Abstract
Rationale
The widely available hallucinogen salvinorin A is a unique example of a plant-derived compound selective for κ-opioid receptors and may produce effects distinct from those of other compounds with classic hallucinogenic or dissociative properties which are also abused in humans.
Objectives
The objective of this study is to characterize the salvinorin A discriminative cue in nonhuman primates with high κ-receptor genetic homology to humans.
Methods
Adult rhesus monkeys (n=3) were trained to discriminate salvinorin A (0.015 mg/kg, s.c.) from vehicle, in a food-reinforced operant discrimination assay. Parallel studies, using unconditioned behavioral endpoints (facial relaxation and ptosis) also evaluated the κ-opioid receptor mediation of salvinorin A in vivo function.
Results
Monkeys trained to discriminate salvinorin A generalized structurally diverse, centrally penetrating κ-agonists (bremazocine, U69,593, and U50,488). By contrast, μ- and δ-opioid agonists (fentanyl and SNC80, respectively) were not generalized, nor were the serotonergic 5HT2 hallucinogen psilocybin or the dissociative N-methyl-D-aspartic acid antagonist, ketamine. The discriminative effects of salvinorin A were blocked by the opioid antagonist quadazocine (0.32 mg/kg), but not by the 5HT2 antagonist ketanserin (0.1 mg/kg). Consistent with these findings, salvinorin and κ-agonists (e.g., U69,593) produce effects in the unconditioned endpoints (e.g., ptosis), whereas psilocybin was inactive.
Conclusions
These findings support the conclusion that the interoceptive/discriminative cue produced by salvinorin A is mediated by agonism at κ-receptors and is mechanistically distinct from that produced by a classic serotonergic hallucinogen.
Keywords: Dynorphin, κ-opioid, Hallucinogen, Opioid, Salvinorin A, Salvia divinorum
Introduction
Salvinorin A, a diterpenoid, is the main active product from the widely available psychoactive plant, Salvia divinorum. Salvinorin A is a structurally unique selective agonist at κ-opioid receptors and also displays particular pharmacodynamic effects at this receptor, including high efficacy, and low propensity to cause receptor desensitization (Chavkin et al. 2004; Wang et al. 2004). Salvinorin A-containing products (including concentrated extracts) are widely available, and their usage for nonmedical purposes in adolescents and young adults has been described in recent surveys (Khey et al. 2008; Lange et al. 2008; Paglia-Boak et al. 2009; Substance Abuse and Mental Health Services Administration 2008). A recent large survey detected prior-year exposure to salvinorin A-containing products in the same order as the “club” drug N-methyl-D-aspartic acid (NMDA) antagonist, ketamine (Paglia-Boak et al. 2009). Also, the most recent “Monitoring the future” survey reported that approximately 6% of 12th graders in a large US sample (n=14,268) reported use of S. divinorum products in the preceding 12 months (Johnston et al. 2009). Salvinorin A is therefore the only high efficacy, selective κ-agonist to have broad diffusion in the population.
Salvinorin A is a highly potent hallucinogen in humans, as determined from descriptive studies and self-reports (Gonzales et al. 2006; Siebert 1994). However, there are no quantitative, laboratory-based reports on its behavioral effects in humans to date. Salvinorin A-containing preparations are in a legislative flux in the USA, with several states moving to regulate these products, widely available on the internet (Griffin et al. 2008; Hoover et al. 2007).
Nonhuman primates are particularly valuable subjects to study the effects of κ-opioids, from a translational perspective. This is due to the relative homology between nonhuman primates and humans, in terms of κ-receptor neuroanatomical populations, OPRK1 (κ-receptor) genetic homology, and ability to study neuroendocrine and behavioral endpoints with translational value (i.e., endpoints that can be easily adapted to human studies; Butelman et al. 2007).
It was originally reported that nonhuman primates trained to discriminate the synthetic κ-agonist U69,593 generalized salvinorin A (Butelman et al. 2004); similar findings were also made in rats (Baker et al. 2009; Willmore-Fordham et al. 2007). Given the uniqueness of salvinorin A as an opioid ligand, and its status as a broadly available psychoactive agent in legislative flux, it is of interest to determine directly whether the discriminative effects of salvinorin A itself are shared by structurally diverse synthetic κ-agonists, other opioids, and by other hallucinogenic/psychotomimetic compounds abused by humans. A recent study reported that rats trained to discriminate salvinorin A do generalize arylacetamide κ-agonists (U69,593 and U50,488; Baker et al. 2009). This study will therefore present a salvinorin A drug discrimination in nonhuman primates, its generalization by structurally diverse κ-agonists (arylacetamide and benzomorphan), as well as the lack of generalization to a classic serotonergic hallucinogen, and to the NMDA antagonist, ketamine, also an abused psychotomimetic agent. We further compared unconditioned behavioral effects of salvinorin A (facial relaxation and ptosis) with those of a classic serotonergic hallucinogen. Facial relaxation and ptosis can be studied in humans (Chernik et al. 1990; Miner et al. 2002) without subject training required for operant variables such as drug discrimination, and therefore, have the potential to aid in the quantitative characterization of the pharmacodynamics of this broadly available hallucinogen.
Methods
Subjects
Three male, adult, captive-bred, gonadally intact rhesus monkeys (Macaca mulatta; age range, 8–14 years old approximately; weights, 7.8, 8.1, and 9.7 kg) were used in the drug discrimination studies. Two other adult males (weights, 10.4 and 12.5 kg) and two adult females (weights, 6.6 and 8.6 kg) were used in the facial relaxation/ptosis studies (n=4). The subjects were housed singly in stable colony rooms maintained at 20–22°C with controlled humidity and a 12:12-h light:dark cycle (lights on at 0700 hours). Subjects in drug discrimination studies were food restricted to approximately 95% of free-feeding weights; they were fed appropriate amounts of primate chow biscuits (PMI Feeds, Richmond, VA, USA) daily, supplemented by appetitive treats. An environmental enrichment plan was in place (with visual and auditory stimuli) in the colony rooms. Water was available ad lib in home cage, via an automatic waterspout. All subjects had limited histories of prior exposure to opioid pharmacological probes, and no history of chronic exposure to any agent or exposure to any psychostimulant. All sessions were carried out (typically 5 days per week), at least 2 h after lights on and 2 h before lights out.
Studies were reviewed by the Rockefeller University Animal Care and Use Committee, in accordance with the Guide for the Care and Use of Animals (National Academy Press; Washington, DC, USA, 1996).
Drug discrimination
Procedure
Three male subjects were trained to discriminate salvinorin A (0.015 mg/kg, s.c.) from vehicle in a two-key (fixed ratio) FR task, in operant conditioning chambers (Med Associates, St. Albans, VT, USA), connected to a computer, via a Med Associates interface.
Training
Subjects were trained in multiple-cycle sessions; each cycle consisted of an initial s.c. injection followed by a 15-min time-out period (time-out responses had no consequence). At the end of the time-out period, a 5-min response period started (signaled by stimulus lights above each lever), during which, monkeys received up to ten sucrose pellets (300 mg; PJ Noyes/Research Diets; New Brunswick, NJ, USA), by responding on the injection-appropriate (“correct”) lever. A “reset” contingency was in place, in that an incorrect response reset the FR requirement to FR8 (one subject) FR12 (two subjects) on the correct lever. A maximum of four cycles occurred within each training session. In multiple-cycle training sessions, the first cycle was always a vehicle cycle. If the training drug (salvinorin A, 0.015 mg/kg, s.c.) was administered, it would always be on the last cycle of the session. Monkeys were typically trained 5 days per week. Criterion responding was defined as at least 90% injection-appropriate responses, and no more than 1× FR responses on the incorrect lever before the first food presentation in any cycle, with response rates of at least 1 response/s. Prior to commencement of testing, each subject reached criterion responding for five consecutive training days. This occurred for the different subjects at 68, 116, and 197 training days, respectively.
Testing
Test sessions were identical to those described above, except that 1× FR consecutive responses on either lever resulted in pellet delivery. Tests followed a cumulative dosing procedure. In these cumulative dosing tests, doses of the compounds were increased in 0.5 log unit steps (each cycle commenced with a 15-min time-out period, followed by a 5-min response period, as mentioned above). Test doses were increased in each subject until either (a) generalization (i.e., at least 90% salvinorin A-appropriate responding), (b) rates of responding were decreased by ≥70% vs. control (the mean of the three most recent consecutive vehicle-training cycles prior to the test session), or (c) the appearance of untoward effects. Tests in the same subject were separated by at least two consecutive training days at criterion performance (i.e., at least 90% injection-appropriate responding and no more than 1× FR responses on the incorrect lever before the first food presentation in any cycle, with responses rates of at least 1 response/s).
Design
Cumulative salvinorin A (0.001–0.01 mg/kg [two subjects], 0.0032–0.01 mg/kg [one subject]) and its vehicle (0.16 ml/kg, in consecutive cycles) were compared to κ-agonists: bremazocine (0.0001–0.001 mg/kg), U50,488 (0.01–0.032), U69,593 (0.001–0.01 mg/kg), and ICI204,448 (0.01–0.1 mg/kg). Other compounds were also compared under identical cumulative dosing conditions: fentanyl (0.001–0.032 mg/kg), SNC80 (0.032–0.32 mg/kg), ketamine (0.1–3.2 mg/kg), and psilocybin (0.032–0.32 mg/kg). In antagonism studies, the cumulative salvinorin A dose-effect curve was re-determined 60 min after pretreatment with quadazocine (0.32 mg/kg) or 30 min after pretreatment with ketanserin (0.1 mg/kg; Butelman et al. 2004; Li et al. 2009). Quadazocine and ketanserin control studies were also completed, (i.e., with the administration of three vehicle test cycles after the appropriate antagonist pretreatment). Each of the above experiments was carried out as one or two determinations per subject.
Data analysis
The main dependent variable was percent drug (salvinorin A)-appropriate responding presented graphically as mean±SEM; 90% drug appropriate was considered the minimum value for generalization in an individual subject (consistent with training criteria). Rate of responding is presented graphically as %control value (mean±SEM). Individual control values for rate of responding were calculated as the mean of the three most recent consecutive vehicle-training cycles prior to each test session.
Unconditioned behavioral effects (facial relaxation and ptosis)
Facial relaxation and ptosis
In separate studies, the effects of salvinorin A and selected compounds studied above were determined in two unconditioned behavioral effects: (a) facial relaxation (partial or severe) and (b) ptosis (partial or complete). These endpoints, as well as their potential translational significance have been described recently (Butelman et al. 2008). Studies were carried out using either a timecourse or cumulative dose-effect curve design (the latter with a 20 min inter-injection interval, similarly to drug discrimination studies above). Discriminative effects of salvinorin A (e.g., at 0.01 mg/kg, s.c.) occurred at doses that were devoid of robust overt behavioral effects, by direct observation (Butelman et al. 2008). In these studies, we therefore compared the effects the largest salvinorin A dose that can be administered s.c. (0.032 mg/kg; due to solubility limitations) and compared it to its effects by the i.v. route (see below).
The cumulative duration of facial relaxation and ptosis behaviors within 1-min windows was separately scored “blind” from videotapes of chaired subjects (i.e., scores could range 0–60 s for facial relaxation or ptosis). Brief events less than 1 s in duration were not scored, in order to avoid baseline behaviors, such as blinking. All time windows were selected a priori, using standardized time and dose points.
Time windows were rated “blind” by a main trained rater, using the Observer XT System (Noldus, Wageningen, Netherlands), as recently described in detail (Butelman et al. 2008). The rater was previously trained and completed a periodic test of scoring reliability during these studies. This resulted in a coefficient of variation of 6% when rating a 1-min time window of peak robust pharmacological activity for salvinorin A, five independent times. Data collection and statistical analyses were completed to the nearest 0.1 s.
Design
All observational studies were carried out with four subjects across conditions, using either a timecourse design (0–60 min after injection) or in cumulative dosing sessions with 20 min inter-injection interval, and scored time windows 14–15 min after each injection (i.e., timing similar to drug discrimination cycles). The order of different experiments was unsystematic, with consecutive experiments in the same subject separated by at least 72 h.
Timecourse studies
The effects of salvinorin A 0.032 mg/kg were compared by the i.v. vs. the s.c.route and compared to i.v. vehicle (standard 1-min time windows were scored at 0–1, 1–2, 4–5, 14–15, 29–30, and 59–60 min after injection). This study determined whether salvinorin A, which is reportedly sensitive to systemic degradation, is more effective by the i.v. vs. the s.c. route, due to its greater predicted bioavailability (Hooker et al. 2008; Schmidt et al. 2005).
Cumulative dosing studies
Consistent with the more robust effects of i.v. vs. s.c. salvinorin A in the above study, all cumulative dosing studies were carried out by the i.v. route. Subjects were studied with intravenous salvinorin A (0.001–0.032 mg/kg), U69,593 (0.001–0.032 mg/kg), ICI204,448 (0.01–0.32 mg/kg), and psilocybin (0.01–0.1 mg/kg). Ketamine was not studied on these dependent variables, due to its known effects on nystagmus, possibly confounding scoring of ptosis (Condy et al. 2005).
Data analysis
Data for the two dependent variables (facial relaxation and ptosis) are presented separately as mean±SEM, expressed as cumulative time of each behavior observed within a 1-min window. When appropriate, data are analyzed by one- or two-way repeated measures analysis of variance (ANOVA) followed by post hoc Newman–Keuls testing (SPSS Sigmastat). The α level was set at p<0.05 throughout, values denoted as significant therefore used this p value as a cutoff.
Drugs (all assays)
Salvinorin A (extracted in the Laboratory of Dr. T.E. Prisinzano) was dissolved in daily aliquots in ethanol:Tween 80:sterile water vehicle (1:1:8 proportion, by volume). U69,593 (Pharmacia, Kalamazoo, MI, USA), quadazocine mesylate (Sanofi-Winthrop, Malvern, PA, USA), ICI204,448 (Tocris Bioscience, Ellisville, MO, USA), and SNC80 (Tocris Bioscience, Ellisville, MO, USA) were dissolved in sterile water with the addition of two drops of lactic acid (further dilutions were made with sterile water). Ketamine HCl (Fort Dodge, IA, USA) was diluted with sterile water from stock solution (100 mg/ml). Ketanserin tartrate was dissolved in 5% DMSO in sterile water (ketanserin dose is expressed as free base). Bremazocine HCl (RBI), U50,488 (Pharmacia, Kalamazoo, MI, USA), fentanyl citrate (Sigma, St. Louis, MO, USA), and psilocybin (kindly supplied by the National Institute on Drug Abuse Research Technology Branch) were dissolved in sterile water. Drug doses are expressed as the forms detailed above, unless stated. All drugs were injected s.c. in volumes of 0.05–0.16 ml/kg in the drug discrimination studies. Drugs were injected either s.c. or i.v. in the facial relaxation/ptosis studies, as indicated in text.
Results
Drug discrimination
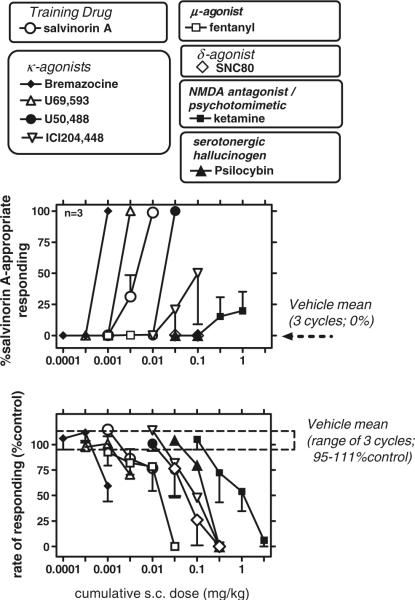
Three subjects were trained to criterion (see “Methods”). Mean response rates in three consecutive vehicle-training cycles were 2.7 responses/s (SEM 0.7). Repeated vehicle, administered as part of a three-cycle test, resulted in vehicle-appropriate responding (i.e., 0% salvinorin A-appropriate responding) in all subjects and did not cause a robust change in response rates (range of mean values in the three cycles of this test was 95–111% of control; see Fig. 1). Cumulative salvinorin A was dose-dependently generalized by all subjects (Fig. 1) (generalization occurred at the 0.01-mg/kg dose in all subjects). Slight response rate-decreasing effects of salvinorin A were detected at the fully generalized dose (Fig. 1).
Fig. 1.
Discriminative effects of salvinorin A and other ligands in subjects trained to discriminate salvinorin A (0.015 mg/kg; n=3) from vehicle. Upper panel: percent salvinorin A-appropriate responding; lower panel: rate of responding. Abscissae: cumulative dose after s.c. administration (milligrams per kilogram). Ordinate (upper panel): percent salvinorin A-appropriate responding; ordinate (lower panel): rate of responding expressed as percentage of control rates (control rate was the mean rate of responding for the three prior vehicle-training cycles for each subject). Data are mean±SEM, selected SEM omitted for clarity
Drug generalization tests
The centrally penetrating κ-agonists bremazocine, U69,593, and U50,488 were generalized by all subjects, albeit with substantially different potency (bremazocine>U69,593>salvinorin A>U50,488; Fig. 1). By contrast, the peripherally selective κ-agonist ICI204,448 was only generalized by one of three subjects, up to doses that caused response rate-decreasing effects. The μ-agonist fentanyl, the δ-agonist SNC80, the serotonergic hallucinogen psilocybin, and the noncompetitive NMDA antagonist ketamine were not generalized by any of the subjects, up to doses that caused rate-decreasing effects (Fig. 1). One subject tested with ketamine emitted a maximum of 50% salvinorin A-appropriate responding, at the highest doses (1 and 3.2 mg/kg).
Antagonism tests
The opioid antagonist quadazocine (0.32 mg/kg) fully prevented the discriminative stimulus effects of salvinorin A in all subjects (Fig. 2). This quadazocine-induced blockade was not surmounted by salvinorin A, up to the largest dose that could be administered by the s.c. route (0.032 mg/kg; due to solubility limitations), in any subject. By contrast, the 5HT2 antagonist ketanserin (0.1 mg/kg) did not cause blockade of the discriminative effects of salvinorin A in any subject (Fig. 2). In control determinations (not shown), each of these antagonists alone (quadazocine or ketanserin) was devoid of effect on responding on three consecutive cycles, each preceded by vehicle administration. Effects of salvinorin A and on unconditioned behavioral effects (facial relaxation and ptosis)
Fig. 2.
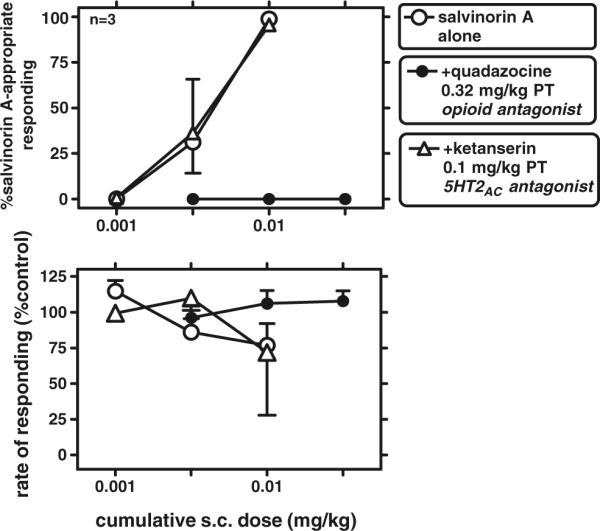
Antagonism of the discriminative effects of salvinorin A, after pretreatment with either quadazocine (0.32 mg/kg) or ketanserin (0.1 mg/kg). Other details as in Fig. 1
Timecourse studies
Pre-injection (baseline) scores for either endpoint (facial relaxation or ptosis) were predominantly 0 s (from a 1-min time window), as were timecourse vehicle determinations after i.v. vehicle (0–60 min; with standard time bins examined; Fig. 3). Salvinorin A (0.032 mg/kg) by the s.c. route caused only slight effects on facial relaxation and ptosis, with peak effects between 5 and 15 min after administration (Fig. 3). By contrast, this salvinorin A dose administered by the i.v. route in the same subjects resulted in more robust effects on both endpoints and a faster onset (robust effects were observed within 1–2 min of i.v. administration). A two-way (time X condition ([vehicle, i.v. salvinorin A, and s.c. salvinorin A]) repeated measures ANOVA for facial relaxation yielded a significant main effect of condition (F[2,6]=5.95; p<0.04). Newman–Keuls post hoc tests showed that i.v. salvinorin A produced greater facial relaxation than either s.c. salvinorin A or vehicle for the time windows 0–1 and 4–5 min after administration. A similar two-way (time X condition (vehicle, i.v. salvinorin, and s.c. salvinorin)) repeated measures ANOVA for ptosis yielded a significant main effect of condition (F[2,6]=18.49; p<0.003) and a time X condition interaction (F[10,30]=3.87; p<0.002). Newman–Keuls post hoc tests showed that i.v. salvinorin A produced greater ptosis than vehicle for the time windows at 0–1, 1–2, 4–5, and 14–15 min after administration. Newman–Keuls post hoc tests also show that s.c. salvinorin A administration produced greater ptosis than vehicle for the time bins 4–5 and 14–15 min after administration.
Fig. 3.
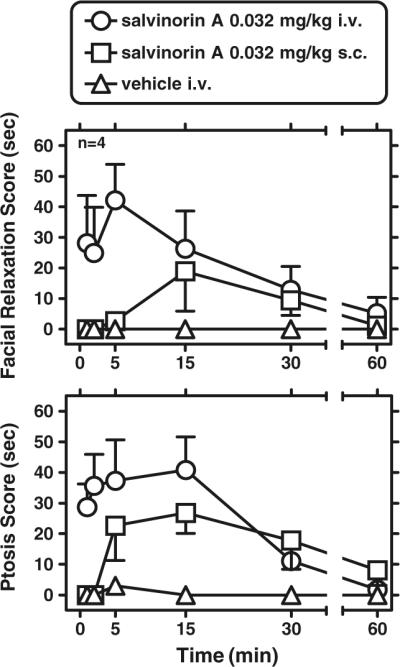
Timecourse of salvinorin A (0.032 mg/kg, n=4) by the intravenous or subcutaneous route, on facial relaxation (upper panel) and ptosis (lower panel). Abscissae: time from the end of injection (minutes). Ordinates: behavioral score for each behavior, within a 1-min bin (scores could therefore range from 0 to 60 s). Data are mean±SEM
Cumulative dose-effect curve studies
Pre-injection time windows for all subjects resulted predominantly in scores of 0 s in either dependent variable, as above. In a vehicle cumulative dosing procedure, with a 20-min inter-injection interval and time windows quantified 14–15 min after each injection, scores of 0 s in either variable were predominantly observed in all subjects (a line depicting mean values for the four vehicle cycles is presented in Fig. 4).
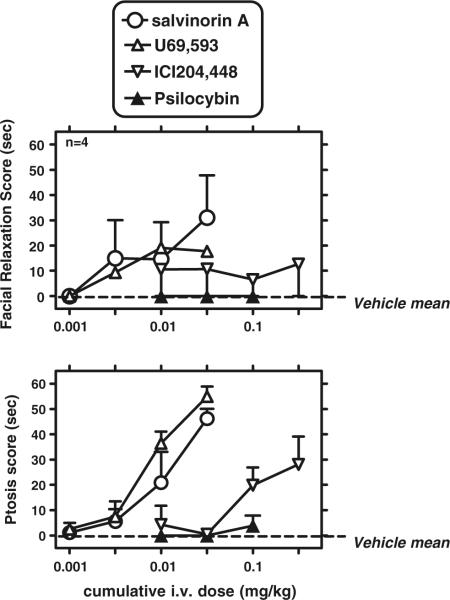
Fig. 4.
Effects of salvinorin A and other ligands on facial relaxation (upper panel) and ptosis (lower panel). Abscissae: cumulative dose after i.v. administration (milligrams per kilogram). Ordinates: behavioral score for each behavior, within a 1-min bin, measured 14–15 min after each injection. Data are mean±SEM
The effects of cumulative salvinorin A, U69,593, ICI204,88, and psilocybin were studied by the i.v. route, since this route resulted in the more robust effects than the s.c. route (see above; Fig. 4). Ketamine was not studied due to its known actions on nystagmus, possibly interfering with ptosis scoring.
Salvinorin A (0.001–0.032 mg/kg) caused facial relaxation and ptosis, with clear dose-dependent effects in the latter measure. A repeated measures ANOVA (F[4,12]=1.43; ns) did not detect an effect of salvinorin A on facial relaxation in this cumulative dosing determination. However, a similar ANOVA (F[4,12]=13.47; p<0.001) revealed a significant effect of salvinorin A dose on ptosis. Newman–Keuls tests revealed that the effects of the largest salvinorin A dose on ptosis (0.032 mg/kg) were significantly different from vehicle and all other salvinorin A doses (0.001–0.01 mg/kg).
U69,593 (0.001–0.032 mg/kg) also caused increases in facial relaxation and ptosis, but again with greater dose-dependent effects in the latter measure. A repeated measures ANOVA for facial relaxation did not detect a significant effect of U69,593 (F[4,12]=1.84; ns). A repeated measures ANOVA (F[4,12]=31.60; p<0.001) revealed a significant effect of U69,593 on ptosis. Newman–Keuls tests revealed that the effects of the two largest U69,593 doses on ptosis (0.01 and 0.032 mg/kg) were significantly different from vehicle and the two smaller U69,593 doses (0.001–0.0032 mg/kg).
ICI204,448 (0.01–0.032 mg/kg) produced robust facial relaxation only in one of four subjects, whereas it caused dose-dependent ptosis in all subjects. A repeated measures ANOVA (F[4,12]=1; ns) did not detect an effect of ICI204,448 on facial relaxation, under these conditions. However, a repeated measures ANOVA (F[4,12]=6.61; p< 0.005) revealed a significant effect of ICI204,448 on ptosis. Newman–Keuls tests revealed that the effects of the largest ICI204,448 dose on ptosis (0.32 mg/kg) was significantly different from vehicle and the two smallest ICI204,448 doses (0.01–0.032 mg/kg).
Psilocybin (0.01–0.1 mg/kg) did not result in facial relaxation in any of the subjects (0 s scores were obtained throughout). Psilocybin likewise did not result in detectable ptosis, under these conditions; the largest effect observed at the largest psilocybin dose (0.1 mg/kg) had a mean value of 3.9 s (compared to a possible maximum of 60 s).
Quadazocine and naltrexone antagonism of salvinorin A-induced unconditioned behaviors
In two separate experiments, the salvinorin A cumulative dose-effect curve was re-determined after quadazocine pretreatment (0.32 mg/kg, s.c.; 60 min pretreatment) or naltrexone pretreatment (0.1 mg/kg, s.c.; 30 min pretreatment; Fig. 5). In both cases, a time window was also examined 19–20 min after each antagonist alone. Values after each antagonist alone were uniformly 0 s for all subjects, for facial relaxation and ptosis (not shown). Quadazocine did not cause robust antagonism of salvinorin A-induced facial relaxation under these conditions, whereas naltrexone depressed the salvinorin A dose-effect curve. However, a two-way (salvinorin A dose X pretreatment condition ([no pretreatment, quadazocine or naltrexone pretreatment]) repeated measures ANOVA did not reveal significant main effect of dose or pretreatment condition, possibly due to the limited effects of salvinorin A alone (e.g., Fig. 3) with these cumulative dosing parameters (not shown).
Fig. 5.
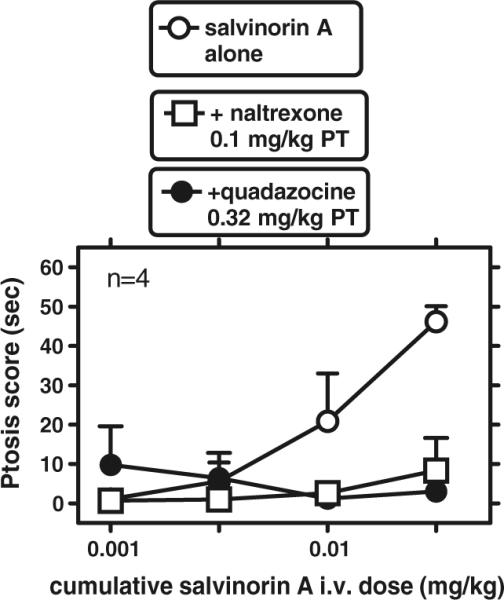
Antagonism of the effects of salvinorin A on ptosis, after pretreatment with either quadazocine (0.32 mg/kg) or naltrexone (0.1 mg/kg). Abscissa: cumulative salvinorin A dose after i.v. administration (milligrams per kilogram). Ordinate: ptosis score. Other details as in Fig. 4
However, both quadazocine and naltrexone caused robust antagonism of the cumulative salvinorin A dose-effect curve on ptosis. A two-way (salvinorin A dose X pretreatment condition ([no pretreatment, quadazocine or naltrexone pretreatment]) repeated measures ANOVA on ptosis revealed the main effects of salvinorin A dose (F[3,9]=12.63; p<0.00), pretreatment (F[2,6]=7.19; p< 0.03), and their interaction (F[6,18]=5.79; p<0.002). Newman–Keuls tests revealed that both quadazocine and naltrexone pretreatment conditions were different from salvinorin A alone.
Discussion
The widely available plant-derived hallucinogen, salvinorin A, is unique due to its high affinity and efficacy at κ-receptors, whereas other abused drugs with hallucinogenic or dissociative/psychotomimetic effects have 5HT2 (especially 5HT2A) agonist or NMDA antagonist effects (Roth et al. 2002). We present herein the first drug discrimination assay using salvinorin A as a training stimulus in nonhuman primates with high κ-receptor genetic homology to humans (Butelman et al. 2007).
Consistent with recent data from rats (Baker et al. 2009), rhesus monkey trained to discriminate salvinorin A generalized two centrally penetrating arylacetamide κ-agonists, U69,593 and U50,488. In addition, we report here that the benzomorphan κ-agonist bremazocine was also generalized in these primates, suggesting that structurally diverse κ-agonists share the discriminative effects of salvinorin A. The potency order of these centrally acting κ-agonists (bremazocine>U69,593>U50,488) is consistent with the potency order in other κ-receptor-mediated endpoints in this species (France et al. 1994). Up to the largest dose that did not decrease responding, the prototypical peripherally selective κ-agonist ICI204,448 was only generalized in one of three subjects. ICI204,448 may only display relative peripheral selectivity, based on behavioral/in vivo data (Butelman et al. 1999; Carey and Bergman 2001; Kumar et al. 2005), and this may likely underlie its partial effectiveness herein. Taken together, these data indicate that central κ-agonist effects are predominantly involved in the discriminative effects of salvinorin A in primates.
Other opioid receptor mechanisms, in addition to κ-receptors, were not apparently implicated in this effect, since the μ-agonist fentanyl or the δ-agonist SNC80 were not generalized in any subject, up to doses that decreased responding. Fentanyl and SNC80 are known to produce operant effects in this species mediated respectively by μ-and δ-agonist mechanisms (Brandt et al. 1999; Gerak and France 1996; Negus et al. 1993). Furthermore, the 5HT2-agonist psilocybin and the NMDA antagonist ketamine, abused compounds with hallucinogenic or dissociative/psychotomimetic effects, were likewise not generalized by any subject. Partial generalization with ketamine (50% salvinorin A-appropriate responding) was observed in one subject. It is known that NMDA antagonists can result in partial generalization in drug discrimination assays, due to nonspecific behavioral changes, rather than qualitative similarities with the relevant training drug (Koek et al. 1995). Therefore, this partial generalization in one subject is not sufficient to suggest a clear similarity between the discriminative stimulus effects of ketamine and salvinorin A.
Overall, the profile of drug discrimination findings supports the conclusion that the interoceptive effects of salvinorin A are mediated by different receptors and are qualitatively different from those of other known hallucinogenic/psychotomimetic compounds in humans. This conclusion is also consistent with the recent report of a lack of generalization of salvinorin A in rhesus monkeys trained to discriminate the 5HT2 hallucinogen DOM (1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (Li et al. 2008).
We also report the first data on the antagonism of the effects of salvinorin A in this drug discrimination. The opioid antagonist quadazocine, at a dose (0.32 mg/kg) known to be sufficient to block κ-receptor-mediated effects in this species (Negus et al. 1993), prevented the discriminative effects of salvinorin A herein. This robust antagonism could not be surmounted, up to the largest dose of salvinorin A that could be administered subcutaneously, due to solubility limitations. Other opioid antagonists (e.g., naltrexone, see below) were not used in the drug discrimination assay to minimize the potential interference with appetitive tasks in nonhuman primates (France and Morse 1989; Williams et al. 1999). By contrast to the opioid antagonist quadazocine, the 5HT2-antagonist ketanserin did not block the discriminative effects of salvinorin A, under similar conditions. The dose of ketanserin employed herein (0.1 mg/kg) is sufficient to block behavioral effects of 5HT2-agonists in this species (Li et al. 2009). Taken together, these antagonist studies are also consistent with the conclusion that opioid (κ) and not serotonergic 5HT2 receptor mechanisms underlie the behavioral effects of salvinorin A, contrasting with classic hallucinogens.
It has been previously reported that κ-agonists have sedative effects in humans, in addition to their psychotomimetic/dysphoric effects (Pfeiffer et al. 1986; Ur et al. 1997). Sedative and postural effects (e.g., postural relaxation) of centrally penetrating κ-agonists have also been reported in rhesus monkeys (Butelman et al. 1999; Dykstra et al. 1987). We have recently demonstrated that particular unconditioned effects related to the above (facial relaxation and ptosis) can be quantified in primates (Butelman et al. 2008) and may be potentially helpful as translational measures that can be applied for the study of salvinorin A (and more generally κ-opioid) effects in humans (Chernik et al. 1990; Miner et al. 2002). Furthermore, actual hallucinogenic effects in nonhuman primates are not easily quantified (Castner and Goldman-Rakic 1999; Davis et al. 1978). For example, visual and hand “tracking” (presumed behavioral correlates of hallucinations in non-verbal species; Castner and Goldman-Rakic 2003) were occasionally observed with salvinorin A in these studies. However, these endpoints were not easily amenable to quantification herein (e.g., in terms of intensity or duration). Therefore, facial relaxation and ptosis may be endpoints particularly suitable for the quantitative translational analysis of behavioral effects of salvinorin A, including its potential ultra-rapid onset (Butelman et al. 2008; Hooker et al. 2008).
We have recently reported that i.v. bolus salvinorin A caused robust facial relaxation in rhesus monkeys, and that these effects could be prevented and reversed by the clinically available opioid antagonist nalmefene but were not blocked by ketanserin (Butelman et al. 2008). We found here that the largest salvinorin A dose (0.032 mg/kg) that could be administered practically only produced relatively small effects on these unconditioned endpoints by the s.c. route and displayed a slower onset than by the i.v. route (e.g., 5–15 vs. 1–2 min). This is the first comparison of the behavioral effects of salvinorin A by these routes, widely used in behavioral pharmacology studies. Overall, findings are consistent with the value of the i.v. route as a useful substitute to quantify the effects of drugs commonly abused by the smoking route, such as those containing salvinorin A, likely due to fast onset and greater bioavailability by the i.v. route (Hooker et al. 2009; Rose et al. 1999; Schmidt et al. 2005; Tanda et al. 2000).
Consistent with the greater effects observed by the i.v. route in the bolus comparison (above), cumulative dose-effect curve studies were designed with salvinorin A and a subset of the compounds studied in the drug discrimination assay (using timing conditions similar to those in the drug discrimination assay). Salvinorin A (as well as U69,593) caused facial relaxation in some subjects in this cumulative dosing determination. This effect did not reach significance, possibly due to the relatively short duration of salvinorin A-induced facial relaxation, partially dissipating by 15 min (i.e., the time window used in the cumulative dosing determination). For comparison, a robust effect of salvinorin A on facial relaxation was observed in bolus i.v. administration in these subjects (e.g., up to 5 min after administration; see above).
Salvinorin A, as well as U69,593 caused robust dose-dependent ptosis in all subjects, in these cumulative determinations, similar to the bolus determination herein and a recent study (Butelman et al. 2008). ICI204,448 was also ineffective in causing facial relaxation herein, but caused ptosis, albeit with considerably less potency than salvinorin A or U69,593. The maximum dose of ICI204,448 used herein is larger than that needed to cause a neuroendocrine effect mediated by κ-receptors outside the blood-brain barrier in this species (prolactin release; Butelman et al. 1999). These data, together with the generalization to ICI204,448 in only one of three subjects in the drug discrimination above, suggest that relatively large doses of ICI204,448 can produce centrally mediated effects. Consistent with this possibility, large doses of ICI204,448 were also able to produce limited sedation in nonhuman primates (Butelman et al. 1999). Other, more recently developed κ-receptor agonists do exhibit greater peripheral selectivity than ICI204,448 itself (Kumar et al. 2005; Shaw et al. 1989).
We also report here that the opioid antagonist quadazocine (which robustly prevented the discriminative effects of salvinorin A above and previously; Butelman et al. 2004) also fully prevented the effects of salvinorin A in ptosis. A similar profile was observed with the clinically available antagonist naltrexone, at a dose (0.1 mg/kg) known to block the behavioral effects of arylacetamide κ-agonists in this species (Ko et al. 1998).
The serotonergic hallucinogen psilocybin did not cause either facial relaxation or ptosis, up to the largest dose administered. The psilocybin doses studied are in the upper range of those studied for operant self-administration studies in this species (Fantegrossi et al. 2004). We have also recently reported that ketanserin did not block the effects of salvinorin A in either facial relaxation or ptosis (Butelman et al. 2008). These findings, taken together with the inactivity of psilocybin and ketanserin in the drug discrimination assay (above), underscore the mechanistic divergence of salvinorin A to that of classic serotonergic hallucinogens.
Overall, these data show for the first time in nonhuman primates that the discriminative cue produced by salvinorin A is predominantly mediated by agonist effects at central κ-receptors and is not shared by agonists acting at other opioid receptors (μ or δ). Furthermore, abused compounds with known classic hallucinogenic (psilocybin) or psychotomimetic/dissociative effects in humans (ketamine) do not share the discriminative cue produced by salvinorin A. Together with prior drug discrimination data (Baker et al. 2009; Butelman et al. 2004; Li et al. 2008; Willmore-Fordham et al. 2007), these findings underscore the functional uniqueness of salvinorin A as a widely available powerful hallucinogen. We further show here that these general conclusions can also be supported from studies with unconditioned endpoints (facial relaxation and ptosis). These endpoints (unlike drug discrimination) do not require extensive subject training and may be of translational value in the study of salvinorin A or, more generally, the κ-opioid system in humans.
Acknowledgements
These studies were funded by NIH-NIDA grants DA017369 (ERB), DA018151 (TEP), and DA05130 (MJK).
Glossary
Abbreviations
- PT
Pretreatment
References
- Baker LE, Panos JJ, Killinger BA, Peet MM, Bell LM, Haliw LA, Walker SL. Comparison of the discriminative stimulus effects of salvinorin A and its derivatives to U69, 593 and U50, 488 in rats. Psychopharmacology. 2009;203:203–211. doi: 10.1007/s00213-008-1458-3. [DOI] [PubMed] [Google Scholar]
- Brandt MR, Negus SS, Mello NK, Furness MS, Zhang X, Rice KC. Discriminative stimulus effects of the nonpeptidic delta-opioid agonist SNC80 in rhesus monkeys. J Pharmacol Exp Ther. 1999;290:1157–1164. [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Kreek MJ. Effects of E-2078, a stable dynorphin A(1–8) analog, on sedation and serum prolactin levels in rhesus monkeys. Psychopharmacology (Berl) 1999;147:73–80. doi: 10.1007/s002130051144. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Kreek MJ. The plant-derived hallucinogen, salvinorin A, produces kappa-opioid agonist-like discriminative effects in rhesus monkeys. Psychopharmacology. 2004;172:220–224. doi: 10.1007/s00213-003-1638-0. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Mandau M, Tidgewell K, Prisinzano TE, Yuferov V, Kreek MJ. Effects of salvinorin A, a kappa-opioid hallucinogen, on a neuroendocrine biomarker assay in non-human primates with high kappa-receptor homology to humans. J Pharmacol Exp Ther. 2007;320:300–306. doi: 10.1124/jpet.106.112417. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Prisinzano TE, Deng H, Rus S, Kreek MJ. Unconditioned behavioral effects of the powerful kappa-opioid hallucinogen salvinorin A in nonhuman primates: fast onset and entry into cerebrospinal fluid. J Pharmacol Exp Ther. 2008;328:588–597. doi: 10.1124/jpet.108.145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey GJ, Bergman J. Enadoline discrimination in squirrel monkeys: effects of opioid agonists and antagonists. J Pharmacol Exp Ther. 2001;297:215–223. [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS. Long-lasting psychotomimetic consequences of repeated low-dose amphetamine exposure in rhesus monkeys. Neuropsychopharmacology. 1999;20:10–28. doi: 10.1016/S0893-133X(98)00050-5. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS. Amphetamine sensitization of hallucinatory-like behaviors is dependent on prefrontal cortex in non-human primates. Biol Psychiatry. 2003;54:105–110. doi: 10.1016/s0006-3223(03)00292-0. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BL. Salvinorin A, an active component of the halucinogenic sage Salvia divinorum, is a highly efficacious kappa opioid receptor agonist: Structural and functional considerations. J Pharmacol Exp Ther. 2004;308:1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, Schwam EM, Siegel JL. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–251. [PubMed] [Google Scholar]
- Condy C, Wattiez N, Rivaud-Pechoux S, Gaymard B. Ketamine-induced distractability: an oculomotor study in monkeys. Biol Psychiatry. 2005;57:366–372. doi: 10.1016/j.biopsych.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Davis WM, Bedford JA, Guinn MM, Hatoum HT, Waters IW, Wilson MC, Braude MC. Acute toxicity and gross behavioral effects of amphetamine, four methoxyamphetamines, and mescaline in rodents, dogs and monkeys. Toxicol Appl Pharmacol. 1978;45:49–62. doi: 10.1016/0041-008x(78)90027-3. [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Gmerek DE, Winger G, Woods JH. Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther. 1987;242:413–420. [PubMed] [Google Scholar]
- Fantegrossi WE, Woods JH, Winger G. Transient reinforcing effects of phenylisopropylamine and indolealkylamine hallucinogens in rhesus monkeys. Behav Pharmacol. 2004;15:149–158. doi: 10.1097/00008877-200403000-00007. [DOI] [PubMed] [Google Scholar]
- France CP, Morse WH. Pharmacological characterization of supersensitivity to naltrexone in squirrel monkeys. J Pharmacol Exp Ther. 1989;250:937–943. [PubMed] [Google Scholar]
- France CP, Medzihradsky F, Woods JH. Comparison of kappa opioids in rhesus monkeys: behavioral effects and receptor binding affinities. J Pharmacol Exp Ther. 1994;268:47–58. [PubMed] [Google Scholar]
- Gerak LR, France CP. Discriminative stimulus effects of nalbuphine in rhesus monkeys. J Pharmacol Exp Ther. 1996;276:523–531. [PubMed] [Google Scholar]
- Gonzales D, Riba J, Bouso JC, Gomez-Jarabo G, Barbanoj MJ. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend. 2006;85:157–162. doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Griffin OH, Miller BL, Khey DN. Legally high? Legal considerations of Salvia divinorum. J Psychoactive Drugs. 2008;40:183–191. doi: 10.1080/02791072.2008.10400629. [DOI] [PubMed] [Google Scholar]
- Hooker JM, Xu Y, Schiffer W, Shea C, Carter P, Fowler JS. Pharmacokinetics of the potent hallucinogen, salvinorin A in primates parallels the rapid onset, short duration of effects in humans. Neuroimage. 2008;41:1044–1050. doi: 10.1016/j.neuroimage.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker JM, Munro TA, Beguin C, Alexoff D, Shea C, Xu Y, Cohen BM. Salvinorin A and derivatives: protection from metabolism does not prolong short-term whole body residence. Neuropharmacology. 2009;57:386–391. doi: 10.1016/j.neuropharm.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover V, Marlowe DB, Patapis NS, Festinger DS, Forman RF. Internet access to Salvia divinorum: implications for policy, prevention and treatment. J Subst Abuse Treat. 2007;35:22–27. doi: 10.1016/j.jsat.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Smoking continues gradual decline among U.S. teens, smokeless tobacco threatens a comeback. 2009. www.monitoringthefuture.org. Press Release, 14 Dec 2009. [Google Scholar]
- Khey DN, Miller BL, Griffin OH. Salvia divinorum use among a college student sample. J Drug Educ. 2008;38:297–306. doi: 10.2190/DE.38.3.g. [DOI] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Traynor JR, Woods JH. Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. J Pharmacol Exp Ther. 1998;285:518–526. [PMC free article] [PubMed] [Google Scholar]
- Koek W, Kleven MS, Colpaert FC. Effects of the NMDA antagonist, dizocilpine, in various drug discriminations: characterization of intermediate levels of drug lever selection. Behav Pharmacol. 1995;6:590–600. [PubMed] [Google Scholar]
- Kumar V, Guo D, Cassel JA, Daubert JD, DeHaven RN, DeHaven Hudkins DL, Gauntner EK, Gottshall SL, Greiner SL, Koblish M, Little PJ, Mansson E, Maycock AL. Synthesis and evaluation of novel peripherally restricted kappa-opioid receptor agonists. Bioorg Med Chem Lett. 2005;15:1091–1095. doi: 10.1016/j.bmcl.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Lange JE, Reed MB, Ketchie Croff JM, Clapp JD. College student use of Salvia divinorum. Drug Alcohol Depend. 2008;94:263–266. doi: 10.1016/j.drugalcdep.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Rice KC, France CP. Discriminative stimulus effects of 1-(2, 5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) in rhesus monkeys. J Pharmacol Exp Ther. 2008;324:827–833. doi: 10.1124/jpet.107.130625. [DOI] [PubMed] [Google Scholar]
- Li JX, Rice KC, France CP. Discriminative stimulus effects of 1-(2, 5-dimethoxy-4-methylphenyl)-2aminopropane (DOM) in rhesus monkeys: antagonism and apparent pA2 analyses. J Pharmacol Exp Ther. 2009;328:976–981. doi: 10.1124/jpet.108.145458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JR, Heegard W, Plummer D. End-tidal carbon dioxide monitoring during procedural sedation. Acad Emerg Med. 2002;9:275–280. doi: 10.1111/j.1553-2712.2002.tb01318.x. [DOI] [PubMed] [Google Scholar]
- Negus SS, Burke TF, Medzihradsky F, Woods JH. Effects of opioid agonists selective for mu, kappa and delta opioid receptors on schedule-controlled responding in rhesus monkeys: antagonism by quadazocine. J Pharmacol Exp Ther. 1993;267:896–903. [PubMed] [Google Scholar]
- Paglia-Boak A, Mann RE, Adlaf EM, Rehm J. Highlights from the 2009 OSDUHS. Centre for Addiction and Mental Health; Toronto: 2009. Drug use among Ontario students, 1977–2009. [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Coleman RE. Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration. Drug Alcohol Depend. 1999;56:99–107. doi: 10.1016/s0376-8716(99)00025-3. [DOI] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: a potent naturally occuring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MD, Schmidt MS, Butelman ER, Harding WW, Tidgewell K, Murry DJ, Kreek MJ, Prisinzano TE. Pharmacokinetics of the plant-derived hallucinogen salvinorin A in nonhuman primates. Synapse. 2005;58:208–210. doi: 10.1002/syn.20191. PMID: 16138318. [DOI] [PubMed] [Google Scholar]
- Shaw JS, Carroll JA, Alcock P, Main BG. ICI204448: a kopioid agonist with limited access to the CNS. Br J Pharmacol. 1989;96:986–992. doi: 10.1111/j.1476-5381.1989.tb11911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert DJ. Salvia divinorum and salvinorin A: new pharmacologic findings. J Ethnopharmacol. 1994;43:53–56. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration The NSDUH report: use of specific hallucinogens: 2006. 2008 Available online: www.oas.samsha.gov/2k8/hallucinogens.pdf.
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Ur E, Wright DM, Bouloux PM, Grossman A. The effects of spiradoline (U-62066E), a kappa-opioid receptor agonist, on neuroendocrine function in man. Br J Pharmacol. 1997;120:781–784. doi: 10.1038/sj.bjp.0700971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DYW, Huang P, Li JG, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of three distinct k-ligands (salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol ExpTher. 2004;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- Williams KL, Pakarinen ED, Woods JH. Quadazocine decreases responding reinforced by ethanol, sucrose, and phencyclidine fluid deliveries in rhesus monkeys: comparison to naltrexone's effects. Psychopharmacology (Berl) 1999;144:316–322. doi: 10.1007/s002130051013. [DOI] [PubMed] [Google Scholar]
- Willmore-Fordham CB, Krall DM, McCurdy CR, Kinder DH. The hallucinogen derived from Salvia divinorum, salvinorin A, has kappa-opioid agonist discriminative stimulus effects in rats. Neuropharmacology. 2007;53:481–486. doi: 10.1016/j.neuropharm.2007.06.008. [DOI] [PubMed] [Google Scholar]