Abstract
Sex differences in Parkinson's disease (PD) have been reported in humans and rodent models, with a higher incidence in men and increased severity in male rodents. The current study examined sex differences and the effects of gonadal steroid hormones in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mouse model of PD. Male (n=51) and female (n=50) mice were gonadectomized and received physiologic replacement with testosterone or estrogen (Experiment 1), or no hormones (Experiment 2). Two weeks later, mice received either MPTP (10mg/kg per day for 5 days) or saline. Higher doses killed female mice. Mice were tested one week after MPTP for motor performance using rotarod, pole and gait tests. In hormone-treated mice, males significantly outperformed females in all three tests (p<0.05). Compared with females, males had a greater overall rotarod performance (ORP: 1317.1±98.3 vs. 988.1±95.6), descended a pole faster (7.1±0.6 vs. 9.6±0.7 sec), and had longer stride lengths (hindlimb 7.3±0.1 vs. 6.8±0.1 cm). By contrast, ovariectomized female mice receiving saline outperformed castrated males on the rotarod (1296.6±83.3 vs. 811.2±113.7, p<0.05) and descended a pole faster (9.7±2.0 vs. 15.6±1.9 sec, p<0.05). MPTP significantly impaired ORP (p<0.05) in hormone-treated males (703.7±65.5) and females (432.8±88.6, p<0.05). After MPTP, stride length was selectively decreased in males (hindlimb 6.6±0.1 cm, p<0.05), and pole test performance was unimpaired in either sex. After gonadectomy, MPTP did not decrease motor performance in males (p>0.05) but significantly reduced ORP in females (975.9±110.3 vs. saline females, p<0.05). Our results show that small, chronic doses of MPTP produce subtle, sexually-dimorphic impairments in motor performance, but without a loss of tyrosine hydroxylase-positive neurons in the substantia nigra. In gonadectomized mice, this sex difference is reversed.
Keywords: Parkinson's disease; striatum; substantia nigra; 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; motor behavior; sex characteristics testosterone, estrogen
1. Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by motor impairments that include resting tremor, rigidity, bradykinesia, and postural instability (reviewed in Jankovic, 2008). Sex differences have been reported for PD, and include a one- to two-fold higher incidence in men (reviewed in Shulman and Bhat, 2006). Sex differences are often driven by gonadal steroid hormones, acting both during development and in the adult. With respect to PD, estrogen is thought to exert neuroprotective effects which lessens both the incidence and phenotype in women (Benedetti et al., 2001; Dluzen, 2000; Haaxma et al., 2007; Shulman and Bhat, 2006; Tsang et al., 2001). In the current study, sex differences and the effects of gonadal steroid hormones were investigated using a mouse model of PD.
Rodent models of PD include the 6-hydroxydopamine (6-OHDA)-lesioned rat and the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mouse (Bove et al., 2005; Jakowec and Petzinger, 2004; Jeon et al., 1995; Schober, 2004). Both models replicate key features of PD by lesioning dopaminergic neurons in the substantia nigra pars compacta (SNC) which project rostrally to the striatum. Thus, after either 6-OHDA or MPTP, striatal dopamine is depleted (Alvarez-Fischer et al., 2008; Jakowec et al., 2004). Furthermore, in both models, dopamine depletion is more severe in males (Miller et al., 1998; Murray et al., 2003; Tamas et al., 2006, Xu et al., 2006). With 6-OHDA, male rats also have an increased loss of dopaminergic neurons in SNC compared to females (Murray et al., 2003; Tamas et al., 2006) and have more severe behavioral deficits post-lesion, including decreased motor activity (Cass et al., 2005; Tamas et al., 2005).
The current study investigated sex differences in motor impairments after MPTP in C57BL/6 mice. 6-OHDA is commonly delivered by intracerebral injection to produce an acute, unilateral depletion of dopaminergic neurons in SNC of rats. In mice, MPTP can be delivered systemically to produce less severe, bilateral lesions to the nigrostriatal dopamine system. We used a subacute dose of MPTP to reveal the potentially subtle sex differences in motor impairment between male and female mice. The behavioral tests measured distinct aspects of motor performance that parallel the motor deficits seen in human Parkinson's disease.
The goals of the current study were two-fold. Experiment 1 determined whether motor impairments after MPTP are sexually dimorphic. The results indicated that motor behavior was impaired in both male and female mice after MPTP, but that motor deficits were more severe in males. In addition, motor impairments in both sexes occurred without the loss of dopaminergic neurons in SNC. In Experiment 2, we determined whether gonadal hormones in adulthood underlie sex differences in motor impairments after MPTP. This was accomplished by comparing motor performance in gonadectomized male and female mice after treatment with either MPTP or saline.
2. Methods
2.1 Animals
Adult male and female C57BL/6 mice (8-12 weeks) were obtained from Charles River Laboratories (Wilmington, MA). Mice were group-housed on a 12:12 LD photoperiod with access to food and water ad libitum. Experimental procedures were approved by USC's Institutional Animal Care and Use Committee and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (DHEW Publication 80-23, revised 1985, Office of Science and Health reports, DRR/NIH, Bethesda, MD 20205).
2.2 Gonadectomy
All mice were gonadectomized under Avertin anesthesia (250 mg/kg). Males were castrated via a mid-line scrotal incision, and females were ovariectomized via bilateral dorsal flank incisions. In Experiment 1, steroids were replaced chronically at physiologic levels by Silastic implant sc (Barkley and goldman, 1977; Jacob et al., 2001). Males received a 5 mm implant (o.d. 2.16 mm, i.d. 1.02 mm, Dow Corning, Midland, MI) filled with crystalline testosterone (Steraloids, Newport, RI). Females received a similar implant of estradiol (1:1 17β-estradiol:cholesterol). Hormone-responsive tissue weights were recorded for males (seminal vesicle: 297.3±9.3g) and females (uterus: 171.7±4.8g). These measurements were consistent with physiogic levels of steroids in both sexes (Froment et al., 2004; Grasso et al, 1998). Seminal vesicle weight in male mice was 297.3±9.3g and uterine weight in females was 171.7±4.8g. In Experiment 2, gonadectomized male and female mice without steroid replacement received blank implants. Mice were allowed to recover from surgery (up to 2 weeks) before behavioral acclimatization and MPTP treatment. This time-course is sufficient to demonstrate decreases in hormone-driven behaviors (e.g. mating) after gonadectomy (Hull et al., 2006; Pfaff et al., 2006).
2.3 MPTP lesion
Male and female mice differ in their systemic tolerance for MPTP, with females being more severely affected (Przedborski et al., 2001). Thus, pilot studies in gonadectomized, hormone-treated males and females established the appropriate dose and inter-injection interval for MPTP. MPTP (free-base, Sigma, St. Louis, MO) was dissolved in 0.9% saline and injected ip. Male and female mice received either (1) four injections of 20 mg/kg MPTP at 2h intervals (n=15 males, n=25 females), (2) one injection of 30 mg/kg MPTP (n=5 females), or (3) one injection of 10 mg/kg MPTP daily for 5 days (n=15 males, n=15 females). Males tolerated all doses and inter-injection intervals well. However, 80-90% of females died after MPTP at 4×20 mg/kg or 1×30mg/kg (see Table 1). With 4×20 mg/kg MPTP, deaths occurred after the second injection. Therefore, to investigate sex differences in motor and neuromorphologic responses to MPTP, male and female mice receiving 10 mg/kg MPTP per day for 5 days were used for Experiment 1. Control male and female mice (n=15 each) received equivalent injections of 0.1 mL saline. Experiment 2 used the same regimen of MPTP or saline in gonadectomized males and females without hormone replacement (n=10-11/group).
Table 1.
Mortality after different doses of MPTP in gonadectomized male and female mice with hormone replacement.
| MPTP Dose | Survival Rate (Male Mice) | Survival Rate (Female Mice) |
|---|---|---|
| 4 × 20mg/kg MPTP q. 2h | 12 of 15 (80%) | 3 of 25 (12%) |
| 1 × 30mg/kg MPTP daily for 5d | 1 of 5 (20%) | |
| 1 × 10mg/kg MPTP daily for 5d | 15 of 15 (100%) | 13 of 15 (87%) |
2.4 Behavioral Tests
Rotarod, pole, and gait tests were used to measure locomotor ability, bradykinesia, and stride length, respectively. Initially, mice were acclimatized to the testing apparatus 1-2 days before lesion, and were tested for motor performance 1 week following the last injection of MPTP or saline. MPTP-induced loss of SNC dopaminergic neurons is complete within one week (Jackson-Lewis et al., 1995). Behavioral testing was conducted under red light conditions during the subjective dark phase.
Rotarod
The latency to fall off a rotating spindle (rat size, diameter 7.3 cm, Columbus Instruments, Columbus, OH) was measured according to the protocol of Rozas et al. (1998). Initially, mice were acclimatized to the rotarod for 10 minutes at 5 rpm. During acclimatization, mice that fell were replaced on the spindle. For behavioral testing, mice were tested at increasing speeds (12, 14, 16, 18, 20, 22, 24, and 26 rpm) and the latency to fall was recorded for each animal. The maximum duration at each speed was 150 seconds, with 150 seconds rest between trials. The overall rotarod performance (ORP) for each group was calculated by plotting the average latency to fall at each speed, and using the trapezoidal method of Rozas et al. (1997) to estimate the area under the curve.
Pole Test
Bradykinesia is measured by the time to a) turn head-down and b) completely descend a metal pole wrapped in cloth tape, according to Matsuura et al. (1997). Mice were acclimatized to the pole (1 cm diameter, 50 cm height) over 5 trials, each separated by 60 seconds. The first trial was limited to 300 seconds and subsequent trials were limited to 120 seconds. Behavioral testing was identical to acclimatization, with the exception that all five trials were capped at 120 seconds. Mice that failed to turn or fell off the pole were assigned a time of 120 seconds. The three best scores were averaged for each mouse. There was no sex difference and no effect of MPTP on the time to turn head-down; these data are not reported.
Gait Test
Stride length was measured according to the methods of Fernagut et al. (2002). Briefly, mice were placed on an illuminated runway (4.5 cm wide, 42 cm long, with walls 12 cm high) and were allowed to run toward a dark goal box (20×17×10 cm). Mice were acclimatized over two trials. Gait was measured in a single trial. To differentiate between forelimb and hindlimb strides, front and back paws were painted with red and black ink, respectively (Winsor and Newton, London, England) as mice ran down the paper-lined runway. Stride length was measured as the distance between successive paw prints. The average of three strides was taken for each animal. Because forelimb and hindlimb stride lengths were similar and demonstrated an equivalent sex difference and effect of MPTP, data are reported for hindlimb stride length only.
2.5 Perfusion and Immunocytochemistry
In gonadectomized mice with hormone replacement (Experiment 1), we determined whether MPTP reduced the number of TH-positive neurons in SNC in males and females. TH is the rate-limiting enzyme in dopamine biosynthesis and is used as a marker for dopamine-producing neurons. Because we found no sex differences and no effect of MPTP, TH neurons were not counted for gonadectomized mice in Experiment 2.
Mice were deeply anesthetized with sodium pentobarbital (150 mg/kg BW) and perfused intracardially with 150 mL of 0.1 M sodium phosphate buffer (PB, pH=7.4) containing 0.9% NaCl and 0.1% NaNO3. The brains were removed, post-fixed in the perfusion fixative overnight and cryoprotected for 5 days at 4°C with 20% sucrose in PB. Brains were rapidly frozen and sectioned coronally at 30 um through the rostro-caudal extent of SNC according to the stereotaxic atlas of the mouse brain (Paxinos and Franklin, 2001). Sections were stored in PB with 0.01% sodium azide at 4°C until processed for TH immunocytochemistry.
Every sixth section was stained for TH. Sections from mice in different groups of the same experiment were stained at the same time. Sections were incubated overnight at room temperature (RT) in polyclonal rabbit anti-TH antibody (1:5000; Chemicon, Temecula, CA) with 4% normal donkey serum and 0.3% Triton X-100 in PB. The following day, sections were incubated in biotinylated donkey anti-rabbit secondary antibody (1:200; Jackson Immunoresearch, West Grove, PA) and the avidin-biotin-horseradish peroxidase complex (Vector Elite Kit; Vector Laboratories, Burlingame, CA), each for 1 hour at RT with extensive washes in between. TH-labeled cells were visualized using NiCl-enhanced 3’3-diaminobenzidine tetrahydrochloride with 0.25% hydrogen peroxide. Sections were mounted onto gelatin-coated slides, air-dried, and counterstained for Nissl to identify neuronal nuclei. Afterwards, the slides were dehydrated in alcohols, cleared in xylenes and coverslipped with permount.
Unbiased stereological counts of TH neurons in SNC were performed on 7-9 animals randomly selected from each experimental group. TH-positive, Nissl-stained cells were counted bilaterally on coded slides by an observer blind to the treatment group using an Olympus BX-50 microscope (Olympus Optical, Tokyo, Japan) with motorized stage and Retiga-cooled CCD camera (Q-imaging, Burnaby, British Columbia, Canada) according to the methods of Petzinger et al. (2007). SNC was outlined in each section at low magnification (10x) using the third cranial nerve and cerebral peduncle as landmarks. A grid was placed over the outlined SNC and counting frames were established at grid intersections. Counting was performed at 80x using the optical fractionator technique assisted by the computer program BioQuant NovaPrime (BioQuant Image Analysis, Nashville, TN). TH-positive cells were counted if they had a visible nucleolus and were located within the counting frame. The total number of TH-positive neurons in SNC was calculated based on the method of Gundersen and Jensen (1987).
2.6 Statistics
All data are reported as Mean±SEM. For both experiments, group differences were analyzed by a factorial analysis of variance (ANOVA). Post-hoc comparisons using the Fisher's LSD test were conducted when statistically significant differences (p < 0.05) were found.
3.0 Results
3.1 Experiment 1: Gonadectomized mice with hormone replacement
In MPTP-treated mice, 100% of males survived the daily 10 mg/kg injection schedule, compared with 87% of females (p<0.05). At the time of sacrifice, saline-treated males weighed 26.7±0.3 g while control females weighed 24.2±0.2 g (p<0.05). Body weight in MPTP treated mice increased over the course of the experiment. Male mice weighed 24.3±0.3g prior to MPTP treatment and 26.0±0.3g at the time of sacrifice (p<0.05). In females, MPTP caused a non-significant increase in body weight (23.2±0.3 g vs. 23.6±0.7 g, p>0.05). Among MPTP treated animals, males outweighed females at the time of sacrifice (26.0±0.3 vs. 23.6±0.7 g).
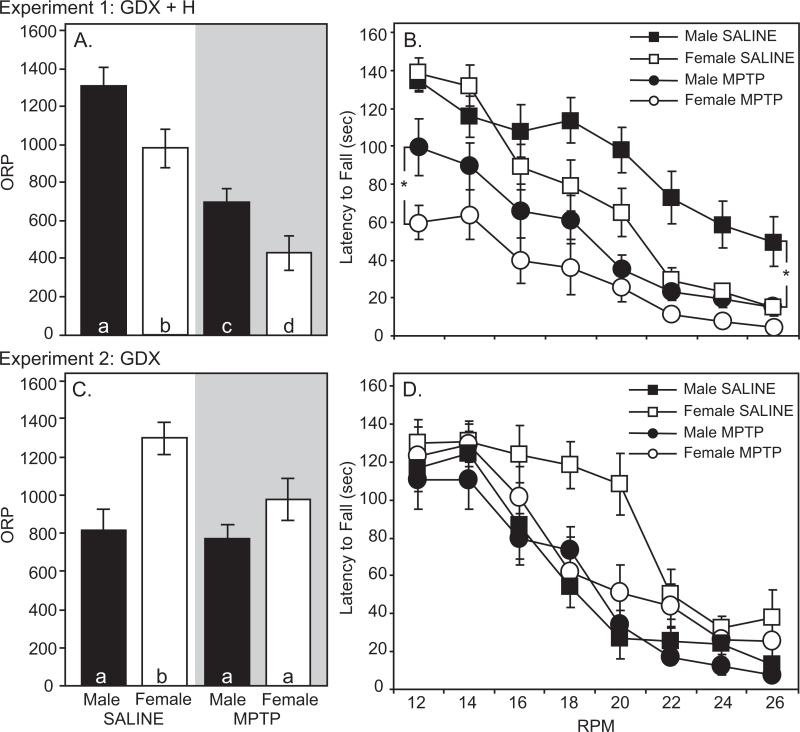
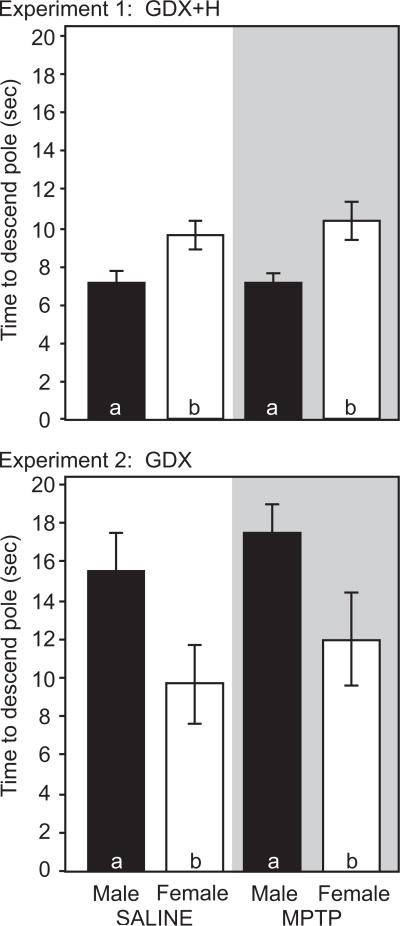
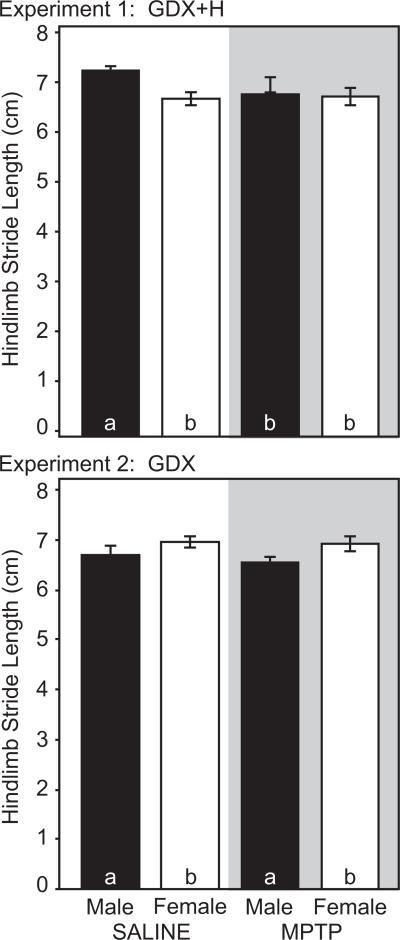
Saline-treated mice exhibited sex differences in motor performance measured by the rotarod, pole and gait tests. For all tests, males outperformed females. On the rotarod, ORP was greater in male mice (1317.0±98.3) than in females (988.1±95.6, p<0.05, Figure 1a). At low speeds (12 rpm), male and female mice had similar latencies to fall (134.3±6.0 vs. 138.1±8.7 sec, p>0.05). However, sex differences emerged at higher speeds. At 26 rpm, males stayed on the rotarod 3 times longer than females (49.6±13.1 vs. 14.9±2.9 sec, p<0.05). On the pole test, male mice descended the pole faster than females (7.1±0.5 vs. 9.6±0.7 sec, p<0.05, Figure 2). On the gait test, males had a longer stride length (hindlimb: 7.3±0.1 cm) than females (6.8±0.1 cm, p<0.05, Figure 3).
Figure 1.
Rotarod performance in gonadectomized male (black) and female (white) mice (n=13-15 per group). Overall rotarod performance (ORP) for saline- (clear) and MPTP-treated (shaded) for GDX+H mice (A) and GDX mice (C). Latency to fall off the rotarod at 12-26 rpm for saline- (squares) and MPTP-treated (circles) for GDX+H mice (B) and GDX mice (D). Bars with different letter subscripts are significantly different. Asterisks represent sex differences (p<0.05) within the same treatment group (MPTP or Saline). Abbreviations: GDX, gonadectomy; GDX+H, gonadectomy with hormone replacement.
Figure 2.
Pole test performance in male (black bars) and female (white bars) mice (n=13-15 per group). Time to completely descend the pole for for saline- (clear) and MPTP-treated (shaded) GDX+H mice (Top) and GDX mice (Bottom). Bars with different letter subscripts are significantly different. Abbreviations: GDX, gonadectomy; GDX+H, gonadectomy with hormone replacement.
Figure 3.
Stride length in male (black bars) and female (white bars) mice (n=13-15 per group). Hindlimb stride lengths for saline- (clear) and MPTP-treated (shaded) GDX+H mice (Top) and GDX mice (Bottom). Bars with different letter subscripts are significantly different. Abbreviations: GDX, gonadectomy; GDX+H, gonadectomy with hormone replacement.
MPTP lesion produced a comparable deficit in rotarod performance in both male and female mice. In males, MPTP reduced ORP by 47% to 703.7±65.5 (p<0.05 vs. unlesioned males, Figure 1a). In MPTP-lesioned female mice, ORP was reduced 56% to 432.8±88.6 (p<0.05 versus unlesioned females). MPTP treatment also altered the relative performance on the rotarod in males and females (Figure 1b). At low speeds, MPTP-lesioned males remained on the rotarod longer than females (99.5±14.8 vs. 59.8±8.8 sec, p<0.05). However, at 26 rpm, the latency to fall for males (14.6±4.4 sec) and females (4.5±1.8 sec) was not significantly different. On the pole test, MPTP did not impair performance in either sex (Figure 2). The time to descend the pole was 7.0±0.5 sec in males and 10.3±1.0 sec in females (p>0.05 vs. same-sex conspecifics, Figure 2). Finally, gait was slightly, but selectively reduced in male mice after MPTP. Hindlimb stride length was 6.9±0.2 cm in MPTP-lesioned males (p<0.05 vs. saline-treated males, Figure 3). By contrast, MPTP-lesioned female mice had comparable stride lengths (6.8±0.2 cm) compared to unlesioned females (p>0.05, Figure 3).
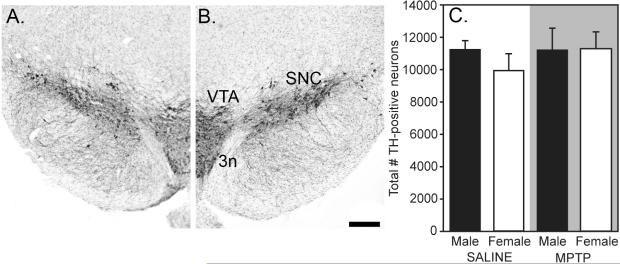
Cell counts of TH-positive, Nissl-stained neurons in SNC are shown in Figure 4. Unlesioned male and female mice had similar numbers of TH-positive neurons in SNC (11,174.1±620.0 vs. 9927.6±978.9, p>0.05, Figure 4). MPTP lesioning did not deplete TH neurons in males (11,192.6±1295.4) or in females (11,246.1±1064.9), and there were no sex differences (Figure 4).
Figure 4.
Photomicrographs of TH-positive neurons in SNC of saline- (A) and MPTP-treated (B) mice. (C) Mean±SEM number of TH-labeled neurons in male (black bars) and female (white bars) mice (n=7-9/group) in SNC. There were no significant differences between groups. Scale bar =200um. Abbreviations: 3n, third cranial nerve; SNC, substantia nigra pars compacta; TH, tyrosine hydroxylase; VTA, ventral tegmental area.
3.2 Experiment 2: Gonadectomized mice without hormone replacement
The 10 mg/kg daily dose of MPTP was well-tolerated by gonadectomized male and female mice, with 100% of mice surviving. Body weights of saline-injected gonadectomized male and female mice were not different at the time of sacrifice (25.1±0.4 vs. 24.8±0.3 g, p>0.05). As in Experiment 1, MPTP treatment increased body weight. Male mice weighed 24.1±0.6 g prior to MPTP and 24.9±0.4 g at the time of sacrifice (p<0.05). Female mice in the MPTP group weighed 21.1±0.2 g prior to MPTP and 24.8±0.3 g at the time of sacrifice (p<0.05). There were no differences in body weight between MPTP-treated male and female mice at the time of sacrifice.
Saline-treated ovariectomized female mice outperformed castrated males on the rotarod and pole tests. On the rotarod, ORP was significantly greater in gonadectomized female mice (1296.6±83.3) than in gonadectomized males (811.2±113.7, p<0.05, Figure 1c). In saline-injected mice, sex differences were most apparent at 16-20 rpm, where ovariectomized females stayed on the rotarod longer than orchidectomized males (Figure 1d). On the pole test, ovariectomized females took significantly less time to completely descend the pole (9.7±2.0 sec) than castrated males (15.6±1.9 sec, p<0.05, Figure 2). Finally, gonadectomized male and female mice did not differ in measures of stride length (Figure 3). Hindlimb stride length in ovariectomized females was 7.0±0.1 cm compared with 6.7±0.2 cm in castrated male mice (p>0.05, Figure 3).
After MPTP, rotarod performance was reduced in gonadectomized female mice. MPTP-injected female mice had an average ORP of 975.9±110.3 (p<0.05 versus saline-injected females, Figure 1c). ORP was similar in MPTP-treated males (771.8±70.6), but was not different from saline-treated males (p>0.05, Figure 1c).
Performance on the pole and gait tests was unaffected by MPTP treatment. MPTP did not increase the time to descend the pole in female (12.0±2.4 sec) or male (17.5±0.5) mice (p>0.05 vs. same sex conspecifics, Figure 2). However, the sex difference persisted after MPTP, with female mice descending the pole faster than males (p<0.05, Figure 2). Finally, stride length was similar in saline- versus MPTP-treated mice. Hindlimb stride length was 6.9±0.1 cm in MPTP-treated females and 6.5±0.1 cm in MPTP-treated males (p>0.05 vs. same-sex conspecifics, Figure 3).
4.0 Discussion
The current study investigated sex differences and the effects of gonadal steroids in the MPTP mouse model of Parkinson's disease using a subacute MPTP treatment (10 mg/kg MPTP per day for five days). Higher doses of MPTP killed female mice. In gonadectomized mice with physiologic hormone replacement, we observed a baseline sex difference in motor performance with male mice outperforming females on the rotarod, pole, and gait tests. MPTP lesioning reduced rotarod performance in both male and female mice but gait was selectively impaired only in males. In SNC, the total number of dopaminergic neurons was similar in all groups. To determine if these sex differences persist in the absence of gonadal steroids, we then tested MPTP lesions in gonadectomized mice. Ovariectomized, saline-treated females outperformed castrated males on the rotarod and pole tests. After MPTP, rotarod performance was reduced in ovariectomized females; castrated males did not show further motor deficits post-MPTP. Taken together, our results show that small, chronic doses of MPTP attenuate locomotor ability without depleting SNC neurons and reveal a sex-selective motor impairment in males. Gonadectomy reversed this sex difference.
Interestingly, the largest MPTP-induced sex difference was in mortality. At 10 mg/kg for 5 days, more females than males died after MPTP. In fact, in our pilot studies using larger doses of MPTP, 80-90% of female mice died. In contrast, male mice have received substantially higher doses of MPTP without mention of mortality (Fredriksson and Archer, 1994; Schmidt and Ferger, 2001). Death following MPTP is likely due to the systemic actions of MPTP on the cardiovascular system (Jackson-Lewis and Przedborski, 2007), but it is unknown why females are particularly susceptible. Based on our pilot studies the lethal threshold for female mice is approximately 30 mg/kg MPTP. A single injection of 30 mg/kg MPTP killed 80% of female mice within 2 hours. However, when females were given 20 mg/kg injections at two-hour intervals, the majority of females died shortly after the second injection.
Although female mortality was the primary reason for selecting the low MPTP dose and prolonged interinjection interval, subacute administration of MPTP at 10mg/kg revealed modest impairments in motor behavior that were sexually dimorphic. By contrast, larger doses of MPTP in males produce considerable motor deficits (reviewed in Sedelis et al., 2001) which may mask potential sex differences. These large doses of MPTP are used to replicate late-stage Parkinsonism (Schober, 2004). In PD patients, symptom severity is sexually dimorphic, with less severe motor impairment in women (Haaxma et al., 2007; Lyons et al., 1998; Shulman and Bhat, 2006). However, by end-stage PD, sex differences are no longer mentioned. Presumably, sex differences in motor behavior that are present in earlier stages of PD disappear as the disease progresses and as patients become more profoundly disabled.
As a model of early PD, our data demonstrate a substantial deficit in motor performance (50% decline in ORP) without destruction of dopaminergic neurons in SNC. This is likely due to the loss of dopaminergic terminals in the striatum and the subsequent reduction of striatal dopamine (Lau and Meredith, 2003; Petroske et al., 2001). In the current study, 10 mg/kg MPTP did not significantly deplete TH-positive neurons in SNC. This is consistent with Petroske et al. (2001) who delivered daily injections of 25 mg/kg MPTP without inducing dopaminergic cell death in SNC. However, unlike Petroske (2001), we observed significant motor deficits on the rotarod in both male and female mice. While Petroske et al. (2001) tested mice on a small-diameter rotarod spindle, we followed the protocol of Rozas et al. (1998), and used a larger, rat-sized spindle (7.3 cm diameter). This spindle distinguishes locomotor ability from balance and/or coordination, and is more effective than smaller spindles at detecting motor deficits after nigrostriatal lesions (Duan and Mattson, 1999; Rozas et al., 1998).
Our data also demonstrate that multiple behavioral tests are necessary to show deficits in motor performance and, more specifically, to reveal subtle sex differences in behavioral impairment after MPTP. Unfortunately, there is little consensus in published literature on motor deficits following MPTP treatment (reviewed in Sedelis et al., 2001). This reflects the tremendous variability in the dose and timing of MPTP administration and in the selection of behavioral assessments. In our study, 10 mg/kg MPTP reduced ORP in hormone-treated males and females, but there was no effect on pole test performance in hormone-treated or in gonadectomized mice. The absence of bradykinesia in the pole test may be related to the low dose of MPTP. Bradykinesia in rodents has been primarily revealed after substantial lesions and destruction of SNC cells and/or dopaminergic terminals in the striatum (Diguet et al., 2005; Ogawa et al., 1985). Given that our dose and timing of MPTP were relatively modest, it is not surprising that mice did not show deficits on the pole test. By contrast, measures of stride length proved to be the most sensitive for detecting sex differences after MPTP in hormone-treated mice. Male mice had a selective reduction in stride length after MPTP. Other studies have produced comparable reductions in gait using male mice, with larger doses of MPTP (Amende et al., 2005; Mohanasundari et al., 2006). The larger body weight of males may account for the longer stride length in vehicle-injected male mice, as shown previously (Heglund et al., 1974; Koopmans et al., 2007; Parker and Clarke, 1990). However, this cannot explain why MPTP decreased stride length only in males.
After establishing a sex difference in motor impairment after MPTP, our next goal was to determine whether gonadal steroids could account for these sex differences. Instead, castrated male mice performed more poorly on motor tests than ovariectomized females. After MPTP, motor impairments were observed in ovariectomized females, but not in castrated males. This is a complete reversal of our findings in hormone-treated mice, where males outperformed females. Perhaps the poor motor performance of castrated males is not surprising, given testosterone's anabolic effects to increase muscle mass and improve motor performance (American College of Sports Medicine, 2006). The implication is that castration has a more profound effect in males, compared with low-dose MPTP. Nonetheless, we cannot directly compare the results of Experiments 1 and 2, since the studies were not conducted at the same time.
A substantial body of evidence suggests that estrogen is neuroprotective in brain, including in the nigrostriatal system (Dluzen, 2000; Garcia-Segurra et al., 2001). The neuroprotective effects of estrogen would be expected to minimize MPTP-induced neuronal damage in females. Instead, female mice in the present study had comparable decreases in motor performance after MPTP, regardless of hormone status. It is important to note that our study is not an appropriate test of estrogen neuroprotection. First, the present study investigated behavioral endpoints, rather than neurologic outcomes. Motor behavior is expressed through the interactions of different physiologic systems (neurologic, cardiovascular, musculoskeletal), all of which respond to gonadal steroid hormones. Furthermore, it is difficult to demonstrate neuroprotection using a dose of MPTP which does not kill cells. It is more appropriate to test the neuroprotective effects of estrogen using higher doses of MPTP in males, as previously reported (Dluzen, 2000).
Ultimately, gonadal steroid hormones are also relevant to understanding sex differences in PD. Symptoms of PD emerge at approximately 60 years of age (Lees et al., 2009). By this age, most women are post-menopausal and testosterone levels are declining in men. Our results suggest that hypogonadism might have minimal effect on motor performance in females but could drastically reduce motor performance in males. In men with PD, the loss of SNC dopamine neurons superimposed over the decline of endogenous testosterone has the potential to increase symptom severity.
Acknowledgements
The authors would like to acknowledge Marta Vuckovic for her assistance on this project. This work was supported by NIH K18-DC009125.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Fischer D, Henze C, Strenzke C, Westrich J, Ferger B, Hoglinger GU, Oertel WH, Hartmann A. Characterization of the striatal 6-OHDA model of Parkinson's disease in wild type and alpha-synuclein-deleted mice. Exp Neurol. 2008;210:182–193. doi: 10.1016/j.expneurol.2007.10.012. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine . Position Statement: Senate hearing on the abuse of anabolic steroids and their precursors by adolescent atheletes. American College of Sports Medicine; 2006. [Google Scholar]
- Amende I, Kale A, McCue S, Glazier S, Morgan JP, Hampton TG. Gait dynamics in mouse models of Parkinson's disease and Huntington's disease. J Neuroeng Rehabil. 2005;2:20. doi: 10.1186/1743-0003-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley MS, Goldman BD. The effects of castration and Silastic implants of testosterone on intermale aggression in the mouse. Horm Behav. 1977;9:32–48. doi: 10.1016/0018-506x(77)90048-4. [DOI] [PubMed] [Google Scholar]
- Benedetti MD, Maraganore DM, Bower JH, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Hysterectomy, menopause, and estrogen use preceding Parkinson's disease: an exploratory case-control study. Mov Disord. 2001;16:830–837. doi: 10.1002/mds.1170. [DOI] [PubMed] [Google Scholar]
- Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson's disease. Neurorx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, Peters LE, Smith MP. Reductions in spontaneous locomotor activity in aged male, but not female, rats in a model of early Parkinson's disease. Brain Res. 2005;1034:153–161. doi: 10.1016/j.brainres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Diguet E, Fernagut PO, Scherfler C, Wenning G, Tison F. Effects of riluzole on combined MPTP + 3-nitropropionic acid-induced mild to moderate striatonigral degeneration in mice. J Neural Transm. 2005;112:613–631. doi: 10.1007/s00702-004-0206-z. [DOI] [PubMed] [Google Scholar]
- Dluzen DE. Neuroprotective effects of estrogen upon the nigrostriatal dopaminergic system. J. Neurocytol. 2000;29:387–399. doi: 10.1023/a:1007117424491. [DOI] [PubMed] [Google Scholar]
- Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Diguet E, Labattu B, Tison F. A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. J Neurosci Methods. 2002;113:123–130. doi: 10.1016/s0165-0270(01)00485-x. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Archer T. MPTP-induced behavioural and biochemical deficits: a parametric analysis. J. Neural Transm. Park. Dis. Dement. Sect. 1994;7:123–132. doi: 10.1007/BF02260967. [DOI] [PubMed] [Google Scholar]
- Froment P, Staub C, Hembert S, Pisselet C, Magistrini M, Delaleu B, Seurin D, Levine JE, Johnson L, Binoux M, Monget P. Reproductive abnormalities in human insulin-like growth factor-binding protein-1 transgenic male mice. Endocrinology. 2004;145:2080–2091. doi: 10.1210/en.2003-0956. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Grasso P, Rozhavskaya M, Reichert LE., Jr. In vivo effects of human follicle-stimulating hormone-related synthetic peptide hFSH-beta-(81-95) on the mouse estrous cycle. Biol Reprod. 1998;58(3):821–825. doi: 10.1095/biolreprod58.3.821. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MW. Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heglund NC, Taylor CR, McMahon TA. Scaling stride frequency and gait to animal size: mice to horses. Science. 1974;186:1112–1113. doi: 10.1126/science.186.4169.1112. [DOI] [PubMed] [Google Scholar]
- Hull EM, Wood RI, McKenna KE. Neurobiology of Male Sexual Behavior. In: Pfaff DW, editor. Knobil and Neill's Physiology of Reproduction. Elsevier Inc; San Francisco: 2006. pp. 1729–1824. [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Jacob DA, Temple JL, Patisaul HB, Young LJ, Rissman EF. Coumestrol antagonizes neuroendocrine actions of estrogen via the estrogen receptor alpha. Exp Biol Med (Maywood) 2001;226:301–306. doi: 10.1177/153537020122600406. [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Nixon K, Hogg E, McNeill T, Petzinger GM. Tyrosine hydroxylase and dopamine transporter expression following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration of the mouse nigrostriatal pathway. J Neurosci Res. 2004;76:539–550. doi: 10.1002/jnr.20114. [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Petzinger GM. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned model of parkinson's disease, with emphasis on mice and nonhuman primates. Comp Med. 2004;54:497–513. [PubMed] [Google Scholar]
- Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jeon BS, Jackson-Lewis V, Burke RE. 6-Hydroxydopamine lesion of the rat substantia nigra: time course and morphology of cell death. Neurodegeneration. 1995;4:131–137. doi: 10.1006/neur.1995.0016. [DOI] [PubMed] [Google Scholar]
- Koopmans GC, Deumens R, Brook G, Gerver J, Honig WM, Hamers FP, Joosten EA. Strain and locomotor speed affect over-ground locomotion in intact rats. Physiol Behav. 2007;92:993–1001. doi: 10.1016/j.physbeh.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Lau YS, Meredith GE. From drugs of abuse to parkinsonism. The MPTP mouse model of Parkinson's disease. Methods Mol Med. 2003;79:103–116. doi: 10.1385/1-59259-358-5:103. [DOI] [PubMed] [Google Scholar]
- Lees AF, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Lyons KE, Hubble JP, Troster AI, Pahwa R, Koller WC. Gender differences in Parkinson's disease. Clin Neuropharmacol. 1998;21:118–121. [PubMed] [Google Scholar]
- Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 1997;73:45–48. doi: 10.1016/s0165-0270(96)02211-x. [DOI] [PubMed] [Google Scholar]
- Miller DB, Ali SF, O'Callaghan JP, Laws SC. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann N Y Acad Sci. 1998;844:153–165. [PubMed] [Google Scholar]
- Mohanasundari M, Srinivasan MS, Sethupathy S, Sabesan M. Enhanced neuroprotective effect by combination of bromocriptine and Hypericum perforatum extract against MPTP-induced neurotoxicity in mice. J Neurol Sci. 2006;249:140–144. doi: 10.1016/j.jns.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Murray HE, Pillai AV, McArthur SR, Razvi N, Datla KP, Dexter DT, Gillies GE. Dose- and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: differential actions of estrogen in males and females. Neuroscience. 2003;116:213–222. doi: 10.1016/s0306-4522(02)00578-x. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Hirose Y, Ohara S, Ono T, Watanabe Y. A simple quantitative bradykinesia test in MPTP-treated mice. Res Commun Chem Pathol Pharmacol. 1985;50:435–441. [PubMed] [Google Scholar]
- Parker AJ, Clarke KA. Gait topography in rat locomotion. Physiol Behav. 1990;48:41–47. doi: 10.1016/0031-9384(90)90258-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106:589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- Petzinger GM, Walsh J, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Fisher BE, Togasaki D, Jakowec M. Effects of Treadmill Exercise on Dopaminergic Transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-(MPTP)-Lesioned Mouse Model of Basal Ganglia Injury. J Neurosci. 2007;27:5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y, Kow L-M, Lee AWL, Easton A. Hormonal, Neural, and Genomic Mechanisms for Female Reproductive Behaviors. In: Pfaff DW, editor. Knobil and Neill's Physiology of Reproduction. Elsevier Inc; San Francisco: 2006. pp. 1825–1920. [Google Scholar]
- Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- Rozas G, Guerra MJ, Labandeira-Garcia JL. An automated rotarod method for quantitative drug-free evaluation of overall motor deficits in rat models of parkinsonism. Brain Res Brain Res Protoc. 1997;2:75–84. doi: 10.1016/s1385-299x(97)00034-2. [DOI] [PubMed] [Google Scholar]
- Rozas G, Lopez-Martin E, Guerra MJ, Labandeira-Garcia JL. The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J Neurosci Methods. 1998;83:165–175. doi: 10.1016/s0165-0270(98)00078-8. [DOI] [PubMed] [Google Scholar]
- Schmidt N, Ferger B. Neurochemical findings in the MPTP model of Parkinson's disease. J Neural Transm. 2001;108:1263–1282. doi: 10.1007/s007020100004. [DOI] [PubMed] [Google Scholar]
- Schober A. Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- Sedelis M, Schwarting RK, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson's disease. Behav Brain Res. 2001;125:109–125. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- Shulman LM, Bhat V. Gender disparities in Parkinson's disease. Expert Rev Neurother. 2006;6:407–416. doi: 10.1586/14737175.6.3.407. [DOI] [PubMed] [Google Scholar]
- Tamas A, Lubics A, Lengvari I, Reglodi D. Effects of age, gender, and gonadectomy on neurochemistry and behavior in animal models of Parkinson's disease. Endocrine. 2006;29:275–287. doi: 10.1385/ENDO:29:2:275. [DOI] [PubMed] [Google Scholar]
- Tamas A, Lubics A, Szalontay L, Lengvari I, Reglodi D. Age and gender differences in behavioral and morphological outcome after 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Behav Brain Res. 2005;158:221–229. doi: 10.1016/j.bbr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Tsang KL, Jiang H, Ramsden DB, Ho SL. The use of estrogen in the treatment of Parkinson's disease. Parkinsonism Relat Disord. 2001;8:133–137. doi: 10.1016/s1353-8020(01)00027-x. [DOI] [PubMed] [Google Scholar]
- Xu K, Xu Y, Brown-Jermyn D, Chen JF, Ascherio A, Dluzen DE, Schwarzschild MA. Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J Neurosci. 2006;26:535–541. doi: 10.1523/JNEUROSCI.3008-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]