Abstract
Capsaicin produces burning pain, followed by nociceptive responses, such as allodynia and hyperalgesia in humans and rodents. In the present study, when administered subcutaneously into the tail of rhesus monkeys, capsaicin (0.01–0.32 mg) dose-dependently produced thermal allodynia manifested as reduced tail-withdrawal latencies in 46°C water, from a maximum value of 20 sec to approximately 2 sec. Coadministration of selective mu opioid agonists, fentanyl (0.003–0.1 mg) and (d-Ala2,N-Me-Phe4, Gly5-ol)-enkephalin (0.001–0.03 mg), dose-dependently inhibited capsaicin-induced allodynia. This local antinociception was antagonized by small doses of opioid antagonists, quadazocine (0.03 mg) and quaternary naltrexone (1 mg), applied locally in the tail. However, these doses of antagonists injected s.c. in the back did not antagonize local fentanyl. Comparing the relative potency of either agonist or antagonist after local and systemic administration confirmed that the site of action of locally applied mu opioid agonists is in the tail. These results provide evidence that activation of peripheral mu opioid receptors can diminish capsaicin-induced allodynia in primates. This experimental pain model could be a useful tool for evaluating peripherally acting antinociceptive agents without central side effects and enhance new approaches to the treatment of inflammatory pain.
States of nociception, such as allodynia and hyperalgesia, to thermal and mechanical stimuli often occur after tissue injury, inflammation and nerve lesions (e.g., Levine and Taiwo, 1994). Allodynia is a condition in which pain is produced by a stimulus that is normally innocuous, whereas hyperalgesia is increased pain reaction induced by a stimulus that is normally noxious (Willis, 1992). In clinics, many nociceptive conditions such as postoperative pain, cancer and arthritis are associated with inflammation. The development of experimental inflammatory pain models is valuable for investigating the mechanisms of allodynia and hyperalgesia and for evaluating potential antinociceptive agents. Traditionally, most clinically used opioids are predominantly mu opioid agonists (Twycross, 1994). In addition to relieving pain, mu agonists induce a wide variety of other biological effects, and some centrally mediated side effects can be clinically undesirable, such as sedation and respiratory depression. Thus, the existence of peripheral opioid receptors becomes more important for developing local analgesics or peripherally selective opioid agonists. To date, several rodent and clinical studies demonstrate that locally administered opioid agonists can produce pronounced antinociceptive effects by interacting with peripheral opioid receptors in inflamed tissue (Stein et al., 1989, 1991; Barber and Gottschlich, 1992; Kinnman et al., 1997).
Endogenous and exogenous compounds, e.g., formalin, bradykinin, prostaglandin E2 and Freund’s adjuvant, have been used to induce nociception after local application (Stein et al., 1989; Dray and Dickenson, 1991; Negus et al., 1993b). Capsaicin, the pungent ingredient in hot chili peppers, is another compound that has been investigated in both human and rodent behavioral and neurophysiological studies. Topical or intradermal administration of capsaicin to human skin produces burning pain, a flare and a hyperalgesia that is characterized by a lowered pain threshold to heat and a tenderness to innocuous mechanical stimulation (Simone et al., 1987, 1989). These temporary sensory symptoms of the capsaicin model in humans resemble those of patients with neuropathic pain. Several studies have demonstrated that nociception induced by capsaicin is mediated in part by activating a subset of sensory neurons including polymodal nociceptors and thermoceptors (Dray and Dickenson, 1991; LaMotte et al., 1992; Kim et al., 1995; Kinnman and Levine, 1995). In addition, capsaicin stimulates the release of glutamate and neuropeptides such as calcitonin gene-related peptide and substance P from the peripheral and central terminals of sensory neurons (Maggi, 1993; Winter et al., 1995). On the other hand, rodent studies have reported that morphine inhibited microvasodilation in capsaicin-induced peripheral nerve trunk inflammation, presumably through an action on local opioid receptors (Zochodne and Ho, 1993; Schaafsma et al., 1997). Clinical studies also have provided evidence that local or systemic administration of mu opioid agonists attenuate capsaicin-induced allodynia and hyperalgesia in humans (Park et al., 1995; Eisenach et al., 1997; Kinnman et al., 1997). Taken together, these findings support the feasibility of pharmacological studies with an intradermal capsaicin model.
This study was initiated to develop a procedure for thermal allodynia in rhesus monkeys by administering capsaicin to induce local transient nociception. Then, the antinociceptive effect of both fentanyl, a synthetic lipophilic mu agonist, and DAMGO, a selective mu peptide, were compared after local and systemic administration. In addition, antagonist studies were performed to further investigate the role of peripheral mu opioid receptors in this procedure. The aim of this study was to provide a useful experimental pain model and to evaluate the hypothesis that local administration of mu opioid agonists can diminish capsaicin-induced nociception in primates.
Methods
Subjects
Six adult male and female rhesus monkeys (Macaca mulatta) with body weights ranging between 7.7 and 12.1 kg (mean weight, 9.6 kg) were used. They were housed individually with free access to water and were fed approximately 25 to 30 biscuits (Purina Monkey Chow) and fresh fruit daily. All monkeys had previous experience in the tail-withdrawal procedure and did not have exposure to opioids for 2 months before the present study.
Warm Water Tail-Withdrawal Assay
Apparatus and procedure
Antinociception was measured with a procedure that was described previously (Dykstra and Woods, 1986). The subjects were seated in restraint chairs and the lower part of the shaved tail (approximately 15 cm) was immersed in warm water maintained at temperatures of 42, 46 and 50°C. Tail-withdrawal latencies were recorded manually by a computerized timer. A maximum cutoff latency (20 sec) was recorded if the subjects failed to remove their tails by this time. A single dosing procedure was used in all test sessions. Each experimental session began with control determinations at each temperature. Subsequent tail-withdrawal latencies were determined at 5, 15, 30, 45 and 60 min after injection. The subjects were tested one to two times at three temperatures in a varying order, with approximately 1- to 2-min intervals between tests. Experimental sessions were conducted one to two times a week, with at least 3 days between sessions.
Experimental design
Nociceptive effects of capsaicin
Time course of the nociceptive effects of capsaicin (0.01–0.32 mg/tail) was determined twice in each subject. Capsaicin was injected subcutaneously (s.c.) into the terminal 1 to 4 cm of the tail, in a constant 0.1 ml volume. In the time-course study only, monkeys were exposed to four temperatures, 38, 42, 46 and 50°C, during the test session.
Antinociceptive effects of mu agonists
From the protocol described above, 0.1 mg of capsaicin was chosen as a standard noxious stimulus for further studies in 46°C water. Fentanyl (0.0032–0.1 mg) or DAMGO (0.001–0.032 mg) was coadministered with capsaicin in the tail to assess local antinociceptive effects of both mu agonists in 46°C water. Maximally effective locally administered doses of both agonists also were administered either in the back against capsaicin or in the tail against 50°C water under normal (noncapsaicin) conditions. In addition, systemic antinociceptive effects of fentanyl (0.01–0.056 mg/kg) or DAMGO (0.01–0.32 mg/kg) were determined by s.c. administration in the midscapular region of the back, immediately after capsaicin injection. As noted below (“Data Analysis”), an attempt was made to compare potency (ED50 values) of locally administered agents with potency of the same agent administered systemically based on the weight of the animals.
Antagonism of mu agonist-induced antinociception
Given that local injection of compounds might have a quick onset, the opioid antagonists, quadazocine (0.001–0.032 mg) or QNTX (0.032–1 mg), were coadministered with capsaicin and fentanyl in the tail to investigate antagonist effects. The highest effective doses of both antagonists were injected s.c. in the back to specify whether the antagonist effects were localized in the tail. In other experiments, quadazocine (0.0032–0.1 mg/kg) or QNTX (0.1–3.2 mg/kg) were given s.c. in the back 30 min before injection of capsaicin and fentanyl. The relative potency of each antagonist was compared by their ID50 values obtained following local and systemic routes. In addition, nor-binaltorphimine (0.032–1 mg), a selective kappa antagonist, and naltrindole (0.01–0.32 mg), a selective delta antagonist, were coadministered with capsaicin and fentanyl in the tail to further investigate the receptor selectivity of the peripheral effects of fentanyl in this procedure.
By use of only one dose of the antagonist, in vivo apparent pKB analysis (Negus et al., 1993a) was applied to measure the potency of quadazocine in blocking the effects of systemic fentanyl. The systemic antinociceptive effects of fentanyl (0.01– 0.1 mg/kg) were determined against either normal noxious stimulus (50°C water) without capsaicin or capsaicin-induced thermal allodynia in 46°C water. After this, the antinociceptive effects of fentanyl (0.1–1 mg/kg) against both stimuli were redetermined 30 min after pretreatment with 0.1 mg/kg quadazocine.
There were two groups of subjects in this study. The first group (n = 3) was used in all experimental sessions. The second group (n = 3) was used to confirm selected experimental data from the first group; in particular, studies of the time course of capsaicin-induced thermal allodynia, local and systemic effective doses of mu agonists against capsaicin, and locally effective doses of antagonists, were replicated.
Data Analysis
Except for the time-course study, the 15-min time point was used for analysis because this was the time of peak effects of both capsaicin and mu agonists. Individual tail-withdrawal latencies were converted to %MPE by the following formula: %MPE = [(test latency – control latency)/(cutoff latency – control latency)] × 100. Individual control latencies were averaged from two determinations after application of 0.1 mg of capsaicin in the tail in 46°C water. Mean ED50 values were obtained after log transformation of individual ED50 values, which were calculated by least-squares regression with the portion of the dose-effect curves spanning the 50% MPE; 95% confidence limits (95% C.L.) also were determined. Mean ID50 values of antagonists were determined in the same manner by defining the dose that inhibited the 50% MPE of local fentanyl (0.1 mg). Comparison of relative potencies of each compound administered locally or systemically was performed by converting the mg/kg units to total mg units based on individual monkey’s body weight (i.e., 0.1 mg/kg corresponds to 1 mg, assuming an approximate monkey weight of 10 kg). In addition, dose ratios (D.R.) were calculated by dividing mean ED50 values in the presence of the antagonist by the base-line ED50 values. A significant difference between dose-effect curves was defined as a lack of overlap in the 95% C.L. of the ED50 values. In the time-course study, a significant reduction in tail-withdrawal latency was determined by use of the Newman-Keuls test (P < .01).
Apparent pKB values were determined for individual antagonist doses by use of a modified equation (Negus et al., 1993a): pKB = − log [B/(D.R. − 1)], where B equals the antagonist dose in moles per kilogram. Mean pKB values ± 95% C.L. also were calculated from individual pKB values for quadazocine. Apparent pKB values were considered to be significantly different when their 95% C.L. values did not overlap.
Drugs
Fentanyl hydrochloride (National Institute on Drug Abuse, Bethesda, MD), DAMGO acetate salt (Sigma, St. Louis, MO), quadazocine methanesulfonate (Sanofi, Malvern, PA), QNTX (MRZ-2663-BR; Dr. H. Merz, Boehringer Ingelheim KG, Germany), nor-binaltorphimine (Dr. H.I. Mosberg, Dept. of Medicinal Chemistry, University of Michigan) and naltrindole hydrochloride (Dr. K.C. Rice, NIH-NIDDK, Bethesda, MD) were dissolved in sterile water. For systemic administration, all compounds were administered s.c. in the back at a volume of 0.1 ml/kg. Capsaicin (Sigma, St. Louis, MO) was dissolved in a solution of Tween 80/ethanol/saline in a ratio of 1:1:8. For local coadministration, all compounds were mixed in capsaicin solution and injected in 0.1 ml volume in the tail.
Results
Control tail-withdrawal latencies
The monkeys used in this study showed a consistent profile in tail-withdrawal responses. They kept their tails in 38, 42 and 46°C water for 20 sec (cutoff latency) and removed their tails from 50°C water rapidly (typically 1–3 sec). The thermal pain thresholds in monkeys used in this study were similar to other studies in primates. For example, it has been reported that monkeys frequently escaped the 51°C stimuli, but almost never the 43 and 47°C temperatures; human subjects have described 43°C as slightly warm, 47°C as distinctly warm but not painful, and 51°C as a clearly painful stimulus (Kupers et al., 1997).
Nociceptive effects of capsaicin
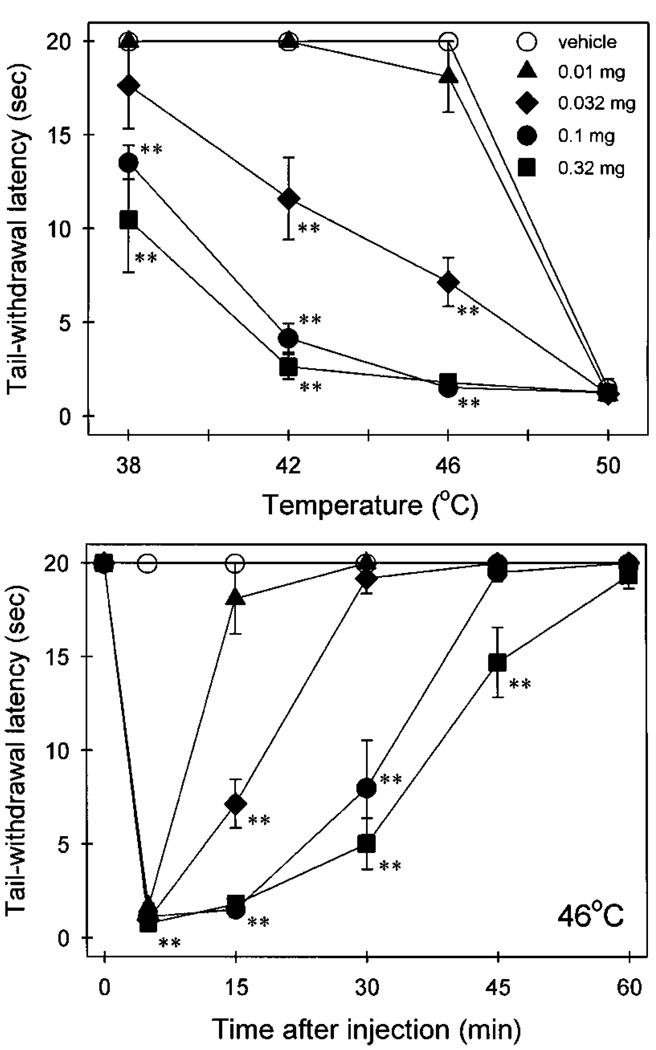
When capsaicin (0.01–0.32 mg) was injected into the tail, it produced a transient nociception (thermal allodynia), which was indicated as reduced tail-withdrawal latencies, both in a dose- and temperature-dependent manner (fig. 1; upper panel). In particular, both 0.1 and 0.32 mg of capsaicin caused rapid tail-withdrawal latencies of approximately 2 sec in 46°C water, 15 min after injection (fig. 1; lower panel). However, all doses of capsaicin caused a general nociception, which was not temperature-dependent, within the first 5 min after administration (data only shown in 46°C water). For our purposes, a standard dose of 0.1 mg capsaicin was chosen to induce allodynia in 46°C water for further studies.
Fig. 1.
Thermal allodynia caused by capsaicin locally applied in the tail. (Upper panel) temperature-effect curves measured at 15 min after injection. (Lower panel) time course of capsaicin-induced thermal allodynia in 46°C water. All points represent the mean ± S.E.M. (n = 6). Asterisks represent significant difference (P < .01) from vehicle.
Antinociceptive effects of mu agonists
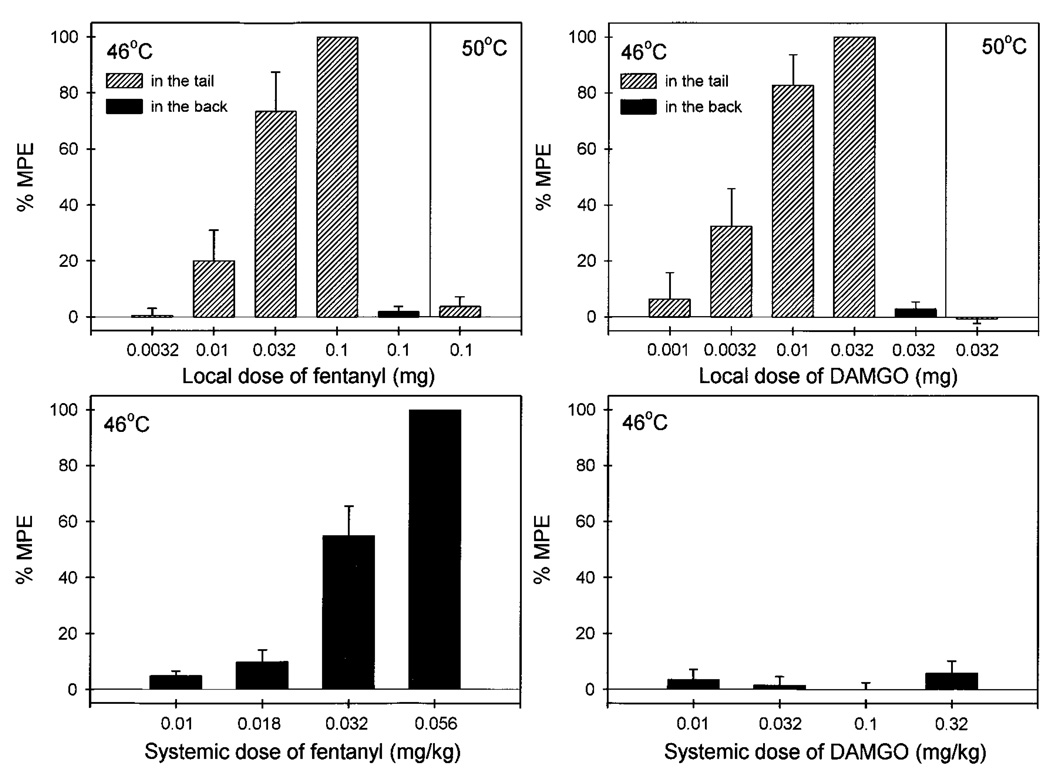
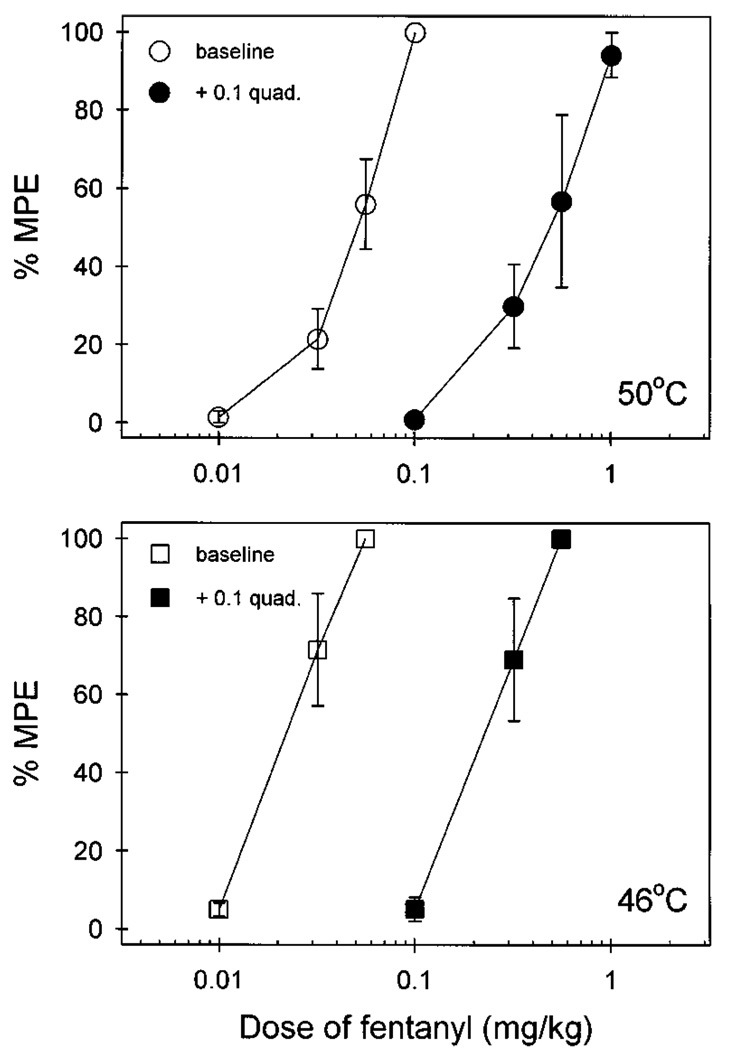
Figure 2 compares the antinociceptive effects of both fentanyl and DAMGO after local and systemic administration. Local administration of fentanyl (0.0032–0.1 mg) dose-dependently inhibited capsaicin (0.1 mg)-induced thermal allodynia in 46°C water (fig. 2, upper left panel). However, the high dose of fentanyl (0.1 mg), when applied in the back, was not effective against capsaicin, and it was not locally effective against a noxious stimulus, 50°C water, in the absence of capsaicin. After systemic administration, fentanyl (0.01–0.056 mg/kg) also dose-dependently inhibited capsaicin-induced allodynia (fig. 2, lower left panel). Comparing the potency of both administration routes, local administration of fentanyl was approximately 15-fold more potent than systemic injection (table 1).
Fig. 2.
Antinociceptive effects of fentanyl (left panels) and DAMGO (right panels) administered locally (hashed bars, in the tail) or systemically (filled bars, in the back) against 46°C water in the presence of 0.1 mg capsaicin or against 50°C water in the absence of capsaicin. Each value represents the mean ± S.E.M. (n = 3–6). Abscissae (all panels): agonist doses in mg or mg/kg (s.c.). Ordinates (all panels): percent of maximum possible effect (%MPE). Each data point was obtained 15 min after injection. See “Methods” for other details.
TABLE 1.
Comparison of potencies of opioid compounds administered locally or systemically
| Agonists | ED50a (95% C.L.) | Relative Potenciesb |
|
|---|---|---|---|
| Local: tail | Systemic: back | ||
| mg | mg/kg | ||
| Fentanyl | 0.019 (0.009–0.041) | 0.029 (0.021–0.038) | 14.7 |
| DAMGO | 0.006 (0.003–0.012) | NAc | >500 |
| Antagonists | ID50a (95% C.L.) | Relative Potenciesb |
|
| Local: tail | Systemic: back | ||
| mg | mg/kg | ||
| Quadazocine | 0.004 (0.001–0.010) | 0.031 (0.014–0.070) | 78.9 |
| QNTX | 0.095 (0.049–0.186) | NAd | >300 |
ED50 or ID50 values were the mean of individual ED50 values (n = 3–6).
Values were the mean of individual relative potencies, which were obtained by converting milligrams per kilogram to total milligram units based on each monkey’s body weight.
Inactive up to 0.32 mg/kg.
Inactive up to 3.2 mg/kg.
Local administration of DAMGO (0.001–0.032 mg) dose-dependently inhibited capsaicin-induced allodynia (fig. 2, upper right panel). Similarly, this local antinociceptive effect of DAMGO was not observed when the high dose of DAMGO (0.032 mg) was applied in the back or when injected into the tail in 50°C water in the absence of capsaicin. In addition, systemic administration of DAMGO (0.01–0.32 mg/kg) did not inhibit capsaicin-induced allodynia (fig. 2, lower right panel). Given that the mean weight of the monkeys was 9.6 kg during this study, 0.32 mg/kg of DAMGO approximately corresponded to 3 mg total dose per monkey. The antinociceptive potency of local DAMGO (ED50 = 0.006 mg) was therefore at least 500-fold higher than systemic DAMGO (table 1).
Antagonism of mu agonist-induced antinociception
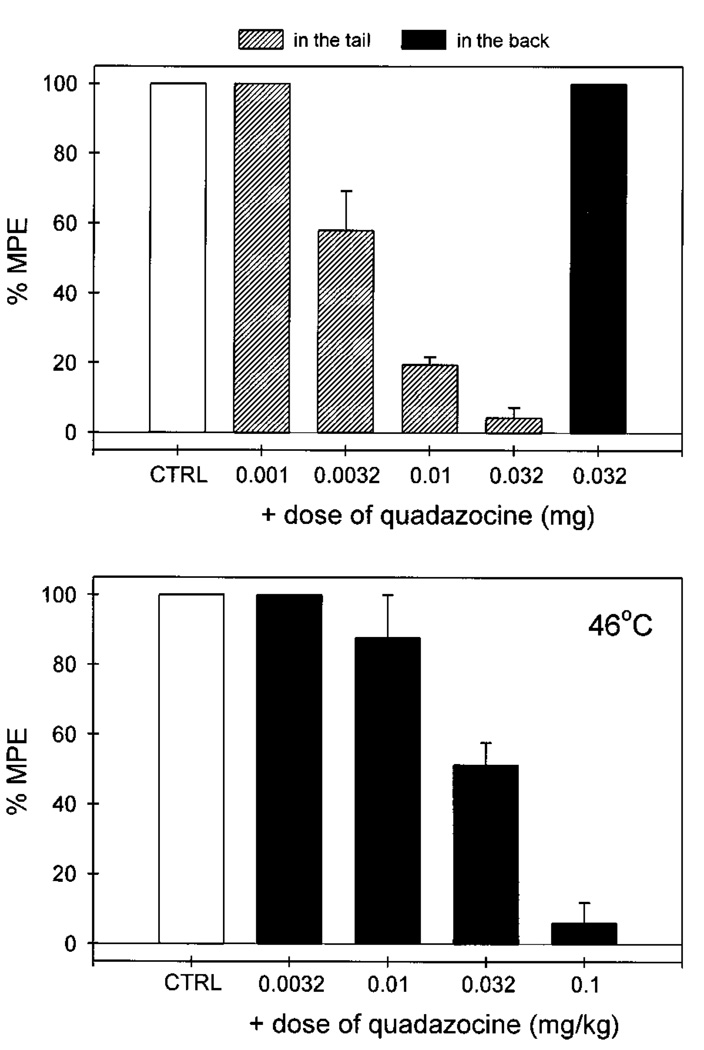
Local administration of quadazocine (0.001–0.032 mg) antagonized the local antinociceptive effects of fentanyl (0.1 mg) against capsaicin in a dose-dependent manner (fig. 3). When the locally effective dose of quadazocine (0.032 mg) was applied in the back, it did not antagonize local fentanyl. This locally effective dose of quadazocine also significantly antagonized local DAMGO (0.032 mg) (data not shown). Although systemic administration of quadazocine (0.0032–0.1 mg/kg) dose-dependently antagonized local fentanyl, the relative antagonist potency of quadazocine was approximately 80-fold higher after local versus systemic injections (table 1).
Fig. 3.
Antagonist effects of quadazocine administered locally (hashed bars, in the tail) and systemically (filled bars, in the back) against local fentanyl in 46°C water in the presence of capsaicin. CTRL represents the effects of coadministration of 0.1 mg of capsaicin and 0.1 mg of fentanyl in the tail. Other details are as in figure 2.
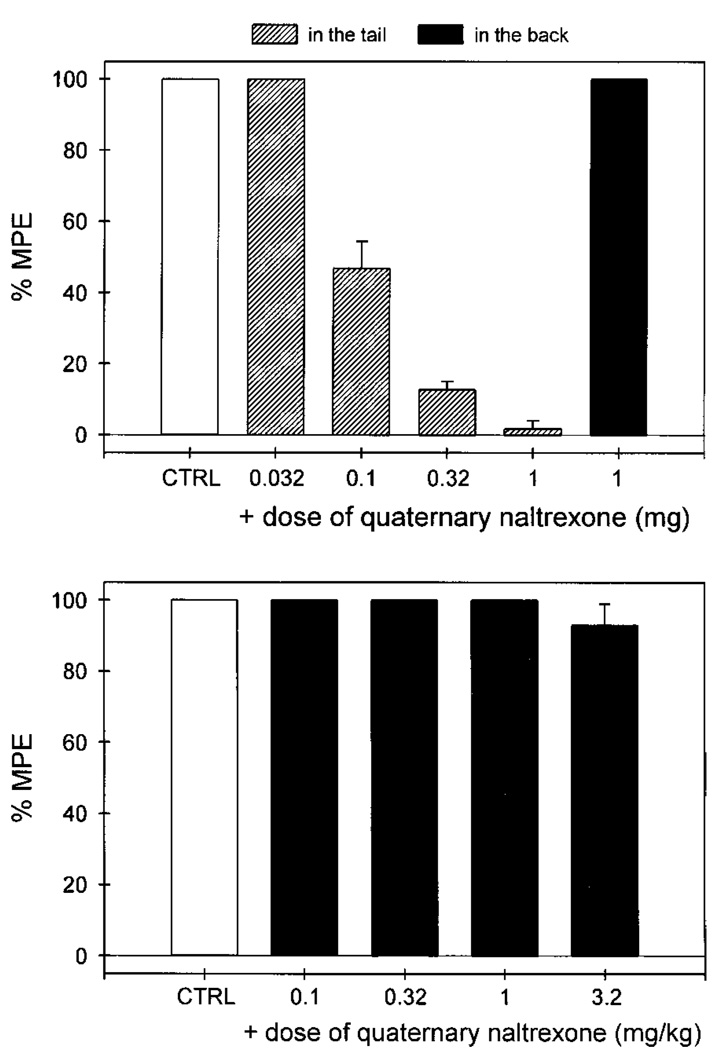
Local administration of quaternary naltrexone (0.032–1 mg) dose-dependently antagonized the local antinociception caused by fentanyl against capsaicin (fig. 4). However, the locally effective dose of QNTX (1 mg), when applied in the back, could not antagonize local fentanyl. After systemic administration, QNTX (0.1–3.2 mg/kg) did not antagonize local fentanyl. A higher dose of QNTX (10 mg/kg) was not assessed because it was reported to have sedative effects (Negus et al., 1993b). Nevertheless, the antagonist potency of local QNTX was at least 300-fold higher than by that of systemic QNTX (table 1). In addition, local administration of both nor-binaltorphimine (0.032–1 mg) and naltrindole (0.01–0.32 mg) did not antagonize the local antinociception of fentanyl against capsaicin (data not shown).
Fig. 4.
Antagonist effects of quaternary naltrexone administered locally (hashed bars, in the tail) and systemically (filled bars, in the back) against local fentanyl in 46°C water in the presence of capsaicin. CTRL represents the effects of coadministration of 0.1 mg of capsaicin and 0.1 mg of fentanyl in the tail. Other details are as in figure 2.
To confirm the profile of the quadazocine potency in antagonizing the systemic fentanyl against two different noxious stimuli (50°C in the absence of capsaicin vs. 46°C in the presence of capsaicin), quadazocine pKB values were calculated in one set of subjects (n = 3). Figure 5 illustrates the similar quadazocine-induced changes in fentanyl potency under both conditions. Systemic fentanyl (0.01–0.1 mg/kg) dose-dependently produced antinociception against 50°C water stimulus without capsaicin and this effect was antagonized by pretreatment with quadazocine (0.1 mg/kg). The pKB value (95% C.L.) was 7.6 (7.3–8.0). Systemic fentanyl (0.01–0.056 mg/kg) also produced antinociception against capsaicin-induced allodynia in 46°C water. Pretreatment with 0.1 mg/kg of quadazocine was equally potent as an antagonist of fentanyl under this condition, as indicated by a similar pKB value, 7.7 (7.6–7.8).
Fig. 5.
Quadazocine potency in antagonizing systemic fentanyl against either 50°C water without capsaicin or 46°C water in the presence of capsaicin. Open symbols represent dose-effect curves determined alone. Closed symbols represent dose-effect curves obtained 30 min after quadazocine (0.1 mg/kg) pretreatment. All points represent the mean ± S.E.M. (n = 3). Abscissae (all panels): fentanyl dose in mg/kg. Ordinates (all panels): %MPE. Each data point was obtained 15 min after fentanyl injection in a single dosing procedure.
Discussion
The present study was designed to develop a model of capsaicin-induced thermal allodynia in rhesus monkeys and to investigate the role of peripheral mu opioid receptors in this model. Capsaicin caused allodynia in the warm water tail-withdrawal assay. Local administration of fentanyl and DAMGO significantly diminished capsaicin-induced allodynia in a dose-dependent manner. This antinociception was antagonized by a small dose of either quadazocine or quaternary naltrexone applied locally. These results support the hypothesis that activation of peripheral mu opioid receptors can relieve nociception caused by capsaicin, which is thought to be mediated by stimulating primary afferent C- and Aδ-fibers (Holzer, 1991; Winter et al., 1995).
Nociceptive effects of capsaicin
It has been suggested that burning pain induced by capsaicin is mediated through the activation of a heat-gated ion channel (Caterina et al., 1997). Exposure of nociceptor terminals to capsaicin leads to excitation of the neuron and local release of inflammatory mediators (Szolcsányi, 1993; Winter et al., 1995). In human and rodent studies, capsaicin has been used widely to evoke nociceptive responses for investigation of antinociceptive agents (Park et al., 1995; Gilchrist et al., 1996; Eisenach et al., 1997). In the present study, s.c. administration of capsaicin (0.1 mg) into the tail produced thermal allodynia in 46°C water. This quick-onset and short-lasting nociceptive effect was mediated locally in the tail, because the same amount of capsaicin, s.c. applied in the back, had no effect on the tail-withdrawal latency (data not shown).
Although repeated application of high doses of capsaicin could lead to desensitization, the rapidity and extent with which desensitization develops is related to the dose of capsaicin, the time of exposure to capsaicin and the interval between consecutive doses (Craft and Porreca, 1992; Winter et al., 1995). With low doses given at appropriate intervals, capsaicin-induced pain reaction and excitation of sensory neurons can be reproduced with each application (Holzer, 1991). In this preparation, 0.1 mg of capsaicin given either once or twice each week did not cause desensitization in tail-withdrawal responses throughout the presently reported experiments. Thus, in the absence of long-term observable changes in nociceptive responses, capsaicin-induced transient thermal allodynia in primates could be a useful model to evaluate systemic and local effects of various antinociceptive agents.
Antinociceptive effects of mu agonists
The present study provides the first behavioral demonstration that local administration of selective mu opioid agonists, fentanyl and DAMGO, dose-dependently inhibited capsaicin-induced thermal allodynia in non-human primates. However, the locally effective doses of both mu agonists, when applied in the back, did not inhibit capsaicin-induced allodynia. This indicates that the site of fentanyl- and DAMGO-induced antinociception against capsaicin is located in the tail. It has been suggested that stimulation of local opioid receptors is likely to inhibit the sensory afferent barrage and vasodilatation by reducing the release of vasoactive substances from capsaicin-sensitive neurons (Barthó et al., 1990, 1992; Zochodne and Ho, 1993; Schaafsma et al., 1997).
When a noxious thermal stimulus of 50°C water was assessed in the absence of capsaicin, local application of fentanyl and DAMGO (up to the doses studied presently) did not produce antinociception. These observations agree with rodent findings, supporting the notion that the antinociceptive potency of opioid agonists is enhanced on the peripheral terminals of nociceptive primary afferents innervating inflamed tissue (Stein et al., 1989; Barber and Gottschlich, 1992; Andreev et al., 1994). There are several factors that may account for the increased effectiveness of locally administered opioids in pain of inflammatory origins. For instance, opioid agonists may have easier access to neuronal opioid receptors because of perineurial disruption (Antonijevic et al., 1995). In addition, enhanced axonal transport of opioid receptors in the periphery also may play a role during inflammation (Laduron, 1984; Hassan et al., 1993). The fact that local administration of mu opioids are more effective in inflamed tissues may prove advantageous, considering that in the clinic, many painful conditions are associated with inflammation (Stein et al., 1991; Stanfa and Dickenson, 1995; Kinnman et al., 1997).
The antinociceptive potency of fentanyl and DAMGO was compared after local and systemic administration. Local fentanyl was approximately 15-fold more potent than systemic fentanyl. In contrast, local DAMGO was at least 500-fold more potent than systemic DAMGO, as shown by its inactivity by the systemic route, up to a dose of 0.32 mg/kg. Such profound differential potencies of fentanyl and DAMGO likely are explained by their distinct pharmacokinetic profiles. For example, fentanyl is highly lipophilic and well-distributed after systemic administration (Roy and Flynn, 1989). At the dose of 0.056 mg/kg fentanyl used in this study, monkeys had observable respiratory depression, which indicates activation of central sites of action. However, DAMGO, a mu opioid peptide, may be degraded rapidly, distributed slowly or both. In particular, at a dose up to 0.32 mg/kg, DAMGO did not produce any overt behavioral changes. This observation strengthens the notion that selective peripherally acting mu opioid agonists can be developed by targeting compounds to act at peripheral sites, while avoiding centrally mediated undesirable effects.
Antagonism of mu agonist-induced antinociception
Local administration of both quadazocine and QNTX dose-dependently antagonized local inhibition of fentanyl against capsaicin-induced allodynia. However, the locally effective dose of both antagonists, when applied in the back, did not antagonize local fentanyl. This observation confirms the local agonist study, which indicates that the site of action of locally applied mu opioids is in the tail. Similarly, a greater relative potency of both antagonists following local versus systemic routes was observed. In particular, local QNTX was at least 300-fold more potent than systemic QNTX, which has been studied up to a dose of 3.2 mg/kg. Quaternary naltrexone is an N-methylated derivative of naltrexone. Although it is an effective opioid antagonist in vitro, systemic QNTX is ineffective in precipitating withdrawal in morphine-dependent rhesus monkeys at doses up to 8000 times larger than the effective dose of naltrexone (Valentino et al., 1983). Such large differential potency could be explained by the fact that most quaternary compounds have poor distribution after systemic administration (Brown and Goldberg, 1985).
The mu selective actions of local and systemic fentanyl were investigated further. Local administration of a selective kappa antagonist, nor-binaltorphimine, and a selective delta antagonist, naltrindole, did not antagonize local fentanyl. The dose range of both antagonists was based on a 30-fold potency difference relative to systemically effective doses in other antinociception studies in rhesus monkeys (Butelman et al., 1993, 1995). These results confirm that the local effect of fentanyl against capsaicin-induced allodynia is exclusively mu receptor-mediated. In addition, the quadazocine pKB values for fentanyl against either 50°C water in the absence of capsaicin or 46°C water in the presence of capsaicin are similar (7.6 –7.7) and close to the quadazocine pA2 value of 7.6 to 7.8 for mu agonists in other behavioral assays (Negus et al., 1993a; Walker et al., 1993). This confirms that systemic fentanyl-induced antinociception against both noxious stimuli mainly occurs through mu receptors.
In summary, the present study illustrates that local application of capsaicin into the tail of rhesus monkeys causes transient thermal allodynia. To our knowledge, it is the first report demonstrating that local administration of mu opioid agonists diminish the nociception induced by capsaicin in non-human primates. This experimental pain model could provide a useful tool for evaluating various antinociceptive agents and expand new approaches to the treatment of inflammatory pain, such as the development of peripherally acting mu opioid agonists without central side effects.
ABBREVIATIONS
- Capsaicin
8-methyl-N-vanillyl-6-nonenamide
- DAMGO
(d-Ala2,N-Me-Phe4, Gly5-ol)-enkephalin
- D.R.
dose ratio
- Quadazocine
WIN 44441–3
- QNTX
quaternary naltrexone
- %MPE
%maximum possible effect
Footnotes
Animals used in these studies were maintained in accordance with the University Committee on the Use and Care of Animals, University of Michigan, and Guidelines of the Committee on the Care and Use of Laboratory Animals of the institute of Laboratory Animal Resources, National Health Council (Department of Health, Education and Welfare, Publication ISBN 0–309-05377–3, revised 1996).
Support for this research was provided by USPHS Grant 00254. Preliminary results were presented at the 16th annual meeting of American Pain Society, New Orleans, LA, October 23–26, 1997.
References
- Andreev N, Urban L, Dray A. Opioids suppress spontaneous activity of polymodal nociceptors in rat paw skin induced by ultraviolet irradiation. Neuroscience. 1994;58:793–798. doi: 10.1016/0306-4522(94)90456-1. [DOI] [PubMed] [Google Scholar]
- Antonijevic I, Mousa SA, Schäfer M, Stein C. Perineurial defect and peripheral opioid analgesia in inflammation. J Neurosci. 1995;15:165–172. doi: 10.1523/JNEUROSCI.15-01-00165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A, Gottschlich R. Opioid agonists and antagonists: An evaluation of their peripheral actions in inflammation. Med Res Rev. 1992;12:525–562. doi: 10.1002/med.2610120505. [DOI] [PubMed] [Google Scholar]
- Barthó L, Stein C, Herz A. Involvement of capsaicin-sensitive neurones in hyperalgesia and enhanced opioid antinociception in inflammation. Naunyn-Schmiedeberg’s Arch Pharmacol. 1990;342:666–670. doi: 10.1007/BF00175710. [DOI] [PubMed] [Google Scholar]
- Barthó L, Ernst R, Pierau FK, Sann H, Faulstroh K, Pethö G. An opioid peptide inhibits capsaicin-sensitive vasodilatation in the pig’s skin. Neuropeptides. 1992;23:227–237. doi: 10.1016/0143-4179(92)90129-k. [DOI] [PubMed] [Google Scholar]
- Brown DR, Goldberg LI. The use of quaternary narcotic antagonists in opiate research. Neuropharmacology. 1985;24:181–191. doi: 10.1016/0028-3908(85)90072-3. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther. 1993;267:1269–1276. [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Gatch MB, Chang KJ, Woods JH. BW373U86, a δ-opioid receptor agonist, reverses bradykinin-induced thermal allodynia in rhesus monkeys. Eur J Pharmacol. 1995;277:285–287. doi: 10.1016/0014-2999(95)00134-7. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen T, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Craft RM, Porreca F. Treatment parameters of desensitization to capsaicin. Life Sci. 1992;51:1767–1775. doi: 10.1016/0024-3205(92)90046-r. [DOI] [PubMed] [Google Scholar]
- Dray A, Dickenson A. Systemic capsaicin and olvanil reduce the acute algogenic and the late inflammatory phase following formalin injection into rodent paw. Pain. 1991;47:79–83. doi: 10.1016/0304-3959(91)90014-O. [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Woods JH. A tail withdrawal procedure for assessing analgesic activity in rhesus monkeys. J Pharmacol Methods. 1986;15:263–269. doi: 10.1016/0160-5402(86)90056-2. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, Hood DD, Curry R, Tong C. Alfentanil, but not amitriptyline, reduces pain, hyperalgesia, and allodynia from intradermal injection of capsaicin in humans. Anesthesiology. 1997;86:1279–1287. doi: 10.1097/00000542-199706000-00008. [DOI] [PubMed] [Google Scholar]
- Gilchrist HD, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67:179–188. doi: 10.1016/0304-3959(96)03104-1. [DOI] [PubMed] [Google Scholar]
- Hassan AHS, Ableitner A, Stein C, Herz A. Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissue. Neuroscience. 1993;55:185–195. doi: 10.1016/0306-4522(93)90465-r. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: Cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Kim YI, Na HS, Han JS, Hong SK. Critical role of the capsaicin-sensitive nerve fibers in the development of the causalgic symptoms produced by transecting some but not all of the nerves innervating the rat tail. J Neurosci. 1995;15:4133–4139. doi: 10.1523/JNEUROSCI.15-06-04133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnman E, Levine JD. Involvement of the sympathetic postganglionic neuron in capsaicin-induced secondary hyperalgesia in the rat. Neuroscience. 1995;65:283–291. doi: 10.1016/0306-4522(94)00474-j. [DOI] [PubMed] [Google Scholar]
- Kinnman E, Nygårds EB, Hansson P. Peripherally administrated morphine attenuates capsaicin-induced mechanical hypersensitivity in humans. Anesth Analg. 1997;84:595–599. doi: 10.1097/00000539-199703000-00024. [DOI] [PubMed] [Google Scholar]
- Kupers RC, Chen CC, Bushnell MC. A model of transient hyperalgesia in the behaving monkey induced by topical application of capsaicin. Pain. 1997;72:269–275. doi: 10.1016/s0304-3959(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Laduron PM. Axonal transport of opiate receptors in capsaicin-sensitive neurones. Brain Res. 1984;294:157–160. doi: 10.1016/0006-8993(84)91322-2. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LER, Torebjörk HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Taiwo Y. Inflammatory pain. In: Wall PD, Melzack R, editors. Textbook of Pain. New York: Churchill Livingstone; 1994. pp. 45–56. [Google Scholar]
- Maggi CA. The pharmacological modulation of neurotransmitter release. In: Wood JN, editor. Capsaicin in the study of Pain. London: Academic Press; 1993. pp. 161–189. [Google Scholar]
- Negus SS, Burke TF, Medzihradsky F, Woods JH. Effects of opioid agonists selective for mu, kappa and delta opioid receptors on schedule-controlled responding in rhesus monkeys: Antagonism by quadazocine. J Pharmacol Exp Ther. 1993a;267:896–903. [PubMed] [Google Scholar]
- Negus SS, Butelman ER, Ai Y, Woods JH. Prostaglandin E2-induced thermal hyperalgesia and its reversal by morphine in the warm-water tail-withdrawal procedure in rhesus monkeys. J Pharmacol Exp Ther. 1993b;266:1355–1363. [PubMed] [Google Scholar]
- Park KM, Max MB, Robinovitz E, Gracely RH, Bennett GJ. Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain. 1995;63:163–172. doi: 10.1016/0304-3959(95)00029-R. [DOI] [PubMed] [Google Scholar]
- Roy SD, Flynn GL. Transdermal delivery of narcotic analgesics: Comparative permeabilities of narcotic analgesics through human cadaver skin. Pharm Res. 1989;6:825–832. doi: 10.1023/a:1015944018555. [DOI] [PubMed] [Google Scholar]
- Schaafsma L, Sun H, Zochodne D. Exogenous opioids influence the microcirculation of injured peripheral nerves. Am J Physiol. 1997;272:H76–H82. doi: 10.1152/ajpheart.1997.272.1.H76. [DOI] [PubMed] [Google Scholar]
- Simone DA, Ngeow JYF, Putterman GJ, LaMotte RH. Hyperalgesia to heat after intradermal injection of capsaicin. Brain Res. 1987;418:201–203. doi: 10.1016/0006-8993(87)90982-6. [DOI] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Stanfa L, Dickenson A. Spinal opioid systems in inflammation. Inflamm Res. 1995;44:231–241. doi: 10.1007/BF01782974. [DOI] [PubMed] [Google Scholar]
- Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther. 1989;248:1269–1275. [PubMed] [Google Scholar]
- Stein C, Comisel K, Haimerl E, Yassouridis A, Lehrberger K, Herz A, Peter K. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N Engl J Med. 1991;325:1123–1126. doi: 10.1056/NEJM199110173251602. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J. Actions of capsaicin on sensory receptors. In: Wood JN, editor. Capsaicin in the study of Pain. London: Academic Press; 1993. pp. 1–26. [Google Scholar]
- Twycross RG. Opioids. In: Wall PD, Melzack R, editors. Textbook of Pain. New York: Churchill Livingstone; 1994. pp. 943–962. [Google Scholar]
- Valentino RJ, Katz JL, Medzihradsky F, Woods JH. Receptor binding, antagonist, and withdrawal precipitating properties of opiate antagonists. Life Sci. 1983;32:2887–2896. doi: 10.1016/0024-3205(83)90325-9. [DOI] [PubMed] [Google Scholar]
- Walker EA, Butelman ER, de Costa BR, Woods JH. Opioid thermal antinociception in rhesus monkeys: Receptor mechanisms and temperature dependency. J Pharmacol Exp Ther. 1993;267:280–286. [PubMed] [Google Scholar]
- Willis WD. Hyperalgesia and allodynia. In: Willis WD, editor. Hyperalgesia and Allodynia. New York: Raven Press; 1992. pp. 1–11. [Google Scholar]
- Winter J, Bevan S, Campbell EA. Capsaicin and pain mechanisms. Br J Anaesth. 1995;75:157–168. doi: 10.1093/bja/75.2.157. [DOI] [PubMed] [Google Scholar]
- Zochodne DW, Ho LT. Evidence that capsaicin hyperaemia of rat sciatic vasa nervorum is local, opiate-sensitive and involves mast cells. J Physiol. 1993;468:325–333. doi: 10.1113/jphysiol.1993.sp019774. [DOI] [PMC free article] [PubMed] [Google Scholar]