Abstract
The antidepressant action of cannabis as well as the interaction between antidepressants and the endocannabinoid system has been reported. This study was conducted to assess the antidepressant-like activity of Δ9-THC and other cannabinoids. Cannabinoids were initially evaluated in the mouse tetrad assay to determine doses that do not induce hypothermia or catalepsy. The automated mouse forced swim (FST) and tail suspension (TST) tests were used to determine antidepressant action. At doses lacking hypothermic and cataleptic effects (1.25, 2.5, and 5 mg/kg, i.p.), both Δ9-THC and Δ8-THC showed a U-shaped dose response with only Δ9-THC showing significant antidepressant-like effects at 2.5 mg/kg (p < 0.05) in the FST. The cannabinoids cannabigerol (CBG) and cannabinol (CBN) did not produce antidepressant-like actions up to 80 mg/kg in the mouse FST, while cannabichromene (CBC) and cannabidiol (CBD) exhibited significant effect at 20 and 200 mg/kg, respectively (p < 0.01). The antidepressant-like action of Δ9-THC and CBC was further confirmed in the TST. Δ9 -THC exhibited the same U-shaped dose response with significant antidepressant-like action at 2.5 mg/kg (p < 0.05) while CBC resulted in a significant dose dependent decrease in immobility at 40 and 80 mg/kg doses (p < 0.01). Results of this study show that Δ9-THC and other cannabinoids exert antidepressant-like actions, and thus may contribute to the overall mood-elevating properties of cannabis.
Keywords: Cannabis, Δ9-Tetrahydrocannabinol, Δ8-Tetrahydrocannabinol, Cannabidiol, Cannabichromene, Cannabigerol, Cannabinol, Antidepressant, Forced swim test, Tail suspension test, Locomotor activity
1. Introduction
Cannabis sativa L. is one of the most widely used plants for both recreational and medicinal purposes. To date a total of 525 natural constituents covering several chemical classes have been isolated and identified from C. sativa (Ahmed et al., 2008a, 2008b; ElSohly and Slade, 2005; Radwan et al., 2008a, 2008b, 2009; Ross and ElSohly, 1995, Turner et al., 1980). The cannabinoids belong to the chemical class of terpenophenolics, of which 85 have been uniquely identified in cannabis, including the most psychoactive cannabinoid, Δ9-tetrahydrocannabinol (Δ9-THC). The most common natural plant cannabinoids (phytocannabinoids) are: Δ9-THC, cannabidiol (CBD), cannabigerol (CBG), cannabichromene (CBC), and cannabinol (CBN). Several of the identified cannabinoids are both chemically and pharmacologically poorly characterized due to insufficient isolated amounts; however, the pharmacology of Δ9-THC has been widely studied, and it is regarded as the main psychoactive constituent of cannabis.
The psychological and physiological effects of cannabis have been extensively characterized, including euphoria, analgesia, sedation, memory and cognitive impairment, appetite stimulation, and anti-emesis. Most of these effects have been primarily attributed to Δ9-THC (Pertwee, 2006). Major advances in the field of cannabinoid research were achieved following the unraveling of the molecular mechanism underlying the actions of Δ9-THC and the discovery of the endocannabinoid system. The endocannabinoid system is regarded as a neuromodulator, and is comprised of cannabinoid receptors (primarily CB1 and CB2 receptors), their endogenous ligands, and enzymes responsible for the synthesis and metabolism of these ligands (Devane et al., 1992; Dinh et al., 2002; Gong et al., 2006; Matsuda et al., 1990; Okamoto et al., 2004; Sugiura et al., 1995).
In addition to the established effects of cannabis, it is well recognized that mood elevation is one of the components of the complex experience elicited by cannabis (Skolnick et al., 2001). Much of our knowledge regarding cannabis effect on mood and anxiety is based on individual reports following cannabis use for medicinal or recreational purposes. Several anecdotal reports describe the antidepressant effect of cannabis, with patients confirming beneficial outcomes from its use in primary or secondary depressive disorders (Grinsponn and Balkar, 1998; Gruber et al., 1996; Johns, 2001). On the other hand, similar increasing literature associate cannabis abuse with bipolar disorders and depression (Bovasso, 2001; Jarvis et al., 2008; Lee et al., 2008; van Rossum et al., 2009). Because of such bidirectional effects of cannabis in humans, recent research has primarily focused on the complex role of the endocannabinoid system in the pathogenesis and treatment of depression (Witkin et al., 2005). Hill et al. (2008) reported a reduction in serum 2-arachidonyl glycerol (2-AG) levels in patients suffering from major depression with the decrease correlating with the duration of depression episodes. The authors also reported a significantly enhanced serum anandamide level in patients with minor depression, while both 2-AG and anandamide were reduced in women suffering from major depression. Similarly, postmortem studies of patients with major depression have revealed a decrease in CB1 receptor density in the glial cells of the brain grey matter (Koethe et al., 2007). The available data thus suggest that changes in the central endocannabinoid system may differ from minor to major depression with down-regulation of the system involved in major depression while an up-regulation is elicited in minor depression.
Contrary to the extensive research done regarding the role of the endocannabinoid system in depression, only a number of studies have examined the effect of exogenous cannabinoids on depression. However, the controversial role of the endocannabinoid system in depression further extends to the evidence collected regarding the antidepressant effect of exogenous cannabinoids. Hill and Gorzalka (2005) reported that stimulation of CB1 receptor activity resulted in antidepressant-like activity in animal models. Direct stimulation of the receptors by administration of the CB1 receptor agonists HU210 or oleamide resulted in antidepressant-like effects in the rat forced swim test (FST) comparable to the tricyclic antidepressant desipramine. Jiang et al. (2005) showed that chronic administration of cannabinoids enhanced adult hippocampal neurogenesis, an effect previously proven to play a key role in antidepressant action. Such data suggest that CB1 activation leads to antidepressant-like properties.
This hypothesis is, however, in conflict with the findings that blockade of CB1 receptors leads to antidepressant-like actions in animal models. The administration of the CB1 receptor antagonists SR141716 and AM251 elicited antidepressant effects in the mice tail suspension test (TST) and the rat FST, respectively (Shearman et al., 2003; Witkin et al., 2005). In accordance with these findings, several studies reported neurochemical changes induced by CB1 receptor antagonists that correspond to antidepressant action. These changes include enhanced efflux of noradrenaline, 5-hydroxytryptamine, and dopamine in various brain regions associated with mood (Tzavara et al., 2001, 2003).
While most of these studies used synthetic CB1 ligands, the antidepressant action exerted by phytocannabinoids have not been examined in detail, and hence impedes full understanding of the antidepressant effect of cannabis. One possible explanation is the lack of sufficient amounts of the isolated phytocannabinoids to conduct proper pharmacological evaluation. Accordingly, the primary objectives of the current study were to isolate the major cannabinoids from cannabis, and to evaluate their antidepressant-like actions using an automated mouse FST followed by the mouse TST. Since typical cannabinoids cause severe catalepsy and hypothermia which may impede escape attempts in these behavioral despair paradigms, the antidepressant evaluation was conducted at doses that did not exhibit these effects as determined by the established mouse tetrad assay. The presented data provide better understanding for the participation of these compounds to the overall antidepressant action of cannabis.
2. Experimental procedures
2.1. Subjects
Experiments were performed using eight week old mice. Male Swiss Webster mice (Harlan, IN, USA) weighing 24–30 g at the time of testing were used for the tetrad and mouse FST, while adult male DBA/2 weighing 19–23 g (Harlan, IN, USA) were used for the TST. Mice were housed in groups of five with a 12 h light/12 h dark cycle. Food and water were provided ad libitum. All mice were randomly selected for each treatment group. Procedures involving animals were performed according to the guidelines approved by the Institutional Animal Care and Use Committee of the University of Mississippi and according to the National Institutes of Health Guide for Care and Use of Laboratory Animals.
2.2. Drugs
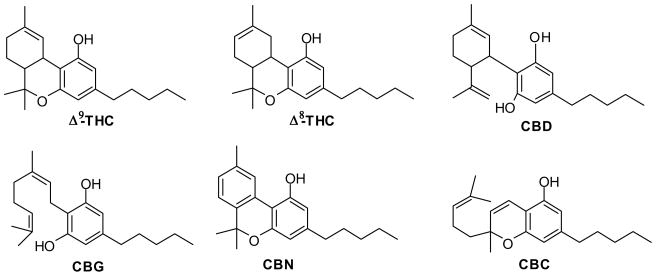
Dersipramine hydrochloride and fluoxetine hydrochloride were purchased from Sigma (St. Louis, MO, USA). The tested cannabinoids were isolated from high potency Cannabis sativa as previously described (Radwan et al., 2008a, 2008b; Ahmed et al., 2008b). Chemical structures for isolated cannabinoids are shown in Fig 1.
Fig. 1.
Chemical structure of cannabinoids: Δ9-tetrahydocannabinol (Δ9-THC), Δ8-tetrahydrocannabinol (Δ8-THC), cannabidiol (CBD), cannabigerol (CBG), cannabinol (CBN), and cannabichromene (CBC).
2.3. Mouse tetrad assay
Twenty four hours prior to testing, Swiss Webster mice were acclimated to experimental settings (ambient temperature 22–24 °C) and rectal probe insertion. At test day, pre-injection control values for rectal temperature, catalepsy, and tail flick latencies were determined. Animals (n=7–10/group) were injected intraperitoneally (i.p.) with either the vehicle control (ethanol/cremphor/saline 1:1:18), or the test compound (1.25–80 mg/kg). Animals were subsequently individually placed in activity chambers (San Diego Instruments, CA, USA) where the locomotor activity was automatically monitored for 30 min. Total activity during the last 10 min was expressed as the total number of interruptions of 16 cell photobeams per chamber. Each mouse was then placed on a ring immobility apparatus and the latency to drop time was recorded with a maximum of 180 s latency. Rectal temperature was recorded by inserting a rectal probe connected to a telethermometer (Physitemp Instruments, Clifton, NJ, USA) to a depth of 2 cm. Change in core temperature was expressed as the difference between basal and post injection temperatures. Finally, tail flick latency was measured with a maximum latency of 15 s to avoid tissue damage.
2.4. Forced Swim Test (FST)
Adult male Swiss Webster mice (n=7–10/group) were injected i.p. with the test compound (1.25–80 mg/kg), vehicle control (ethanol/cremphor/saline 1:1:18), or control antidepressant (desipramine or fluoxetine, 10–40 mg/kg). Locomotor activity was measured using an automated activity monitoring system (San Diego Instruments, CA, USA). Each mouse was immediately placed in a Plexiglas enclosure and locomotor activity monitored for 30 min. The data during the last 10 min of the testing period was analyzed. Immediately following the locomotor measurements (30 min post treatment), the mice were subject to the FST. The animals were individually placed in clear plastic cylinders (height 23 cm, internal diameter 10 cm) filled with 8 cm of deionized water at 23–25 °C. Individual mice were videotaped from above for a total of 6 min and the digital video output analyzed using SMART II Video Tracking System Software (San Diego Instruments, CA, USA). The software determined immobility in the 6 min session, but only data from the last 4 min were used to determine the effect. The immobility time was defined as the time spent by the mouse moving at a velocity below 2 cm/s. Such threshold velocity was chosen based on previously published data (Crowley et al. 2004) and our validation of the automated system (unpublished data) where this threshold produced immobility scores similar to those determined from manually scored tapes.
2.5. Tail suspension test (TST)
Adult male DBA/2 mice were injected i.p. with either the test compound (1.25–80 mg/kg), control antidepressant (desipramine, 20 mg/kg), or vehicle control (ethanol/cremphor/saline 1:1:18) 30 min prior to behavioral testing. Each mouse was placed in the photobeam activity monitoring system, and locomotor activity monitored for 30 minutes. Activity in the last 10 min was used for analysis. Mice were then tested in the TST paradigm by hanging each mouse upside down by the tail and fastened with clear packing tape (2–4 cm from the tip of the tail) to a metal bar attached to a ring-stand. The mice were suspended 35 cm above a protective sponge material (5 cm thick), with the animals at least 15 cm from any object.
Individual mice were recorded with a video camera, which was positioned at the same level as the mouse, for a total of 6 min. Quantification of immobility time during the last 4 min of each testing session was conducted by three independent raters. Immobility was operationally defined as the mice hanging motionless (Steru et al., 1985).
2.6. Data analysis
Statistical analysis was performed using Graphpad Prism Version 5.0. All values were presented as mean ± SEM with n = 7–10 animals/group. Antinociception in the tail flick assay was expressed as the percent maximal possible effect [% MPE = (post drug latency − basal latency/15 sec-basal latency) X 100]. All data were analyzed using One Way ANOVA followed by Dunnett’s post hoc test to determine significant difference from vehicle control at p < 0.05. Tukey’s multiple comparisons post hoc test was used to determine statistical differences among different experimental groups.
3. Results
3.1. Mouse tetrad assay
It is well established that the psychoactive properties exerted by Δ9-THC and other cannabinoids manifest in experimental animals as classical cannabimimetic activity in the mouse tetrad assay (Pertwee et al., 2007; Varvel et al., 2005).
3.1.1. Δ9-THC
As shown in Table 1, Δ9-THC exerted typical cannabinoid-like activity whereby it caused significant reduction in locomotor activity (F[6,75] = 21.11; p < 0.0001), increase in catalepsy (F[6,74] = 5.76; p < 0.0001), significant hypothermic effect (F[6,75] = 27.96; p < 0.0001), as well as antinociceptive action in the tail flick assay (F[6,84] = 6.11; p < 0.0001). Dunnett’s post hoc comparison revealed that the decrease in locomotor activity caused by 10 (q = 4.90, p < 0.001), 20 (q = 5.47, p < 0.001), and 40 (q = 5.06, p < 0.001) mg/kg doses was statistically significant compared to the vehicle control, while the 1.25 mg/kg dose significantly increased (q = 3.87, p < 0.01) locomotor activity. Post hoc comparisons of individual doses showed that the increase in catalepsy latency induced by the 20 (q = 4.32, p < 0.001) and 40 (q = 3.62, p < 0.01) mg/kg doses were statistically significant. Similarly, both the 20 (q = 6.43, p < 0.001) and 40 (q = 7.46, p < 0.001) mg/kg doses of Δ9-THC significantly reduced the animals’ core body temperature. Δ9-THC caused a dose dependent increase in tail-withdrawal latency confirming its antinociceptive effect, with the 20 (q = 2.95, p < 0.05) and 40 (q = 4.75, p < 0.001) mg/kg doses statistically significant from the vehicle control.
Table 1.
Behavioral effects of the isolated cannabinoids in the mouse tetrad assay.
| Treatment | Locomotor activity | Catalepsy (sec) | Decrease in rectal temperature (°C) | Tail flick latency (%MPE) |
|---|---|---|---|---|
| Δ9-Tetrahydrocannabinol (mg/kg) | ||||
| 0 | 1329 ± 175.9 | 1.44 ± 0.26 | −0.73 ± 0.11 | 1.50 ± 1.85 |
| 1.25 | 2098 ± 216.9 ** | 1.70 ± 0.42 | −0.22 ± 0.09 | 2.05 ± 4.25 |
| 2.5 | 876.9 ± 260.9 | 1.00 ± 0.00 | −0.22 ± 0.06 | 5.50 ± 4.37 |
| 5 | 786.4 ± 179.0 | 5.00 ± 0.93 | −1.47 ± 0.31 | 5.94 ± 1.45 |
| 10 | 190.9 ± 33.78 *** | 14.40 ± 4.16 | −2.05 ±33333 | 6.52 ± 2.02 |
| 20 | 260.4 ± 41.39 *** | 47.88 ± 20.04 *** | −3.87 ± 0.45 *** | 28.24 ± 11.12 * |
| 40 | 194.0 ± 49.18 *** | 37.70 ± 14.64 ** | −4.80 ± 0.28 *** | 51.96 ± 13.22 *** |
| Δ8-Tetrahydrocannabinol (mg/kg) | ||||
| 0 | 2091 ± 209.3 | 2.10 ± 0.61 | −0.56 ± 0.21 | 3.58 ± 0.84 |
| 1.25 | 1668 ± 245.4 | 1.10 ± 0.10 | −0.22 ± 0.09 | 3.55 ± 3.45 |
| 2.5 | 1606 ± 240.6 | 1.80 ± 0.47 | −0.22 ± 0.07 | 10.96 ± 3.62 |
| 5 | 1480 ± 184.3 | 1.60 ± 0.60 | −0.12 ± 0.09 | 8.01 ± 5.88 |
| 20 | 377.4 ± 169.7 *** | 5.25 ± 2.68 | −1.96 ± 0.37 *** | 5.97 ± 1.63 |
| Cannabigerol (mg/kg) | ||||
| 0 | 1833 ± 177.6 | 2.10 ± 0.61 | −0.58 ± 0.27 | 0.84 ± 3.58 |
| 20 | 1614 ± 290.4 | 2.63 ± 1.22 | −0.24 ± 0.11 | 7.26 ± 4.67 |
| 40 | 1825 ± 278.4 | 1.60 ± 0.34 | −0.34 ± 0.22 | 7.29 ± 7.11 |
| 80 | 2019 ± 185.2 | 1.80 ± 0.51 | −0.14 ± 0.08 | 2.33 ± 3.76 |
| Cannabichromene (mg/kg) | ||||
| 0 | 1609 ± 148.9 | 1.50 ± 0.50 | −0.34 ± 0.07 | 6.31 ± 2.86 |
| 20 | 1606 ± 256.8 | 1.00 ± 0.00 | −0.067 ± 0.042 | 3.02 ± 2.45 |
| 40 | 1416 ± 219.1 | 1.00 ± 0.00 | −0.32 ± 0.13 | 5.31 ± 2.36 |
| 80 | 780.8 ± 276.6 * | 1.83 ± 0.83 | −0.45 ± 0.08 | 0.47 ± 3.22 |
| Cannabinol (mg/kg) | ||||
| 0 | 1609 ± 148.9 | 1.50 ± 0.50 | −0.34 ± 0.07 | 6.31 ± 2.86 |
| 20 | 1214 ± 170.5 | 1.00 ± 0.00 | −0.35 ± 0.06 | 1.06 ± 5.37 |
| 40 | 972.1 ± 202.7 * | 1.33 ± 0.33 | −0.38 ± 0.12 | 1.70 ± 2.80 |
| 80 | 657.0 ± 227.3 ** | 2.17 ± 1.17 | −0.30 ± 0.08 | 5.69 ± 6.66 |
| Cannabidiol (mg/g) + Δ9-Tetrahydrocannabinol (20 mg/kg) | ||||
| 0 | 1470 ± 166.0 | 1.47 ± 0.27 | −0.73 ± 0.12 | 1.50 ± 1.85 |
| 20 | 293.7 ± 16.40 *** | 21.88 ± 14.33 | −3.68 ± 0.32 *** | 46.02 ± 17.43 * |
| 100 | 219.8 ± 76.04 *** | 5.13 ± 1.99# | −3.68 ± 0.35 *** | 22.03 ± 4.18 |
| 200 | 206.1 ± 38.55 *** | 9.38 ± 3.64 | −2.93 ± 0.24 *** | 15.97 ± 7.28 |
Values are mean ± SEM. n = 7–10 per group.
p < 0.001 versus control (0 mg/kg) (ANOVA followed by Dunnett’s posthoc test), and
p < 0.05 versus Δ9-THC (ANOVA followed by Dunnett’s posthoc test)
3.1.2. Δ8-THC
Δ8-THC produced some cannabimimetic-like actions in the tetrad assay leading to significant reduction in locomotor activity (F[4,42] = 7.87; p < 0.0001), significant hypothermic effect (F[4,49] = 12.12; p < 0.0001), as well as antinociceptive action in the tail flick assay (F[4,40] = 3.22; p = 0.028). No significant cataleptic effect was observed (F[4,43] = 2.02; p = 0.11) (Table 1). Post hoc comparisons of different groups versus the vehicle control showed that administration of Δ8-THC at only the 20 mg/kg dose caused significant hypolocomotive (q = 5.42, p < 0.001) and hypothermic (q = 5.06, p < 0.001) effects. However, no individual dose proved to possess a significant antinociceptive action. Such data confirm the decreased cannabimimetic potency of Δ8-THC as compared to Δ9-THC.
3.1.3. CBG, CBC, and CBN
As expected, the cannabinoids CBG, CBC, and CBN did not exhibit the typical cannabinoid-like action in the tetrad assay (Table 1). CBG did not cause significant change in locomotor activity (F[3,27] = 0.55; p = 0.65), catalepsy (F[3,34] = 0.39; p = 0.76), decrease in core body temperature (F[3,34] = 0.99; p = 0.41), or antinociceptive action in the tail flick assay (F[3,34] = 1.37; p = 0.27). CBC caused significant decrease in locomotor activity (F[3,23] = 4.54; p = 0.01) at 80 mg/kg dose (q = 3.12, p < 0.05) and an overall significant reduction in core body temperature (F[3,24] = 3.19; p = 0.04). However, it did not cause any catalepsy (F[3,24] = 0.57; p = 0.64) or change in tail flick latency (F[3,24] = 2.49; p = 0.09). Similar to CBC, CBN caused a dose dependent significant reduction in locomotor activity (F[3,43] = 5.17; p = 0.004) at 40 (q = 2.53, p<0.05) and 80 (q = 3.63, p < 0.01) mg/kg. It did not exhibit any effect on catalepsy (F[3,24] = 0.52; p = 0.67), core body temperature (F[3,24] = 0.15; p = 0.93), or tail flick latency (F[3,23] = 0.69; p = 0.56)
3.1.4. CBD
While CBD did not exert any cannabimimetic-like action in the mouse tetrad assay (data not shown), it significantly mitigated the cataleptic and antinociceptive actions exerted by Δ9-THC. The groups pretreated with 200 mg/kg CBD followed by 20 mg/kg of Δ9-THC showed cataleptic (q = 2.41, non significant) and nociceptive responses (q = 3.77, non significant) that were not statistically different from the vehicle control (Table 1).
3.2. Mouse FST
The FST is a model of behavioral despair whereby mice placed in an inescapable situation (in this case a cylinder of water) usually exhibit behavioral despair within 2 min of a 6 min session. An antidepressant-like effect is elicited as a reduction in immobility duration and sustained escape attempts (swimming and climbing) (Cryan et al., 2005a). Coupled to the FST, the effect of the test compound on locomotor activity was monitored in order to avoid any false positives resulting from stimulant action.
3.2.1. Control antidepressants
The tricyclic antidepressant desipramine exerted a significant decrease in the immobility time in the FST (F[3,45] = 9.68; p < 0.0001) (Table 2). Dunnett’s analysis showed that the effect caused by the 20 (q = 3.56, p < 0.01) and 40 (q = 4.75, p < 0.001) mg/kg doses were statistically significant from the vehicle control. Desipramine caused a significant reduction in locomotor activity (F[3,46] = 16.56; p < 0.0001) at 10 (q = 3.83, p < 0.01), 20 (q = 3.78, p < 0.01), and 40 (q = 6.73, p < 0.001) mg/kg. The selective serotonin re-uptake inhibitor fluoxetine exhibited a dose dependent reduction in immobility time in the FST (F[3,46] = 5.03; p = 0.004), with the effect significantly different from the vehicle control at 40 mg/kg (q = 3.54, p < 0.01). A significant locomotor depressant action (F[3,46] = 19.84; p < 0.0001) was also observed for fluoxetine at 40 mg/kg (q = 6.74, p < 0.001).
Table 2.
Effects of control antidepressants on immobility time in the automated mouse forced swim test and locomotor activity.
| Treatment | Immobility Time (sec) | Locomotor Activity |
|---|---|---|
| Vehicle | ||
| 120.5 ± 7.37 | 1618 ± 142 | |
| Fluoxetine (mg/kg) | ||
| 10 | 90.8 ± 10.00 | 1898 ± 132 |
| 20 | 90.4 ± 6.82 | 1293 ± 243 |
| 40 | 75.8 ± 12.89 ** | 143 ± 34 *** |
| Desipramine (mg/kg) | ||
| 10 | 111.8 ± 6.63 | 763 ± 112 ** |
| 20 | 81.44 ± 4.93 ** | 776 ± 265 ** |
| 40 | 70.30 ± 8.91 *** | 117 ± 43 *** |
Values are mean ± SEM. n = 7–10 per group.
p < 0.01,
p < 0.001 versus control (0 mg/kg) (ANOVA followed by Dunnett’s posthoc test)
3.2.2. Cannabinoids
All cannabinoids were tested in the FST at doses that did not cause hypothermia or catalepsy as determined by the tetrad assay. Such choice was made to guard against any behavioral impairment that might impede the animal’s ability to attempt escape thus masking any potential antidepressant effect.
3.2.2.1. Δ9-THC, Δ8-THC, and CBD
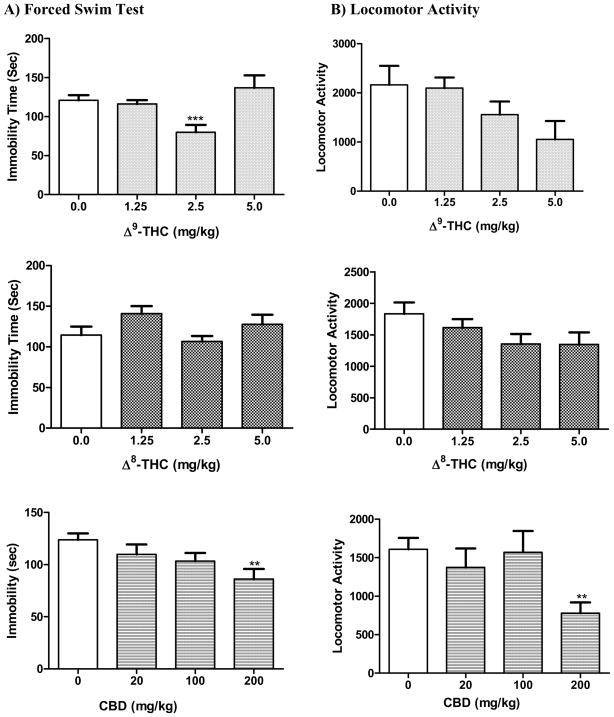
Δ9-THC showed a U-shaped dose response when tested in the FST (Fig. 2). One Way ANOVA showed a significant overall reduction in immobility time (F[3,35] = 8.32; p = 0.0003). Dunnett’s post hoc comparison of individual doses to the vehicle control showed that Δ9-THC was significantly different from control only at 2.5 mg/kg (q = 4.48, p < 0.001). Δ8-THC had no significant effect on immobility time (F[3,44] = 2.14; p = 0.11). Similar to Δ9-THC, a U-shaped effect on the immobility time was observed, with the 2.5 mg/kg dose causing a non-significant 7% reduction in immobility. Evaluation of CBD in the FST revealed a significant decrease in immobility time (F[3,42] = 3.89; p = 0.015) indicative of potential antidepressant-like action. The observed effect was significant only at 200 mg/kg (q = 3.30, p < 0.01).
Fig. 2.
Effects of the cannabinoids Δ9-THC, Δ8-THC, and CBD on A) immobility time in the mouse forced swim test and B) mouse locomotor activity. Data represented are the mean ± SEM. n = 7–10 mice per dose. ** P < 0.01, *** P < 0.001 compared with vehicle control (0.0 mg/kg group) (ANOVA followed by Dunnett’s test).
3.2.2.2. CBG, CBC, and CBN
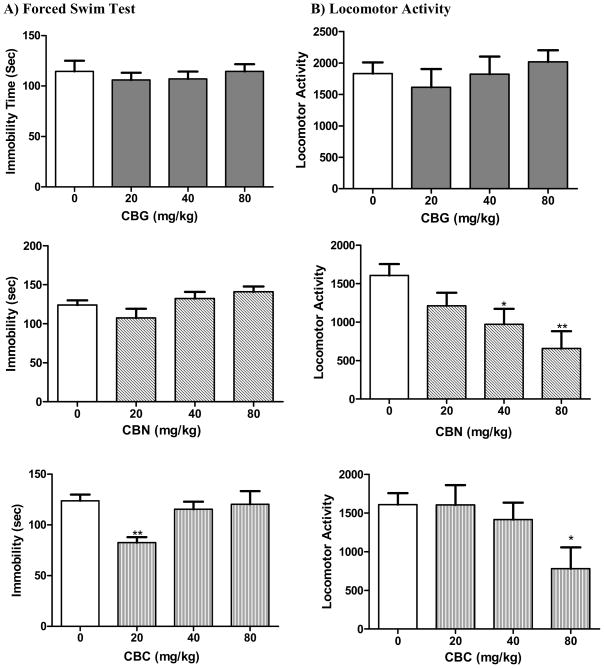
The non psychotropic cannabinoid CBG did not show any antidepressant-like action as is evident in the FST(F[3,30] = 0.31; p = 0.82) (Fig. 3). The compound did not exert any significant change in the locomotor activity of the animals either (F[3,27] = 0.55; p = 0.65. Similarly, CBN did not alter the FST immobility time as compared to the control (F[3,43] = 2.45; p = 0.076) indicating lack of antidepressant-like action at the tested doses (Figure 3). However, the locomotor activity of the animals was significantly reduced (F[3,43] = 5.17; p = 0.004) at 40 (q = 2.53, p < 0.05) and 80 mg/kg (q = 3.63, p < 0.01). CBC, however, showed a significant overall reduction in immobility time (F[3,40] = 4.85; p = 0.006). Dunnett’s post hoc analysis showed that only the 20 mg/kg dose was statistically significant from the vehicle control (q = 3.72, p < 0.01), however, such antidepressant-like effect was not maintained at 40 or 80 mg/kg. CBC caused a significant reduction in locomotor activity (F[3,42] = 2.88; p = 0.047) at 80 mg/kg (q = 2.81, p < 0.05).
Fig. 3.
Effects of the cannabinoids CBG, CBN, and CBC on A) immobility time in the mouse forced swim test and B) mouse locomotor activity. Data represented are the mean ± SEM. n = 7–10 mice per dose. * P < 0.05 and ** P < 0.01 compared with vehicle control (0.0 mg/kg group) (ANOVA followed by Dunnett’s test).
3.3. Mouse TST
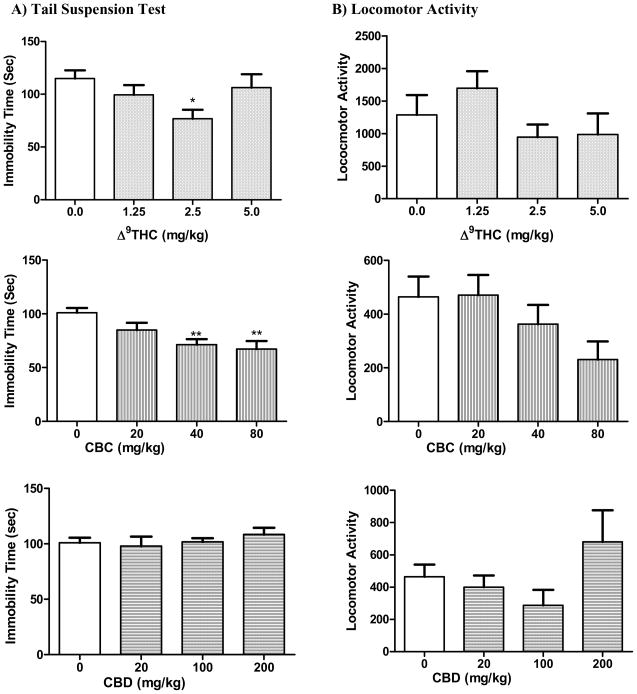
Adult male DBA/2 mice were selected for the TST based on the research of Liu and Gershenfeld (2001) and Crowley et al. (2005). The studies demonstrated robust strain differences in the response to various antidepressant drugs in the TST and confirmed the high responsiveness of DBA/2 mice in this test. As demonstrated in Figure 4, treatment with Δ9-THC resulted in significant decrease in immobility time (F[3,32] = 3.29; p = 0.033). Dunnett’s post hoc analysis revealed that the 33% decrease in immobility caused by the 2.5 mg/kg dose is statistically different from the control vehicle group (q = 2.90, p < 0.05). The non psychotropic CBC elicited a significant dose-dependent reduction in immobility indicative of antidepressant-like action (F[3,33] = 6.24; p = 0.002). Such action was significant at the 40 (q = 3.54, p < 0.01) and 80 (q=3.82, p < 0.01) mg/kg doses of CBC. CBD did not cause a significant change in immobility time at any of the doses (F[3,33] = 0.59; p = 0.623). None of the compounds altered the locomotor activity of the animals, suggesting that the observed antidepressant action is not associated with a significant change in general locomotion.
Fig. 4.
Effects of the cannabinoids Δ9-THC, CBC, and CBD on A) immobility time in the mouse tail suspension test and B) mouse locomotor activity. Data represented are the mean ± SEM. n = 7–10 mice per dose. * P < 0.05 and ** P < 0.01 compared with vehicle control (0.0 mg/kg group) (ANOVA followed by Dunnett’s test).
4. Discussion
The main finding of the current study is that phytocannabinoids display antidepressant-like actions in established models of behavioral despair, namely the FST and TST as demonstrated by the significant reductions in immobility time. The FST is among the most established animal models for assessing the potential clinical antidepressant activity of drugs (Cryan et al., 2003; Cryan et al., 2005a, 2005b). It was originally described using a rat model and was later implemented for use with mice (Porsolt et al., 1977a, 1977b). The TST was subsequently developed as an additional measure of antidepressant-like activity in mice (Steru et al., 1985). A plethora of research reports have shown that both the FST and TST procedures are highly predictive of antidepressant actions, whereby various classes of therapeutically employed antidepressants have shown robust antidepressant action in both tests (Bourin et al., 2005; Crowley et al., 2005; Cryan et al., 2002, 2005a, 2005b; Porsolt et al., 1977a). The current study employed these tests to determine the potential antidepressant-like effect of phytocannabinoids. The effect on locomotor activity was also evaluated to demonstrate that reductions in immobility time were not a secondary consequence of non-specific stimulant actions of the test compounds. Results collected show that the tested cannabinoids either did not significantly alter locomotor activity or caused a significant reduction. No stimulant action was recorded, suggesting that it is very unlikely that the observed antidepressant effects are false positives. The observed antidepressant-like action was not restricted to Δ9-THC, the major psychoactive component in cannabis. In fact, both CBD and CBC displayed significant antidepressant-like effect in the used animal models. These results confirm previous reports that phytocannabinoid analogs of Δ9-THC can modulate the endocannabinoid system, thus providing additional potential therapeutic drug leads (Grotenhermen, 2003).
This study shows that Δ9-THC exerts a significant antidepressant-like action at 2.5 mg/kg dose in both the FST and TST. At such dose, Δ9-THC does not cause any impairment of locomotor activity, catalepsy, or change in body temperature as determined by the tetrad assay. The observed action shows a U-shaped dose response, with the antidepressant-like effect lacking at both the lower and higher doses of Δ9-THC, similar to reported dose-dependent biphasic behavior of endocannabinoids, particularly anandamide (Sulcova et al., 1998) as well reported anxiolytic effect of Δ9-THC and other cannabinoids (Onaivi et al., 1990; Valjent et al., 2002). The complex picture of cannabinoid-induced response in this study and its function of the dose administered highly mimics the various emotional responses in humans following cannabis use, with users reporting both mood elevation as well as depressive symptoms (Leweke and Koethe, 2008). A possible explanation for the observed U-shaped dose response is the activation of various pathways at different doses. Mechanistic studies are thus needed to delineate the underlying mechanisms. As seen in this study, it is well established that Δ9-THC exerts a typical cannabimimetic action in the tetrad assay inducing a dose-dependent antinociception, catalepsy, hypothermia, and reduced locomotor activity (Burkey et al., 1997; Varvel et al., 2005). These behavioral effects are mediated via binding to cannabinoid receptors, with the CB1 receptors as the primary mediator of behavioral actions. Pharmacological studies have shown that Δ9-THC acts as a partial agonist at the CB1 receptors (Sim et al., 1996). Whether the observed antidepressant-like action is due to binding to CB1 receptors is still under investigation; however, several lines of evidence suggest that enhancement of CB1 activity results in antidepressant-like effect. Hill and Gorzalka (2005) reported that direct stimulation of CB1 receptors by administration of the CB1 agonists HU210 and oleamide results in antidepressant-like action in the rat FST. The CB1 agonist arachidonyl-2-chloroethylamide has similarly demonstrated antidepressant-like properties in the mouse FST (Rutkowska and Jachimezuk, 2004). Additionally, indirect stimulation of the CB1 receptors via administration of the uptake inhibitor AM404 also elicited antidepressant-like effect (Hill and Gorzalka, 2005). Likewise, Gobbi et al. (2005) have demonstrated antidepressant-like actions exerted by chronic administration of the fatty acid amide hydrolase inhibitor URB597 in a rat chronic mild stress model. However, such data are in contrast with the study reported by Naidu et al. (2007) whereby URB597 administration failed to demonstrate antidepressant-like action in either the FST or TST.
The current study shows that Δ8-THC does not exhibit significant antidepressant-like effect at any of the tested doses, although it behaves similar to Δ9-THC in the tetrad assay. The cannabimimetic effects displayed by Δ8-THC in the tetrad test confirm its decreased potency as compared to Δ9-THC. This is in accordance with previous in vitro assays that showed a threefold lower CB1 binding affinity of Δ8-THC compared to Δ9-THC (Compton et al., 1993). Although Δ8-THC failed to stimulate [35S]-GTPγS binding in rat cerebellar membranes (Griffin et al., 1999), in vivo data in this study support a possible agonist effect on the CB1 receptors as evident from the tetrad assay.
One interesting result of this is that the non-psychoactive cannabinoid CBD exhibited a dose dependent antidepressant-like effect in the FST animal model. Unlike Δ9-THC, CBD has low affinity for both CB1 and CB2 receptors (Pertwee, 1999). However, in vitro studies have reported that CBD acts as a potent antagonist of CB1 and CB2 receptors agonists (Pertwee, 2005; Thomas et al., 2007). It also displayed high inverse agonist efficacy of [35S]GTPγS binding at micromolar concentrations (Thomas et al., 2007). Similar to previous findings, CBD alone did not exert any cannabimimetic action in the tetrad assay. However, it blocked the Δ9-THC-induced catalepsy at high doses (100–200 mg/kg, i.p.). No interactive effect was observed between CBD and Δ9-THC in the antinociceptive assay in contrast to published data (Varvel et al., 2006). Such discrepancy might be attributed to the differences in dosage range and route of administration used in the studies. To our knowledge, this is the first report of activity of CBD in the mouse FST. In this behavioral despair model, CBD caused a significant dose dependent reduction in immobility. However, such effect was not extended to the TST. The discrepancy between the two tests might be attributed to the inherent differences between both tests, the use of different mice strains in each, or the mechanism of antidepressant action exerted by CBD. Several hemodynamic, behavioral, physiological, and pharmacological studies suggest that the TST is considerably less stressful than the behavioral despair paradigm in the FST. The added hypothermia induced in the FST when the animal is immersed in water is lacking in the TST and augments the stress level of the model (Thierry et al., 1986). Such differences extend to biochemical and neurochemical mechanisms involved in the two models (Renard et al., 2003). Previous studies have reported compounds that showed antidepressant action in the FST but not the TST. Atypical antidepressants such as rolipram and levoprotiline have been reported to reduce the immobility time in the FST but not in TST (Porsolt and Lenegre, 1992). In addition, it has been shown that antagonists or gene knockouts of the GABAB receptor results in an antidepressant-like effect in the FST with no effect seen in the TST (Mombereau et al., 2004). Hence further mechanistic studies are needed to fully understand the antidepressant potential of CBD. A confounding factor is the multiple mechanisms involved in actions of CBD. In addition to its low affinity to CB1 and CB2 receptors, it blocks the enzymatic hydrolysis and uptake of the endocannabinoid anandamide (Bisogno et al., 2001). Moreover, CBD interacts with systems other the endocannabinoid one. It stimulates vanilloid VR1 receptors (Bisogno et al., 2001), acts as an agonist on the human serotonin 5-HT1A receptors (Russo et al., 2005), and enhances adenosine signaling via uptake inhibition (Carrier et al., 2006). The conflicting data presented in this study that both a CB1 agonist (Δ9-THC) and an antagonist (CBD) result in antidepressant-like action is not uncommon. In fact previous research groups have reported similar findings. As mentioned earlier, several reports advocate the hypothesis that enhancement of CB1 receptor activity results in antidepressant effect. On the other hand, numerous studies reported antidepressant action following blockade of CB1 receptors. Shearman et al. (2003) described a dose-dependent reduction in immobility in the TST elicited by the CB1 inverse agonist AM251. The effect was not observed in CB1 receptor knockout mice suggesting that such action is mediated by CB1 receptors. Similarly, the CB1 antagonist SR141716A was reported to increase monoamine release in mouse prefrontal cortex and exert antidepressant-like action in the FST in both mice and rats (Griebel et al., 2005; Tzavara et al., 2003). An explanation of these conflicting results can only be resolved by detailed systematic investigation of the mechanism of the observed antidepressant action in each case. It is highly possible that the actions of cannabinoid receptor ligands regarding mood are mediated by cannabinoid receptor subtypes that have not yet been characterized.
While both CB1 agonists and antagonists seem to elicit antidepressant-like actions in behavioral despair models, CBC showed significant antidepressant-like effect in both the FST and TST. Interestingly, this compound does not have binding affinity to the CB1 receptor (Booker et al., 2009). Such data add to the complexity of the mechanism by which phytocannabinoids exert antidepressant-like action. It is evident that multiple mechanisms play a role in such action, and that a thorough investigation of these potential mechanisms is warranted.
In conclusion, our results show that phytocannabinoids, including Δ9-THC, CBD, and CBC, exert antidepressant-like actions in animal models of behavioral despair. The exact mechanism underlying such activity is still unclear and confounded by the fact that these compounds have varying binding profiles to the established cannabinoid CB1 as well as to non CB1 receptors. The results support the effect of phytocannabinoids on mood disorders and provide potential leads for further studies.
Acknowledgments
The project described was supported in part by Grant No. 5P20RR021929 from the National Center for Research Resources. The project has been funded in part with Federal funds from the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, under Contract No. N01DA-05-7746. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SA, Ross SA, Slade D, Radwan M, Zulfiqar F, ElSohly MA. Cannabinoid ester constituents from high potency Cannabis sativa L. J Nat Prod. 2008a;71:536–42. doi: 10.1021/np070454a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SA, Ross SA, Slade D, Radwan MM, Khan IA, ElSohly MA. Absolute configuration of cannabichromene derivatives from high potency Cannabis sativa. Tetrahedron Lett. 2008b;49:6050–53. doi: 10.1016/j.tetlet.2008.07.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde D, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogs: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–52. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker L, Naidu PS, Razdan RS, Mahadevan A, Lichtman AH. Evaluation of prevalent phytocannabinoids in the acetic acid model of visceral nociception. Drug Alc Dependence. 2009;105:42–7. doi: 10.1016/j.drugalcdep.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovassa GB. Cannabis abuse as a risk factor for depressive symptoms. Am J Psychiatry. 2001;158:2033–37. doi: 10.1176/appi.ajp.158.12.2033. [DOI] [PubMed] [Google Scholar]
- Bourin M, Chenu F, Ripoll N, David DJP. A proposal of decision tree to screen putative antidepressants using forced swim and tail suspension tests. Behav Brain Res. 2005;164:266–9. doi: 10.1016/j.bbr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Burkey TH, Quock RM, Consroe P, Roeske WR, Yamamura HI. Delta9-tetrahydrocannabinol is a partial agonist of cannabinoid receptors in mouse brain. Eur J Pharmacol. 1997;323:R3–R4. doi: 10.1016/s0014-2999(97)00146-5. [DOI] [PubMed] [Google Scholar]
- Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci USA. 2006;103:7895–900. doi: 10.1073/pnas.0511232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. Cannabinoid structure activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–26. [PubMed] [Google Scholar]
- Crowley JJ, Jones MD, O’Leary OF, Lucki I. Automated tests for measuring the effects of antidepressants in mice. Pharmacol Biochem and Behav. 2004;78:269–74. doi: 10.1016/j.pbb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology. 2005;183:257–64. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–45. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry. 2003;54:49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Differential behavior effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. J Psychopharmacology. 2005a;182:335–44. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav R. 2005b;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Devane W, Hanus L, Breuer A, Pertwee R, Stevenson L, Griffin G, Gibson D, Manelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptors. Science. 1992;258:1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dinh T, Carpenter D, Leslie F, Freund T, Katona I, Sensi S, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. PNAS. 2002;99:10819–24. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly MA, Slade D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005;78:539–48. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockage of anandamide hydrolysis. PNAS. 2005;102:18620–5. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Onaivi E, Ishigura HLQ, Tagliaferro P, Brusco A, Uhi G. Cannabinoid CB2 receptors immunohistochemical localization in rat brain. Brain Res. 2006;1071(1):10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist Rimonabant in models of emotional reactivity in rodents. Biol Psychiatry. 2005;57:261–7. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Griffin G, Wray EJ, Rorrer WK, Crocker PJ, Ryan WJ, Saha B, Razdan RK, Martin BR, Abood ME. An investigation into the structural determinants of cannabinoid receptor ligand efficacy. Br J Pharmacol. 1999;126:1575–84. doi: 10.1038/sj.bjp.0702469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsponn L, Balkar J. The use of cannabis as a mood stabilizer in bipolar disorder: Anecdotal evidence and the need for clinical research. J Psychoactive Drugs. 1998;30:171–7. doi: 10.1080/02791072.1998.10399687. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Drug Disposition. 2003;42:327–60. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Gruber A, Pope H, Brown M. Do patients use marijuana as an antidepressant? Depression. 1996;4:77–80. doi: 10.1002/(SICI)1522-7162(1996)4:2<77::AID-DEPR7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Hill M, Miller G, Ho W, Gorzalka B, Hillard C. Serum endocannabinoid content is altered in females with depressive disorders: a prelominary report. Pharmacopsychiatry. 2008;41(2):48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol. 2005;15(6):593–9. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Jarvis K, DelBello MP, Mills N, Elman I, Strakowski SM, Adler CM. Neuroanatomic comparison of bipolar adolescents with and without cannabis use disorders. J Child Adolesc Psychopharmacol. 2008;18:557–63. doi: 10.1089/cap.2008.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji S, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus meurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–16. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns A. The psychiatric effects of cannabis. Br J Psychiatry. 2001;178:116–122. doi: 10.1192/bjp.178.2.116. [DOI] [PubMed] [Google Scholar]
- Koethe D, Llenos I, Dulay J, Hoyer C, Torrey E, Leweke F, Weis S. Expression of CB1 cannabinoid receptor in the anterior cinguate cortex in schizophrenia, bipolar disorder, and major depression. J Neural Transm. 2007;114(8):1055–63. doi: 10.1007/s00702-007-0660-5. [DOI] [PubMed] [Google Scholar]
- Lee KS, Clough AR, Jaragba MJ, Conigrave KM, Patton GC. Heavy cannabis use and depressive symptoms in three Aboriginal communities in Arnhem Land, Northern Territory. Med J Aust. 2008;188:605–8. doi: 10.5694/j.1326-5377.2008.tb01803.x. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Koethe D. Cannabis and psychiatric disorders: it is not only addiction. Addict Biol. 2008;13(2):274–75. doi: 10.1111/j.1369-1600.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biol Psychiatry. 2001;49:575–81. doi: 10.1016/s0006-3223(00)01028-3. [DOI] [PubMed] [Google Scholar]
- Matsuda L, Lolait S, Brownstein M, Young A, Bonner T. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetics and pharmacological evidence of a role for GABAB receptors in the modulation of anxiety and antidepressant-like behavior. Neuropsychopharmacology. 2004;29:1050–62. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Yarvel SA, Ahn K, Cravatt BF, Martin BR. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology. 2007;192:65–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Green MR, Martin BR. Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther. 1990;253:1002–9. [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–24. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147:S163–71. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Thomas A, Stevenson LA, Ross RA, Varvel SA, Lichtman AH, Martin BR, Razdan RK. The psychoactive plant cannabinoid Δ9-tetrahydrocannabinol is antagonized by Δ8- and Δ9-tetrahydrocannabivarin in mice in vivo. Br J Pharmacol. 2007;150:586–94. doi: 10.1038/sj.bjp.0707124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977a;229:327–36. [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977b;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Lenegre A. Behavioral models of depression. In: Eliott J, Heal D, Mardesen C, editors. Experimental approaches to anxiety and depression. London: Wiley; 1992. pp. 73–85. [Google Scholar]
- Radwan MM, ElSohly MA, Slade D, Ahmed SA, Ross SA. Biologically active cannabinoids from high potency Cannabis sativa. J Nat Prod. 2009;72:906–11. doi: 10.1021/np900067k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan MM, Ross SA, Slade D, Ahmed S, Zulfiqar F, ElSohly MA. Isolation and characterization of new cannabis constituents from high potency variety. Planta Medica. 2008a;74:267–72. doi: 10.1055/s-2008-1034311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan M, ElSohly MA, Slade D, Ahmed SA, Wilson L, El-Alfy AT, Khan IA, Ross SA. Non- cannabinoid constituents from a high potency Cannabis sativa variety. J Nat Prod. 2008b;69:2627–33. doi: 10.1016/j.phytochem.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard CE, Dailly E, David DJP, Hascoet M, Bourin M. Monoamine metabolism changes following the mouse forced swim test but not the tail suspension test. Fundam Clin Pharmacol. 2003;17:449–55. doi: 10.1046/j.1472-8206.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- Ross SA, ElSohly MA. Constituents of Cannabis sativa L. XXVII. A review of the natural constituents 1980–1994. Zagazig J Pharm Sci. 1995;4:1–10. [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1A receptors. Neurochem Res. 2005;30:1037–43. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- van Rossum I, Boomsma M, Tenback D, Reed C, van Os J. Does cannabis use affect treatment outcome in bipolar disorder? A longitudinal analysis. J Nerv Ment Dis. 2009;197(1):35–40. doi: 10.1097/NMD.0b013e31819292a6. [DOI] [PubMed] [Google Scholar]
- Rutkowska M, Jachimezuk O. Antidepressant-like properties of ACEA (arachidonyl-2-chloroethylamide), the selective agonist of CB1 receptors. Acta Pol Pharm. 2004;61:165–7. [PubMed] [Google Scholar]
- Shearman L, Rosko K, Fleicher R, Wang J, Xu S, Tong X, Rocha B. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav Pharmacol. 2003;14(8):573–82. doi: 10.1097/00008877-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S] GTPS autoradiography in rat brain 13. J Neurosci. 1996;16:8057–66. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, Legutko B, Li X, Bymaster F. Current perspectives on the development of non biogenic amine-based antidepressants. Pharmacol Res. 2001;43:411–2. doi: 10.1006/phrs.2000.0806. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P, Porsolt R. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashitaand A, Waku K. 2-Arachidonylglycerol, a possible endogenous receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Sulcova E, Mechoulam R, Fride E. Biphasic effects of anandamide. Pharmacol Biochem Behav. 1998;59:347–52. doi: 10.1016/s0091-3057(97)00422-x. [DOI] [PubMed] [Google Scholar]
- Thierry B, Steru L, Simon P, Porsolt RD. The tail suspension: ethical considerations. Psychopharmacology. 1986;90:284–5. doi: 10.1007/BF00181261. [DOI] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–23. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE, ElSohly MA, Boeren EG. Constituents of Cannabis sativa L. XVII. A review of natural constituents. J Nat Prod. 1980;43:169–70. doi: 10.1021/np50008a001. [DOI] [PubMed] [Google Scholar]
- Tzavara E, Perry K, Rodriguez D, Bymaster F, Nomikos G. The cannabinoid CB(1) receptor antagonist SR141716A increases norepinephrine outflow in the rat anterior hypothalamus. Eur J Pharmacol. 2001;426(3):R3–R4. doi: 10.1016/s0014-2999(01)01228-6. [DOI] [PubMed] [Google Scholar]
- Tzavara E, Davis R, Perry K, Li X, Salhoff C, Bymaster F, Witkin J, Nomikos G. The CB1 receptor antagonist SR14176 selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic action. Br J Pharmacol. 2003;138(4):544–53. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Rogard M, Besson MJ, Maldonado R, Caboche J. Delta-9 tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur J Neurosci. 2001;14(2):342–52. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH. Delta9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J Pharmacol Exp Ther. 2005;314:329–37. doi: 10.1124/jpet.104.080739. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, Martin BR. Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology. 2006;186:226–34. doi: 10.1007/s00213-006-0356-9. [DOI] [PubMed] [Google Scholar]
- Witkin J, Tzavara E, Davis R, Li X, Nomikos G. A therapeutic role for cannabinoid CB1 receptor antagonists in major depressive disorders. Trends Pharmacol Sci. 2005;26:609–17. doi: 10.1016/j.tips.2005.10.006. [DOI] [PubMed] [Google Scholar]