Abstract
Objectives
The age-associated decline in sex hormone levels in men is paralleled by an increase in cardiovascular disease and associated risk factors including low grade chronic inflammation. The objective of this analysis was to investigate the association between sex hormone levels and C-reactive protein (CRP) in a population-based sample of men.
Design
Population-based, cross-sectional observational survey.
Participants
A multistage stratified design was used to recruit a random sample of 2,301 racially and ethnically diverse men age 30–79 years. Blood samples were obtained on 1,899 men. Analyses were conducted on 1,559 men with complete data on CRP and sex hormone levels.
Measurements
High sensitivity CRP levels. The association between CRP and sex hormone levels was assessed using multiple linear regression models.
Results
An inverse association was observed, in both bivariate and multivariate analyses, between CRP and total testosterone, free testosterone, and sex hormone-binding globulin (SHBG) levels. These associations remained statistically significant after adjusting for age, body mass index (BMI), comorbid conditions, and lifestyle factors. A positive trend between estradiol (total and free) and CRP levels was not statistically significant.
Conclusions
A robust, inverse dose-response correlation between testosterone and SHBG levels with CRP levels provides further evidence of a potential role of androgens in inflammatory processes.
Keywords: sex hormones, C-reactive protein, epidemiology
Introduction
The age-associated decline in sex hormone levels in men is paralleled by an increase in cardiovascular disease and associated risk factors.1 Low grade chronic inflammation has emerged as an independent predictor of cardiovascular events,2 and an increase in inflammatory status with age has been reported.3 Although a role of sex hormones in inflammatory processes has been hypothesized,4 few studies have investigated the association between sex hormone levels and inflammatory markers, and reported results have not been consistent. Cross-sectional data from the InCHIANTI study show an inverse association between total testosterone levels and soluble interleukin-6 receptor (sIL6r), but not with IL-6, TNF-α or IL-1β.5 In contrast, a recent cross-sectional analysis of 400 men age 40–80 years reports a positive association of C-reactive protein (CRP) levels with estradiol (E2) but not with total or bioavailable testosterone levels.6 Randomized trials of testosterone supplementation have shown a decrease in TNF-α and IL-1β levels,7 but no effect on CRP.8
Using data from the Boston Area Community Health (BACH) Survey, the objectives of this analysis were to investigate the association between CRP and sex hormone levels in a racially and ethnically diverse population-based sample of men.
Materials and Methods
Overall Design
The BACH survey is a population-based epidemiologic survey of a broad range of urologic symptoms and risk factors in a randomly selected sample. Detailed methods have been described elsewhere.9 In brief, BACH used a multi-stage stratified random sample to recruit approximately equal numbers of subjects according to age (30–39, 40–49, 50–59, 60–79 years), gender, and race/ethnic group (African American (Black), Hispanic, and Caucasian (White)). The BACH sample was recruited from April 2002 through June 2005. Interviews were completed with 63.3% of eligible subjects, resulting in a total sample of 5504 adults (2301 men, 3203 women, 1767 Black, 1877 Hispanic, 1859 White respondents). All protocols and informed consent procedures were approved by the New England Research Institutes’ Institutional Review Board. All subjects provided written informed consent.
Data collection
Data were obtained during a 2-hour in-person interview, conducted by a trained (bilingual) phlebotomist/interviewer, generally in the subject’s home. A venous blood sample (20 ml) was obtained and height, weight, and hip and waist circumference were measured along with self-reported information on medical and reproductive history, major comorbidities, lifestyle and psychosocial factors, and symptoms of urogynecological conditions. Two blood pressure measurements were obtained during the interview and were averaged. BACH participants were asked to gather all prescription, over-the-counter and alternative medications in the home used by them over the past 4 weeks for recording of the label information by the interviewer. Additionally, participants were asked separately if they were taking medications for specific indications, such as high cholesterol and high blood pressure.
Hormones
Non-fasting blood samples were collected close to waking time (median time since awakening 3 h 38 min) to control for diurnal variation in hormone levels. Serum testosterone (T) and sex hormone-binding globulin (SHBG) levels were measured by competitive electrochemiluminescence immunoassays on the 2010 Elecsys system (Roche Diagnostics, Indianapolis, IN). All assays were previously approved by the Food and Drug Administration for clinical use. The lower limits of detection for T and SHBG were 0.07 nmol/L (2 ng/dL) and 3 nmol/L, respectively. Reference ranges are 9–27.8 nmol/L (260–801 ng/dL) for T and 14.5–48.4 nmol/L for SHBG. The inter-assay coefficients of variation (CV) for T at concentrations of 0.8, 9.5 and 24.3 nmol/L (24, 275, and 700 ng/dL) were 7.4, 2.2, and 1.7%, respectively. For SHBG at 16.5, 25, and 64 nmol/L, inter-assay CVs were 3.9, 2.4, and 2.2%, respectively. Estradiol (E2) was measured by the Mayo Clinic Core Laboratory (Rochester, MN) with liquid chromatography-tandem mass spectrometry. The lower limit of detection was 46 pmol/L (12.5 pg/mL). To reliably measure E2 levels in the low range, E2 values less than 46 pmol/L (12.5 pg/mL) were calculated by manual integration of chromatograms. The inter-assay CVs for E2 concentrations 4.6–220 pmol/L (1.25–60 pg/mL) ranged between 13.4–6.0%. Free testosterone (FT) and estradiol (FE2) concentrations were calculated from total T (TT) or E2 and SHBG concentrations using mass action equations assuming a fixed albumin concentration.10
High-sensitivity C-Reactive Protein (CRP) levels
The concentration of CRP was determined using an immunoturbidimetric assay on the Hitachi 917 analyzer (Roche Diagnostics - Indianapolis, IN), using reagents and calibrators from DiaSorin (Stillwater, MN). In this assay, an antigen-antibody reaction occurs between CRP in the sample and an anti-CRP antibody that has been sensitized to latex particles, and agglutination results. This antigen-antibody complex causes an increase in light scattering, which is detected spectrophotometrically, with the magnitude of the change being proportional to the concentration of CRP in the sample. Assays were performed at the Children’s Hospital Medical Center Research Laboratories, Boston, MA, with a reported a sensitivity of 0.28 nmol/L (0.03 mg/L). The coefficients of variation at concentrations of 8.67, 29.24, and 127.43 nmol/L (0.91, 3.07, 13.38 mg/L) are 2.81, 1.61 and 1.1%, respectively.
Covariates
Potential confounders included in the analysis include sociodemographic, lifestyle factors, and comorbid conditions. Age was categorized by decades: 30–39, 40–49, 50–59, 60–69, and 70–79 years. Self reported race/ethnicity was defined as Black, Hispanic, or White. Body mass index (BMI) was categorized as <25.0, 25.0–29.9, and ≥30.0 kg/m2. Physical activity was measured using the Physical Activity Scale for the Elderly (PASE) and was categorized as low (<100), medium (100–250), and high (>250). Alcohol consumption was defined as alcoholic drinks including beer, wine and hard liquor consumed per day: 0, <1, 1–2.9, ≥3 drinks per day. Smoking was defined as never smokers (smoked <100 cigarettes lifetime and not currently smoking), former smokers (smoked ≥100 cigarettes lifetime and currently non-smoker), and current smoker (smoked ≥100 cigarettes and currently a smoker). The socioeconomic status (SES) index was calculated using a combination of education and household income. SES was categorized as low (lower 25% of the distribution of the SES index), middle (middle 50% of the distribution), and high (upper 25% of the distribution). Comorbid conditions included in the analysis were heart disease, type 2 diabetes, hypertension, and depression. The presence of comorbidities was defined as a yes response to “Have you ever been told by a health care provider that you have or had….”? Heart disease was defined by self-report of myocardial infarction, angina, congestive heart failure, coronary artery bypass, or angioplasty stent. Participants reporting five or more depressive symptoms (out of 8) using the abbreviated Center for Epidemiological Studies – Depression (CES-D) scale were considered to have depressive symptoms. Medications included in the analysis were anti-inflammatory and other medications (both prescription and over the counter) that could affect CRP levels.11
Statistical analysis
Descriptive statistics, proportions for categorical variables and mean and standard deviations (SD) for continuous variables, were used to describe the analysis sample. As the distribution of CRP levels was skewed, log (base 10) transformations of CRP levels were used. Log10(CRP) levels were analyzed as a continuous variable. Additionally, CRP levels were categorized into three groups: <9.5 nmol/L (low cardiovascular risk), 9.5–28.5 nmol/L (moderate cardiovascular (CVD) risk), >28.5 nmol/L (high CVD risk).2 Similarly, sex hormone levels were log10 transformed. Serum hormone levels were analyzed as continuous variables. Scatter plots of CRP levels against sex hormone levels with locally weighted scatterplot smoothing (LOESS, smoothing parameter = 0.8) were used to assess graphically the linearity of the association. Multiple linear regression models were used to assess the association between sex hormones and CRP and to adjust for potential confounders.
Of the 2,301 men in BACH, blood samples were obtained for 1,899 (82.5%). A total of 12 men with missing or extreme values for T and SHBG were excluded from the analysis. Additionally, 281 men were missing E2. Of the remaining 1,607 men, 48 had at least one outlying hormone value with the largest groups outlying on FT (N=10) or SHBG (N=10). Analyses were conducted on a sample of 1,559 men with complete data on sex hormones and CRP. As results for T and SHBG were similar when conducted on the larger group of men with these measures available compared to the subgroup of men with complete data on all hormones and CRP, results are presented for the subgroup of 1,559 men. Of the 1,559 men included in the analysis, 87 (5.6%) had missing data on one or more covariates. Twenty-five multiple imputations were performed separately by race/ethnicity using all relevant variables to obtain plausible values for missing data on covariates included in the analysis. Observations were weighted as inversely proportional to their probability of selection. Weights were post-stratified to the Boston population according to the 2000 census. Analyses were conducted in version 9.1 of SAS (SAS Institute, Cary, NC, USA) and version 9.0.1 of SUDAAN (Research Triangle Institute, Research Triangle Park, NC, USA).
Results
Characteristics of the men included in the analysis are presented in Table 1 and descriptive statistics on sex hormones and CRP levels are included in Table 2. About 40% of men were overweight (BMI 25.0–29.9) and one-third were obese (BMI ≥30). About 30% of men were current smokers and 25% reported no alcohol consumption. Over half of the analysis sample reported use of anti-inflammatory or other medications that could affect CRP levels. Prevalence of heart disease and type 2 diabetes was similar at around 10%, while both depressive symptoms (14%) and hypertension (25%) were more common. One-third of men were at moderate cardiovascular risk (CRP levels 9.5–28.5 nmol/L) while almost 20% were at high risk (CRP >28.5 nmol/L).
Table 1.
Characteristics of the analysis sample (N=1,559).
| N (weighted %) | |
|---|---|
| Age (years) | |
| 30–39 | 418 (37.1%) |
| 40–49 | 454 (25.5%) |
| 50–59 | 353 (17.9%) |
| 60–69 | 220 (12.4%) |
| 70–79 | 114 (7.0%) |
| Race/Ethnicity* | |
| Black | 429 (27.5%) |
| Hispanic | 515 (33.0%) |
| White | 615 (39.5%) |
| Socioeconomic Status | |
| Low | 632 (22.8%) |
| Middle | 641 (48.3%) |
| High | 286 (28.9%) |
| Body Mass Index (kg/m2) | |
| <25.0 | 407 (26.8%) |
| 25.0–29.9 | 612 (39.5%) |
| 30.0+ | 541 (33.7%) |
| Physical Activity (PASE) | |
| Low (<100) | 434 (25.4%) |
| Middle (100–250) | 731 (47.2%) |
| High (250+) | 394 (27.5%) |
| Smoking | |
| Never | 600 (39.4%) |
| Former | 455 (29.4%) |
| Current | 503 (31.2%) |
| Alcohol Consumption (drinks/day) | |
| None | 499 (24.8%) |
| <1 | 575 (41.1%) |
| 1–2.9 | 311 (25.2%) |
| 3+ | 174 (8.9%) |
| Heart disease (% Yes) | 152 (9.4%) |
| Diabetes (% Yes) | 190 (9.7%) |
| High blood pressure (% Yes) | 481 (25.8%) |
| Depression (% Yes) | 259 (14.1%) |
| Anti-inflammatory medication** (% Yes) | 732 (53.5%) |
| Current cancer treatment (% Yes) | 16 (1.4%) |
| Taking hormone medication (% Yes) | 25 (1.4%) |
unweighted
Anti-inflammatory and other medications that may could affect CRP levels: Blood form & coagulant/anti-platelet agent (clopidogrel, ticlopidine); Cardiovascular/antilipemics/hmg-coa reductase inhibitors (statins); Non-statin anti-cholesterol drugs; Beta-blockers; Calcium channel blockers; Select ACE inhibitors (captopril, ramipril, fosinopril); Rosiglitazone, pioglitazone; Angiotensin II receptor agonists (Losartan, Valsartan, Irbesartan, Olmesartan, Candesartan, Telmisartan); Aspirin; Naproxen; Ibuprofen
Table 2.
Descriptive statistics for sex hormones and C-reactive protein (CRP) levels.
| Geometric Mean ± SD or Median (Interquartile range) or N (%) | ||
|---|---|---|
| Total T (nmol/L) | Geometric Mean ± SD | 13.75 ± 1.55 |
| Median (Interquartile range) | 14.47 (10.44, 18.98) | |
| Free T (nmol/L) | Geometric Mean ± SD | 0.29 ± 1.52 |
| Median (Interquartile range) | 0.29 (0.23, 0.38) | |
| SHBG (nmol/L) | Geometric Mean ± SD | 0.29 ± 1.52 |
| Median (Interquartile range) | 30.47 (22.16, 41.53) | |
| Estradiol (pmol/L) | Geometric Mean ± SD | 79.62 ± 1.52 |
| Median (Interquartile range) | 82.23 (61.31, 108.29) | |
| Free estradiol (pmol/L) | Geometric Mean ± SD | 2.26 ± 1.53 |
| Median (Interquartile range) | 2.34 (1.75, 3.09) | |
| CRP (nmol/L) | Geometric Mean ± SD | 10.69 ± 3.22 |
| Median (Interquartile range) | 10.29 (4.67, 22.95) | |
| <9.5 nmol/L | 688 (47.2%) | |
| 9.5–28.5 nmol/L | 533 (33.1%) | |
| >28.5 nmol/L | 338 (19.7%) |
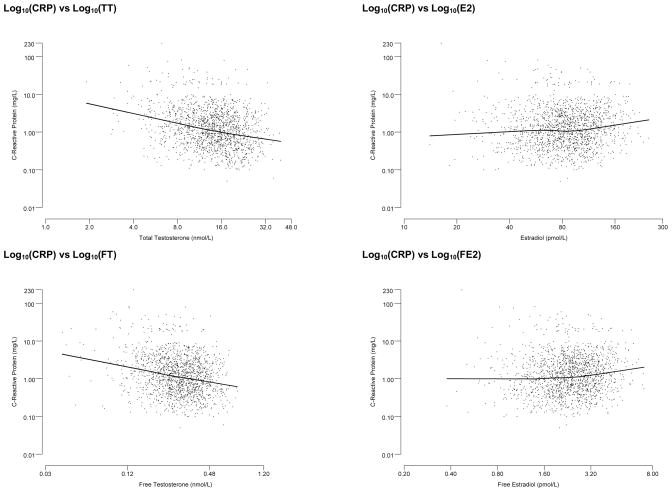
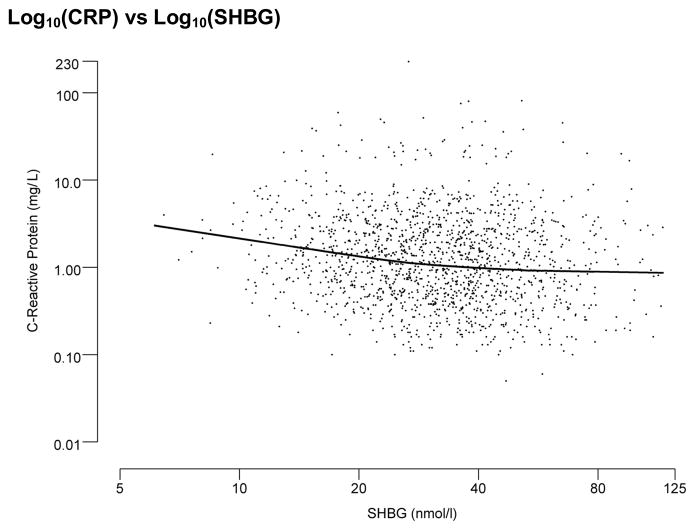
Figure 1 presents scatterplots of CRP against sex hormone levels (TT, SHBG, and E2). These figures show an inverse association of CRP levels with both TT and SHBG. A similar association was observed with FT. In contrast, a slight trend in increased CRP was observed with increasing E2 and FE2 levels. Scatterplots presented in Figure 1 show fairly linear associations between CRP and sex hormone levels on the log scale. Table 3 presents crude and adjusted correlations between sex-hormones and CRP levels. Testosterone (both total and free) and SHBG levels were negatively associated with CRP levels (age- and BMI adjusted r = −0.176 for total T, r = −.132 for free T, and r = −0.157 for SHBG). E2 and free E2 were positively associated with CRP levels, but these associations were not statistically significant. These observed associations were robust and remained statistically significant in multivariate models. BMI had the largest impact on the association of CRP with T and SHBG level with changes of 35–40% in the regression coefficients after adding BMI to the model. Adjusting for waist circumference instead of BMI, similar results were observed. Additional covariates did not further attenuate the observed association. In contrast, no association was observed between E2 and CRP levels in either unadjusted or adjusted models. We also examined whether the ratio of TT to E2 was associated with CRP, the result was similar to the association between TT and CRP.
Figure 1.
Scatter plots of Log10 transformed C-reactive protein levels by Log10 transformed sex hormone levels with LOESS curve
Table 3.
Association of log10 transformed sex-hormones and log10 transformed CRP levels. Pearson correlations and 95% confidence intervals. (N=1,559)
| Crude | p-value | Age-adjusted | p-value | Age and BMI-adjusted | p-value | Multivariate-adjusted* | p-value | |
|---|---|---|---|---|---|---|---|---|
| Total T | −0.282 (−0.368, −0.196) | <0.001 | −0.277 (−0.366, −0.187) | <0.001 | −0.176 (−0.266, −0.087) | <0.001 | −0.184 (−0.259, −0.110) | <0.001 |
| Free T | −0.234 (−0.315, −0.154) | <0.001 | −0.221 (−0.318, −0.124) | <0.001 | −0.132 (−0.223, −0.040) | 0.005 | −0.129 (−0.207, −0.051) | 0.001 |
| SHBG | −0.164 (−0.241, −0.088) | <0.001 | −0.263 (−0.347, −0.180) | <0.001 | −0.157 (−0.235, −0.078) | <0.001 | −0.176 (−0.245, −0.107) | <0.001 |
| E2 | 0.065 (−0.042, 0.173) | 0.24 | 0.061 (−0.047, 0.168) | 0.27 | 0.040 (−0.060, 0.139) | 0.44 | 0.027 (−0.066, 0.120) | 0.57 |
| Free E2 | 0.092 (−0.017, 0.201) | 0.099 | 0.103 (−0.007, 0.213) | 0.07 | 0.063 (−0.038, 0.164) | 0.22 | 0.054 (−0.038, 0.147) | 0.25 |
adjusted for age, BMI, SES, smoking status, alcohol intake, heart disease, high blood pressure, depression, diabetes, current cancer treatment, anti-inflammatory medication
Discussion
Results from the BACH study demonstrate, in a community-based sample of men, an inverse association of CRP with total and free testosterone as well as SHBG. These associations remained statistically significant after adjusting for age, obesity, comorbid conditions, lifestyle factors, and prescription medications use. An association of E2 and CRP levels was not observed. These findings support the hypothesis that higher androgen levels may have an anti-inflammatory effect.
Results from previous observational studies of the association of sex hormone levels and inflammatory markers have not been consistent. Data from a study of 1,896 men with the metabolic syndrome but not type 2 diabetes conducted in Finland show an inverse association of T and SHBG with CRP levels after adjusting for age, BMI, comorbid conditions, and lifestyle factors.12 Similarly, a study of 70 men with type 2 diabetes reports an inverse association between bioavailable T and CRP levels.13 Data from the InCHIANTI study, a cross-sectional study of 497 men 65 yr and older, show an inverse association of between both total and bioavailable T and sIL6r but not with IL-6, TNF-α, IL-1β, or CRP levels.5 Data from the HAMLET (Hormonal changes in the Ageing MaLe: and Epidemiologic Taskforce) study on 400 men 40–80 years conducted in Germany reported a different pattern of association between sex hormones and CRP levels.6 The inverse association between T (total, free, or DHEAS) and CRP levels was not statistically significant while increased levels of both E2 and FE2 were associated with higher CRP levels. The estradiol and CRP association was attenuated but remained significant in multivariate models; however, this association was further attenuated and statistically non-significant after adjusting for intra-abdominal fat. A recent report from the InCHIANTI study shows a similar null result for the E2 and CRP association; however, a positive and statistically significant association was observed between estradiol and IL-6 levels.14 Finally, in an occupation-based sample of 715 middle-aged men (35–59 yrs), no association was observed between CRP and sex hormone levels, including TT, FT, SHBG, and E2.15
Data from studies of the effect of testosterone replacement therapy (TRT) on levels of inflammatory markers are not always consistent. A study of TRT among older men with androgen deficiency (TT <15 nmol/l) found a decrease in TNF-α and IL-1β levels after 1 month of treatment but no changes in CRP levels.7 A randomized trial among healthy older men (age>60 years) did not find a difference in inflammatory marker levels after 3 months of therapy.16 Similar results were reported by a randomized trial of TRT among hypogonadal men with type 2 diabetes after 3 months of treatment despite a baseline association of TT and FT with IL-6 and CRP levels.17 Finally a larger trial of men age 60–80 years with TT levels <13.7 nmol/l reported no change in CRP levels after testosterone supplementation for 26 weeks.8 While TRT seems to be associated with either a decrease or no change in levels of inflammatory markers, estrogen treatment in men with prostate cancer has been reported to increase CRP concentrations.18
The immunosuppressive effect of androgens is well established and the greater incidence of immune-mediated diseases in women and hypogonadal men is attributed to androgens down-regulating pro-inflammatory cytokines.4, 7 Although the association of sex hormone levels and cardiovascular events remains controversial,19 there is evidence from longitudinal studies showing low sex hormone levels as predictive of the development of the metabolic syndrome (a constellation of cardiovascular risk factors including dyslipidemia, hypertension, abdominal obesity, and insulin resistance) and type 2 diabetes.20, 21 Similar data on the association of E2 and cardiometabolic risk factors or outcomes is sparse. Elevated E2 levels have been associated with type 2 diabetes and increased insulin levels,22 however, the association with the metabolic syndrome has not been consistent.23, 24 While data from the Framingham Heart Study have suggested that men with higher E2 are at lower risk of cardiovascular events,25 other longitudinal studies have found either no association between E2 and CVD (Rancho-Bernardo and Caerphilly studies)26, 27 or reported an increase in the risk of stroke (Honolulu-Asia Aging Study)28 or progression of atherosclerosis (Atherosclerosis and Insulin Resistance study).29 The robust inverse association between testosterone and CRP levels observed in the present study provides further evidence supporting the hypothesis that modulation of inflammatory processes may be one of the pathways by which androgens affect cardiometabolic risk. As BACH is presently a cross-sectional study, the temporal sequence of causality or specific mechanisms of action cannot be assessed by means of cross-sectional data alone. Thus, we cannot exclude the alternative hypothesis that low androgen levels may be a consequence of inflammation. Longitudinal studies as well as investigation of the association of sex hormones and additional inflammatory markers are warranted to confirm the role of androgens in inflammatory processes.
Strengths of the BACH study include a community-based random sample across a wide age range (30–79 years), inclusion of large numbers of minority participants representative of Black and Hispanic populations, and a wide range of covariates including sociodemographic, lifestyle, and health variables, which can be adjusted for in the analysis. Although the effect of anti-inflammatory medications are controlled for in the present analysis, the effect of the treatment of comorbid conditions, which could have further attenuated CRP levels, are not accounted for in this analysis. Additionally, information on recent hospitalization was not collected. However, a full analysis of the potential influence of medication use in general on CRP levels is beyond the scope of this paper. The potential confounding effect of insulin, a negative modulator of SHBG, on the androgens and CRP association could not be assessed as insulin levels were not measured in the present study. History of comorbid conditions was assessed by self-report with the potential for reporting and/or recall bias; however, previous research has demonstrated the reliability and validity of self-report for heart disease, diabetes, and hypertension.30 The BACH study was limited geographically to the Boston area. However, comparison of sociodemographic and health-related variables from BACH with other large regional (Boston Behavioral Risk Factor Surveillance System) and national (National Health Interview Survey) surveys have shown that the BACH estimates are comparable to national trends on key health related variables. Although BACH measured demographics, medications and comorbidities in detail, residual confounding in this observational study cannot be excluded as the explanation for these associations.
In summary, results from the present study demonstrate a robust inverse correlation of T (both total and free) and SHBG with CRP levels. Although cross-sectional, these findings provide further evidence supporting the hypothesis that modulation of inflammatory processes is a potential pathway by which androgens could affect cardiometabolic risk and associated conditions such as the metabolic syndrome, diabetes, and cardiovascular disease.
Acknowledgments
Funding: This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) DK 56842. Analyses for the current manuscript were supported through an unrestricted educational grant to New England Research Institutes, Inc. from GlaxoSmithKline. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. The Corresponding Author retains the right to provide a copy of the final manuscript to the NIH upon acceptance for publication, for public archiving in PubMed Central as soon as possible but no later than 12 months after publication.
References
- 1.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. The Journal of Clinical Endocrinology and Metabolism. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. Journal of the American College of Cardiology. 2007;49:2129–2138. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 3.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khosla S, Atkinson EJ, Dunstan CR, O’Fallon WM. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. The Journal of Clinical Endocrinology and Metabolism. 2002;87:1550–1554. doi: 10.1210/jcem.87.4.8397. [DOI] [PubMed] [Google Scholar]
- 5.Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, Valenti G, Ling SM, Ferrucci L. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. The Journal of Clinical Endocrinology and Metabolism. 2006;91:345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- 6.Nakhai Pour HR, Grobbee DE, Muller M, van der Schouw YT. Association of endogenous sex hormone with C-reactive protein levels in middle-aged and elderly men. Clinical Endocrinology. 2007;66:394–398. doi: 10.1111/j.1365-2265.2007.02745.x. [DOI] [PubMed] [Google Scholar]
- 7.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. The Journal of Clinical Endocrinology and Metabolism. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 8.Nakhai-Pour HR, Grobbee DE, Emmelot-Vonk MH, Bots ML, Verhaar HJ, van der Schouw YT. Oral testosterone supplementation and chronic low-grade inflammation in elderly men: a 26-week randomized, placebo-controlled trial. American Heart Journal. 2007;154(1228):e1221–1227. doi: 10.1016/j.ahj.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 9.McKinlay JB, Link CL. Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) Survey. European Urology. 2007;52:389–396. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. Journal of Steroid Biochemistry. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 11.Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovascular Drug Reviews. 2006;24:33–50. doi: 10.1111/j.1527-3466.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 12.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Salonen R, Rauramaa R, Salonen JT. Sex hormones, inflammation and the metabolic syndrome: a population-based study. European Journal of Endocrinology. 2003;149:601–608. doi: 10.1530/eje.0.1490601. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia V, Chaudhuri A, Tomar R, Dhindsa S, Ghanim H, Dandona P. Low testosterone and high C-reactive protein concentrations predict low hematocrit in type 2 diabetes. Diabetes Care. 2006;29:2289–2294. doi: 10.2337/dc06-0637. [DOI] [PubMed] [Google Scholar]
- 14.Maggio M, Ceda GP, Lauretani F, Bandinelli S, Metter EJ, Artoni A, Gatti E, Ruggiero C, Guralnik JM, Valenti G, Ling SM, Basaria S, Ferrucci L. Estradiol and inflammatory markers in older men. The Journal of Clinical Endocrinology and Metabolism. 2009;94:518–22. doi: 10.1210/jc.2008-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Pottelbergh I, Braeckman L, De Bacquer D, De Backer G, Kaufman JM. Differential contribution of testosterone and estradiol in the determination of cholesterol and lipoprotein profile in healthy middle-aged men. Atherosclerosis. 2003;166:95–102. doi: 10.1016/s0021-9150(02)00308-8. [DOI] [PubMed] [Google Scholar]
- 16.Ng MK, Liu PY, Williams AJ, Nakhla S, Ly LP, Handelsman DJ, Celermajer DS. Prospective study of effect of androgens on serum inflammatory markers in men. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22:1136–1141. doi: 10.1161/01.atv.0000022167.80130.a6. [DOI] [PubMed] [Google Scholar]
- 17.Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. European Journal of Endocrinology. 2007;156:595–602. doi: 10.1530/EJE-06-0737. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs A, Henriksson P, Hamsten A, Wallen H, Bjorkegren J, Tornvall P. Hormonal regulation of circulating C-reactive protein in men. Clinical Chemistry. 2005;51:911–913. doi: 10.1373/clinchem.2004.046169. [DOI] [PubMed] [Google Scholar]
- 19.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocrine Reviews. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 20.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 21.Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–494. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- 22.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA: the Journal of the American Medical Association. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 23.Maggio M, Lauretani F, Ceda G, Bandinelli S, Basaria S, Paolisso G, Giumelli C, Luci M, Najjar SS, Metter EJ, Valenti G, Guralnik JM, Ferrucci L. Estradiol and Metabolic Syndrome in Older Italian Men: The InCHIANTI Study. Journal of Andrology. 2008 Dec 4; doi: 10.2164/jandrol.108.006098. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. The Journal of Clinical Endocrinology and Metabolism. 2005;90:2618–2623. doi: 10.1210/jc.2004-1158. [DOI] [PubMed] [Google Scholar]
- 25.Arnlov J, Pencina MJ, Amin S, Nam BH, Benjamin EJ, Murabito JM, Wang TJ, Knapp PE, D’Agostino RB, Sr, Bhasin S, Vasan RS. Endogenous sex hormones and cardiovascular disease incidence in men. Annals of Internal Medicine. 2006;145:176–184. doi: 10.7326/0003-4819-145-3-200608010-00005. [DOI] [PubMed] [Google Scholar]
- 26.Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. 1988;78:539–545. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 27.Yarnell JW, Beswick AD, Sweetnam PM, Riad-Fahmy D. Endogenous sex hormones and ischemic heart disease in men. The Caerphilly prospective study. Arteriosclerosis and Thrombosis: a Journal of Vascular Biology/American Heart Association. 1993;13:517–520. doi: 10.1161/01.atv.13.4.517. [DOI] [PubMed] [Google Scholar]
- 28.Abbott RD, Launer LJ, Rodriguez BL, Ross GW, Wilson PW, Masaki KH, Strozyk D, Curb JD, Yano K, Popper JS, Petrovitch H. Serum estradiol and risk of stroke in elderly men. Neurology. 2007;68:563–568. doi: 10.1212/01.wnl.0000254473.88647.ca. [DOI] [PubMed] [Google Scholar]
- 29.Tivesten A, Hulthe J, Wallenfeldt K, Wikstrand J, Ohlsson C, Fagerberg B. Circulating estradiol is an independent predictor of progression of carotid artery intima-media thickness in middle-aged men. The Journal of Clinical Endocrinology and Metabolism. 2006;91:4433–4437. doi: 10.1210/jc.2006-0932. [DOI] [PubMed] [Google Scholar]
- 30.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. Journal of Clinical Epidemiology. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]