers from the TKA and PNA captured fractions respectively. Almost all of these proteins were membrane proteins. Differential expression was verified by Western blotting analysis of 6 interesting proteins, including the up-regulated Receptor-type tyrosine-protein phosphatase zeta, Tenascin-C, Chondroitin sulfate proteoglycan NG2, Podocalyxin-like protein 1 and CD90, and the down-regulated CD44. An improved understanding of these proteins may be important for earlier diagnosis and better therapeutic targeting of glioblastoma.
Keywords: glycoprotein, biomarker, lectin microarray, glioblastoma, stem-like cells, LC-MS/MS
Introduction
Glioblastoma (GBM) is the most common malignant brain tumor in adults. Even with advanced technology in surgery, in vivo imaging, chemotherapy and radiotherapy, the overall survival rate of GBM patients was only 17.7% at one year, and 3.3% at two years.1–2 Therefore, new strategies to treat GBM are urgently needed. There is emerging evidence showing that cancer stem cells within tumors are responsible for tumor formation and ongoing growth.3 Cancer stem cells have the ability to self-renew and drive tumorigenesis. The term cancer stem cells reflects the “stem-like” properties of these cells.4 Such stem-like cells have been found in various solid tumors such as breast, colon, head and neck, pancreas and liver. Their existence has also been demonstrated in brain tumors, including GBM.5–8 We have demonstrated recently that Notch pathway blockade depletes cancer stem-like cells in medulloblastoma and GBM.9–11 Therefore, new therapeutic methods targeting cancer stem-like cells may bring hope for GBM patients. Identification of markers of GBM stem cells will be indispensable, since this may lead to earlier diagnosis and improved therapeutic targeting of the disease. Most of the known markers of cancer stem-like cells in solid tumors are cell surface glycoproteins.4 The discovery of unique glycan expression patterns on the surface of cancer stem-like cells is an important step to identify novel surface glycoprotein markers of these cells.
The lectin microarray is a powerful tool to analyze the glycan structures of glycoproteins.12–15 Recently, the technology has been employed to study diverse cell processes by profiling cell surface glycans of live cells.16–19 Using lectin microarray, Chen et al.17 evaluated glycan expression patterns on the surface of several human breast cancer cell lines and observed significant differences that related to cancer metastasis. Tao et al.19 analyzed the cell surface glycan signature of a human breast cancer cell line MCF7 and found that the Lycopersicon esculentum lectin (LEL) may be used as a biomarker of this cell line. The investigation of cell surface glycan changes and the discovery of specific glycan-binding lectins facilitate the identification of cell surface glycoprotein markers. In a recent study, Lee et al.20 found that the Griffonia simplicifolia lectin II (GS II) selectively bound to rat fibroblast cells but not to rat endothelial cells, and therefore they captured glycoproteins from fibroblast cells using GS II and identified several glycoprotein markers by LC-MS/MS. This work provided a useful strategy that combined lectin microarray and mass spectrometry to identify cell surface glycoprotein biomarkers.
In our work, high-sensitive fluorescence-assisted lectin microarrays were used to discover the cell surface glycan expression patterns of a human GBM stem-like cell line and a traditional adherent GBM cell line as a control. Two lectins TKA and PNA which can distinguish the two cell lines were used to capture the glycoproteins by affinity chromatography. The LC-MS/MS analysis and label-free quantification resulted in the identification of 12 and 11 potential glycoprotein markers from the TKA and PNA captured fractions respectively. Western blotting analysis of 6 selected proteins confirmed the differential expression. Future investigations of these proteins may be helpful for identifying cancer stem-like cells from GBM.
Materials and Methods
Cell culture
The human GBM-derived stem-like cell line HSR-GBM1 was established by Vescovi and colleagues and maintained in serum-free Neurocult medium (Stem Cell Technologies) supplemented with 20 ng/ml of both epidermal growth factor (EGF) and fibroblast growth factor.6, 21 Human GBM cell line U373 was maintained in DMEM supplemented with 10 % fetal bovine serum. Cells growing exponentially were harvested and washed twice with PBS.
Lectin microarray
Sixteen lectins were used in this study. The carbohydrate specificities of these lectins were listed in Table 1. Each lectin was dissolved in PBS buffer (0.01 M phosphate, 0.15 M NaCl, pH 7.4) to a concentration of 1 mg/ml and printed on SuperAmine slides (Arrayit, Sunnyvale, CA, USA) using a piezoelectric noncontact printer (Nano plotter; GeSiM, GmbH, Germany). Three spots per lectin were printed in each block and 12 blocks were printed per slide. The total volume of each spot was 2.5 nL, which resulted from spotting of 500 pL for 5 times. The slides were incubated in a humidity-controlled incubator (> 45% humidity) overnight to allow lectin immobilization. After incubation, the slides were blocked with 1% BSA/PBS for 1 h and washed three times with PBST (0.1% Tween 20 in PBS). U373 and HSR-GBM1 cells were labeled with 10 µM CFSE cell-tracing dye (Invitrogen, Carlsbad, CA, USA) for 15 min in darkness. Each lectin blot on the slide was seeded with 2 × 105 CFSE-labeled cells in PBS with 0.5 mM CaCl2 and 1% BSA. Cells were incubated with lectin microarrays at room temperature for 40 min in darkness. After being washed with PBS for 5 min, the slides were air-dried and scanned with a microarray scanner (Genepix 4000A; Axon). Genepix 6.0 was used to analyze the images.
Table 1.
Carbohydrate specificities of the 16 lectins used for lectin microarray.
| Lectin | Abbreviation | Carbohydrate specificity | Source |
|---|---|---|---|
| Concanavalin A | Con A | Branched and terminal α-linked Man | Vector Laboratories Inc. |
| Ulex europaeus lectin I | UEA-1 | α-linked Fuc | EY Laboratories Inc. |
| Sambucus nigra lectin II | SNA-2 | GalNAcβ > Galβ | EY Laboratories Inc. |
| Wheat germ agglutinin | WGA | GlcNAcβ | EY Laboratories Inc. |
| Sambucus nigra lectin | SNA | Neu5Acα (2–6)Gal | Vector Laboratories Inc. |
| Lycopersicon esculentum (Tomato) lectin |
LEL | GlcNAc (prefers trimer and tetramer) | Vector Laboratories Inc. |
| Helix pomatia agglutinin | HPA | GalNAcα (Terminal) | EY Laboratories Inc. |
| Phaseolus vulgaris leucoagglutinin |
PHA-L | Galβ (1–4)GlcNAcβ (1–6)Man | EY Laboratories Inc. |
| Maackia amurensis lectin II | MAL-2 | Neu5Acα (2–3) | Vector Laboratories Inc. |
| Aleuria aurentia lectin | AAL | Terminal α-linked Fuc | Vector Laboratories Inc. |
| Lens culinaris agglutinin | LCA | α-linked mannose, Fucα (1–6)GlcNAc | Vector Laboratories Inc. |
| Solanum tuberosum lectin | STL | GlcNAcβ (1–4)GlcNAc | Vector Laboratories Inc. |
| Bauhinia purpurea lectin | BPL | Galβ (1–3)GalNAc, GalNAc | Vector Laboratories Inc. |
| Trichosanthes kirilowii | TKA | Galβ | EY Laboratories Inc. |
| Peanut agglutinin | PNA | Galβ (terminal) | Vector Laboratories Inc. |
| Soybean agglutinin | SBA | Terminal α- or β-linked GalNAc | Vector Laboratories Inc. |
Man, mannose; Fuc, fucose; GalNAc, N-acetyl-D-galactosamine; Gal, galactose; GlcNAc, N-acetyl-D-glucosamine; Neu5Ac, N-acetylneuraminic acid (a member of the sialic acid family).
Protein extraction
Cells were washed once with PBS and then suspended in 1 ml of lysis buffer (1% octyl-β-D-glucopyranoside, 20 mM Tris-HCl, pH7.4, 150 mM NaCl and 1% protease inhibitor mixture (Sigma-Aldrich). The cells were homogenized with 30 strokes in a Dounce glass homogenizer with a tight-fitting pestle. The cell lysate was centrifuged at 40,000g for 30 min at 4 °C. The supernatant was collected and the protein concentration was determined by the Bradford method.22
Capture of glycoproteins by lectin affinity
A Pierce centrifuge column was packed with 1.5 mL of agarose-bound peanut agglutinin (PNA) or trichosanthes kirilowii (TKA). The column was first washed with 3 volume of binding buffer (20 mM Tris-HCl, pH7.4, 150 mM NaCl, 1mM MgCl2, 1mM CaCl2 and 1mM MnCl2). Cell lysate containing 800 µg of protein was diluted four times with binding buffer, and passed through the column twice. The column was washed with 6 mL of binding buffer to remove the nonspecific binding proteins. Finally, the captured glycoproteins were released with 3 mL of elution buffer (200 mM D-galactose in binding buffer, pH 7.4). This step was repeated twice, and the eluted fractions were pooled. For mass spectrometric analysis, the samples were concentrated with Microcon YM-10 centrifugal filter devices, reduced with 5mM disulfide bond reducing agent Tris(2-carboxyethyl)phosphine (TCEP) for 30 min, alkylated with 25 mM iodoacetamide for 20 min, and digested with trypsin and PNGase F (Sigma-Aldrich).
Mass Spectrometry
The resulting tryptic peptides were analyzed by LC-MS/MS using an LTQ mass spectrometer (Thermo Finnigan, San Jose, CA). Chromatographic separation of peptides was performed on a Paradigm MG4 micropump system (Michrom Biosciences Inc., Auburn, CA) equipped with a C18 separation column (0.1 mm × 150 mm, C18 AQ particles, 5 µm, 120 Å, Michrom Biosciences Inc., Auburn, CA). Peptides were separated with a linear gradient of acetonitrile/water containing 0.1% formic acid at a flow rate of 300 nl/min. A 90 min linear gradient from 5 to 40% acetonitrile was used. The MS instrument was operated in positive ion mode. The ESI spray voltage was set at 2.5 kV, and the capillary voltage at 30 V. The ion activation was achieved by utilizing helium at a normalized collision energy of 35%. The data were acquired in data-dependent mode using the Xcaliber software. For each cycle of one full mass scan (range of m/z 400−2000), the three most intense ions in the spectrum were selected for tandem MS analysis, unless they appeared in the dynamic or mass exclusion lists.
Western blotting
Western blotting was performed essentially as described before.23 Briefly, 20 µg of proteins from HSR-GBM1, U373, U87 and T98G GBM cells were separated by 4–20% SDS-PAGE and then transferred to PVDF membranes (Bio-Rad, USA). U87 and T98G cell lysates were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The membranes were blocked for 2 h, and then incubated with various primary antibodies for 4 h or overnight. Anti-NG2 and anti-CD44 were obtained from Millipore (Billerica, MA, USA); anti-PODXL and anti-beta actin were from Abcam (Cambridge, MA, USA); anti-CD90 and anti-Tenascin-C were from Abnova (Taipei, China); anti-Receptor-type tyrosine-protein phosphatase zeta (PTPRZ1) was from Sigma-Aldrich (St. Louis, MO, USA). After being washed three times, the membranes were incubated with peroxidase-conjugated IgG (H+L) for 1 h, washed three times, and detected by Immobilon Western Chemiluminescent HRP Substrate Kit (Millipore, Billerica, MA, USA).
Data analysis
All MS/MS spectra were searched against the IPI database (IPI.human.v3.39). The search was performed using SEQUEST algorithm version 27 incorporated in Bioworks software version 3.1 SR1 (Thermo Finnigan). The search parameters were as follows: (1) Fixed modification, Carbamidomethyl of C; (2) variable modification, oxidation of M; (3) allowing two missed cleavages; (4) peptide ion mass tolerance 1.50 Da; (5) fragment ion mass tolerance 0.0 Da; (6) peptide charges +1, +2, and +3. The identified peptides were processed by the Trans-Proteomic Pipeline (TPP).24 This software includes both the PeptideProphet and ProteinProphet programs. The database search results were first validated using the PeptideProphet software, and then the peptides were assigned for protein identification using the ProteinProphet software. In this study, both the PeptideProphet probability score and the ProteinProphet probability score were set to be higher than 0.9. This resulted in an overall false positive rate below 1%.25
We performed a comparative analysis between two cell lines using the spectral counting method.26 Spectral counting is an isotope label-free quantitation method to measure relative abundance between proteins in a complex protein mixture. The spectral count of individual proteins in both samples is acquired after TPP analysis. Spectral count fold-change was calculated as the ratio of the average spectral count for the target protein. A 2-fold change was considered as a significant difference between the two samples.
Results and discussion
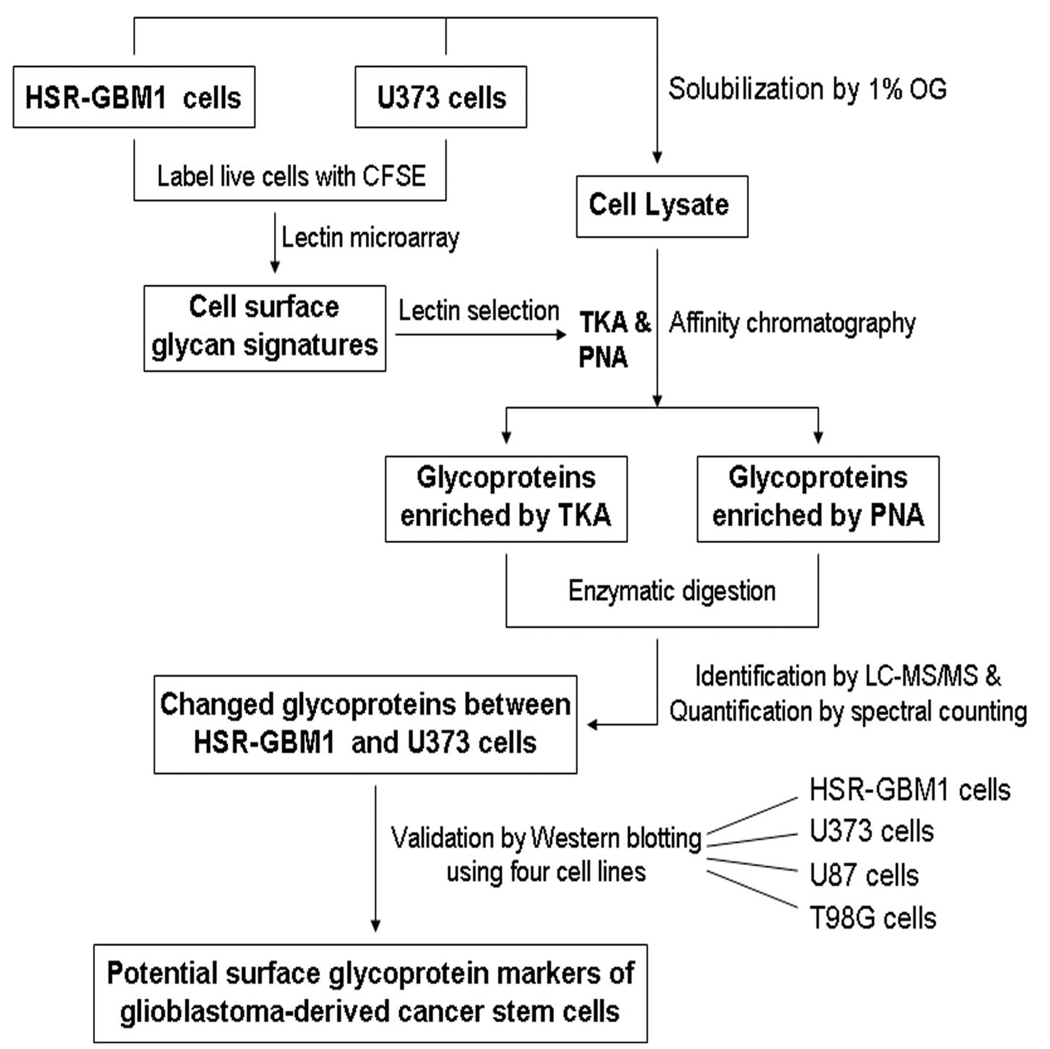
In the present work, we employed a method combining lectin microarray, lectin affinity chromatography and LC-MS/MS to identify the cell surface glycoprotein markers for a GBM-derived stem-like cell line. A diagram of the workflow of this method is shown in Figure 1. The cell surface glycan expression patterns of the stem-like cell line and a control cell line were first explored by fluorescence-assisted lectin microarray, and then two lectins TKA and PNA were selected to capture the glycoproteins of the two cell lines by affinity chromatography. Subsequently, the glycoproteins were identified by LC-MS/MS and quantified by the spectral counting method. Finally, the differentially expressed glycoproteins were further verified by Western blotting analysis.
Figure 1. Workflow of lectin microarray and LC-MS/MS based identification of cell surface glycoprotein markers.
The HSR-GBM1 and U373 live cells were first labeled with a fluorescent dye CFSE, and then their cell surface glycan signatures were profiled by lectin microarray. According to the binding patterns of the two cell lines to the arrays, two lectins TKA and PNA were selected for subsequent enrichment of glycoproteins by affinity chromatography. The glycoproteins were identified by LC-MS/MS and quantified by spectral counting. Finally, the changed glycoproteins between the two cell lines were further validated by Western blotting using four cell lines including the human GBM-derived stem-like cell line HSR-GBM1 and three traditional adherent human GBM cell lines U373, U87 and T98G.
Illustration of cell surface glycan signatures by lectin microarray
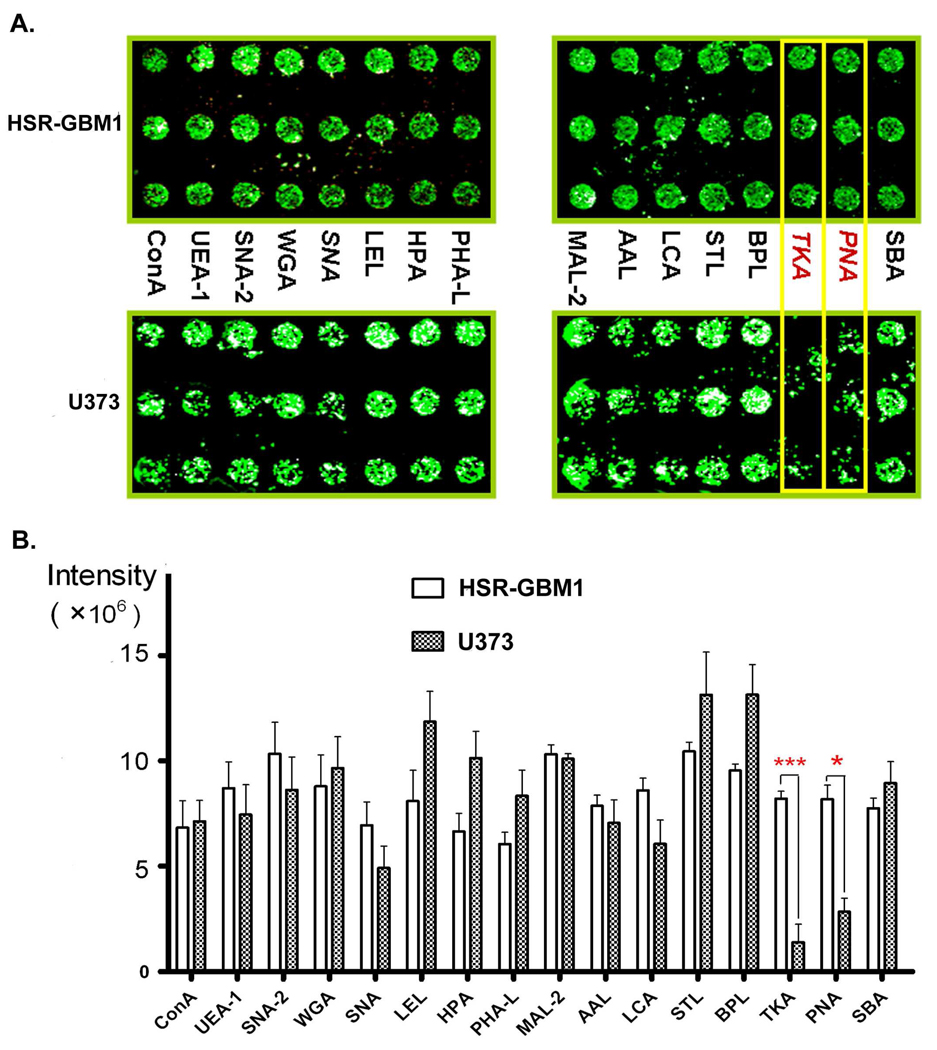
Lectin microarrays consisting of 16 lectins were used to profile the surface glycan signature of the HSR-GBM1 GBM neurosphere. As shown in Table 1, the carbohydrate specificities of the 16 lectins range extensively. The HSR-GBM1 cell line is a GBM neurosphere cell line derived from human glioblastoma, and it displayed self-renewal and multi-lineage differentiation features of neural stem cells.6 To investigate the specific glycan patterns on the surface of this undifferentiated cell line, a widely used traditional adherent GBM cell line U373 was chosen as a control. Figure 2A shows the binding patterns of fluorescently labeled live cells to the lectin microarrays. Note that the lectins TKA and PNA had much stronger binding capacity to HSR-GBM1 cells than to U373 cells. Both of the two lectins showed significant differences in fluorescent intensities, while all the other 14 lectins showed similar intensities (Figure 2B). This indicates TKA and PNA may be used to distinguish the two cell lines.
Figure 2. Lectin microarray.
(A) A typical lectin microarray image depicts the binding patterns of two cell lines to the arrays of 16 lectins. Note that TKA and PNA lectins captured many more HSR-GBM1 cells than U373 cells. Each lectin was spotted in triplicate. (B) Fluorescent intensities of the 16 lectins. Spot intensities were the average of three values. *p<0.05; ***p<0.001
Interestingly, both TKA and PNA have binding specificities to β-galactose (Table 1), which implies much higher expression of galactosylated glycoproteins on the surface of the GBM-derived stem-like cells as compared with traditional adherent GBM cells. In a previous study, the galactosylated glycoproteins were found to be expressed at higher level in glioma compared with normal brains.27 Our findings suggest that the increase of galactosylation in glioma brain tissues might be contributed by cancer stem-like cells.
As shown in Table 1, the lectin Sambucus nigra lectin II (SNA-2) also has binding specificity to β-galactose, but it primarily binds to β-GalNAc. Therefore, we did not observe a significant difference in fluorescent intensities for SNA-2 (Figure 2).
Preparation and identification of membrane glycoproteins
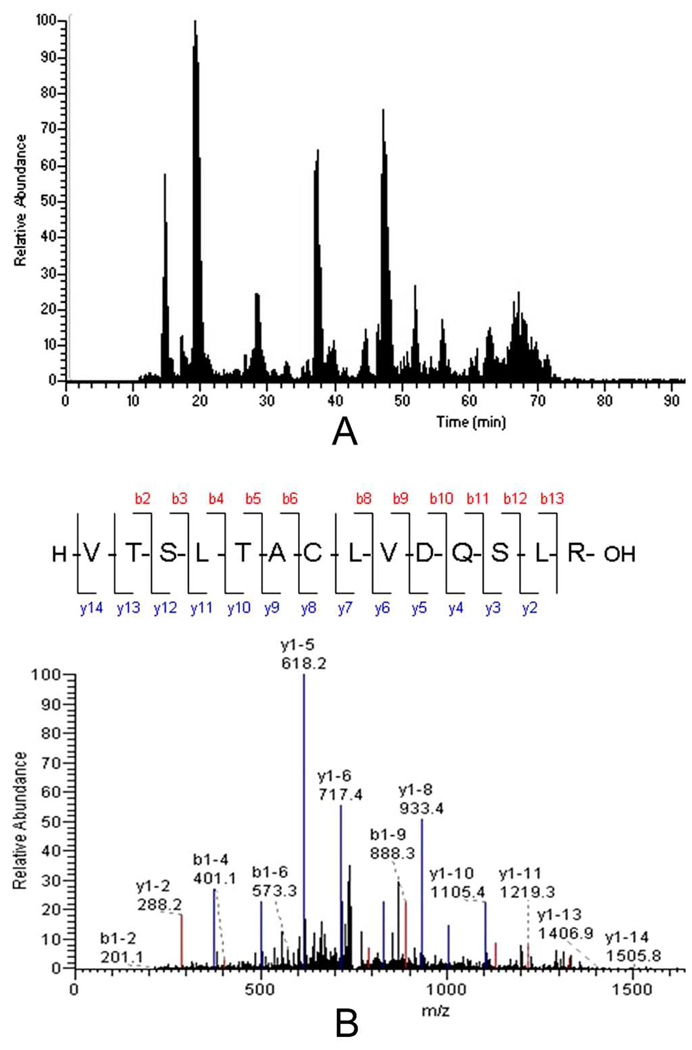
The binding patterns of TKA and PNA (Figure 2A) suggest that their binding partners on the cell surface were potential markers to distinguish the two cell lines. To discover these glycoprotein markers, we applied lectin affinity chromatography to capture glycoproteins from the HSR-GBM1 and U373 cells. A non-ionic detergent octyl-β-D-glucopyranoside was used to lyse cells. This class of detergents can improve the solubility of membrane proteins.28–29 Agarose-bound TKA and PNA were packed into a column to capture the glycoproteins. The eluted glycoproteins from three independent enrichment experiments were digested and analyzed by LC-MS/MS using an LTQ mass spectrometer. As an example, Figure 3A shows a representative base peak chromatogram of TKA captured fraction from HSR-GBM1 cells, and Figure 3B is a typical MS/MS spectrum.
Figure 3. LC-MS/MS analysis.
(A) A representative base peak chromatogram of TKA captured fraction from HSR-GBM1 cells. (B) MS/MS spectrum of a peptide. The sequence of the peptide was identified as VTSLTACLVDQSLR, which was from CD90 antigen.
The LTQ analysis resulted in the identification of 43 glycoproteins from TKA enriched fractions (Supplementary Table S1) and 48 glycoproteins from PNA enriched fractions (Supplementary Table S2), wherein 24 glycoproteins overlapped by the two lectins. Consequently, a total of 67 glycoproteins were identified from the two cell lines after PNA and TKA enrichment. The detailed information of these glycoproteins is listed in Supplementary Tables S3–S6. The difference observed in protein identification from TKA and PNA enriched fractions may result from the minor difference in the carbohydrate specificities of the two lectins. Although TKA and PNA have similar binding specificities to Galβ, PNA can only bind to terminal Galβ, preferentially to terminal Galβ (1–3)GalNAcα, while TKA binds both terminal Galβ and internal Galβ (1–3)GalNAcα.
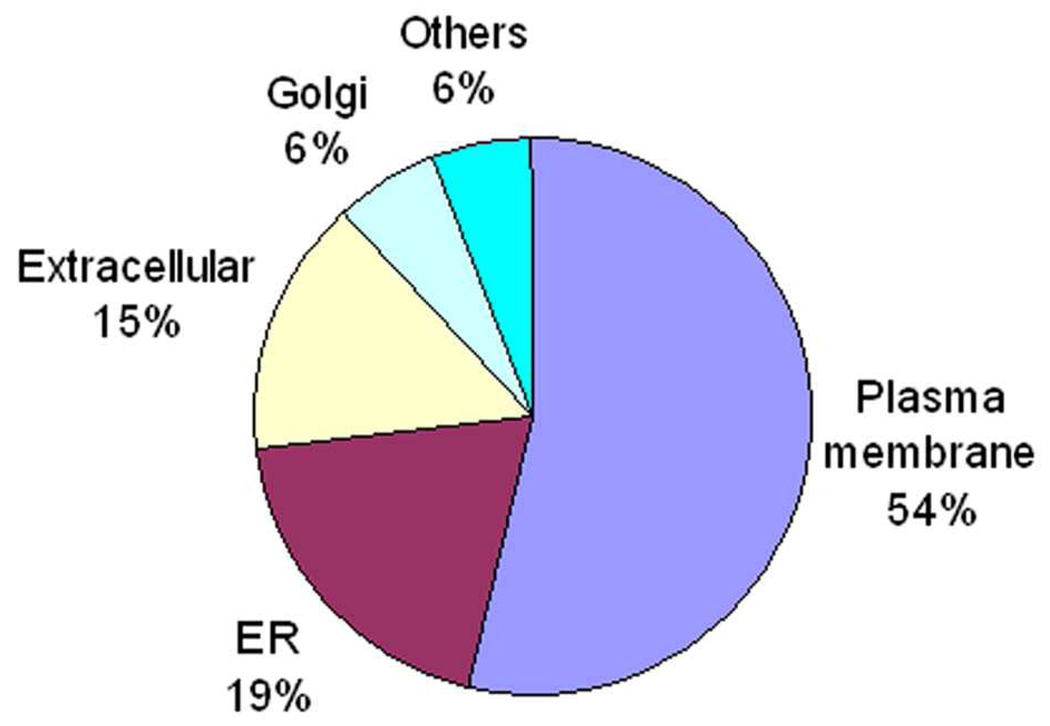
The subcellular locations of the 67 glycoproteins were annotated according to the UniProtKB database. The majority of these glycoproteins were localized to the plasma membrane, ER and extracellular (Figure 4). Approximately 54% of these glycoproteins were plasma membrane proteins.
Figure 4. Subcellular location of glycoproteins identified from HSR-GBM1 and U373 cells after TKA and PNA enrichment.
The number of glycoproteins identified after TKA and PNA enrichment in this work was relatively low compared to previous studies using other lectins to capture glycoproteins from cells.29–31 To confirm that the difference was caused by different lectins rather than our working system, we captured glycoproteins from the HSR-GBM1 and U373 cells using Concanavalin A (Con A) and analyzed these proteins by LC-MS/MS, resulting in the identification of 83 and 100 glycoproteins from the two cell lines respectively (Supplementary Tables S7 and S8). The numbers are comparable to that from similar studies.30–31 The glycoproteins bound by either TKA or PNA were less than half of those bound by Con A. This indicates that both TKA and PNA have a more narrow specificity than Con A.
A concern of the method used in this work was the reproducibility. We used R2 values from linear regression for spectral counts to investigate the reproducibility of three replicate experiments. Here 33 and 40 glycoproteins were identified from HSR-GBM1 cells after TKA and PNA enrichment respectively (Supplementary Table S3 and Table S5), and the average R2 values were around 0.7 – 0.8 for the three independent experiments of the two lectins, which are comparable to the values in some similar studies.32–33 This indicates that the experiments from cell lysis to glycoprotein enrichment, and to LTQ analysis were reproducible in the present work. Scatter plots for each TKA or PNA experiment pairs of HSR-GBM1 cells are available in Supplementary Figure S1. Consistent with the lectin microarray analysis (Figure 2), less glycoproteins were identified from U373 cells after TKA and PNA enrichment (only 23 and 10 glycoproteins were identified respectively, see Supplementary Table S4 and Table S6), and most of the spectral counts for individual proteins were less than 5. Therefore, the U373 data sets were not suitable to evaluate the reproducibility.
Differentially expressed glycoproteins analyzed by the label-free spectral counting method
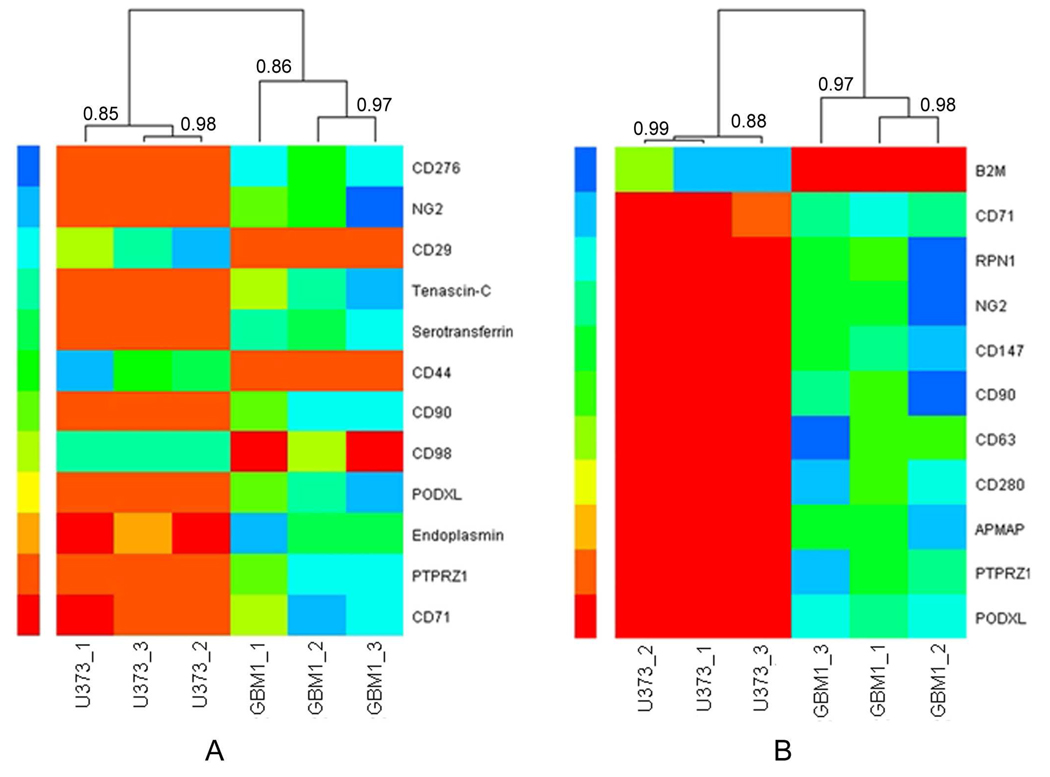
The glycoproteins identified by LC-MS/MS were analyzed by the spectral counting method to quantify the relative protein abundance.26, 34 As a result, 12 differentially expressed glycoproteins between HSR-GBM1 and U373 cells were identified from the TKA enriched fractions (Table 2), and 11 differentially expressed glycoproteins were identified from the PNA enriched fractions (Table 3), wherein 5 proteins overlapped. As shown in Table 2 and Table 3, the majority of these proteins were membrane proteins, and a large part of them were CD antigens. Note that most of these glycoproteins were highly expressed in HSR-GBM1 cells. This is consistent with the results of lectin microarray, where both TKA and PNA showed higher binding capacity to HSR-GBM1 cells than to U373 cells (Figure 2). Figure 5 shows heat maps based on the spectral counts of differentially expressed glycoproteins enriched by TKA (Figure 5A) and PNA (Figure 5B) from HSR-GBM1 and U373 cells. In this figure, protein abundance increased from red to blue color. The differential expression of these proteins was remarkable between the two cell lines. Hierarchical clustering revealed good correlation between each experimental replicate.
Table 2.
Differentially expressed glycoproteins from TKA captured fractions.
| Accession | Protein name | Gene | Location | Ratioa | p-valueb |
|---|---|---|---|---|---|
| IPI00022462 | CD71 antigen | TFRC | membrane | 12.89 | 0.0011 |
| IPI00748312 | Receptor-type tyrosine-protein phosphatase zeta |
PTPRZ1 | membrane | NAc | 0.0019 |
| IPI00027230 | Endoplasmin | HSP90B1 | ER | 2.78 | 0.0020 |
| IPI00299116 | Podocalyxin-like protein 1 | PODXL | membrane | NA | 0.0033 |
| IPI00027493 | CD98 antigen | SLC3A2 | membrane | 0.15 | 0.0058 |
| IPI00022892 | CD90 antigen | THY1 | membrane | NA | 0.0074 |
| IPI00297160 | CD44 antigen | CD44 | membrane | 0 | 0.011 |
| IPI00022463 | Serotransferrin | TF | extracellular | NA | 0.019 |
| IPI00031008 | Tenascin-C | TNC | extracellular | NA | 0.023 |
| IPI00217563 | CD29 antigen | ITGB1 | membrane | 0 | 0.039 |
| IPI00019275 | CD276 antigen | CD276 | membrane | NA | 0.045 |
| IPI00019157 | Chondroitin sulfate proteoglycan NG2 | CSPG4 | membrane | NA | 0.045 |
The ratio of the spectral count of a target protein identified from HSR-GBM1 cells to that identified from U373 cells.
p-value: statistical significance of differentially expressed proteins between HSR-GBM1 and U373 cells. The values were generated by Student’s t-test for three independent experiments.
NA means the protein was only identified from HSR-GBM1 cells.
Table 3.
Differentially expressed glycoproteins from PNA captured fractions.
| Accession | Protein name | Gene | location | Ratioa | p-valueb |
|---|---|---|---|---|---|
| IPI00299116 | Podocalyxin-like protein 1 | PODXL | membrane | NAc | 0.00005 |
| IPI00748312 | Receptor-type tyrosine-protein phosphatase zeta |
PTPRZ1 | membrane | NA | 0.00027 |
| IPI00031131 | Adipocyte plasma membrane-associated protein |
APMAP | membrane | NA | 0.00089 |
| IPI00005707 | CD280 antigen | MRC2 | membrane | NA | 0.00096 |
| IPI00215998 | CD63 antigen | CD63 | membrane | NA | 0.0015 |
| IPI00022892 | CD90 antigen | THY1 | membrane | NA | 0.0016 |
| IPI00019906 | CD147 antigen | BSG | membrane | NA | 0.0023 |
| IPI00019157 | Chondroitin sulfate proteoglycan NG2 | CSPG4 | membrane | NA | 0.0027 |
| IPI00025874 | Dolichyl-diphosphooligosaccharide-- protein glycosyltransferase subunit 1 |
RPN1 | ER | NA | 0.0036 |
| IPI00022462 | CD71 antigen | TFRC | membrane | 6.10 | 0.021 |
| IPI00004656 | Beta-2-microglobulin | B2M | extracellular | 0 | 0.029 |
The ratio of the spectral count of a target protein identified from HSR-GBM1 cells to that identified from U373 cells.
p-value: statistical significance of differentially expressed proteins between HSR-GBM1 and U373 cells. The values were generated by Student’s t-test for three independent experiments.
NA means the protein was only identified from HSR-GBM1 cells.
Figure 5. Heat maps with dendrograms of hierarchical clustering for triplicate experiments.
The heat maps were based on the spectral counts of differentially expressed glycoproteins identified from TKA (A) and PNA (B) enriched fractions of HSR-GBM1 and U373 cells. Each column indicates an independent experiment of each cell line, and each row indicates a glycoprotein identified by LC-MS/MS. The number of spectral count increased from red color to blue color. The Pearson correlation coefficients were indicated at each branching point. Both of the heat maps were generated with R language. GBM1 represents HSR-GBM1.
Western blotting analysis of selected proteins
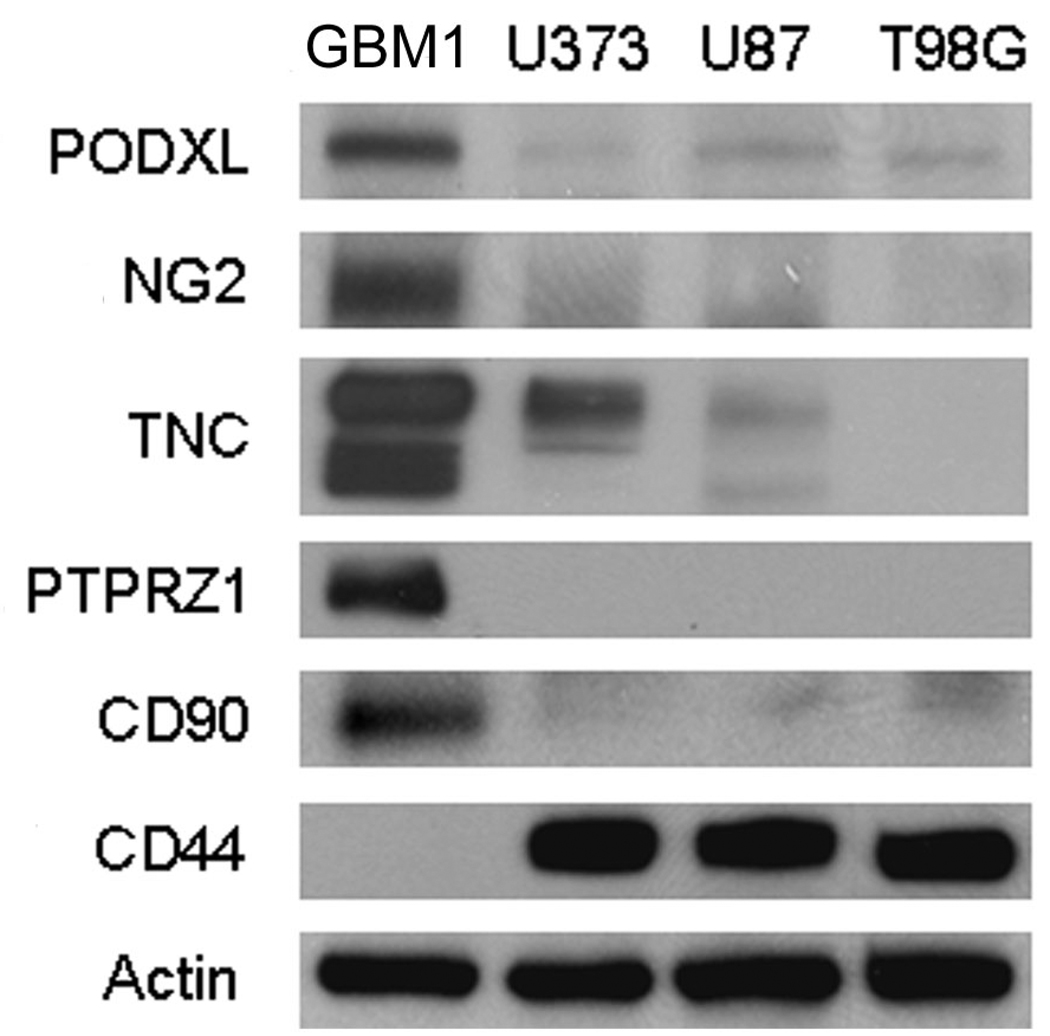
To make the previous results more confident, we applied Western blotting analysis to evaluate the changes of a suite of differentially expressed glycoproteins (Figure 6). To verify that these changes were universal between the HSR-GBM1 glioblastoma-derived stem-like cells and the GBM cells from traditional cultured adherent cell lines, we selected four cell lines including the HSR-GBM1 neurosphere line and three human GBM cell lines U373, U87 and T98G. Five glycoproteins including Podocalyxin-like protein 1 (PODXL), Chondroitin sulfate proteoglycan NG2 (NG2), Tenascin-C (TNC), Receptor-type tyrosine-protein phosphatase zeta (PTPRZ1) and CD90 were expressed at much higher levels in the HSR-GBM1 neurosphere cell line than in the three adherent GBM cell lines. CD44 antigen was undetectable in the HSR-GBM1 neurosphere cell line, while it was highly expressed in all of the other three cell lines (Figure 6). The trends in expression of all of the six proteins shown in Figure 6 were consistent with the previous MS results shown in Table 2 and Table 3.
Figure 6. Western blotting analysis of selected proteins.
Twenty micrograms of proteins from HSR-GBM1, U373, U87 and T98G cells were separated by 4–20% SDS-PAGE and transferred to PVDF membranes. The blots were probed with antibodies against Podocalyxin-like protein 1 (PODXL), Chondroitin sulfate proteoglycan NG2 (NG2), Tenascin-C (TNC), Receptor-type tyrosine-protein phosphatase zeta (PTPRZ1), CD90, CD44 and Beta-actin. GBM1 represents HSR-GBM1.
Noteworthy, PTPRZ1 was detected only in the HSR-GBM1 neurosphere cells, as shown in Figure 6. Similar to our results, PTPRZ1 was highly expressed in the undifferentiated embryonic stem cells compared with differentiated cells.35 PTPRZ1 was also found to be overexpressed in human glioblastomas compared to normal brains, and knockdown study of this protein established its function in regulating cell motility.36 Therefore, the overexpression of PTPRZ1 in cancer stem-like cells may play important roles in cell migration and cancer metastasis.
PTPRZ1 has been found to interact with a glycoprotein Tenascin-C in vivo.37–38 Tenascin-C was originally discovered as a glioma-mesenchymal extracellular matrix antigen that is ubiquitously expressed in glioblastomas but not in normal brains.39 In a recent work, this protein has been identified as a novel target gene for Notch signaling in GBM.40 Tenascin-C contributes to the generation of a stem cell niche, which is important to the development of neural stem cells.41–42 In the present work, the overexpression of Tenascin-C in the HSR-GBM1 neurosphere cell line compared to the other three adherent GBM cell lines (Figure 6) indicates that the protein is mainly expressed in the stem-like cells in GBM. Tenascin-C has previously been shown to interact with Chondroitin sulfate proteoglycan NG2 43, which was also highly expressed in the HSR-GBM1 cells (Figure 6). NG2 has been widely used as a marker of multiponent neural progenitor cells.44–46
The interactions among PTPRZ1, Tenascin-C and NG2 may play important roles in the development and metastasis of GBM. These glycoproteins may be used as markers for GBM stem-like cells, and they may be targeted for drug treatment of GBM patients. An anti-Tenascin monoclonal antibody has been studied for clinical treatment of malignant brain tumors.47 PTPRZ1 and NG2 may also be used for the similar clinical studies.
The changes of three other glycoproteins Podocalyxin-like protein 1, CD90 and CD44 were also confirmed by Western blotting analysis. Podocalyxin-like protein 1 belongs to the CD34 family, and it has been used as a marker of embryonic and hematopoietic stem cells.48–49 CD90 has been identified as a marker of cancer stem cells in human liver cancer. 50 CD44 is the receptor for hyaluronic acid, and it has been used to fractionate cancer stem-like cells from several kinds of solid tumors including breast 51, head and neck 52, pancreatic 53 and colorectal 54 cancers. However, this protein was not detectable in the GBM-derived stem-like cells in our work (Figure 6).
Conclusions
We employed a method combining lectin microarray and LC-MS/MS to discover the cell surface glycoprotein markers of a GBM-derived cancer stem-like cell line. The cell surface glycan expression patterns of a GBM-derived stem-like neurosphere line HSR-GBM1 and a traditional adherent GBM cell line U373 were first explored by lectin microarrays consisting of 16 lectins. Two galactose-specific lectins TKA and PNA showed much stronger binding capacity to HSR-GBM1 neurosphere cells than to U373 cells, indicating higher expression of galactosylated glycoproteins on the surface of GBM stem-like cells. Agarose-bound TKA and PNA were then used to capture the glycoproteins of the two cell lines by affinity chromatography, followed by protein identification by LC-MS/MS and quantification by spectral counting. Consequently, 12 differentially expressed glycoproteins between HSR-GBM1 and U373 cells were identified from the TKA enriched fractions, and 11 were identified from the PNA enriched fractions. Of these proteins, 5 were overlapped, making it a total of 18 differentially expressed glycoproteins. The changes of six interesting glycoproteins were further verified by Western blotting analysis using four cell lines including the HSR-GBM1 neurosphere line and three adherent GBM cell lines U373, U87 and T98G. These proteins include the overexpressed Receptor-type tyrosine-protein phosphatase zeta, Tenascin-C, Chondroitin sulfate proteoglycan NG2, Podocalyxin-like protein 1 and CD90, and the down-regulated CD44. A better understanding of these proteins may bring an exciting opportunity for identifying markers for cancer stem-like cells in GBM.
Supplementary Material
Acknowledgement
This work was funded under the National Institute of Health Grant No. R01 49500 (D.M.L.) and the National Cancer Institute Grant No. R21 CA134623 (D.M.L.). We would like to acknowledge grant support to Dr. Fan from Accelerate Brain Cancer Cure Project Award, American Brain Tumor Association Translational Grant, and Voices Against Brain Cancer Research Grant.
Footnotes
Supporting Information Available: Glycoproteins identified from TKA-captured fractions (Table S1) and PNA-captured fractions (Table S2). Detailed information of the glycoproteins identified from HSR-GBM1 and U373 cells after TKA, PNA or Con A enrichment (Table S3 – S8). Scatter plots for each Con A experiment pairs (Figure S1). This material is available free at http://pubs.acs.org.
Contributor Information
Jintang He, Email: jintang@umich.edu.
Yashu Liu, Email: yashul@umich.edu.
Xiaolei Xie, Email: xlxie@umich.edu.
Thant Zhu, Email: thant@umich.edu.
Mary Soules, Email: mfield@med.umich.edu.
Francesco DiMeco, Email: fdimeco1@jhmi.edu.
Angelo L. Vescovi, Email: vescovi@tin.it.
Xing Fan, Email: xingf@umich.edu.
David M. Lubman, Email: dmlubman@umich.edu.
References
- 1.Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schuler D, Probst-Hensch NM, Maiorka PC, Baeza N, Pisani P, Yonekawa Y, Yasargil MG, Lutolf UM, Kleihues P. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64(19):6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 2.Reardon DA, Rich JN, Friedman HS, Bigner DD. Recent advances in the treatment of malignant astrocytoma. J Clin Oncol. 2006;24(8):1253–1265. doi: 10.1200/JCO.2005.04.5302. [DOI] [PubMed] [Google Scholar]
- 3.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 4.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 5.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39(3):193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 6.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 7.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 8.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353(8):811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 9.Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, Eberhart CG. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66(15):7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 10.Fan X, Eberhart CG. Medulloblastoma stem cells. J Clin Oncol. 2008;26(17):2821–2827. doi: 10.1200/JCO.2007.15.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J, Nikkhah G, Dimeco F, Piccirillo S, Vescovi AL, Eberhart CG. Notch Pathway Blockade Depletes CD133-Positive Glioblastoma Cells and Inhibits Growth of Tumor Neurospheres and Xenografts. Stem Cells. 2009 doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirabayashi J. Lectin-based structural glycomics: glycoproteomics and glycan profiling. Glycoconj J. 2004;21(1–2):35–40. doi: 10.1023/B:GLYC.0000043745.18988.a1. [DOI] [PubMed] [Google Scholar]
- 13.Kuno A, Uchiyama N, Koseki-Kuno S, Ebe Y, Takashima S, Yamada M, Hirabayashi J. Evanescent-field fluorescence-assisted lectin microarray: a new strategy for glycan profiling. Nat Methods. 2005;2(11):851–856. doi: 10.1038/nmeth803. [DOI] [PubMed] [Google Scholar]
- 14.Pilobello KT, Mahal LK. Lectin microarrays for glycoprotein analysis. Methods Mol Biol. 2007;385:193–203. doi: 10.1007/978-1-59745-426-1_14. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, He J, Li C, Benitez R, Fu S, Marrero J, Lubman DM. Identification and confirmation of biomarkers using an integrated platform for quantitative analysis of glycoproteins and their glycosylations. J Proteome Res. 2010;9(2):798–805. doi: 10.1021/pr900715p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng T, Peelen D, Smith LM. Lectin arrays for profiling cell surface carbohydrate expression. J Am Chem Soc. 2005;127(28):9982–9983. doi: 10.1021/ja0505550. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Zheng T, Shortreed MR, Alexander C, Smith LM. Analysis of cell surface carbohydrate expression patterns in normal and tumorigenic human breast cell lines using lectin arrays. Anal Chem. 2007;79(15):5698–5702. doi: 10.1021/ac070423k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee MR, Park S, Shin I. Protein microarrays to study carbohydrate-recognition events. Bioorg Med Chem Lett. 2006;16(19):5132–5135. doi: 10.1016/j.bmcl.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Tao SC, Li Y, Zhou J, Qian J, Schnaar RL, Zhang Y, Goldstein IJ, Zhu H, Schneck JP. Lectin microarrays identify cell-specific and functionally significant cell surface glycan markers. Glycobiology. 2008;18(10):761–769. doi: 10.1093/glycob/cwn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JE, Mirza SP, Didier DN, Scalf M, Olivier M, Greene AS, Smith LM. Identification of cell surface markers to differentiate rat endothelial and fibroblast cells using lectin arrays and LC-ESI-MS/MS. Anal Chem. 2008;80(21):8269–8275. doi: 10.1021/ac801390b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun P, Xia S, Lal B, Eberhart CG, Quinones-Hinojosa A, Maciaczyk J, Matsui W, Dimeco F, Piccirillo SM, Vescovi AL, Laterra J. DNER, an epigenetically modulated gene, regulates glioblastoma-derived neurosphere cell differentiation and tumor propagation. Stem Cells. 2009;27(7):1473–1486. doi: 10.1002/stem.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.He J, Liu Y, He S, Wang Q, Pu H, Ji J. Proteomic analysis of a membrane skeleton fraction from human liver. J Proteome Res. 2007;6(9):3509–3518. doi: 10.1021/pr070197v. [DOI] [PubMed] [Google Scholar]
- 24.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74(20):5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 25.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75(17):4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76(14):4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 27.Xu S, Zhu X, Zhang S, Yin S, Zhou L, Chen C, Gu J. Over-expression of beta-1,4-galactosyltransferase I, II, and V in human astrocytoma. J Cancer Res Clin Oncol. 2001;127(8):502–506. doi: 10.1007/s004320100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garner AE, Smith DA, Hooper NM. Visualization of detergent solubilization of membranes: implications for the isolation of rafts. Biophys J. 2008;94(4):1326–1340. doi: 10.1529/biophysj.107.114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald CA, Yang JY, Marathe V, Yen TY, Macher BA. Combining results from lectin affinity chromatography and glycocapture approaches substantially improves the coverage of the glycoproteome. Mol Cell Proteomics. 2009;8(2):287–301. doi: 10.1074/mcp.M800272-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Ao X, Vuong H, Konanur M, Miller FR, Goodison S, Lubman DM. Membrane glycoproteins associated with breast tumor cell progression identified by a lectin affinity approach. J Proteome Res. 2008;7(10):4313–4325. doi: 10.1021/pr8002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez-Manilla G, Warren NL, Atwood JA, Dalton S, Orlando R, Pierce M. Glycoproteomic analysis of embryonic stem cells: identification of potential glycobiomarkers using lectin affinity chromatography of glycopeptides. J Proteome Res. 2009 doi: 10.1021/pr8007489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niittyla T, Fuglsang AT, Palmgren MG, Frommer WB, Schulze WX. Temporal analysis of sucrose-induced phosphorylation changes in plasma membrane proteins of Arabidopsis. Mol Cell Proteomics. 2007;6(10):1711–1726. doi: 10.1074/mcp.M700164-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Yu H, Wakim B, Li M, Halligan B, Tint GS, Patel SB. Quantifying raft proteins in neonatal mouse brain by 'tube-gel' protein digestion label-free shotgun proteomics. Proteome Sci. 2007;5:17. doi: 10.1186/1477-5956-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang B, VerBerkmoes NC, Langston MA, Uberbacher E, Hettich RL, Samatova NF. Detecting differential and correlated protein expression in label-free shotgun proteomics. J Proteome Res. 2006;5(11):2909–2918. doi: 10.1021/pr0600273. [DOI] [PubMed] [Google Scholar]
- 35.Prokhorova TA, Rigbolt KT, Johansen PT, Henningsen J, Kratchmarova I, Kassem M, Blagoev B. Stable isotope labeling by amino acids in cell culture (SILAC) and quantitative comparison of the membrane proteomes of self-renewing and differentiating human embryonic stem cells. Mol Cell Proteomics. 2009;8(5):959–970. doi: 10.1074/mcp.M800287-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller S, Kunkel P, Lamszus K, Ulbricht U, Lorente GA, Nelson AM, von Schack D, Chin DJ, Lohr SC, Westphal M, Melcher T. A role for receptor tyrosine phosphatase zeta in glioma cell migration. Oncogene. 2003;22(43):6661–6668. doi: 10.1038/sj.onc.1206763. [DOI] [PubMed] [Google Scholar]
- 37.Grumet M, Milev P, Sakurai T, Karthikeyan L, Bourdon M, Margolis RK, Margolis RU. Interactions with tenascin and differential effects on cell adhesion of neurocan and phosphacan, two major chondroitin sulfate proteoglycans of nervous tissue. J Biol Chem. 1994;269(16):12142–12146. [PubMed] [Google Scholar]
- 38.Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J Cell Biol. 1994;127(6 Pt 1):1703–1715. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourdon MA, Wikstrand CJ, Furthmayr H, Matthews TJ, Bigner DD. Human glioma-mesenchymal extracellular matrix antigen defined by monoclonal antibody. Cancer Res. 1983;43(6):2796–2805. [PubMed] [Google Scholar]
- 40.Sivasankaran B, Degen M, Ghaffari A, Hegi ME, Hamou MF, Ionescu MC, Zweifel C, Tolnay M, Wasner M, Mergenthaler S, Miserez AR, Kiss R, Lino MM, Merlo A, Chiquet-Ehrismann R, Boulay JL. Tenascin-C is a novel RBPJkappa-induced target gene for Notch signaling in gliomas. Cancer Res. 2009;69(2):458–465. doi: 10.1158/0008-5472.CAN-08-2610. [DOI] [PubMed] [Google Scholar]
- 41.Garcion E, Halilagic A, Faissner A, ffrench-Constant C. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development. 2004;131(14):3423–3432. doi: 10.1242/dev.01202. [DOI] [PubMed] [Google Scholar]
- 42.von Holst A. Tenascin C in stem cell niches: redundant, permissive or instructive? Cells Tissues Organs. 2008;188(1–2):170–177. doi: 10.1159/000112848. [DOI] [PubMed] [Google Scholar]
- 43.Burg MA, Tillet E, Timpl R, Stallcup WB. Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix molecules. J Biol Chem. 1996;271(42):26110–26116. doi: 10.1074/jbc.271.42.26110. [DOI] [PubMed] [Google Scholar]
- 44.Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61(5):471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 45.Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24(1):39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- 46.Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia. 2007;9(11):882–892. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akabani G, Reardon DA, Coleman RE, Wong TZ, Metzler SD, Bowsher JE, Barboriak DP, Provenzale JM, Greer KL, DeLong D, Friedman HS, Friedman AH, Zhao XG, Pegram CN, McLendon RE, Bigner DD, Zalutsky MR. Dosimetry and radiographic analysis of 131I-labeled anti-tenascin 81C6 murine monoclonal antibody in newly diagnosed patients with malignant gliomas: a phase II study. J Nucl Med. 2005;46(6):1042–1051. [PubMed] [Google Scholar]
- 48.Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci. 2008;121(Pt 22):3683–3692. doi: 10.1242/jcs.037507. [DOI] [PubMed] [Google Scholar]
- 49.Doyonnas R, Nielsen JS, Chelliah S, Drew E, Hara T, Miyajima A, McNagny KM. Podocalyxin is a CD34-related marker of murine hematopoietic stem cells and embryonic erythroid cells. Blood. 2005;105(11):4170–4178. doi: 10.1182/blood-2004-10-4077. [DOI] [PubMed] [Google Scholar]
- 50.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13(2):153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 54.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.