Abstract
Receptor tyrosine kinases of the Eph family become tyrosine phosphorylated and initiate signaling events upon binding of their ligands, the ephrins. Eph receptors such as EphA2 and EphB4 are highly expressed but poorly tyrosine phosphorylated in many types of cancer cells, suggesting a limited interaction with ephrin ligands. Nevertheless, decreasing the expression of these receptors affects the malignant properties of cancer cells, suggesting that Eph receptors may influence cancer cells independently of ephrin stimulation. Ligand-independent activities of Eph receptors in cancer, however, have not been demonstrated. By using siRNA interference to downregulate EphB4 in MCF7 and MDA-MB-435 cancer cells, we found that EphB4 inhibits integrin-mediated cell substrate adhesion, spreading, and migration and reduces β1 integrin protein levels. Low expression of the EphB4 preferred ligand, ephrin-B2, and minimal contact between cells in these assays suggest that cell contact-dependent stimulation of EphB4 by the transmembrane ephrin-B2 ligand does not play a role in these effects. Indeed, inhibitors of ephrin-B2 binding to endogenous EphB4 did not influence cell substrate adhesion. Increasing EphB4 expression by transient transfection inhibited cell substrate adhesion, and this effect was also independent of ephrin stimulation because it was not affected by single amino acid mutations in EphB4 that impair ephrin binding. The overexpressed EphB4 was tyrosine phosphorylated, and we found that EphB4 kinase activity is important for inhibition of integrin-mediated adhesion while several EphB4 tyrosine phosphorylation sites are dispensable. These findings demonstrate that EphB4 can affect cancer cell behavior in an ephrin-independent manner.
Keywords: ligand-independent, receptor tyrosine kinase, β1 integrin, cell attachment, cell spreading, cell migration
INTRODUCTION
High expression of the EphB4 receptor has been documented in a large variety of human cancers, including breast, ovarian and prostate cancer, early colorectal cancer and others [1-9]. In the corresponding cancer cell lines, EphB4 is also widely expressed but its level of tyrosine phosphorylation is often low [3-6, 9, 10], suggesting low levels of activation by its preferred ligand, ephrin-B2. Consistent with this, ephrin-B2 is poorly expressed in many of the cancer cell lines. Whether other mechanisms may also contribute to low EphB4 activation by ephrin-B2 in cancer cells remains to be investigated. For example, loss of E-cadherin in cancer cells has been shown to impair EphA2 activation by the GPI-linked ephrin-A1 ligand, suggesting a requirement for stable cell-cell contacts [11].
Interestingly, we found that inducing activation of EphB4 with a soluble form of ephrin-B2 (ephrin-B2 Fc) inhibits the tumorigenicity of MCF7, MDA-MB-231 and MDA-MB-435 cancer cells through a pathway that involves Abl family cytoplasmic tyrosine kinases and the adaptor protein Crk [10]. This tumor suppressor pathway appears to be operational in different types of cancer cells because MCF7 and MDA-MB-231 are breast cancer cells, whereas the currently used MDA-MB-435 cell line has been recently shown to be derived from the M14 melanoma cell line [12]. Downregulation of EphB4 using RNA interference or antisense oligonucleotides has also provided evidence for a functional role of EphB4 in a variety of cancer cell types where this receptor appears to be phosphorylated (activated) at low levels [3, 4, 7, 9].
EphB4 is part of the large Eph family of receptor tyrosine kinases, which comprises EphA and EphB receptors. Prototypical Eph receptor activation takes place upon cell contact-dependent interaction with the ephrin ligands, which comprise the GPI-linked A-ephrins and the transmembrane B-ephrins [13]. Binding to ephrins on adjacent cells triggers Eph receptor autophosphorylation and activation of the kinase domain, initiating Eph receptor signaling activities. However, the functional importance of Eph receptors in cancer cells where they do not appear to be extensively activated by their ephrin ligands has led to the hypothesis that these receptors might influence the properties of cancer cells through ephrin-independent mechanisms [13-15]. A few reports raise the intriguing possibility that the Eph receptors might indeed be able to influence cell behavior independently of ephrin binding. For example, genetic experiments have suggested an ephrin-independent function for the C. elegans Eph receptor, VAB-1, in gonadal sheath cell contraction [16]. Furthermore, forced expression of the EphA8 receptor in a neural cell line has been shown to promote MAP kinase activation and neurite outgrowth even when the receptor lacks the amino terminal ephrin-binding domain [17]. Very recently, ligand-independent activities have been reported for the EphA4 receptor in neural cells, where cytoplasmic fragments generated by γ-secretase or caspase cleavage affect dendritic spine morphogenesis and cell survival, respectively [18, 19]. However, ligand-independent activities of Eph receptors in cancer have not been demonstrated. Here we report that the EphB4 receptor can modulate integrin-mediated adhesion in cancer cells independently of ephrin binding.
EXPERIMENTAL
Cell lines and transfections
MDA-MB-435p (parental), MDA-MB-435c (clonal) and 293 human embryonal kidney (HEK) cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS). MDA-MB-435c is a clonal cell line derived from MDA-MB-435p that expresses predominantly EphB4 among the EphB receptors [10]. MCF7 cells were grown in Modified Eagle’s Medium (MEM) with 10 μg/ml insulin and 10% FBS.
To knock down human EphB4, we tested Dharmacon siGENOME individual siRNAs (Dharmacon RNA Technologies) and the Dharmacon SMARTpool of the four siRNAs. Individual siRNAs 1 through 4 correspond respectively to Dharmacon siGENOME duplex-5 (sense sequence GGACAAACACGGACAGUAUUU and antisense sequence 5′-PAUACUGUCCGUGUUUGUCCUU, where P indicates phosphorylation), duplex-6 (sense sequence GUACUAAGGUCUACAUCGAUU and antisense sequence 5′-PUCGAUGUAGACCUUAGUACUU), duplex-7 (GGAGAGAAGCAGAAUAUUCUU and antisense sequence 5′-PGAAUAUUCUGCUUCUCUCCUU), duplex-8 (sense sequence GCCAAUAGCCACUCUAACAUU and antisense sequence 5′-PUGUUAGAGUGGCUAUUGGCUU). siRNA that engages the RISC complex but does not match any mouse or human genes (Dharmacon siCONTROL non-targeting siRNA) was used as a control. The amount of siRNA and transfection protocol were optimized for each cell line. For 6 cm dishes, MDA-MB-435p and MDA-MB-435c were transfected with 0.3 nanomoles of the siRNAs used individually or with 0.075 nanomoles of each siRNA in the SMARTpool (95 nM final total concentration) using OligofectAMINE (Invitrogen Life Technologies). For the experiments shown in Fig. 1 and Fig. 2A,B, MCF7 cells were transfected with 0.1 nanomoles siRNA (29 nM final concentration) using Lipofectamine 2000 (Invitrogen Life Technologies) according to manufacturer’s instructions. For the experiments shown in Fig. 2C, Fig. 3A, and Suppl. Figs. 1 and 2, MCF7 cells were transfected with 0.172 nanomoles siRNA (50 nM final concentration).
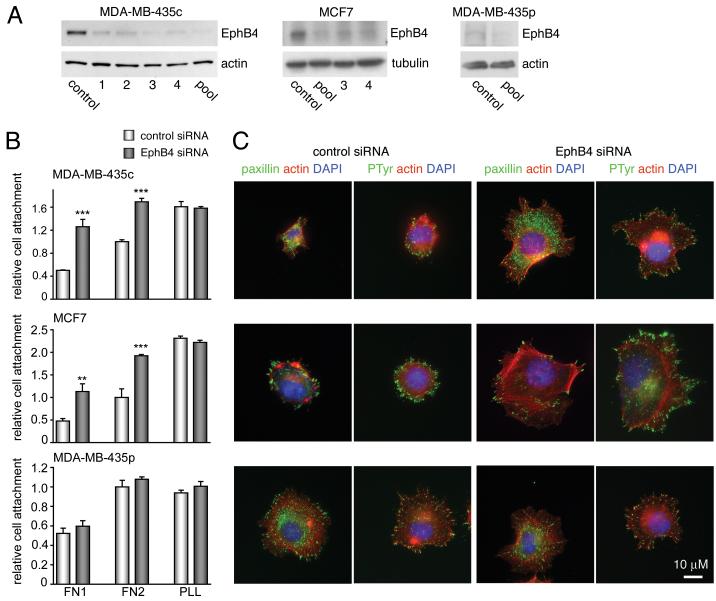
Figure 1. EphB4 siRNA knock down decreases EphB4 expression and increases cancer cell adhesion to fibronectin.
(A) Lysates from control transfected cells and from cells transfected with 4 individual (1 through 4) or pooled EphB4 siRNAs were probed by immunoblotting for EphB4 72 hours after transfection. Anti-actin or anti-tubulin antibodies were used as protein loading controls. (B) The indicated cell lines were transfected with control or pooled EphB4 siRNAs and allowed to attach to fibronectin for 15 min (FN 1) or 30 min for the MDA-MB-435 cells and 1 hour for the MCF7 cells (FN 2). As a control, cells were allowed to attach to poly-l-lysine (PLL) for 1 hour. The histogram shows average numbers of attached cells ± SEM from 3 coverslips. **P<0.01 or ***P<0.001 compared to control siRNA using one-way ANOVA and Bonferroni’s post-hoc test. (C) siRNA transfected cells were triple labeled to visualize focal adhesions (green: paxillin or phosphotyrosine), the actin cytoskeleton (red: rhodamine phalloidin) and nuclei (blue: DAPI). The scale bar represents 10 μM.
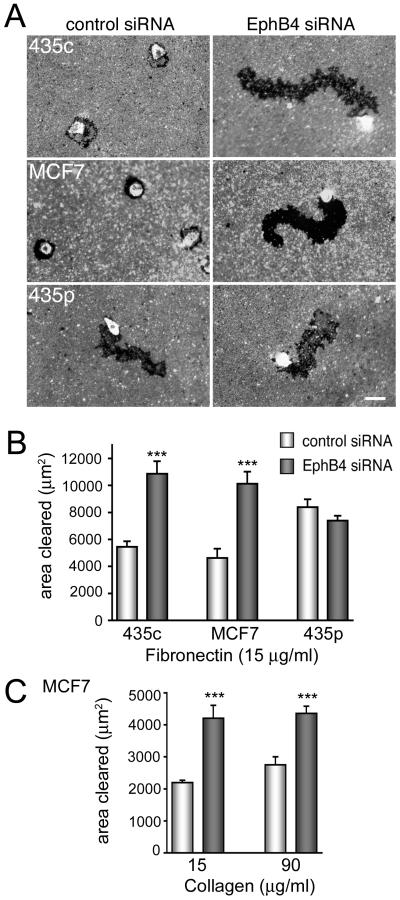
Figure 2. EphB4 siRNA knock down increases cancer cell motility.
(A) The indicated cell lines were transfected with control siRNA or the EphB4 siRNA pool and after 3 days the cells were allowed to migrate on wells coated with 25 μg/ml fibronectin and fluorescent beads. The dark area cleared by the migrating cells, which are very bright because they have engulfed the fluorescent beads, was measured using Image J. The scale bar represents 50 μm. (B) The histogram shows averages ± SEM from quadruplicate measurements of the areas cleared by cells plated on fibronectin. (C) The histogram shows averages ± SEM from triplicate measurements of the areas cleared by cells plated on the indicated concentrations of collagen. ***P<0.001 compared to control siRNA using one-way ANOVA and Tukey’s post-hoc test. Furthermore, P<0.05 for the comparison of control siRNA-transfected cells migrating on wells coated with 15 versus 90 μg/ml collagen; no significant difference for the comparison of EphB4 siRNA-transfected cells migrating on wells coated with 15 versus 90 μg/ml collagen; and P<0.001 for the comparison between EphB4 siRNA-transfected cells plated on 15 μg/ml collagen and control siRNA-transfected cells plated on 90 μg/ml collagen.
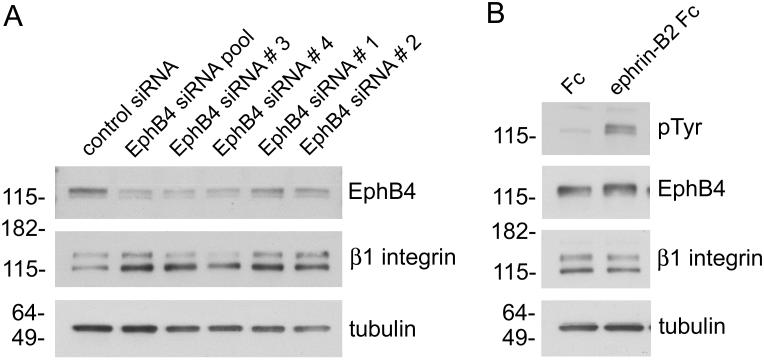
Figure 3. EphB4 expression decreases β1 integrin levels whereas ligand-dependent EphB4 activation does not.
(A) MCF7 breast cancer cells were transfected with a control siRNA, a pool of 4 EphB4 siRNAs, or 4 individual EphB4 siRNAs for 72 hours. Lysates of cells transfected with the EphB4 siRNAs show increased levels of β1 integrin concomitant with the expected downregulation of EphB4 expression; the blots were also probed for β-tubulin as a loading control. (B) Stimulation of MCF7 cells with ephrin-B2 Fc increases tyrosine phosphorylation (PTyr) in lysates without affecting β1 integrin levels.
The pcDNA3-EphB4 plasmid was a gift from D.T. Scaddon and pEGFP is from Clonetech. The EphB4 590/594E mutant has been described [20] and the other EphB4 mutants were generated by site directed mutagenesis with the QuikChange® site-directed mutagenesis kit (Stratagene) using pcDNA3-EphB4 as the template. For transfections of the plasmids in 293 HEK cells, 6 cm plates of cells were transfected with 5 μg DNA and 15 μl FuGENE (Roche Applied Science). Plasmids were transfected in MDA-MB-435p cells with Lipofectamine/PLUS according to manufacturer’s instructions. The cells were used 2 days after transfection.
Adhesion assays
For the experiments shown in Fig. 1, coverslips and culture dishes for adhesion assays were coated with 25 μg/ml fibronectin or 10 μg/ml poly-L-lysine (Sigma) for 1 hour at 37°C and then blocked with 1% bovine serum albumin in DMEM. For the experiments shown in Suppl. Fig. 2A,B, coverslips were coated with various concentrations of fibronectin (2-40 μg/ml) or collagen (15-90 μg/ml) and for those shown in Suppl. Fig. 2C, coverslips were coated with 5 μg/ml fibronectin. Cells transfected with siRNA or plasmids were used 3 and 2 days after transfection, respectively.
In the experiments shown in Fig. 4, cells were washed with phosphate buffered saline (PBS), detached in 0.2 mM EDTA in PBS, washed twice with DMEM, kept in suspension for 30 min in 0.5% bovine serum albumin in DMEM, and then plated in the same medium. For the experiments shown in Fig. 4, cells were kept in suspension for 10 min and then treated in suspension for 20 min with either 15 μg/ml EphB4 Fc, Fc control (R&D Systems), or 50 μM TNYL-RAW antagonistic peptide [21]. Untreated cells were used as a further control. For the experiments shown in Fig. 4D, cells were then stimulated for 10 min with 5 μg/ml ephrin-B2 Fc or control human Fc, pre-clustered with 25 μg/ml anti-human Fc polyclonal antibody, in the continued presence of the inhibitors. To calculate relative cell attachment, the number of attached cells was normalized to that of control attached cells. In experiments including different substrate concentrations or time points, the control plated with the highest substrate concentration or for longest time was used for normalization.
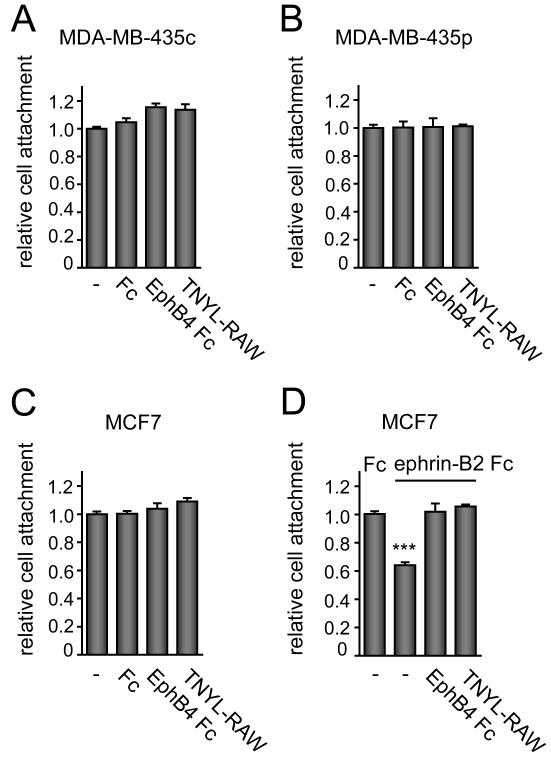
Figure 4. Cancer cells treated with EphB4 Fc or an antagonistic peptide attach to fibronectin similarly to untreated cells.
(A-C) The indicated cells in suspension were left untreated (–), treated for 20 min with 15 μg/ml EphB4 Fc or Fc as a control, or treated with 50 μM TNYL-RAW peptide. The cells were then allowed to attach for 15 min in the continued presence of Fc proteins or peptide. Attached cells were stained with DAPI and counted. There was no significant difference between controls and treatments using one-way ANOVA and Tukey’s post-hoc test. (D) MCF7 cells in suspension were left untreated (–), treated for 20 min with 15 μg/ml EphB4 Fc, or treated with 50 μM TNYL-RAW peptide. The cells were then allowed to attach for 10 min the presence of Fc or ephrin-B2 Fc and EphB4 Fc or TNYL-RAW. Attached cells were stained with DAPI and counted. The histograms show averages ± SEM from triplicate measurements. ***P<0.001 compared to control Fc treated using one-way ANOVA and Bonferroni’s post-hoc test.
Immunofluorescence labeling
Cells plated on glass coverslips were fixed in 3.7% formaldehyde in PBS, permeabilized in 0.5% Triton-X-100 in TBS and incubated with 0.7 μg/ml anti-paxillin monoclonal antibody (BD Biosciences) or 0.5 μg/ml anti-phosphotyrosine monoclonal antibody (BD Biosciences), and subsequently incubated with Alexa conjugated anti-mouse secondary antibodies from Molecular Probes. Cells were also labeled with rhodamine phalloidin and DAPI (Molecular Probes).
Immunoprecipitation and immunoblotting
For ephrin-B2 Fc stimulation experiments, adherent or suspended MDA-MB-435c cells were stimulated for 15 min with 3 μg/ml ephrin-B2 Fc (R&D Systems) pre-clustered by incubation with 0.3 μg/ml anti-human Fc polyclonal antibody (Jackson ImmunoResearch; Fig. 6C), for 20 min with 1.5 μg/ml ephrin-B2 Fc pre-clustered with 4.5 μg/ml anti-Fc antibody (Fig. 6D), or for 20 min with 5 μg/ml ephrin-B2 Fc pre-clustered by incubation with 25 μg/ml anti-human Fc antibody (Fig. 3B). In the experiments shown in Fig. 6C,D, cells were serum-starved overnight prior to stimulation. For immunoprecipitation, MDA-MB-435c cells and transiently transfected 293 HEK cells were lysed in modified RIPA buffer (50 mM TrisHCl, pH 7.6, 150 mM NaCl, 1% TritonX-100, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM EDTA with protease and phosphatase inhibitors) and EphB4 was immunoprecipitated with anti-EphB4 polyclonal antibodies [10]. The immunoprecipitates were separated by SDS-PAGE and probed by immunoblotting with anti-phosphotyrosine antibodies (PY20, BD Biosciences), anti-EphB4 polyclonal antibodies [10], anti-EphB4 monoclonal antibodies (Invitrogen/Zymed) or antibodies to the Y590 and Y594 juxtamembrane phosphorylation sites [22]. Lysates from siRNA- or plasmid-transfected cells were lysed in RIPA buffer and analyzed by SDS-PAGE followed by immunoblotting with anti-EphB4 antibodies, anti-β1 integrin (BD Bioscience), anti-actin and anti-tubulin antibodies (Sigma). It should be noted that some transfected EphB4 mutants tended to be expressed at lower levels than wild-type EphB4. Therefore, the amounts of cell lysates used for immunoprecipitation or immunoblotting were adjusted to obtain comparable levels of EphB4.
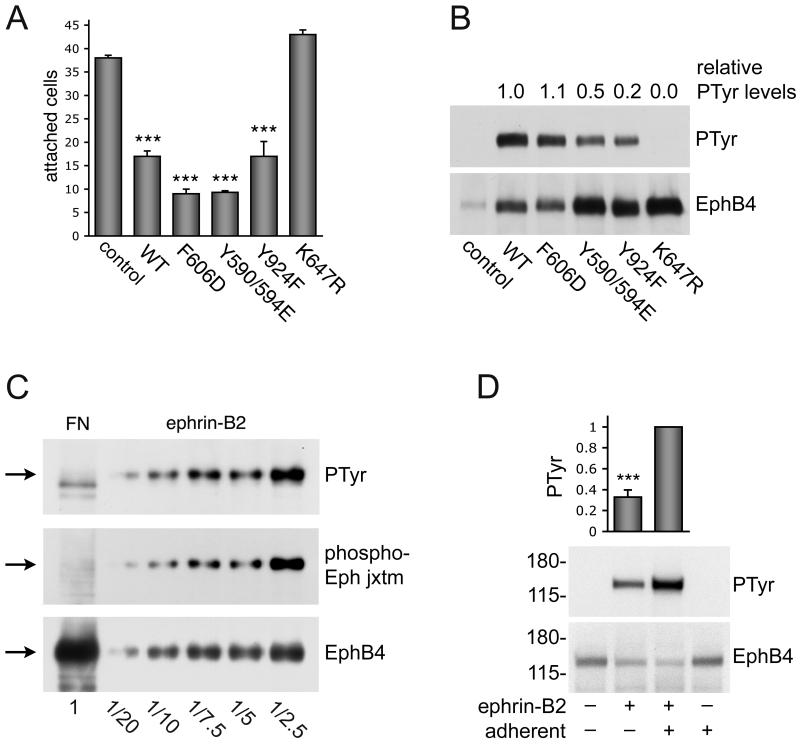
Figure 6. Inhibition of cell adhesion by EphB4 requires kinase activity.
(A) MDA-MB-435p cells, which express low levels of EphB4, were transfected with the indicated EphB4 constructs or pcDNA3 control vector at a 10:1 ratio with EGFP. Forty-eight hours after transfection, the cells were detached and allowed to adhere to fibronectin for 15 min. EGFP-positive cells that had attached to fibronectin were counted under a fluorescence microscope. The histogram shows averages ± SEM from triplicate measurements.***P<0.001 compared to vector control using one-way ANOVA and Tukey’s post-hoc test. Furthermore, the EphB4 F606D and Y590/594E mutants inhibited cell attachment slightly more than EphB4 wild-type (*P<0.05). (B) 293 HEK cells were transfected with the indicated EphB4 constructs or vector control. EphB4 immunoprecipitates were probed with anti-phosphotyrosine antibodies (PTyr) and reprobed with anti-EphB4 antibodies. The tyrosine phosphorylation levels were quantified from immunoblots and normalized to the amount of immunoprecipitated EphB4. The numbers above the blot represent phosphorylation levels compared to wild-type EphB4 averaged from 2 independent experiments. (C) EphB4 immunoprecipitates from MDA-MB-435c cells plated on fibronectin (FN) or treated with 3 μg/ml clustered ephrin-B2 Fc for 15 min were probed with antibodies to the two conserved Eph receptor juxtamembrane tyrosine phosphorylation sites (phospho-Eph jxtm), reprobed with anti-phosphotyrosine antibodies (PTyr) and then reprobed with anti-EphB4 antibodies. Different fractional amounts of the immunoprecipitate from ephrin-B2-treated cells were loaded, as indicated at the bottom, to show that even the low levels of phosphorylated EphB4 present in a small portion (1/20) of the immunoprecipitate are detectable. (D) Integrin-mediated adhesion potentiates ephrin-induced tyrosine phosphorylation of EphB4. Serum-starved MDA-MB-435c cells were kept adherent or detached and kept in suspension for 30 min. Cells were then stimulated for 20 min with 1.5 μg/ml clustered ephrin-B2 Fc. EphB4 immunoprecipitates were probed with anti-phosphotyrosine antibodies and reprobed with anti-EphB4 antibodies. The histogram shows average relative levels of EphB4 phosphorylation normalized to the amount of immunoprecipitated EphB4 from 6 independent experiments. Error bars represent SEMs. ***P<0.001 by Student’s t-test.
Cell motility assays
We used the Cell Motility HitKit™ from Cellomics, Inc. to measure random migration of cells. Black μClear flat bottom 96-well plates (Greiner Bio-One) were coated with the indicated concentrations of collagen or 25 μg/ml fibronectin, and subsequently coated with a blue fluorescent bead monolayer. Three days after transfection with siRNA, 500 cells/well were allowed to migrate in the presence of 5% FBS for 18 hrs, and then were fixed and stained with rhodamine phalloidin to verify the location of the cells. Beads and cells were visualized using a fluorescence microscope, 5 10X pictures were taken from 4 wells per condition, and 22-48 cells were counted per well. Only the blue channel, showing the beads fluorescence, is shown in the images in Fig. 2A. The area cleared by migrating cells, including the area occupied by the cells, was measured using Image J.
ELISA assays
To examine the ability of wild-type and mutant EphB4 to bind ephrin-B2, protein-A-coated 96 well plates (Pierce) were incubated with 1 μg/ml anti-EphB4 polyclonal antibodies or rabbit IgG control in 3% BSA in TBST (Tris buffered saline, 0.01% Tween) for 1 hour at room temperature. Wells were washed 3 times with wash buffer (Tris buffered saline, 0.01% Tween, 1 mM CaCl2) and then incubated with lysates from 293 HEK cells transfected with wild-type EphB4 or the indicated EphB4 mutants for 1.5 hours. Wells were washed and incubated with ephrin-B2 fused to alkaline phosphatase for 1 hour [21]. Ephrin-B2 alkaline phosphatase remaining bound after washing was detected by measuring alkaline phosphatase activity using the substrate p-nitrophenyl phosphate. Absorbance at 405 nm was measured using an ELISA plate reader.
RESULTS
EphB4 knock down increases cancer cell adhesion, spreading and migration
Substantial evidence shows that Eph receptors regulate integrin activity when stimulated by ephrin ligands [23-25]. To investigate whether in cancer cells EphB4 might affect integrin-mediated cell substrate adhesion and migration independently of activation by ephrin-B2, we downregulated EphB4 expression using RNA interference. Four different EphB4 siRNAs that were transfected all effectively knocked down EphB4 expression in the MDA-MB-435c clonal cell line, which expresses high levels of EphB4 [10] (Fig. 1A). A pool of the 4 siRNAs yielded even greater EphB4 downregulation. The siRNA pool also effectively reduced EphB4 levels in the MCF7 breast cancer cell line, which also has high levels of EphB4 (Fig. 1A). EphB4 expression was already decreased 24 and 48 hours after siRNA transfection, and better downregulation was observed at 72 hours (Suppl. Fig. 1). The parental MDA-MB-435p cell line, which has very low levels of EphB4 [10], was used as a control to identify possible off-target effects (Fig. 1A).
We measured cell attachment to fibronectin and collagen, two extracellular matrix ligands for β1 integrins [26], which are a class of integrins known to be regulated by Eph receptors [23-25] and are well expressed in both MDA-MB-435 and MCF7 cells [27, 28]. We found that MDA-MB-435c and MCF7 cells transfected with the EphB4 siRNA pool adhere faster than control siRNA-transfected cells to surfaces coated with a range of fibronectin and collagen concentrations (Fig. 1B; Suppl. Fig. 2A,B). On the other hand, adhesion to poly-L-lysine, which is not mediated by integrins, was not affected by EphB4 expression (Fig. 1B; Suppl. Fig. 2A). The increased adhesion observed at 30 min reflects a faster attachment rate of the cells in which EphB4 is downregulated, while maximal levels of attachment at longer time points were similar in EphB4 knockdown and control cells (Suppl. Fig. 2C). Immunofluorescence labeling of the cells for phosphotyrosine, the focal adhesion marker paxillin and the actin cytoskeleton revealed that the cells with decreased levels of EphB4 also spread faster on fibronectin compared to cells transfected with control siRNA (Fig. 1C). Interestingly, the MDA-MB-435p cells, where EphB4 expression is already low, also spread faster than the MDA-MB-435c cells (Fig. 1B,C). Furthermore, the EphB4 siRNAs did not significantly affect MDA-MB-435p attachment and spreading (Fig. 1B,C).
In lateral migration assays on fibronectin, EphB4 siRNA-mediated knockdown increased the migration of MDA-MB-435c and MCF7 cells but did not have significant effects on the control MDA-MB-435p cells (Fig. 2A,B). Increased migration was also observed for EphB4 siRNA-transfected MCF7 cells plated on collagen (Fig. 2C). Interestingly, EphB4 downregulation did not affect cell migration on 15 versus 90 μg/ml collagen, whereas cell attachment was increased by the higher collagen concentration (Suppl. Fig. 2B), suggesting a different dependence of cell attachment and migration on substrate concentration. Although EphB4 knockdown has been reported to reduce MCF7 cell growth [4], under our assay conditions we did not detect significant changes in the proliferation of MCF7 and MDA-MB-435c cells transfected for 48 or 72 hours with EphB4 siRNAs compared to cells transfected with control siRNA (data not shown).
Probing lysates from EphB4 siRNA-transfected MCF7 cells with anti-β1 integrin antibodies revealed increased β1 integrin levels (Fig. 3A), suggesting that the presence of endogenous EphB4 may inhibit cell attachment and movement by decreasing β1 integrin levels. In contrast, EphB4 activation by ephrin-B2 Fc did not affect β1-integrin expression (Fig. 3B), consistent with previous findings with other activated Eph receptors [24, 25].
Endogenous EphB4 inhibits cell attachment and spreading independently of ephrin binding
The increase in substrate adhesion, spreading and migration observed after EphB4 downregulation in cells with low ephrin-B2 expression and low EphB4 phosphorylation [10] suggests that these activities of EphB4 are either stimulated by low levels of ephrin-B2 or independent of ephrin binding. To distinguish between these two possibilites, we performed cell attachment assays in the presence of a soluble form of the EphB4 extracellular domain fused to Fc (EphB4 Fc) to inhibit the possible interaction between endogenous EphB4 and ephrin-B2. We also used the TNYL-RAW antagonistic peptide, which blocks the high affinity ephrin-binding site of EphB4 [21, 29]. Both EphB4 Fc and the TNYL-RAW peptide have been shown to effectively inhibit activation of EphB4 by endogenous ephrin-B2 in cultured cells [10]. Hower, they did not affect cancer cell attachment (Fig. 4A-C) and spreading on fibronectin (data not shown). These results are consistent with the notion that EphB4 can inhibit cell adhesion independently of its ability to bind ephrin-B2.
EphB4 activation by ephrin-B2 Fc inhibited MCF7 cell attachment (Fig. 4D), as reported for activation of other Eph receptors [22-25, 30], and both EphB4 Fc and the TNYL-RAW peptide effectively neutralized the effect of ephrin-B2 (Fig. 4D). Because ephrin-B2 Fc stimulation did not affect β1-integrin levels (Fig. 3B), inhibition of cell substrate attachment by ephrin-B2 likely involves inhibition of integrin activity rather than expression.
EphB4 mutants deficient in ephrin binding inhibit cell attachment and spreading
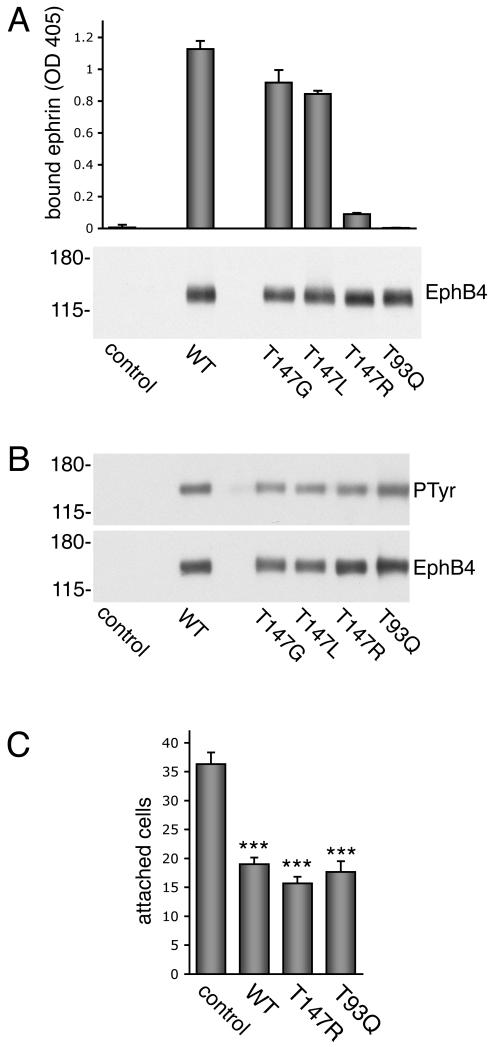
We performed additional experiments to investigate the role of ephrin binding in the effects of EphB4 on cell adhesion. We designed EphB4 mutants with impaired ligand binding ability based on the results of prior mutagenesis experiments with the related EphA3 receptor as well as on available structural information for EphB2 in complex with ephrin-B2 and EphB4 in complex with the TNYL-RAW peptide [29, 31, 32]. We targeted threonine residues 93 and 147 in the high affinity ephrin-binding site of EphB4 because they were shown to play an important role in ligand binding in the prior studies. Lysates from 293 human embryonal kidney (HEK) cells transiently transfected with wild-type and mutant EphB4 were incubated in ELISA wells coated with antibodies to the EphB4 carboxy-terminal region to capture EphB4. By measuring binding of ephrin-B2 alkaline phosphatase to the immobilized EphB4 receptor in ELISA assays, we found that mutation of threonine 147 to either glycine (T147G) or leucine (T147L) does not substantially affect interaction with ephrin-B2 (Fig. 5A), even though the T147G mutation inhibited binding of the TNYL-RAW peptide (data not shown). Mutation of the same threonine to arginine (T147R), however, drastically reduced ephrin-B2 binding to EphB4 (Fig. 5A). Furthermore, mutation of threonine 93 to glutamine (T93Q) eliminated any detectable association of EphB4 with ephrin-B2. The T147R and T93Q mutants also did not bind the TNYL-RAW peptide (data not shown). Binding of ephrin-B1 alkaline phosphatase to EphB4 was not detectable in these assays (data not shown), consistent with the known low binding affinity of EphB4 for ephrin-B1 and other ephrin ligands except ephrin-B2 [33]. The transfected EphB4 mutants with defective ephrin-B2 binding ability exhibited similar levels of tyrosine phosphorylation as the wild-type EphB4 (Fig. 5B), indicating that the observed phosphorylation of overexpressed EphB4 does not depend on ephrin stimulation and may be induced as a result of ligand-independent clustering occuring at high receptor levels [34] or of EphB4 crosstalk with receptors activated by serum growth factors [9].
Figure 5. Transfected EphB4 mutants defective in ephrin binding inhibit cell attachment as effectively as wild-type EphB4.
(A) Lysates from 293 HEK cells transfected with pcDNA3 control vector, wild-type EphB4 or EphB4 with the indicated amino acid mutations in the ephrin-binding domain were incubated in ELISA wells coated with anti-EphB4 antibodies to capture EphB4 and measure its ability to bind its preferred ligand, ephrin-B2, fused to alkaline phosphatase. The histogram shows averages ± SD from triplicate measurements. Background with control wells coated with non-immune antibodies was subtracted. In the lower panel, the transfected lysates used for ELISA assays were probed by immunoblotting with anti-EphB4 polyclonal antibodies. (B) EphB4 immunoprecipitates from transfected 293 HEK cells were probed with anti-phosphotyrosine antibodies (PTyr) and reprobed with anti-EphB4 antibodies. The mutations that disrupt ephrin-B2 binding do not affect basal EphB4 phosphorylation in the transiently transfected cells. (C) EphB4 constructs or pcDNA3 control vector were transfected at a 10:1 ratio with EGFP into MDA-MB-435p cells, which express low levels of EphB4. Transfected cells were plated on fibronectin-coated coverslips for 15 min and EGFP-positive attached cells were visualized using a fluorescence microscope and counted. The histogram shows averages ± SEM from triplicate measurements. *** P<0.001 for EphB4 constructs compared to vector control using one-way ANOVA and Tukey’s post-hoc test. There was no significant difference in adhesion to fibronectin between wild-type EphB4 and the T147R and T93Q mutants.
To examine the importance of ephrin-B2 binding in the effects of transfected EphB4 on cell attachment, we used MDA-MB-435p cells, which have low levels of endogenous EphB4. Transient transfection of wild-type EphB4 inhibited cell adhesion to fibronectin (Fig. 5C) as well as cell spreading (data not shown). The transfected T93Q and T147R mutants, which have impaired ephrin-B2 binding ability, inhibited cell substrate adhesion as effectively as wild-type EphB4 (Fig. 5C), indicating that ephrin-B2 binding is not required for the effects of transfected EphB4 on MDA-MB-435 cell attachment and spreading on fibronectin.
Kinase activity is required for ligand-independent inhibition of cell attachment and spreading by transfected EphB4
We next investigated whether EphB4 kinase activity and tyrosine phosphorylation play a role in the observed inhibition of cell adhesion to fibronectin. Transfection of an EphB4 mutant with an inactive kinase domain (K647R) did not inhibit cell attachment (Fig. 6A). Furthermore, mutation of tyrosine 606 to aspartic acid (Y606D) and of tyrosines 590 and 594 to glutamic acid (Y590/594E) slightly increased the ability of EphB4 to inhibit cell attachment and spreading (Fig. 6A and data not shown), consistent with the fact that these mutations facilitate catalytic activity by destabilizing the inhibitory interaction of the juxamembrane domain with the kinase domain [35-37]. Tyrosine 590 and 594 in the juxtamembrane region of EphB4 are conserved in all Eph receptors and represent major ephrin-dependent autophosphorylation sites as well as binding sites for SH2 domain-containing proteins [23]. However, we found that mutation of these tyrosines to glutamic acid, which does not support binding of SH2 domains [38], did not reduce the ability of EphB4 Y590/594E to inhibit integrin-mediated MDA-MB-435c cell adhesion (Fig. 6A). Mutation of another tyrosine phosphorylation site in the carboxy-terminal sterile alpha motif (SAM) domain of EphB4 (Y924F) also did not reduce inhibition of cell adhesion by EphB4 (Fig. 6A). Thus, the binding of SH2 or PTB domain-containing effector proteins to the tyrosine 590, 594 or 924 phosphorylated motifs in EphB4 is not critical for modulation of cell adhesion by EphB4.
Interestingly, inhibition of cell adhesion did not seem to correlate with the tyrosine phosphorylation levels of overexpressed EphB4 because the EphB4 Y924F mutant inhibited adhesion as well as wild-type EphB4 while being much less phosphorylated (Fig. 6B). Phosphorylation of the EphB4 Y590/594E mutant was also lower than wild-type, but this is likely due to the fact that the two mutated juxtamembrane tyrosines are major Eph receptor autophosphorylation sites [39].
We did not detect tyrosine phosphorylation of endogenous EphB4 in cells attaching to a fibronectin substrate using either an anti-phosphotyrosine antibody or a phosphospecific antibody recognizing the phosphorylated tyrosines 590 and 594 (Fig. 6C, FN lane). In contrast, ephrin stimulation caused prominent phosphorylation of these juxtamembrane tyrosines, as expected (Fig. 6C, ephrin-B2 lanes). Furthermore, we detected lower levels of EphB4 tyrosine phosphorylation when the MDA-MB-435c cells were stimulated with ephrin-B2 Fc while in suspension compared to adherent cells (Fig. 6D). Thus, integrin-mediated attachment enhances ephrin-dependent EphB4 tyrosine phosphorylation, whereas EphB4 kinase activity inhibits integrin-dependent cell adhesion.
DISCUSSION
Ligand-independent functions of Eph receptors in cancer have been hypothesized in order to reconcile some of the results reported in the literature [9, 13-15]. However, there has been no conclusive evidence demonstrating ligand-independent activities of Eph receptors in cancer. We show here that the EphB4 receptor expressed in cancer cells can inhibit cell-substrate adhesion and cell spreading independently of stimulation by its ligand, ephrin-B2.
It is well documented that Eph receptor activation by ephrins can inhibit cell substrate adhesion by regulating the activity of β1 and other integrins through signaling effectors such as the Ras family GTPases, R-Ras and Rap1 [23-25, 40]. Eph receptors may also inhibit cell attachment by promoting dephosphorylation of components of integrin signaling complexes, such as p130Cas and Fak, or by sequestering them away from nascent adhesion complexes [10, 24, 41, 42]. We now show that EphB4 can also inhibit cell substrate attachment independently of ephrin binding, concomitant with regulation of β1 integrin protein levels. Furthermore, we found that integrin-mediated adhesion potentiates ephrin-B2-induced EphB4 tyrosine phosphorylation. This suggests that integrins can in turn modulate the ligand-dependent activities of EphB4, as is the case for several other receptor tyrosine kinases [26, 43-45].
We used several approaches to demonstrate that the EphB4 effects observed are ligand independent. First, in the attachment, spreading and migration assays the cells were kept sparse to minimize contact between cells. Thus, EphB4 could not be substantially activated through ephrin binding at sites of cell-cell contact. In any case, the levels of endogenous ephrin-B2 in the MCF7 and MDA-MB-435 cells used are low to undetectable [10]. Second, inhibitors of ephrin-B2-EphB4 interaction such as EphB4 Fc and the TNYL-RAW competitive antagonistic peptide did not influence integrin-mediated adhesion. These results suggest that ligand binding does not play a role in the effects we observed in cells expressing endogenous EphB4. Third, the EphB4 T93Q and T147R mutants, which we have shown to be severely defective in ligand binding, were as effective as EphB4 wild-type in inhibiting cell attachment when expressed in cells with low EphB4 levels.
Given the high binding affinity of the Eph receptor-ephrin association and the large size of the ephrin binding pocket [46], it is remarkable that changing a single amino acid is sufficient to disrupt ephrin binding. That single amino acid mutations in the high affinity ephrin-binding domain of an Eph receptor can impair ephrin binding was first proposed based on the phenotypes of C. elegans mutants [47, 48] and was more recently conclusively demonstrated using biochemical approaches [32]. Threonines 93 and 147 are located in the high affinity ephrin-binding pocket of EphB4 and contribute important interactions with ligands such as ephrin-B2 and the TNYL-RAW peptide [29, 31, 49]. Furthermore, these two residues appear to have a conserved role in the binding of different ephrins to Eph receptors because mutation of one of the corresponding amino acids of an EphA receptor (T102A and F152L of EphA3) was also shown to disrupt binding of an ephrin-A ligand [32].
Importantly, the T102A and F152L mutations have been shown to essentially abrogate ephrin binding without disrupting the overall conformation of the EphA3 receptor [32]. Thus, the corresponding T93Q and T147R mutations in EphB4 are unlikely to disrupt the overall three-dimensional structure of the ephrin-binding domain. It has also been recently reported that another single amino acid mutation (L95R) drastically reduces the binding affinity of EphB4 for ephrin-B2 and the TNYL-RAW peptide [49]. All of these mutants will be useful to further characterize ephrin-independent activities of the EphB4 receptor in cancer cells.
Higher β1 integrin levels in cancer cells where endogenous EphB4 is downregulated likely explain, at least in part, the observed increases in cell adhesion, spreading and migration. It will be interesting in the future to determine whether the ephrin-independent regulation of β1-integrin levels by EphB4 occurs at the transcriptional or post-transcriptional level, and to elucidate the mechanism involved in this novel form of Eph receptor/integrin crosstalk.
A possible model to explain our findings is that integrin-mediated cell attachment promotes EphB4 kinase activity, causing a negative feedback loop that in turn limits integrin expression. Several other receptor tyrosine kinases are known to interact with integrins and/or become activated upon cell attachment in a ligand independent manner [26, 43-45]. However, the adhesion-dependent activation of receptor tyrosine kinases typically correlates with their increased tyrosine phosphorylation, whereas we did not detect phosphorylation of endogenous EphB4 during cell attachment to fibronectin. Furthermore, we did not detect EphB4 immunolabeling in forming focal contacts and focal adhesions marked with paxillin or phosphotyrosine antibodies (data not shown), suggesting that a mechanism involving physical interaction of EphB4 with integrins in focal adhesions is also unlikely. On the other hand, integrin-mediated adhesion may affect only a subset of EphB4 molecules located in strategic position to in turn regulate integrin levels [50], while overall EphB4 phosphorylation remains too low for detection. Availability of a selective EphB4 kinase inhibitor would greatly help determine whether kinase activity is important in the regulation of cell attachment by endogenous EphB4, as observed for transfected EphB4.
A requirement for kinase activity in the inhibition of cell adhesion by a transiently transfected Eph receptor has been previously reported, but the importance of ephrin binding was not investigated [38, 40]. It is conceivable that Eph receptor clustering due to high levels of expression or crosstalk with activated growth factor receptors may lead to increased kinase activity even in the absence of ephrin-B2 binding [9, 13]. In addition, there may be an equilibrium where in a fraction of the EphB4 molecules the juxtamembrane and kinase domains are not engaged in autoinhibitory interactions even if unphosphorylated [51], resulting in a basal level of kinase activity under unstimulated conditions. Molecules such as PI3 kinase p110γ, Ephexin, and possibly others whose association with the Eph receptor juxtamembrane or kinase domain is independent of ephrin binding and receptor phosphorylation [52, 53], may contribute to destabilize the autoinhibited conformation of the receptor. Additional work will be needed to conclusively elucidate the precise mechanisms underlying the ephrin-independent inhibition of cell-substrate adhesion and spreading by endogenous and transfected EphB4, and whether similar or different mechanisms are involved. Given the low transfection efficiency of MDA-MB-435p cells, we could not reliably determine the effect of transiently transfected EphB4 on overall β1 integrin levels.
Regardless, a decrease in integrin-mediated adhesion in cancer cells expressing EphB4 would be expected to inhibit their malignant properties. Recent genetic data have demonstrated that β1 integrin activity is absolutely required for tumorigenesis in a mouse mammary tumor model [54]. Furthermore, integrins have been shown to be important for the survival, migratory and invasive properties of many cancer cells [45, 55]. On the other hand, positive effects of EphB4 on the growth and directional migration/invasion of various cancer cell lines have also been reported using RNA interference and antisense oligonucleotide approaches [3-7, 9]. Under the conditions of our cell culture experiments, however, we did not detect inhibition of MDA-MB-435 and MCF7 cell growth due to EphB4 depletion. In addition, we found that random migration was increased in the absence of EphB4. Additional studies will be needed to elucidate the range of EphB4 receptor activities that do not require ephrin stimulation and their combined impact on the malignant properties of different types of cancer cells. However, investigating the overall effect of the ephrin-independent activities of EphB4 on tumor growth and metastasis in vivo will be challenging due to the interplay between EphB4 expressed in cancer cells and ephrin-B2 expressed in tumor blood vessels and the ability of both to signal through their cytoplasmic domains when bound to each other [56]. Nevertheless, the ephrin-independent effects that we have uncovered for EphB4 yield novel insight into the mode of action of Eph receptors in cancer cells and represent a new facet in the complexities of Eph receptor function.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Bingcheng Wang for the antibodies to the Eph receptor juxtamembrane phosphorylation sites and Iryna Ethell for the EphB4 Y590/594E construct.
FUNDING
This work was supported by NIH grant CA116099 and a grant from MedImmune (E.B.P.), a postdoctoral fellowship from the DOD Breast Cancer Program (N.K.N.), and a Doris A. Howell undergraduate research scholarship (M.S.).
Abbreviations used
- DMEM
Dulbecco’s Modified Eagle’s Medium
- FBS
fetal bovine serum
- HEK
human embryonal kidney
- MEM
Modified Eagle’s Medium
REFERENCES
- 1.Wu Q, Suo Z, Risberg B, Karlsson MG, Villman K, Nesland JM. Expression of Ephb2 and Ephb4 in breast carcinoma. Pathol. Oncol. Res. 2004;10:26–33. doi: 10.1007/BF02893405. [DOI] [PubMed] [Google Scholar]
- 2.Batlle E, Bacani J, Begthel H, Jonkeer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T, Gallinger S, Pals S, Clevers H. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- 3.Xia G, Kumar SR, Masood R, Zhu S, Reddy R, Krasnoperov V, Quinn DI, Henshall SM, Sutherland RL, Pinski JK, Daneshmand S, Buscarini M, Stein JP, Zhong C, Broek D, Roy-Burman P, Gill PS. EphB4 expression and biological significance in prostate cancer. Cancer Res. 2005;65:4623–4632. doi: 10.1158/0008-5472.CAN-04-2667. [DOI] [PubMed] [Google Scholar]
- 4.Kumar SR, Singh J, Xia G, Krasnoperov V, Hassanieh L, Ley EJ, Scehnet J, Kumar NG, Hawes D, Press MF, Weaver FA, Gill PS. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am. J. Pathol. 2006;169:279–293. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masood R, Kumar SR, Sinha UK, Crowe DL, Krasnoperov V, Reddy RK, Zozulya S, Singh J, Xia G, Broek D, Schonthal AH, Gill PS. EphB4 provides survival advantage to squamous cell carcinoma of the head and neck. Int. J. Cancer. 2006;119:1236–1248. doi: 10.1002/ijc.21926. [DOI] [PubMed] [Google Scholar]
- 6.Xia G, Kumar SR, Stein JP, Singh J, Krasnoperov V, Zhu S, Hassanieh L, Smith DL, Buscarini M, Broek D, Quinn DI, Weaver FA, Gill PS. EphB4 receptor tyrosine kinase is expressed in bladder cancer and provides signals for cell survival. Oncogene. 2006;25:769–780. doi: 10.1038/sj.onc.1209108. [DOI] [PubMed] [Google Scholar]
- 7.Kumar SR, Masood R, Spannuth WA, Singh J, Scehnet J, Kleiber G, Jennings N, Deavers M, Krasnoperov V, Dubeau L, Weaver FA, Sood AK, Gill PS. The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals and predicts poor outcome. Br. J. Cancer. 2007;96:1083–1091. doi: 10.1038/sj.bjc.6603642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam SM, Fujimoto J, Jahan I, Sato E, Tamaya T. Coexpression of EphB4 and ephrinB2 in tumour advancement of ovarian cancers. Br. J. Cancer. 2008;98:845–851. doi: 10.1038/sj.bjc.6604216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar SR, Scehnet JS, Ley EJ, Singh J, Krasnoperov V, Liu R, Manchanda PK, Ladner RD, Hawes D, Weaver FA, Beart RW, Singh G, Nguyen C, Kahn M, Gill PS. Preferential induction of EphB4 over EphB2 and its implication in colorectal cancer progression. Cancer Res. 2009;69:3736–3745. doi: 10.1158/0008-5472.CAN-08-3232. [DOI] [PubMed] [Google Scholar]
- 10.Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat. Cell. Biol. 2006;8:815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- 11.Zantek ND, Azimi M, Fedor-Chaiken M, Wang BC, Brackenbury R, Kinch MS. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth & Differentiation. 1999;10:629–638. [PubMed] [Google Scholar]
- 12.Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 melanoma cells--a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res. Treat. 2007;104:13–19. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- 13.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Noren NK, Pasquale EB. Paradoxes of the EphB4 receptor in cancer. Cancer Res. 2007;67:3994–3997. doi: 10.1158/0008-5472.CAN-07-0525. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Zhuang G, Frieden L, Debinski W. Eph receptors and Ephrins in cancer: common themes and controversies. Cancer Res. 2008;68:10031–10033. doi: 10.1158/0008-5472.CAN-08-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MA, Ruest PJ, Kosinski M, Hanks SK, Greenstein D. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 2003;17:187–200. doi: 10.1101/gad.1028303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu C, Shim S, Shin J, Kim J, Park J, Han K, Park S. The EphA8 receptor induces sustained MAP kinase activation to promote neurite outgrowth in neuronal cells. Oncogene. 2005;24:4243–4256. doi: 10.1038/sj.onc.1208584. [DOI] [PubMed] [Google Scholar]
- 18.Furne C, Ricard J, Cabrera JR, Pays L, Bethea JR, Mehlen P, Liebl DJ. EphrinB3 is an anti-apoptotic ligand that inhibits the dependence receptor functions of EphA4 receptors during adult neurogenesis. Biochim. Biophys. Acta. 2009;1793:231–238. doi: 10.1016/j.bbamcr.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue E, Deguchi-Tawarada M, Togawa A, Matsui C, Arita K, Katahira-Tayama S, Sato T, Yamauchi E, Oda Y, Takai Y. Synaptic activity prompts gamma-secretase-mediated cleavage of EphA4 and dendritic spine formation. J. Cell Biol. 2009;185:551–564. doi: 10.1083/jcb.200809151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang NY, Pasquale EB, Owen LB, Ethell IM. The EphB4 Receptor-tyrosine Kinase Promotes the Migration of Melanoma Cells through Rho-mediated Actin Cytoskeleton Reorganization. J. Biol. Chem. 2006;281:32574–32586. doi: 10.1074/jbc.M604338200. [DOI] [PubMed] [Google Scholar]
- 21.Koolpe M, Burgess R, Dail M, Pasquale EB. EphB receptor-binding peptides identified by phage display enable design of an antagonist with ephrin-like affinity. J. Biol. Chem. 2005;280:17301–17311. doi: 10.1074/jbc.M500363200. [DOI] [PubMed] [Google Scholar]
- 22.Miao H, Strebhardt K, Pasquale EB, Shen TL, Guan JL, Wang B. Inhibition of Integrin-mediated Cell Adhesion but Not Directional Cell Migration Requires Catalytic Activity of EphB3 Receptor Tyrosine Kinase. J. Biol. Chem. 2005;280:923–932. doi: 10.1074/jbc.M411383200. [DOI] [PubMed] [Google Scholar]
- 23.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol. Cell. Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 24.Bourgin C, Murai KK, Richter M, Pasquale EB. The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways. J. Cell Biol. 2007;178:1295–1307. doi: 10.1083/jcb.200610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang X, Wu D, Jin H, Stupack D, Wang JY. Induction of cell retraction by the combined actions of Abl-CrkII and Rho-ROCK1 signaling. J. Cell Biol. 2008;183:711–723. doi: 10.1083/jcb.200801192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM, Sansoucy BG, Sawyer TK, Languino LR. The integrin-growth factor receptor duet. J. Cell Physiol. 2007;213:649–653. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- 27.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 28.Kirshner J, Hardy J, Wilczynski S, Shively JE. Cell-cell adhesion molecule CEACAM1 is expressed in normal breast and milk and associates with beta1 integrin in a 3D model of morphogenesis. J. Mol. Histol. 2004;35:287–299. doi: 10.1023/B:HIJO.0000032360.01976.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chrencik JE, Brooun A, Recht MI, Kraus ML, Koolpe M, Kolatkar AR, Bruce RH, Martiny-Baron G, Widmer H, Pasquale EB, Kuhn P. Structure and thermodynamic characterization of the EphB4/Ephrin-B2 antagonist peptide complex reveals the determinants for receptor specificity. Structure. 2006;14:321–330. doi: 10.1016/j.str.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Clifford N, Smith LM, Powell J, Gattenlohner S, Marx A, O’Connor R. The EphA3 receptor is expressed in a subset of rhabdomyosarcoma cell lines and suppresses cell adhesion and migration. J. Cell. Biochem. 2008;105:1250–1259. doi: 10.1002/jcb.21926. [DOI] [PubMed] [Google Scholar]
- 31.Himanen JP, Rajashankar KR, Lackmann M, Cowan CA, Henkemeyer M, Nikolov DB. Crystal structure of an Eph receptor-ephrin complex. Nature. 2001;414:933–938. doi: 10.1038/414933a. [DOI] [PubMed] [Google Scholar]
- 32.Smith FM, Vearing C, Lackmann M, Treutlein H, Himanen J, Chen K, Saul A, Nikolov D, Boyd AW. Dissecting the EphA3/Ephrin-A5 interactions using a novel functional mutagenesis screen. J. Biol. Chem. 2004;279:9522–9531. doi: 10.1074/jbc.M309326200. [DOI] [PubMed] [Google Scholar]
- 33.Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Ann. Rev. Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 34.Wimmer-Kleikamp SH, Janes PW, Squire A, Bastiaens PI, Lackmann M. Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J. Cell Biol. 2004;164:661–666. doi: 10.1083/jcb.200312001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zisch AH, Kalo MS, Chong LD, Pasquale EB. Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region. Oncogene. 1998;16:2657–2670. doi: 10.1038/sj.onc.1201823. [DOI] [PubMed] [Google Scholar]
- 36.Wybenga-Groot LE, Baskin B, Ong SH, Tong JF, Pawson T, Sicheri F. Structural basis for autoinhibition of the EphB2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell. 2001;106:745–757. doi: 10.1016/s0092-8674(01)00496-2. [DOI] [PubMed] [Google Scholar]
- 37.Holmberg J, Genander M, Halford MM, Anneren C, Sondell M, Chumley MJ, Silvany RE, Henkemeyer M, Frisen J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151–1163. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 38.Zisch AH, Pazzagli C, Freeman AL, Schneller M, Hadman M, Smith JW, Ruoslahti E, Pasquale EB. Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses. Oncogene. 2000;19:177–187. doi: 10.1038/sj.onc.1203304. [DOI] [PubMed] [Google Scholar]
- 39.Kalo MS, Pasquale EB. Multiple in vivo tyrosine phosphorylation sites in EphB receptors. Biochemistry. 1999;38:14396–14408. doi: 10.1021/bi991628t. [DOI] [PubMed] [Google Scholar]
- 40.Zou JX, Wang B, Kalo MS, Zisch AH, Pasquale EB, Ruoslahti E. An Eph receptor regulates integrin activity through R-Ras. Proc. Natl. Acad. Sci. U S A. 1999;96:13813–13818. doi: 10.1073/pnas.96.24.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat. Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- 42.Moeller ML, Shi Y, Reichardt LF, Ethell IM. EphB receptors regulate dendritic spine morphogenesis through the recruitment/phosphorylation of focal adhesion kinase and RhoA activation. J. Biol. Chem. 2006;281:1587–1598. doi: 10.1074/jbc.M511756200. [DOI] [PubMed] [Google Scholar]
- 43.Yamada KM, Even-Ram S. Integrin regulation of growth factor receptors. Nat. Cell Biol. 2002;4:E75–76. doi: 10.1038/ncb0402-e75. [DOI] [PubMed] [Google Scholar]
- 44.Comoglio PM, Boccaccio C, Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol. 2003;15:565–571. doi: 10.1016/s0955-0674(03)00096-6. [DOI] [PubMed] [Google Scholar]
- 45.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell. Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 46.Himanen JP, Saha N, Nikolov DB. Cell-cell signaling via Eph receptors and ephrins. Curr. Opin. Cell Biol. 2007;19:534–542. doi: 10.1016/j.ceb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.George SE, Simokat K, Hardin J, Chisholm AD. The VAB-1 Eph receptor tyrosine kinase functions in neural and epithelial morphogenesis in C. elegans. Cell. 1998;92:633–643. doi: 10.1016/s0092-8674(00)81131-9. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Roy PJ, Holland SJ, Zhang LW, Culotti JG, Pawson T. Multiple ephrins control cell organization in C. elegans using kinase-dependent and - independent functions of the VAB-1 Eph receptor. Mol. Cell. 1999;4:903–913. doi: 10.1016/s1097-2765(00)80220-8. [DOI] [PubMed] [Google Scholar]
- 49.Chrencik JE, Brooun A, Kraus ML, Recht MI, Kolatkar AR, Han GW, Seifert JM, Widmer H, Auer M, Kuhn P. Structural and biophysical characterization of the EphB4-ephrinB2 protein-protein interaction and receptor specificity. J. Biol. Chem. 2006;281:28185–28192. doi: 10.1074/jbc.M605766200. [DOI] [PubMed] [Google Scholar]
- 50.Schneller M, Vuori K, Ruoslahti E. Alphavbeta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volkman BF, Lipson D, Wemmer DE, Kern D. Two-state allosteric behavior in a single-domain signaling protein. Science. 2001;291:2429–2433. doi: 10.1126/science.291.5512.2429. [DOI] [PubMed] [Google Scholar]
- 52.Gu C, Park S. The EphA8 Receptor Regulates Integrin Activity through p110γ Phosphatidylinositol-3 Kinase in a Tyrosine Kinase Activity-Independent Manner. Mol. Cell. Biol. 2001;21:4579–4597. doi: 10.1128/MCB.21.14.4579-4597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, Greenberg ME. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 54.White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, Muller WJ. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 55.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Noren NK, Lu M, Freeman AL, Koolpe M, Pasquale EB. Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proc. Natl. Acad. Sci. U S A. 2004;101:5583–5588. doi: 10.1073/pnas.0401381101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.