Abstract
Rationale
Kappa agonists can attenuate reinstatement of cocaine-seeking behavior induced by cocaine priming. The mechanisms underlying this effect have not been characterized fully, but may have a serotonergic component as kappa agonists also increase the release of serotonin (5-HT).
Objectives
This study investigated the role of kappa opioid receptor and 5-HT mechanisms in kappa agonist-induced attenuation of cocaine priming in monkeys.
Methods
Squirrel monkeys were trained to self-administer cocaine (0.18-0.3 mg/kg/injection) under a second-order schedule in which drug seeking was maintained jointly by cocaine injections and a cocaine-paired visual stimulus. In extinction sessions, saline was substituted for cocaine and the cocaine-paired stimulus was omitted. During test sessions, only saline was available for self-administration and response-contingent presentations of the cocaine-paired stimulus were restored.
Results
Priming injections of cocaine (0.1–1.0 mg/kg) induced reinstatement of drug seeking. Maximal levels of responding were similar to those maintained by active cocaine self-administration. Pretreatment with the kappa agonists enadoline (0.01 mg/kg) and spiradoline (0.3 mg/kg) or the 5-HT transport inhibitors fluoxetine (5.6 mg/kg) and citalopram (10.0 mg/kg) attenuated the priming effects of cocaine, shifting the cocaine dose-response function rightward and downward. Inhibition of cocaine-induced reinstatement of drug seeking by spiradoline and fluoxetine was reversed by 8-OH-DPAT (0.03 mg/kg), a 5HT1A agonist that inhibits 5-HT release. The effects of spiradoline also were reversed by the kappa antagonist norbinaltorphimine (10.0 mg/kg).
Conclusions
Results suggest that the capacity of kappa opioid agonists to increase extracellular 5-HT levels may at least partially underlie kappa agonist-induced modulation of cocaine seeking.
Keywords: Cocaine, Reinstatement, Relapse, Kappa opioid agonist, Serotonin, Squirrel monkey (Saimiri sciureus)
Although stimulation of the kappa opioid/dynorphin system has been identified as a key mediator of stress responses that can enhance the reinforcing effects of cocaine in a conditioned place preference procedure in mice (McLaughlin et al. 2003; 2006), and inhibition of kappa opioid receptors can inhibit cocaine place-conditioning in a similar procedure (Carey et al. 2007; Redila and Chavkin 2008), other preclinical studies have shown that stimulation of this system with kappa opioid agonists can inhibit the abuse-related effects of cocaine. For example, pretreatment with the kappa opioid agonists enadoline and U50,488H attenuated the discriminative stimulus effects of cocaine in squirrel monkeys trained to discriminate cocaine from vehicle (Spealman and Bergman 1992; 1994). Similarly, administration of these and other kappa opioid agonists also have been found to reduce cocaine self-administration in rhesus monkeys and rats, an effect that could be reversed by the kappa opioid antagonist norbinaltorphimine (nor-BNI; Glick et al. 1995; Negus et al. 1997; Mello and Negus 1998; Schenk et al. 1999). Lastly, in reinstatement studies in rats, the capacity of cocaine to reinstate extinguished drug seeking behavior was blunted by pretreatment with the kappa opioid agonist U69593 (Schenk et al. 1999).
The mechanism underlying the capacity of kappa opioid agonists to attenuate the effects of cocaine is often presumed to be related to their capacity to inhibit dopamine (DA) neurotransmission, as kappa opioid agonists can inhibit DA release from the nucleus accumbens (Di Chiara and Imperato 1988; Spanagel 1990; Thompson et al. 2000), decrease striatal DA levels (Devine at al. 1993) and inhibit firing of DA neurons (Walker et al. 1987). However, results from in vitro and in vivo studies suggest that kappa opioid agonists also can modulate the serotonin (5-HT) system. For example, application of U50,488H to both human and rat brain slices enhanced the outflow of electrically-evoked and K+-evoked [3H]5-HT (Ho and Takemori 1990; Berger et al. 2006). In vivo, i.c.v. administration of U50,488H produced analgesia and induced supraspinal release of 5-HT in mice (Ho and Takemori 1989). Moreover, kappa opioid agonist-induced analgesia was attenuated by 5-HT depletion with p-chlorophenylalanine as well as by several 5-HT antagonists (Von Voigtlander et al. 1984; Ho and Takemori 1989). In drug discrimination studies in pigeons and rats, the discriminative stimulus effects of U50,488H were attenuated following 5-HT depletion as well as after pretreatment with several 5-HT ligands including the 5-HT1A receptor agonist 8-OH-DPAT, the 5-HT2 receptor antagonist ketanserin, and the 5-HT3 receptor antagonist MDL72222 – all of which inhibit serotonergic activity (Bronson et al. 1993; Powell et al. 1994). Lastly, the kappa opioid agonist U69593 attenuated drug seeking induced by priming injections of cocaine and RTI-55 (a cocaine analog with greater affinity for the 5-HT transporter compared to the DA transporter), but not drug seeking induced by the DA uptake inhibitor GBR 12909 or by WIN 35,428 (a cocaine analog with greater affinity for the DA transporter compared to the 5-HT transporter) (Schenk et al. 2000). Together, these findings support the idea that 5-HT release and the subsequent stimulation of 5-HT receptors may be an important mechanism underlying the neurochemical and behavioral effects of kappa opioid agonists.
The idea that kappa opioid agonists may attenuate the abuse-related effects of cocaine via 5-HT mechanisms is supported by evidence of a general inhibitory role for 5-HT in modulating the behavioral effects of cocaine, especially in nonhuman primates. For example, in squirrel monkeys, 5-HT transport inhibitors attenuated the discriminative stimulus effects of cocaine in some cases (Spealman 1993, but see Schama et al. 1997) and reduced the reinforcing effects of cocaine (Howell and Byrd 1995, Czoty et al. 2002). In vivo microdialysis studies in monkeys have shown that 5-HT transport inhibitors also lessened cocaine-induced increase of extracellular DA in the caudate nucleus (Czoty et al. 2002).
The purpose of the present study was to investigate the capacity of kappa opioid agonists to attenuate cocaine priming-induced reinstatement of drug-seeking behavior in squirrel monkeys. Because these agonists can have effects that are mediated by non-opioid receptor mechanisms, we directly evaluated the role of kappa opioid receptors in their cocaine-attenuating effects with the kappa opioid receptor antagonist nor-BNI. To evaluate the potential role of 5-HT mechanisms in the cocaine-blunting effects of kappa opioid agonists, attenuation of reinstatement by the kappa opioid agonists was compared directly with attenuation induced by representative 5-HT transport inhibitors. Finally, to assess the involvement of 5-HT1A receptor mechanisms in the cocaine-antagonizing effects of both kappa opioid agonists and 5-HT transport inhibitors, the capacity of the 5-HT1A-agonist 8-OH-DPAT to reverse the effects of spiradoline and fluoxetine was determined.
Materials and Methods
Subjects
Adult squirrel monkeys (Saimiri sciureus) weighing 0.7 to 1.0 kg were housed individually in a climate-controlled vivarium, where they had access to water ad libitum and received a nutritionally balanced diet of monkey chow (Teklad Monkey Diet) supplemented with fresh fruit. A total of seven monkeys were studied, with groups of at least four monkeys serving as subjects in each experiment (see below). Monkeys used in these studies had participated in an earlier study looking at the contribution of DA and noradrenergic mechanisms to cocaine-induced reinstatement (cf. Platt et al. 2007). Monkeys used in this study were maintained in accordance with the guidelines of the Committee on Animals of the Harvard Medical School and the “Guide for the Care and Use of Laboratory Animals” of the Institute of Laboratory Animal Resources, National Research Council, Department of Health, Education and Welfare Publication No. (NIH) 85-23, revised 1996. Research protocols were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Surgery
Indwelling venous catheters (polyvinyl chloride; i.d. 0.38 mm; o.d. 0.76 mm were implanted in each monkey using aseptic surgical procedures as described by Platt et al. (2005). Briefly, monkeys were anesthetized with isoflurane, and one end of the catheter was passed by way of either a jugular or femoral vein to the level of the right atrium. The distal end of the catheter was passed subcutaneously and exited in the mid-scapular region. Catheters were flushed daily with 0.9% saline solution and were sealed with stainless steel obturators when not in use. Monkeys wore nylon-mesh jackets (Lomir Biomedical, Toronto, Canada) at all times to protect the catheter.
Apparatus
Experimental sessions were conducted in ventilated sound-attenuating chambers which were provided with white noise to mask external sounds. Within the chamber, monkeys were seated in a Plexiglas chair facing a panel that was equipped with a response lever and colored stimulus lights above the lever (Med Associates, Inc., Georgia, VT). Catheters were connected to syringe pumps (Med Associates, Inc., Georgia, VT) located outside the chamber. Each operation of the pump delivered a 1-s infusion of 0.18 ml of vehicle or drug solution into the catheter.
Second-order schedule of cocaine injection
Monkeys were trained to self-administer cocaine under a second-order fixed interval (FI), fixed ratio (FR) schedule of i.v. drug injection similar to the schedule described by Khroyan et al. (2000). Briefly, in the presence of a white light, completion of every 10th or 30th response (FR10 or FR30, depending on the particular monkey) during a 10-min FI resulted in a 2-s change in illumination from white to red. Completion of the first FR after expiration of the FI resulted in an i.v. injection of cocaine simultaneous with the onset of the red light (cocaine-paired stimulus:S). A 60-s time out (TO) period, during which all lights were off and responses had no scheduled consequences, followed each injection. If the FR requirement was not completed within 8 min following the expiration of the FI, the component ended automatically without an injection and was followed by a 60-s TO period. Daily sessions ended after completion of five cycles of the second-order schedule (maximum of 90 min). Initially, the dose of cocaine was varied over a 10-fold range (0.03 – 0.3 mg/kg/injection) to determine the dose that maintained maximum rates of responding for each monkey. For six monkeys, 0.18 mg/kg/injection maintained maximum response rates, whereas for the remaining monkey, 0.3 mg/kg/injection maintained maximum response rates.
Extinction and reinstatement of cocaine seeking
Following a 6- to 8-month period of stable cocaine self-administration, responding was extinguished by substituting saline for cocaine and omitting presentations of the cocaine-paired stimulus. Extinction sessions, including session and TO durations, were otherwise identical to those described above. Extinction sessions were conducted daily until responding declined and stabilized at ≤10% of the response rate maintained by cocaine self-administration (3–10 sessions depending on the subject).
Following extinction, a range of doses of noncontingent priming injections of cocaine (0.1 – 1.0 mg/kg), as well as saline vehicle, were assessed for their capacity to reinstate drug-seeking behavior during test sessions in which only saline was available for self-administration. Response-contingent presentations of the cocaine-paired stimulus also were restored during these test sessions because earlier studies showed that reinstatement of cocaine-seeking behavior was greatest when cocaine priming was accompanied by restoration of the cocaine-paired stimulus (Spealman et al. 2004). Priming injections of cocaine were administered i.v. immediately before the session. Different doses of cocaine were tested on different days, with each test session separated by one or more extinction sessions as described above. The order in which each priming dose of cocaine was tested varied across monkeys. After completion of these experiments, cocaine self-administration was re-established using the procedures described previously until responding stabilized and there were no systematic upward or downward trends in response rates over at least three consecutive sessions. This re-establishment of cocaine self-administration occurred at least monthly, after completion of one part of the study and before testing another set of drugs.
Effects of kappa opioid ligands on reinstatement of cocaine seeking
Following completion of the test sequence with priming doses of cocaine, re-establishment of stable cocaine self-administration and subsequent extinction of responding, the effects of kappa opioid agonists on cocaine-induced drug-seeking were determined. The kappa opioid agonists used in this experiment included: spiradoline (U62066) and enadoline (Cl 977 HCl). Agonists were given i.v. immediately prior to i.v. administration of cocaine priming doses (range: 0.1 – 1.0 mg/kg). Spiradoline was tested at a dose of 0.3 mg/kg and enadoline at a dose of 0.01 mg/kg. These doses were found to be maximally effective at reinstating cocaine-seeking behavior when tested alone in an earlier study with squirrel monkeys (Valdez et al. 2007) and did not consistently reduce rates of responding in other procedures in squirrel monkeys (e.g., drug discrimination: Carey & Bergman 2001; FI stimulus-shock termination: Powell & Holtzman 1999; shock titration: Pitts & Dykstra 1994, Powell & Dykstra 1995). At the end of experiments with each kappa opioid agonist, the priming effect of 1.0 mg/kg cocaine was re-evaluated to assess potential shifts in baseline levels of responding.
Subsequently, an antagonism study was conducted with the kappa opioid antagonist nor-BNI. Nor-BNI (10.0 mg/kg, i.m.) was administered at least one day before i.v. priming with cocaine alone or spiradoline plus cocaine. These parameters were selected on the basis of a previous study showing that nor-BNI exhibits selective kappa opioid receptor antagonist effects beginning one day after administration and lasting at least 25 days in squirrel monkeys (Carey and Bergman 2001). As in reinstatement tests described above, only saline was available for self-administration, and response-contingent presentations of the cocaine-paired stimulus were restored during the test.
Effects of serotonergic ligands on reinstatement of cocaine seeking
After completion of experiments involving kappa opioid ligands and a one week washout period, the effects of the 5-HT transport inhibitors fluoxetine and citalopram on extinguished drug-seeking were determined using the testing procedures described above. Initially, fluoxetine (0.3 – 5.6 mg/kg, i.v.) or citalopram (0.3 – 10.0 mg/kg, i.v.) was administered alone immediately prior to the test session. Dose ranges for each drug were selected on the basis of earlier studies in squirrel monkeys (Spealman 1993; Howell and Byrd 1995). To evaluate the effects of the 5-HT transport inhibitors on cocaine-seeking behavior, the highest doses of fluoxetine (5.6 mg/kg) and citalopram (10.0 mg/kg) were administered in combination with a range of cocaine priming doses (0.1 – 1.0 mg/kg). Importantly, these doses of the 5-HT transport inhibitors do not consistently alter rates of responding in squirrel monkeys in other procedures (e.g., drug discrimination: Spealman 1993, Schama et al. 1997; FI stimulus-shock termination: Spealman 1993; Howell & Byrd 1995). At the end of experiments with each 5-HT transport inhibitor, the priming effect of 1.0 mg/kg cocaine was re-evaluated to assess potential shifts in baseline levels of responding.
In order to assess the involvement of 5-HT release in the attenuation of cocaine seeking by kappa agonists and 5-HT transport inhibitors, 0.03 mg/kg of 8-OH-DPAT, a 5-HT1A agonist, was administered i.m. 10 min prior to the infusion of spiradoline (0.3 mg/kg) or fluoxetine (5.6 mg/kg) combined with the maximally effective dose of cocaine (1.0 mg/kg). The dose and pretreatment interval for 8-OH-DPAT was based on an earlier study by Bronson et al. (1993). We also determined the capacity of 8-OH-DPAT (0.003 – 0.1 mg/kg) to reinstate cocaine-seeking behavior in the absence of cocaine priming and to alter the effects of cocaine priming in the absence of either spiradoline or fluoxetine. As in reinstatement testing with cocaine alone, only saline was available for self-administration, and response-contingent presentations of the cocaine-paired stimulus were restored.
Data analysis
The rate of responding in individual monkeys was computed for each session by dividing the total number of responses by the total elapsed time (excluding responses and time during TO periods). For each experimental condition, the mean response rate ± S.E.M. was calculated for groups of four to seven monkeys. Statistical analysis was conducted using SigmaStat 3.0. Data were analyzed by one-way or two-way repeated measures analysis of variance (ANOVA) and Bonferroni t-tests, when appropriate, to assess differences between control conditions and test drugs. The control conditions were either vehicle priming tests for experiments in which the drugs were tested alone, or cocaine priming alone for experiments in which cocaine was combined with other drugs.
Drugs
Cocaine hydrochloride, enadoline hydrochloride, nor-BNI dihydrochloride and citalopram hydrobromide were dissolved in 0.9% saline solution. Fluoxetine hydrochloride, 8-OH-DPAT [R(+)8-hydroxy-2-(di-n-propylamino)tetralin] hydrobromide and spiradoline mesylate were dissolved in sterile water. Compounds were purchased from Tocris Cookson (Ellisville, MO) and Sigma Aldrich (St. Louis, MO).
Results
Cocaine self-administration, extinction, and priming-induced reinstatement
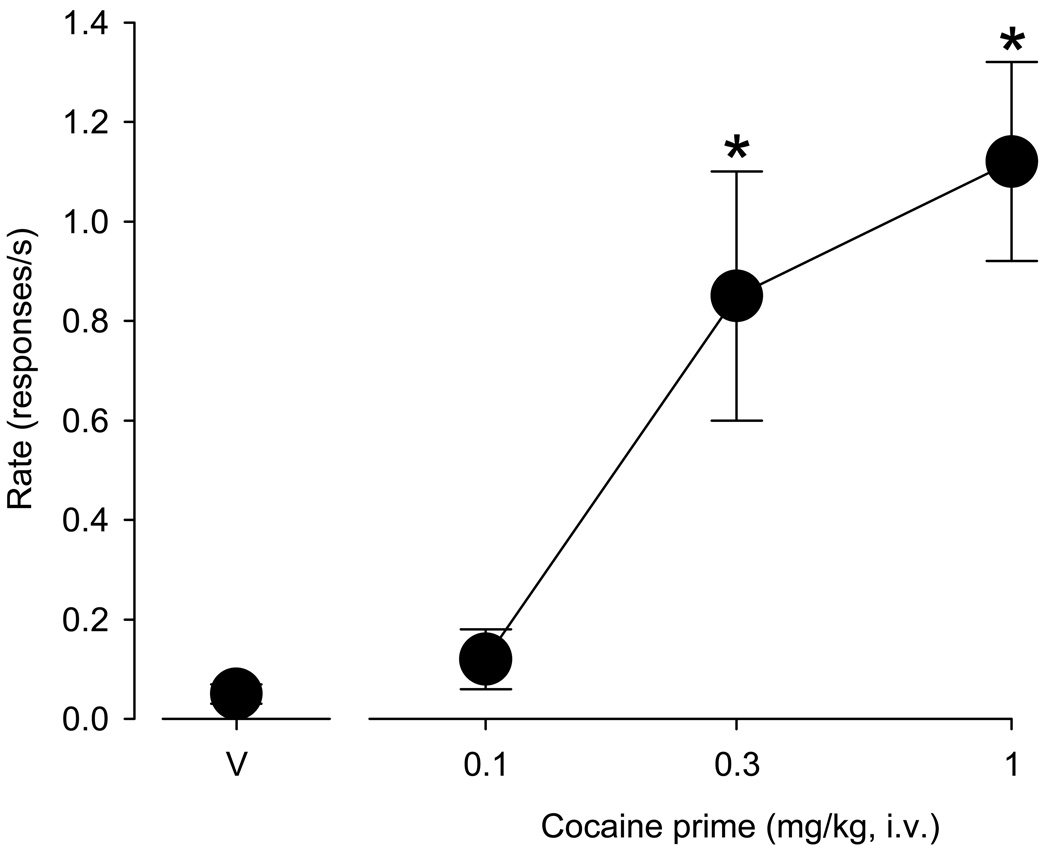
In all monkeys, self-administered cocaine maintained high rates of responding under the second-order schedule of i.v. drug injection, ranging from 0.5 – 2.2 responses/s in individual monkeys (group mean: 1.2 ± 0.27 responses/s, n=7). During extinction sessions, in which only saline was available for self-administration and the cocaine-paired stimulus was omitted, responding declined and stabilized at low rates, ranging from 0 – 0.19 responses/s, depending on the individual monkey (group mean: 0.045 ± 0.015 responses/s). As depicted in Figure 1, priming injections of cocaine (0.1 – 1.0 mg/kg) prior to test sessions in which the cocaine-paired stimulus was restored but only saline was available for self-administration produced a dose-dependent reinstatement of extinguished cocaine-seeking behavior (F(3, 18)=18.03, p < 0.001). Average response rates after administration of 0.3 and 1.0 mg/kg cocaine primes were significantly higher than rates obtained after administration of vehicle (p<0.001 each, Bonferroni t-tests) and approached response rates maintained by active cocaine self-administration. Priming with a lower dose of cocaine (0.1 mg/kg) did not reinstate extinguished drug seeking above levels observed during extinction or following vehicle injections.
Figure 1.
Dose-dependent reinstatement of extinguished drug-seeking behavior induced by cocaine combined with restoration of the cocaine-paired stimulus. Point above “V” shows response rate following a vehicle prime. Data are means ± S.E.M. from seven monkeys. * indicates significant differences relative to vehicle (p < 0.05).
Effects of kappa opioid agonists on reinstatement of cocaine seeking
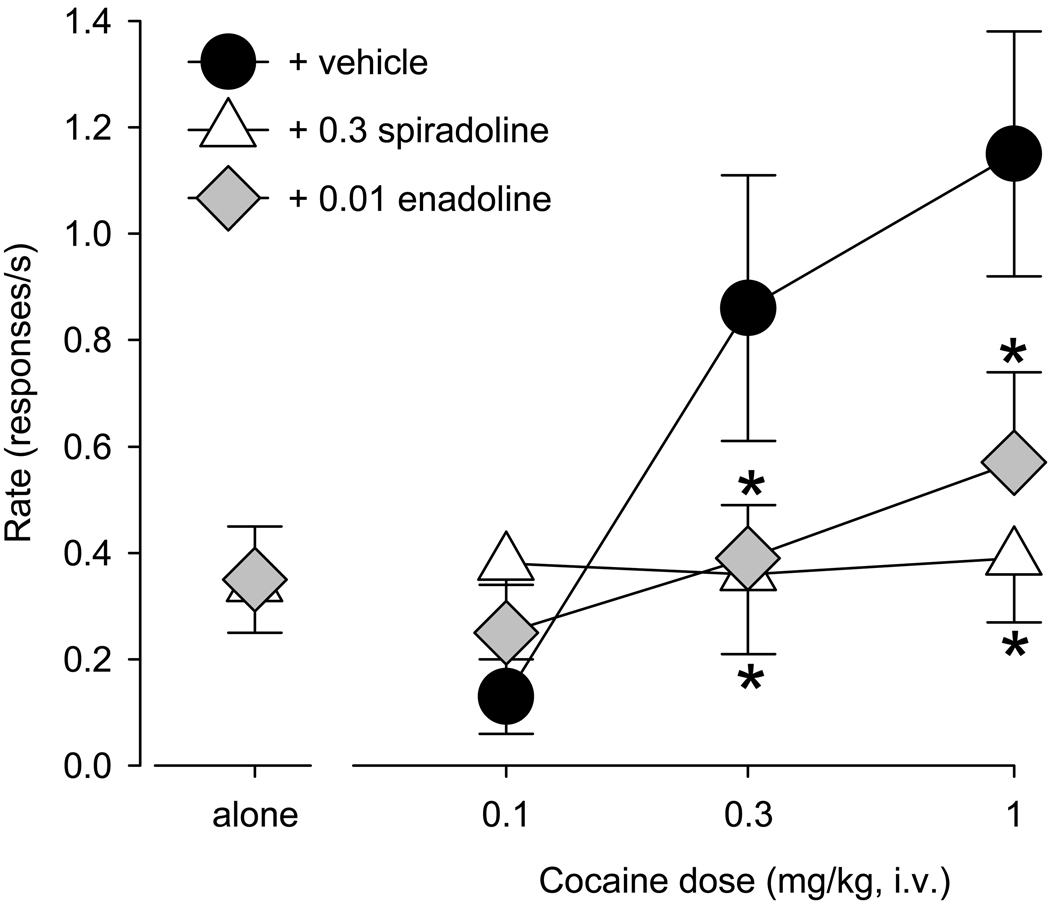
Although both kappa agonists induced some cocaine-seeking behavior on their own (Fig. 2, points above “alone”), they also attenuated cocaine-induced reinstatement, shifting the cocaine priming dose-response function downward (Fig. 2). Pretreatment with 0.3 mg/kg of spiradoline before the two highest priming doses of cocaine significantly attenuated reinstatement (0.3 mg/kg cocaine: p < 0.026; 1.0 mg/kg cocaine: p < 0.002, Bonferroni t-tests), as reflected by a significant spiradoline X cocaine prime interaction (F(2,10)=12.04, p < 0.002; Fig. 2, white triangles). Similar to the findings with spiradoline, enadoline also blocked reinstatement induced by the two highest doses of cocaine (0.3 mg/kg cocaine: p <0.013; 1.0 mg/kg cocaine: p < 0.004, Bonferroni t-tests), as reflected by a significant enadoline X cocaine prime interaction (F(2,10)=8.82, p<0.006; Fig. 2, gray diamonds).
Figure 2.
Attenuation of cocaine priming effects on extinguished drug-seeking behavior after pretreatment with either 0.3 mg/kg spiradoline (white triangles) or 0.01 mg/kg enadoline (gray diamonds) compared to vehicle pretreatment. Points above “alone” show response rates following the kappa agonists alone. Data are means ± S.E.M. from six monkeys. * indicates significant differences relative to vehicle (p < 0.05).
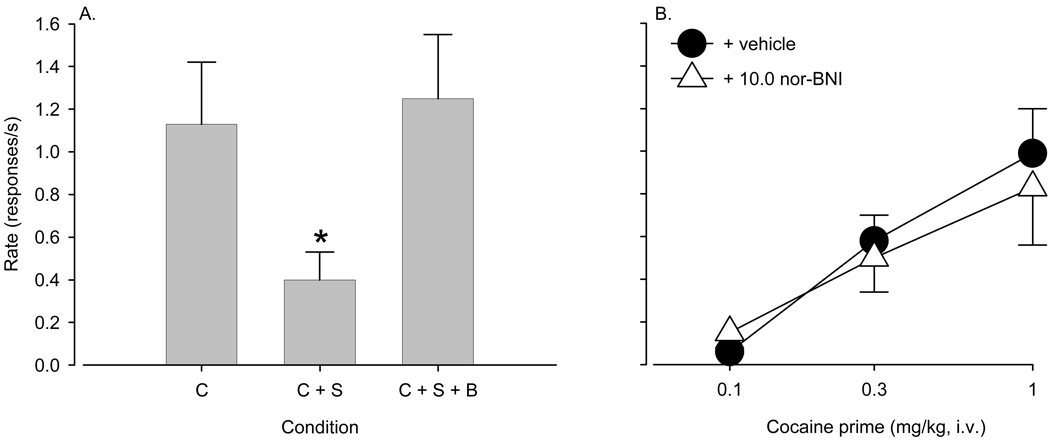
Reversal of the effects of spiradoline by nor-BNI
Compared to 1.0 mg/kg cocaine priming alone, pretreatment with 0.3 mg/kg spiradoline significantly reduced reinstatement of cocaine-seeking behavior (F(2,6)=1.26, p < 0.025; p < 0.05, Bonferroni t-test; Fig. 3A). Pretreatment with 10.0 mg/kg nor-BNI at a minimum of 24 hours before reinstatement testing reversed the effect of spiradoline on cocaine-induced reinstatement of drug seeking. As shown in Figure 3, the rate of responding returned to the level induced by cocaine priming alone. Pretreatment with 10.0 mg/kg nor-BNI before cocaine priming in the absence of spiradoline had no significant effect on the rate of responding (p > 0.31; Fig. 3B).
Figure 3.
Reversal of the cocaine-attenuating effects of A) spiradoline (0.3 mg/kg) by nor-BNI (10 mg/kg). The maximally effective priming dose of cocaine was used (1.0 mg/kg). nor-BNI did not alter the priming effects of B) cocaine alone. Data are means ± S.E.M. from four to five monkeys. * indicates significant difference relative to cocaine priming (p < 0.05). C = cocaine, S = spiradoline, B = nor-BNI.
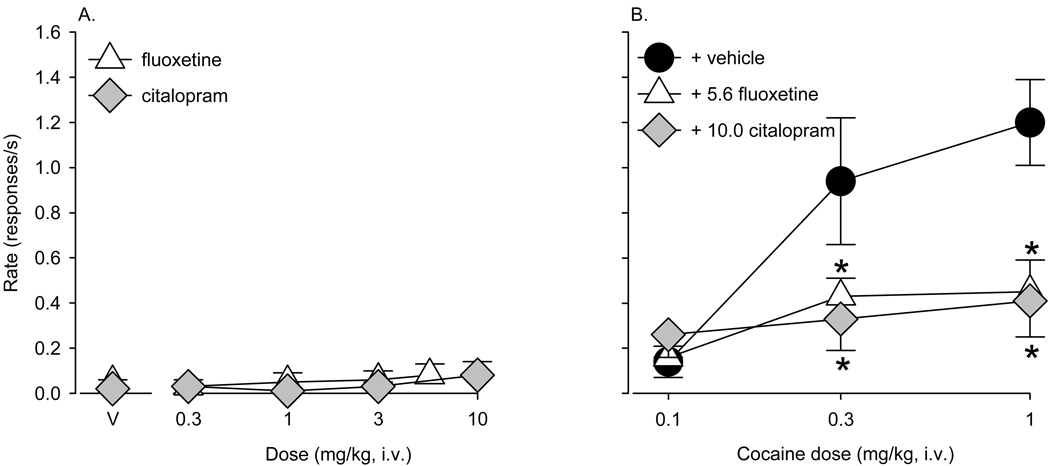
Effects of 5-HT transport inhibitors on reinstatement of cocaine seeking
When various doses of fluoxetine or citalopram were given as primes at the beginning of a reinstatement session, there was no difference in rates of responding relative to a vehicle prime (fluoxetine: p > 0.47; citalopram: p > 0.60; Fig. 4A). However, both 5-HT transport inhibitors attenuated cocaine-induced reinstatement of drug seeking, shifting the cocaine priming dose-response function downward (Figure 4B). Pretreatment with 5.6 mg/kg fluoxetine before cocaine priming significantly attenuated reinstatement of cocaine-seeking behavior compared to vehicle pretreatment (F(1,10)=8.30, p < 0.035, Fig. 4B, white triangles). There was a significant fluoxetine X cocaine prime interaction (F(2,10)=6.71, p < 0.014), reflecting the fact that there was no difference between vehicle and fluoxetine pretreatment administered before a 0.1 mg/kg cocaine prime (p>0.9, Bonferroni t-test), but significant treatment differences with 0.3 (p < 0.02, Bonferroni t-test) and 1.0 mg/kg cocaine (p < 0.002, Bonferroni t-test).
Figure 4.
A) Fluoxetine and citalopram do not reinstate cocaine seeking, B) but do attenuate cocaine priming effects on extinguished drug-seeking behavior (5.6 mg/kg fluoxetine: white triangles; 10.0 mg/kg citalopram: gray diamonds) compared to vehicle pretreatment. Data are means ± S.E.M. from six (fluoxetine) or four (citalopram) monkeys. * indicates significant differences compared to vehicle (p < 0.05).
Similarly, when 10.0 mg/kg citalopram was given as a pretreatment before cocaine priming, there was a significant reduction in reinstatement of cocaine seeking relative to vehicle pretreatment (F(1,6)=15.74, p < 0.029; Fig. 4B, gray diamonds). As with fluoxetine pretreatment, citalopram attenuated reinstatement induced by 0.3 (p < 0.006, Bonferroni t-test) and 1.0 mg/kg cocaine (p < 0.001, Bonferroni t-test). This finding was supported by a significant citalopram X cocaine prime interaction (F(2,10)=19.57, p < 0.002).
Reversal of the effects of fluoxetine and spiradoline by 8-OH-DPAT
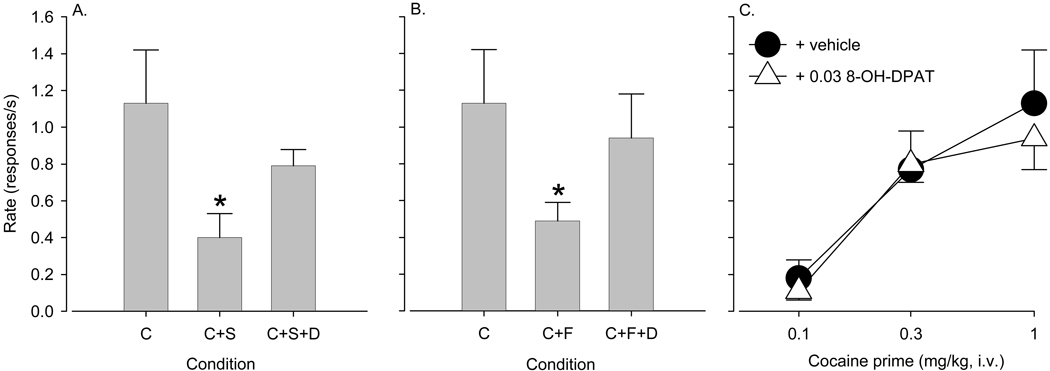
Pretreatment with 0.3 mg/kg spiradoline before cocaine priming (1.0 mg/kg) significantly reduced reinstatement of drug seeking compared to cocaine priming in the absence of spiradoline (F(2,6)=5.22, p < 0.05; p < 0.04, Bonferroni t-test; Fig. 5A). When 8-OH-DPAT (0.03 mg/kg) was administered as a pretreatment before the spiradoline-cocaine combination, the attenuation of reinstatement was reversed (Bonferroni t-test, p > 0.36; Fig. 5A). Similarly, when fluoxetine (5.6 mg/kg) was given as a pretreatment before cocaine priming (1.0 mg/kg), cocaine-induced reinstatement was significantly lower than with cocaine alone (F(2,6)=9.95, p < 0.012; p < 0.01, Bonferroni t-test; Fig. 5B). When 8-OH-DPAT was given as a pretreatment before fluoxetine and cocaine, the attenuation of reinstatement was reversed (Bonferroni t-test, p > 0.43; Fig. 5B).
Figure 5.
Pretreatment with 0.03 mg/kg 8-OH-DPAT reverses the attenuation of the cocaine priming effect induced by A) 0.3 mg/kg spiradoline and B) 5.6 mg/kg fluoxetine but does not alter C) cocaine priming. Data are means ± S.E.M. from four monkeys. * indicates significant differences compared to cocaine priming (p < 0.05). C = cocaine, S = spiradoline, D = 8-OH-DPAT, F = fluoxetine.
Priming with 8-OH-DPAT alone (0.003 – 0.1 mg/kg) did not reinstate drug seeking, and response rates did not differ from those observed with vehicle priming (vehicle: 0.06 ± 0.03 responses/s, 8-OH-DPAT: 0.05 – 0.07 responses/s; p > 0.54). Pretreatment with 0.03 mg/kg 8-OH-DPAT before cocaine priming (0.1 – 1.0 mg/kg, i.v.) had no significant effect on reinstatement compared to cocaine priming alone (p > 0.15), irrespective of the cocaine priming dose (Fig. 5C).
Discussion
In the present study, pretreatment with the kappa opioid agonists spiradoline and enadoline significantly attenuated cocaine priming-induced reinstatement of drug seeking in squirrel monkeys previously trained to self-administer cocaine. The attenuation induced by the kappa opioid agonists appeared to be kappa opioid receptor mediated, because the attenuation could be reversed with the kappa opioid antagonist nor-BNI. These results support and extend earlier studies in rats and nonhuman primates in which administration of kappa opioid agonists attenuated the discriminative stimulus effects of cocaine, reduced cocaine self-administration and blunted reinstatement of drug seeking-behavior after cocaine priming (Spealman & Bergman 1992; 1994; Glick et al. 1995; Negus et al. 1997; Mello & Negus 1998; Schenk et al. 1999).
Previous work from our laboratory has shown that the kappa opioid agonists, at the doses evaluated in the present study, also reinstate cocaine seeking on their own (Valdez et al. 2007). Interestingly, unlike the effects observed in the present study, kappa opioid agonist-induced reinstatement could not be blocked by nor-BNI, only by relatively high doses of naltrexone. The priming effects of kappa opioid agonists also were attenuated by a corticotrophin-releasing factor antagonist as well as an alpha2-adrenoceptor agonist. These data raise the possibility that different subpopulations of kappa opioid receptors interact with different neurotransmitter systems to mediate the reinstatement-inducing vs. reinstatement-inhibiting effects of these ligands.
The inhibitory effects of kappa opioid agonists with respect to the abuse-related effects of cocaine have frequently been attributed to their capacity to modulate the DA system. Kappa opioid agonists have been shown to inhibit DA release in the nucleus accumbens (Di Chiara and Imperato 1988; Spanagel 1990; Thompson et al. 2000), decrease striatal DA levels (Devine at al. 1993) and inhibit firing of DA neurons (Walker et al. 1987). However, several lines of evidence indicate that kappa opioid agonists also can alter the 5-HT system. For example, kappa opioid agonists have been found to enhance 5-HT release (Ho and Takemori 1989; 1990; Berger et al. 2006) and induce behavioral effects that can be reversed by 5-HT depletion or with 5-HT antagonists (Von Voigtlander et al. 1984; Ho and Takemori 1989; Bronson et al. 1993; Powell et al. 1994). In nonhuman primates, 5-HT appears to play a general inhibitory role in modulating the reinforcing and discriminative stimulus effects of cocaine (Spealman 1993; Howell and Byrd 1999; Czoty et al. 2002). In the present study, high doses of the 5-HT transport inhibitors fluoxetine and citalopram also attenuated the priming effects of cocaine. These results support the idea that a common mechanism related to an increased concentration of 5-HT in the synapse, whether via kappa opioid agonist-induced release or blockade of the transporter by 5-HT transport inhibitors, may underlie the similar capacity of kappa opioid agonists and 5-HT transport inhibitors to attenuate the priming and other abuse-related effects of cocaine.
Our finding that pretreatment with high doses of 5-HT transport inhibitors attenuated cocaine priming-induced reinstatement in monkeys differs from results of a similar study in rats. Burmeister and colleagues (2003) showed that reinstatement of cocaine-seeking behavior induced by cocaine priming was unaltered following pretreatment with either fluoxetine or the 5-HT transport inhibitor/releaser d-fenfluramine; consistent with other studies showing that 5-HT transport inhibitors also fail to attenuate cocaine self-administration and the discriminative stimulus effects of cocaine in rats, except at toxic doses (Cunningham and Callahan 1991; Tella 1995; Walsh and Cunningham 1997). In another study in rats, however, cocaine priming-induced reinstatement was enhanced following depletion of brain 5-HT levels with the tryptophan hydroxylase inhibitor para-chlorophenylalanine (Tran-Nguyen et al. 2001). This latter finding raises the possibility of an as yet to be determined role for 5-HT neurotransmission in cocaine priming-induced drug seeking in rats.
The reasons underlying the differences in monkey vs. rat studies is not clear. One possibility is the dose of the 5-HT transport inhibitor studied. As mentioned above, we evaluated only a single high dose of fluoxetine and citalopram. Another difference is the route by which the 5-Ht transport inhibitor was administered (i.v. vs. i.p.). Finally, there is the possibility of a species difference in the behavioral effects of 5-HT transport inhibitors reflecting neurogenetic and/or neuropharmacological differences in 5-HT systems in rodents compared to primates. For example, even though the coding sequences of monoamine transporters are relatively conserved across mammalian species, the greatest deviation in homology between rodents and primates was observed for the 5-HT transporter relative to the DA and norepineprine transporter (Miller et al. 2001). In addition, using 5-HT uptake and radioligand binding assays, Barker and colleagues (1994) demonstrated that 5-HT transporter ligands can display differing potencies and efficacies at rodent compared to primate 5-HT transporters. These differences in the 5-HT system, as well as procedural differences, may form the basis for the different effects of 5-HT transport inhibitors in primates versus rodents.
It is perhaps not surprising that kappa opioid agonists are able to modulate 5-HT release. Anatomically, it has been shown that kappa opioid receptors are localized on serotonergic neurons in the ventral medulla (Kalyuzhny and Wessendorf 1999) and on terminals that release excitatory amino acids on serotonergic neurons in the dorsal raphé nucleus (Pinnock 1992). To explore further the role of 5-HT in kappa agonist-induced attenuation of cocaine priming, we made use of the 5-HT1A receptor agonist 8-OH-DPAT. 8-OH-DPAT decreases 5-HT release by preferentially activating 5-HT1A autoreceptors (Hjorth and Magnusson 1988; Portas et al. 1996). Thus, if kappa opioid agonists produced some of their cocaine-inhibiting effects by releasing 5-HT, 8-OH-DPAT should attenuate these effects. In the present study, results showed that spiradoline- as well as fluoxetine-induced inhibition of cocaine reinstatement was reversed by a relatively low, presumably autoreceptor-selective, dose of 8-OH-DPAT. The capacity of 8-OH-DPAT to antagonize both kappa opioid agonist- and 5-HT transport inhibitor-induced behavioral effects has been observed previously. For example, 8-OH-DPAT dose-dependently attenuated the discriminative stimulus and rate-decreasing effects of U50,488H in pigeons trained to discriminate the kappa opioid agonist from vehicle (Bronson et al. 1993) and blocked conditioned nausea induced by fluoxetine in rats (Limebeer et al. 2009). Collectively, these results support the idea that modulation of 5-HT can be an important mechanism underlying the capacity of kappa opioid agonists to attenuate the abuse-related effects of cocaine.
A potential issue with the use of 8-OH-DPAT in combination with cocaine priming is previous findings showing that 8-OH-DPAT can enhance the reinforcing effects of low doses of cocaine in monkeys trained under a choice schedule (Czoty et al. 2005) and that 8-OH-DPAT can potentiate cocaine-induced hyperlocomotion in rats (Müller et al. 2003; Szumlinski et al. 2004). These results raise the possibility that the increases in cocaine-seeking behavior observed when 8-OH-DPAT was given as a pretreatment in the present study were due simply to behavioral stimulant effects. However, this does not appear to be the case. Pretreatment with our selected dose of 8-OH-DPAT did not alter the effects of either low or high priming doses of cocaine nor did it induce cocaine-seeking behavior on its own across the dose range tested.
In summary, our results show that relatively high doses of kappa opioid agonists can attenuate reinstatement of cocaine-seeking behavior in monkeys, as they have been shown to do in rats. This finding extends previous work in monkeys demonstrating that kappa opioid agonists can attenuate other abuse-related effects of cocaine, including the reinforcing and discriminative stimulus effects. The kappa opioid agonist-induced inhibition of drug seeking was similar to the inhibition of drug seeking induced by relatively high doses of 5-HT transport inhibitors, indicating that increasing synaptic 5-HT is sufficient to blunt the priming effects of cocaine. Finally, the inhibitory effects of both kappa opioid agonists and 5-HT transport inhibitors could be reversed with a selected dose of 8-OH-DPAT, a 5-HT1A agonist that has been shown to decrease 5-HT release. Taken together, our findings provide support for the idea that kappa opioid agonist-induced 5-HT release may at least partially underlie the capacity of these drugs to modulate the characteristic abuse-related effects of cocaine.
Acknowledgments
We thank Kristen Bano, Annemarie Duggan and Donna Reed for expert technical assistance. This research was supported by DA11928, DA11054 and RR00168.
References
- Barker EL, Kimmel HL, Blakely RD. Chimeric human and rat serotonin transporters reveal domains involved in recognition of transporter ligands. Mol Pharmacol. 1994;46:799–807. [PubMed] [Google Scholar]
- Berger B, Rothmaier AK, Wedekind F, Zentner J, Feuerstein TJ, Jackisch R. Presynaptic opioid receptors on noradrenergic and serotonergic neurons in the human as compared to the rat neocortex. Br J Pharmacol. 2006;148:795–806. doi: 10.1038/sj.bjp.0706782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson ME, Lin YP, Burchett K, Picker MJ, Dykstra LA. Serotonin involvement in the discriminative stimulus effects of kappa opioids in pigeons. Psychopharmacology. 1993;111:69–77. doi: 10.1007/BF02257409. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Neisewander JL. Effects of fluoxetine and d-fenfluramine on cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:146–154. doi: 10.1007/s00213-002-1307-8. [DOI] [PubMed] [Google Scholar]
- Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey GJ, Bergman J. Enadoline discrimination in squirrel monkeys: Effects of opioid agonists and antagonists. J Pharmacol Exp Ther. 2001;297:215–223. [PubMed] [Google Scholar]
- Cunningham KA, Callahan PM. Monoamine reuptake inhibitors enhance the discriminative state induced by cocaine in the rat. Psychopharmacology. 1991;104:177–180. doi: 10.1007/BF02244175. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:831–837. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Effects of the 5-HT1A agonist (±)-8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on cocaine choice in cynomolgus monkeys. Behav Pharmacol. 2005;16:187–191. doi: 10.1097/00008877-200505000-00008. [DOI] [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D, Wise RA. Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: In vivo microdialysis studies. J Pharmacol Exp Ther. 1993;266:1236–1246. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Raucci J, Archer S. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–152. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Magnusson T. The 5-HT1A receptor agonist, 8-OH-DPAT, preferentially activates cell body 5-HT autoreceptors in rat brain in vivo. Naunyn-Schmeideberg’s Arch Pharmacol. 1988;338:463–471. doi: 10.1007/BF00179315. [DOI] [PubMed] [Google Scholar]
- Ho BY, Takemori AE. Serotonergic involvement in the antinociceptive action of and the development of tolerance to the kappa-opioid receptor agonist, U-50,488H. J Pharmacol Exp Ther. 1989;250:508–514. [PubMed] [Google Scholar]
- Ho BY, Takemori AE. Release by U-50,488H of [3H]serotonin from brain slices and spinal cord synaptosomes of U-50,488H-tolerant and nontolerant mice. J Pharmacol Exp Ther. 1990;254:8–12. [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Serotonergic modulation of the behavioural effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther. 1995;275:1551–1559. [PubMed] [Google Scholar]
- Kalyuzhny AE, Wessendorf MW. Serotonergic and GABAergic neurons in the medial rostral ventral medulla express kappa-opioid receptor immunoreactivity. Neuroscience. 1999;90:229–234. doi: 10.1016/s0306-4522(98)00376-5. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behaviour: Effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- Limebeer CL, Litt DE, Parker LA. Effect of 5-HT3 antagonists and a 5-HT1A agonist on fluoxetine-induced conditioned gaping reactions in rats. Psychopharmacology. 2009;203:763–770. doi: 10.1007/s00213-008-1421-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioural responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughling JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of kappa opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. J Pharmacol Exp Ther. 1998;286:812–824. [PubMed] [Google Scholar]
- Miller GM, Yatin SM, De La Garza R, II, Goulet M, Madras BK. Cloning of dopamine, norepinephrine and serotonin transporters from monkey brain: Relevance to cocaine sensitivity. Mol Brain Res. 2001;87:124–143. doi: 10.1016/s0169-328x(00)00288-6. [DOI] [PubMed] [Google Scholar]
- Müller CP, Carey RJ, Salloum JB, Huston JP. Serotonin1A-receptor agonism attenuates the cocaine-induced increase in serotonin levels in the hippocampus and nucleus accumbens but potentiates hyperlocomotion: An in vivo microdialysis study. Neuropharmacology. 2003;44:592–603. doi: 10.1016/s0028-3908(03)00046-7. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- Pinnock RD. Activation of kappa-opioid receptors depresses electrically evoked excitatory postsynaptic potentials on 5-HT-sensitive neurones in the rat dorsal raphé nucleus in vitro. Brain Res. 1992;583:237–246. doi: 10.1016/s0006-8993(10)80029-0. [DOI] [PubMed] [Google Scholar]
- Pitts RC, Dykstra LA. Antinociceptive and response rate-altering effects of kappa opioid agonists, spiradoline, enadoline and U69,593, alone and in combination with opioid antagonists in squirrel monkeys. J Pharmacol Exp Ther. 1994;271:1501–1508. [PubMed] [Google Scholar]
- Platt DM, Carey GJ, Spealman RD. Intravenous self-administration techniques in monkeys. In: Enna S, Williams M, Ferkany J, Kenakin T, Porsolt R, Sullivam J, editors. Current Protocols in Neuroscience. New York: Wiley; 2005. pp. 9.21.1–9.21.15. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Noradrenergic mechanisms in cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2007;322:894–902. doi: 10.1124/jpet.107.121806. [DOI] [PubMed] [Google Scholar]
- Portas CM, Thakkar M, Rainnie D, McCarley RW. Microdialysis perfusion of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. J Neurosci. 1996;16:2820–2828. doi: 10.1523/JNEUROSCI.16-08-02820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KR, Picker MJ, Dykstra LA. Serotonin involvement in the discriminative stimulus effects of mu and kappa opioids in rats. Behav Pharmacol. 1994;5:255–264. [PubMed] [Google Scholar]
- Powell KR, Dykstra The role of serotonin in the effects of opioids in squirrel monkeys responding under a titration procedure: I. Kappa opioids. J Pharmacol Exp Ther. 1995;274:1305–1316. [PubMed] [Google Scholar]
- Powell KR, Holtzman SG. Differential antagonism of the rate-decreasing effects of κ-opioid receptor agonists by naltrexone and norbinaltorphimine. Eur J Pharmacol. 1999;377:21–28. doi: 10.1016/s0014-2999(99)00394-5. [DOI] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology. 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schama KF, Howell LL, Byrd LD. Serotonergic modulation of the discriminative-stimulus effects of cocaine in squirrel monkeys. Psychopharmacology. 1997;132:27–34. doi: 10.1007/s002130050316. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology. 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. Reinstatement of extinguished drug-taking behavior in rats: Effect of the kappa-opioid receptor agonist, U69593. Psychopharmacology. 2000;151:85–90. doi: 10.1007/s002130000476. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: An in vivo microdialysis study. J Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Modification of the behavioral effects of cocaine by selective serotonin and dopamine uptake inhibitors in squirrel monkeys. Psychopharmacology. 1993;112:93–99. doi: 10.1007/BF02247368. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Bergman J. Modulation of the discriminative stimulus effects of cocaine by mu and kappa opioids. J Pharmacol Exp Ther. 1992;261:607–615. [PubMed] [Google Scholar]
- Spealman RD, Bergman J. Opioid modulation of the discriminative stimulus effects of cocaine: Comparison of μ, κ and δ agonists in squirrel monkeys discriminating low doses of cocaine. Behav Pharmacol. 1994;5:21–31. doi: 10.1097/00008877-199402000-00003. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Lee B, Tiefenbacher S, Platt DM, Rowlett JK, Khroyan TV. Triggers of relapse: Nonhuman primate models of reinstated cocaine seeking. In: Bevins RA, Bardo MT, editors. Motivational Factors in the Etiology of Drug Abuse. Lincoln: University of Nebraska Press; 2004. pp. 57–84. [PubMed] [Google Scholar]
- Szumlinski KK, Frys KA, Kalivas PW. Dissociable roles for the dorsal and median raphé in the facilitatory effect of 5-HT1A receptor stimulation upon cocaine-induced locomotion and sensitization. Neuropsychopharmacology. 2004;29:1675–1687. doi: 10.1038/sj.npp.1300473. [DOI] [PubMed] [Google Scholar]
- Tella SR. Effects of monoamine reuptake inhibitors on cocaine self-administration in rats. Pharmacol Biochem Behav. 1995;51:687–692. doi: 10.1016/0091-3057(94)00438-o. [DOI] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB, Jr, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modified dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen LTL, Bellew JG, Grote KA, Neisewander JL. Serotonin depletion attenuates cocaine seeking but enhances sucrose seeking and the effects of cocaine priming on reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;157:340–348. doi: 10.1007/s002130100822. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Rüedi-Bettschen D, Spealman RD. κ agonist-induced reinstatement of cocaine seeking in squirrel monkeys: A role for opioid and stress-related mechanisms. J Pharmacol Exp Ther. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- Von Voigtlander PF, Lewis RA, Neff GL. Kappa opioid analgesia is dependent on serotonergic mechanisms. J Pharmacol Exp Ther. 1984;231:270–274. [PubMed] [Google Scholar]
- Walker JM, Thompson LA, Frascella J, Friederich MW. Opposite effects of mu and kappa opiates on the firing-rate of dopamine cells in the substantia nigra of the rat. Eur J Pharmacol. 1987;134:53–59. doi: 10.1016/0014-2999(87)90130-0. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology. 1997;130:41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]