Synopsis
Arboviruses continue to be a major cause of encephalitis in North America and West Nile virus neuroinvasive disease is now the dominant cause of encephalitis. Transmission to humans of North American arboviruses occurs by infected mosquitoes or ticks. Most infections are asymptomatic or produce a flu-like illness. Elderly, immunosuppressed individuals and infants for some arboviruses have the highest incidence of severe encephalitis. Rapid serum or CSF IgM antibody capture ELISA assays are now available to diagnosis the acute infection for all North American arboviruses. Unfortunately, no antiviral drugs are approved for the treatment of arbovirus infection and current therapy is supportive.
Keywords: arbovirus, togavirus, flavivirus, bunyavirus, encephalitis, myelitis
“Arbovirus” is an informal term that refers to viruses that are transmitted by biting arthropods including mosquitoes, ticks, and flies. Arboviruses are more formally classified in different families and genera, based on their morphology (size, shape, capsid symmetry, presence or absence of an envelope), physical properties (genome structure and antigens) and biological properties (mode of replication and transmission, host range, pathogenicity). All arboviruses causing human CNS disease fall within four families—Togaviridae, Flaviviridae, Bunyaviridae, and Reoviridae (table 1). Over 500 different arboviruses are distributed around the world, mainly in the tropics. However, a much smaller number cause human disease, and only a subset of these involve the CNS and cause meningitis, encephalitis, or myelitis (“neuroinvasive disease”). In North America, there are fewer than ten arboviruses that cause significant neurological disease (table 2).
Table 1.
Families and genera that contain North American arboviruses that cause encephalitis
| Family | Genera |
|---|---|
| Togaviridae | Alphavirus |
| - Eastern Equine encephalitis | |
| - Western equine encephalitis | |
| - St. Louis encephalitis | |
| - Venezuelan equine encephalitis | |
| Flaviviridae | Flavivirus |
| - West Nile | |
| - Powassan | |
| Bunyaviridae | Bunyavirus |
| - La Crosse | |
| - California group viruses | |
| Reoviridae | Coltivirus |
| - Colorado tick fever | |
Table 2.
Common arboviruses of North America
| Virus | Distribution | Insect vector | Intermediate vertebrate host |
|---|---|---|---|
| West Nile | U.S., Canada, Mexico | Mosquito (Culex sp.) | Passiform birds (jays, blackbirds, crows, finches, sparrows) |
| St. Louis encephalitis | U.S., Canada, Mexico | Mosquito (Culex sp.) | Passerine birds (sparrows, house finches), |
| Eastern equine encephalitis | Atlantic and Gulf coast states, upper New York, Michigan, eastern Canada | Mosquito (Culex sp.) | Fresh water swamp birds |
| Western equine encephalitis | Western U.S., Canada | Mosquito (Culex sp.) | Passerine birds Jackrabbit |
| Venezuelan encephalitis | Mexico, Florida, Texas | Mosquito (Culex and Aedes sp.) | Rodents, aquatic birds |
| Powassan or La Crosse encephalitis | North Central and northeast U.S. | Mosquito (Aedes triseriatus) | Chipmunks, squirrels |
| Colorado tick fever | Rocky mountain states, Canada | Tick (Dermacentor andersoni) | Chipmunks, squirrels, small mammals |
| Dengue | Mexico and Florida | Mosquito (Aedes sp.) | Humans and non-human primates |
All arboviruses are small spherical RNA viruses ranging in size from 40–50 nm for Togaviridae and Flaviviridae to 80–120 nm for Bunyaviridae [1]. Alphaviruses and flaviviruses are enveloped spherical viruses whose genome consists of positive-sense (+), single stranded (ss) RNA molecule of 10–12 kb. Bunyaviridae are enveloped spherical viruses whose genome consists of 3 linear minus–sense (−) ssRNA segments of ~11 kb, and Reoviridae are non-enveloped icosahedral shaped viruses that can contain up to 12 double-stranded linear RNA segments totaling 19–32kb [2]. All arboviruses, except those belonging to the family Reoviridae, possess a lipid envelope derived from host cells in which several envelope or membrane proteins are inserted and play an essential role in viral attachment and cell entry. In the case of the Reoviridae, these proteins are part of the virion’s outer protein shell. Antibodies to these proteins form the basis for host immunity and serological diagnosis of infections [3].
In the majority of cases, human infection by arboviruses results in an asymptomatic infection associated with seroconversion, and such cases typically vastly outnumber cases of neuroinvasive disease by 100–1000:1. Symptomatic disease generally results in one of three syndromes: (1) a self-limited flu- or dengue-like illness, (2) hemorrhagic fever, or (3) CNS disease. This article focuses exclusively on arbovirus-induced CNS disease, with an emphasis on those agents important and endemic in North America.
It has been difficult to predict when and where arbovirus outbreaks or epidemics will appear, but several environmental factors increase the probability of human infections. With the exception of infections caused by Colorado Tick Fever Virus (CFTV) and Powassan, which are tick-borne, the overwhelming majority of arboviral infections in the U.S. are caused by mosquito vectors. Outside the U.S., sandflies (Phlebotomus sp.) are an important vector for arboviruses such as Toscana, a bunyavirus that is an important cause of summertime meningitis in Mediterranean Europe. For an epidemic to occur, local mosquito vectors must be reasonably abundant, capable of becoming infected, and capable of transmitting virus to mammalian hosts [4]. Wet warm spring seasons tend to enable mosquitoes to expand early, and increase the potential for infection of newly hatched birds, which are typically more susceptible to infection than their adult counterparts. A period of heavy precipitation followed by dry weather facilitates development of small stagnant water pools ideal for mosquito breeding and enhances the potential for arbovirus epidemics [4].
Most arboviruses are maintained in nature in bird or small mammal populations; therefore, local bird or rodent populations must be susceptible to viral infection. Although high levels of immunity generated by prior arbovirus exposure would be expected to reduce the availability of bird or mammal reservoirs, from a practical viewpoint this situation has not yet developed to the point that it significantly affects patterns of arbovirus infection in the U.S. Finally, the amplifying cycle of infection must be within the transmission zone of populations of susceptible humans who are exposed to the infected mosquitoes.
Although human seroprevalence rates against arboviruses increase in regions affected by epidemics, there are currently no areas in the U.S. where these rates approach levels that would have a significant impact on the spread or development of arbovirus epidemics. The ability of public health departments and organizations to rapidly identify encephalitis outbreaks and to track their progression in real-time has improved dramatically in the past several years. The Centers for Disease Control and Prevention (CDC) and state public health departments all participate in ArboNet and contribute data on arbovirus infections derived from trapping of mosquito pools, susceptible animals that can serve as sentinels for human infection (e.g. birds, horses) and human disease. This data for the United States is available through the U.S. Geological Survey website (http://diseasemaps.usgs.gov). This site provides real-time data at county level resolution for human, mosquito, bird, veterinary and sentinel infections due to West Nile (WNV), St. Louis encephalitis (SLEV), Lacrosse (LAC), Western equine encephalitis (WEE), Eastern equine encephalitis (EEE), and Powassan (POW) viruses.
Pathogenesis
All arboviruses have geographical and ecological limitations imposed by the host ranges of their vectors and natural reservoirs, as well as by the temporal features of vector life cycles and breeding patterns. Additionally, arboviruses exhibit variations in replicative capacity between different species of vectors. The enzootic life-cycle of arboviruses usually does not involve humans, which are considered dead-end hosts because the level of viremia in infected humans is seldom high enough to allow continued transmission of infection to mosquitoes or ticks. Exceptions to this general rule include dengue and Venezuelan equine encephalitis, both of which may generate high titer viremia in humans. Human to human transmission of other arbovirus infections can occur when blood or organs from an infected individual are transfused or transplanted into a naïve host [5,6]. Perinatal and transplacental human-to-human transmission of arbovirus infection can also occur [7,8].
West Nile virus infection of Culex pipiens and quinquefasciatus mosquitoes provides an example of the replication of arboviruses in arthropods. Following ingestion of infected blood by a susceptible female mosquito, viral replication first occurs in the midgut around day 2 and rapidly expands until almost all midgut epithelial cells, adjacent muscle, and fat cells are infected [9]. The virus then appears in salivary glands and accumulates in salivary gland ducts by day 14. Virus also appears in abdominal, thoracic and cephalic ganglia and persists in all infected organs. Virus inoculation into a host rapidly occurs after the proboscis breaks the skin and biting can inject up to 104 plaque-forming units of virus into dermis, subcutaneous tissue and directly into blood vessels [10].
Mechanisms of arbovirus survival through winter months (overwintering) in arthropod populations are incompletely understood [11]. Not all mosquitoes die with onset of cold weather, and some infected adult mosquitoes instead become dormant, becoming active again with warmer weather [12]. Infected female mosquitoes can also transmit virus to their eggs (transovarian transmission), which survive through winter and then hatch into infected pupa which mature into infected adults [13]. Like infected mosquitoes, infected ticks can also overwinter, and transmit virus via transovarian transmission with infected larvae developing into infected nymphs and infected adults (transtadial transmission) [14].
Although arthropods are required for efficient transmission of arboviruses, the major natural reservoir for these viruses is species of birds or small mammals. These animals share the common trait of being able to develop and sustain sufficient viremia to transmit infection back to arthropods when bitten. For most arboviruses, replication in the natural host occurs without causing illness or death. However, West Nile virus (WNV) infection of passeriform birds (crows, jays, etc) results in viremias as high as 1010 plaque forming units/mL, with associated multi-organ system pathology and high morbidity and mortality. A viremia of this magnitude leads to subsequent efficient transmission in more than 80% of biting mosquitoes [15]. The duration of viremia in birds is variable, but frequently lasts weeks and even months allowing for repeated cycles of mosquito infection [16]. Infected birds can also shed considerable amounts of virus from the cloaca and nasopharynx allowing for viral spread between birds independent of mosquitoes [17]. In passeriform birds, WNV infects the heart, kidney, spleen, gut, adrenal glands, liver, and brain, and these tissues are then infectious to raptors who may prey on these birds [18]. In birds, infection is associated with development of protective immune responses resulting in immunity to repeat challenges of virus. Consequently, new populations of naïve birds are required to maintain viral endemicity.
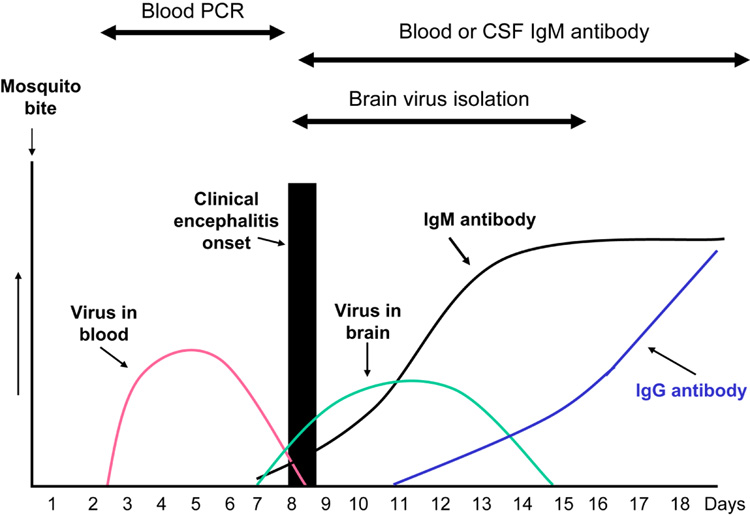
In humans, once WNV is inoculated into the dermis, viral replication begins in Langerhans dendritic cells, which then migrate to regional lymph nodes. The primary viremia disseminates virus to the entire reticuloendothelial system where subsequent replication augments the viremia. A low level viremia can be detected as early as 1–2 days post-inoculation, persists for about one week, and terminates in association with the appearance of serum IgM neutralizing antibodies (figure 1) [19]. When viremia is rapidly and efficiently cleared by the host’s immune system, the patient experiences either an asymptomatic infection or a flu-like syndrome without encephalitis. When immune clearance of the viremia is delayed, as may occur in the elderly or immunosuppressed, the risk of virus entering the central nervous system increases [6]. The precise method by which WNV enters the CNS is unknown, but other flaviviruses have been shown to enter the CNS following infection of cerebral microvascular endothelial cells or by infection of the olfactory bulb with subsequent transneuronal spread to the brain [19].
Figure 1.
Typical time course for development of West Nile virus in blood and brain and occurrence of IgG and IgM antibodies in humans after West Nile virus infection
Once an arbovirus reaches the CNS, it causes widespread, patchy, infection of neurons in the cerebral cortex, basal ganglia, brainstem, cerebellum, and occasionally spinal cord [20,21]. The specific topographic patterns of infection vary between viruses, although there is substantial overlap. The mechanism by which arboviruses kill infected neurons is incompletely understood, although several flaviviruses including WNV and Japanese encephalitis virus can induce apoptosis in infected neurons [22]. Arboviruses do not generally infect oligodendrocytes, so acute demyelination is not a typical feature of arbovirus encephalitis, although rare cases of post-infectious acute disseminated encephalomyelitis have been reported after dengue and St. Louis encephalitis and following immunization with the vaccine strain of Japanese B encephalitis virus [23,24].
The amount of brain inflammation varies by arbovirus but seldom is overwhelming. More severe parenchymal inflammatory responses generally parallel the degree of pleocytosis induced in the CSF. The leptomeninges appear normal grossly but histologically show a lymphocytic infiltration. The brain may appear grossly normal, edematous, or may be congested with focal petechiae in cortical gray matter, deep gray matter and white matter. Multiple focal areas of brain softening may be present, which histologically demonstrate perivascular cuffing, infiltration of mononuclear and polymorphonuclear cells, and neuronophagia of infected cells. The inflammatory cells are mainly CD4+ and CD8+ cells with occasional B cells. In fatal cases, a heavy concentration of viral lesions is often seen in the brainstem [20]. When the spinal cord is involved, viral antigen, neuronophagia, neuronal disappearance, perivascular cuffing and microglia proliferation are seen in the anterior horns of the lumbar and cervical areas [25].
Studies of experimental WNV infection in mice indicate humoral, cell mediated, and innate immune responses all contribute to control and clearance of virus from infected organs including brain. Mice deficient in their ability to develop IgG, IgM, CD4 or CD8 immune responses all show increased severity of WNV infection, as do mice deficient in IFN, IFN receptor, and complement. [26–31].
Syndromes caused by arboviruses
Asymptomatic infections
The overwhelming majority of individuals infected with arboviruses develop asymptomatic infection, although the exact ratio of neuroinvasive disease cases to asymptomatic infection varies for specific viruses. For most arboviruses, asymptomatic infections occur mainly in children and young adults. The resulting infection produces prolonged immunity, except in the case of dengue. It is important to remember that asymptomatically infected individuals can still transmit infection to others through blood transfusion or less commonly organ transplantation. Blood donors are typically asymptomatic at the time of donation, nonetheless in 2003, 877 units of WNV-infected blood were identified among the 2.5 million units screened by the American blood centers [32]. Currently, American blood banks screen pooled lots of donated blood units by polymerase chain reaction (PCR) assay for WNV nucleic acid. Positive pools are then subjected to individual unit by unit assay with positive units discarded. Screening has dramatically reduced the number of cases of transfusion-associated WNV disease; however, rare cases still occur likely reflecting the failure of the available nucleic acid amplification tests to detect low levels of virus that may still be adequate to initiate infection.
Flu-like syndrome
A flu-like syndrome without CNS involvement is the most common clinical presentation of arbovirus infection and typically develops after an incubation period of 3–8 days and as late as 28 days. Patients experience abrupt onset of fever, headache, and fatigue. A non-pruritic maculopapular rash is variably present but may be faint and easily missed. Patients often have malaise, anorexia, nausea, myalgia, cough, sore throat, diarrhea, abdominal pain, lymphadenopathy, and arthralgias. Patients often do not seek medical attention and clinically improve over several weeks, but many patients report residual headaches and malaise for several months following convalescence.
Encephalitis-meningitis syndrome (Neuroinvasive disease)
Patients presenting with headache, fever, and stiff neck without focal weakness or altered mental status are classified as having meningitis. Patients presenting with clinical or laboratory evidence of brain parenchymal involvement are classified as encephalitis.
Patients with encephalitis have signs and symptoms of both a flu-like syndrome and encephalitis. Encephalitic signs and symptoms can include trouble concentrating, memory problems, marked sleepiness, confusion, dizziness, delirium, nausea, vomiting, lethargy, stupor, tremors, myoclonic jerks, photophobia, dysarthria, dysphagia, nystagmus, imbalance and trouble walking, limb or facial pains and weakness, paresthesias, sensory loss, and seizures [21,33]. Extrapyramidal signs including Parkinsonism, increased muscle tone, and tremors occur with variable frequency in patients with alphavirus and flavivirus encephalitis. Alteration in mental status is a hallmark of all viral encephalitides; however, development of coma occurs most frequently in patients with Eastern equine encephalitis and less frequently with other arboviruses. Chorioretinitis can occur with West Nile virus infection [34].
The acute encephalitis phase lasts 1–3 weeks but convalescence is slow. Depending on the severity of the encephalitis, signs and symptoms may persist for months.
Acute flaccid paralysis (AFP)/myelitis syndrome
Many arboviruses also infect neurons in the spinal cord, particularly anterior horn neurons. In a few patients the severity of the anterior horn cell death results in an acute flaccid paralysis syndrome similar to poliomyelitis. WNV infections have been the best studied and it has been estimated that AFP occurs in 5–10% of patients with neuroinvasive disease [35]. Most patients with AFP also have clinical evidence of encephalitis but those signs may be mild or overshadowed by the paralysis. This syndrome has been recognized in several other arboviruses (Dengue, St Louis encephalitis, Powassan, Eastern equine encephalitis) but occurs less frequently than with WNV.
Muscle weakness can develop concurrently with encephalitis or up to a week following clinical evidence of neuroinvasion. The weakness is often abrupt, asymmetrical, and involves one or more limbs, especially leg muscles [35]. Areflexia is common and loss of bladder or bowel control occasionally occurs. Objective sensory loss in the limb is rare but limb paresthesias are common. Some affected patients, including those with brainstem or cervical cord involvement, develop respiratory muscle weakness severe enough to require intubation and mechanical ventilation.
Routine blood tests in patients with arbovirus infection usually show evidence of a mild leukocytosis, although leukopenia and thrombocytopenia can occur, especially following infection with dengue. Hyponatremia (<135 mM/L) is common and due to a syndrome of inappropriate antidiuretic hormone (SIADH) secretion, and liver transaminases (aspartate aminotransferase and alanine aminotransferase) are elevated in about 1/4th of patients.
The CSF may exhibit a normal or elevated opening pressure. There is typically a pleocytosis of 10–300 WBC/mm3, but the CSF occasionally may lack WBCs early in the first one to two days of the encephalitis. Lymphocytes usually predominate but in 1/3rd of the cases, neutrophils predominate early in the illness. Neutrophil predominance and persistence is more common with WNV infection [36] than in other arboviruses. CSF glucose is almost always normal. CSF protein is normal or moderately elevated. Oligoclonal bands are rare in CSF obtained at onset of illness but may be seen in convalescent specimens.
MRI may be normal or demonstrate foci of increased signal intensity on T2-weighted images in the basal ganglia, thalamus, and brainstem [21]. The frequency of acute MRI abnormalities in WNV is <50%, with the yield increasing if FLAIR and diffusion weighted imaging techniques are utilized in addition to standard T1 weighted enhanced and unenhanced and T2 weighted sequences. The frequency of abnormalities also increases when late (2–4 weeks post onset) studies are performed in addition to acute ones.
Electroencephalography (EEG) is abnormal demonstrating diffuse or focal slowing and occasionally areas of sharp waves. Anteriorly predominant slowing has been suggested to be a potential clue to the diagnosis of WNV encephalitis [37]. In distinction to herpes simplex encephalitis, seizures and periodic EEG patterns such as periodic lateralizing epileptiform discharges occur less frequently in arbovirus infections. Electromyography in patients with acute flaccid paralysis (AFP) is normal acutely but after several weeks demonstrates evidence of muscle denervation (fibrillation potentials, positive sharp waves, decreased recruitment of motor unit action potentials). Compound muscle action potentials are reduced in amplitude and sensorineural action potentials are preserved consistent with involvement of anterior horns or roots. Demyelination is not a typical feature of arbovirus infections and as a result conduction velocities are not slowed and conduction block does not occur [38]. Although cases of WNV associated AFP were originally attributed to a Guillain-Barre like syndrome (GBS), it is now generally believed that >80% of WNV-associated AFP cases in fact represent as an anterior poliomyelitis. Well documented WNV-associated GBS cases are rare.
Diagnosis of arbovirus encephalitis
Diagnosis of almost all arbovirus infections is most commonly based on serum and/or CSF serology. In acute infections, virus-specific IgM antibodies can be detected by IgM capture ELISA [39]. For many arboviruses, over 50% of patients with neuroinvasive disease will have IgM antibody present at the time of hospitalization and over 90% will have the antibody by one week of symptoms (figure 1). Closely related arboviruses share epitopes, and antibody responses developed following infection with one virus may cross-react with antigens from other related viruses (heterologous antibody response) [39]. Diagnosis of the specific virus involved may require performance of ELISAs against several candidate viruses. The offending virus typically is the one with the highest titer, although variations in the methods for coating ELISA plates, and other technical aspects of assay performance makes this conclusion a relative rather than absolute one. More traditional assays for arboviral antibodies (hemagglutination inhibition, complement fixation, indirect immunofluorescence and neutralization assays) are only performed by the CDC or qualified reference laboratories as they require use of live virus under appropriate biosafety containment conditions. Diagnosis of a specific arbovirus infection using these assays is generally established by detecting IgM antibodies or a four-fold rise in IgG antibody titer between acute and convalescent serum. Unlike herpesviruses, which are ubiquitous agents with generally high basal seroprevalence levels in the general population, seroprevalence levels for individual arboviruses in the U.S. are generally low. However, following infection arbovirus antibodies persist for life, so the mere presence of IgG antibody in the absence of a rising titer or IgM antibody may simply be indicative of past exposure rather than acute infection. Although IgM antibodies generally reflect acute infection, cases of IgM antibodies against WNV persisting in serum or CSF for greater than a year have been documented [40]. In some cases serial determination of both IgG and IgM antibody titers may be needed to differentiate between acute and prior infection. It is important to recognize that individuals who have been immunized against Japanese encephalitis virus or Yellow fever virus may develop heterologous cross-reacting antibodies to WNV and St. Louis encephalitis virus that may confound diagnosis.
As a general rule the presence of IgM antibodies in CSF is indicative of intrathecal synthesis and the associated presence of the inciting antigen in the CNS, and as such are diagnostic not only of infection but of neurological disease. An elevated CSF/serum antibody ratio, when corrected for breakdown of the blood brain barrier through use of concomitant CSF/serum albumin ratios, can also be indicative of intrathecal antibody synthesis and CNS infection.
Isolation of arboviruses in culture is difficult and not routinely performed. Human viremia is usually of low titer and is terminated by IgM antibody that appears around the onset of the clinical symptoms as shown in figure 1. Thus, for most patients, virus is rarely isolated from blood or CSF and not usually detected by PCR assay from blood, with the exception of infections due to Venezuelan equine encephalitis virus and dengue virus. However, in individuals who are donating blood with asymptomatic West Nile viral infections, PCR assays successfully detect the viremia in most individuals [32].
The sensitivity of CSF PCR for diagnosis of arboviral infections is significantly below that reported for both enteroviruses and herpesviruses, in which this technique is the diagnostic procedure of choice. Comparative data is available predominantly for WNV infection, for which CSF PCR sensitivity is generally around 70% when compared to demonstration of CSF IgM as a diagnostic standard. CSF PCR and serological tests should be considered complementary rather than competing diagnostic methods. CSF PCR may be particularly useful in immunocompromised patients who may have attenuated or delayed antibody responses and in the early stages after onset of illness, when antibody responses may not have fully developed. The specificity of CSF PCR approaches 100%, and the amplification of arboviral nucleic acid from CSF in an appropriate clinical setting by an established laboratory is generally diagnostic of infection. Isolation of virus from brain tissue or the demonstration of arbovirus nucleic acid by PCR or antigen by immunohistochemistry is also diagnostic [20].
Management and prognosis
Currently, there is no anti-viral treatment of proven efficacy available for any arbovirus infection. The few drugs that have shown efficacy in experimental animals and infected cell culture, particularly when administered early after viral infection include interferon [41], ribavirin [42], monoclonal antibodies [43,44], immune globulin [45], and antisense oligomers that bind to arboviral RNA and inhibit translation of viral proteins and viral RNA replication [46,47].
In the absence of specific antiviral therapy the mainstay of treatment for arboviral encephalitides remains optimal supportive care. Patients with encephalitis should be hospitalized as disease can progress for several days to a week or more following presentation. To avoid contributing to cerebral edema and increased intracranial pressure, patients require careful monitoring of fluid balance, and should not receive hypotonic solutions. Accurate ICP monitoring in severely ill patients may require placement of an intracranial pressure bolt. Seizures can complicate many types of encephalitis, although their frequency in arbovirus infections is generally low enough that anticonvulsants are not given prophylactically, but only therapeutically. Patients should receive appropriate prophylaxis for prevention of pressure sores and deep venous thrombosis. Alimentary support should be provided for patients who are unable to sustain adequate oral caloric and nutritional intake. Intubation may be required for airway protection or for insuring adequate oxygenation in patients with depressed consciousness or respiratory muscle weakness. Because of the limited risk of human-to-human transmission of infection, patient isolation is not required for individuals with arbovirus encephalitis although universal precautions should be observed as both blood and CSF are potentially infectious. Stool, urine, and respiratory secretions are generally not infectious. Many patients with encephalitis require treatment in rehabilitation facilities following acute hospitalization.
Prevention
There are currently no human vaccines available for prevention of infection by arboviruses prevalent in the U.S. However, effective vaccines for Japanese encephalitis virus and yellow fever virus exist. It is unclear whether vaccination for these 2 flaviviruses offers cross protection against the North American flaviviruses [48,49]. Thus, prevention of arbovirus infections in the United States focuses on preventing the infection. Health departments in many states and Canada employ methods to identify mosquitoes and birds that are infected with an arbovirus. The earliest warning system comes from mosquito traps with subsequent PCR identification of arboviruses. Later warning systems include sentinel bird flocks and monitoring and assaying dead birds for arboviruses. Finally, veterinarians participate in late warning systems by identifying arbovirus horse infections such as West Nile, Eastern equine, and Western equine viruses. Other public health measures include the elimination of standing water pools that harbor mosquito larvae or administration of larvicides or adulticides. Even urban areas may support infected mosquitoes. In Phoenix, Arizona, abandoned swimming pools with stagnant water were found to serve as a breeding ground for mosquitoes [50]. Finally, aerial insecticide spraying of rural and urban communities can dramatically reduce the number of infected adult mosquitoes. Costs to conduct surveillance can be high. Various insecticide applications can cost as much as $1.4 million [51].
Personal protection for individuals comes from fixing house window screens to prevent mosquitoes from entering, draining neighborhood collections of standing water, clearing brush for tick removal, wearing long sleeved shirts and long pants outdoors, minimizing being outdoors from dusk to dawn, use of mosquito nets during sleep outdoors, spraying permethrin on clothing when hiking in tick or mosquito infested areas, and applying insect repellants such as DEET and Picaridin on exposed skin or wearing permethrin treated clothing [51,52].
West Nile virus (WNV) (Family: Flaviviridae)
Epidemiology
WNV has been recognized in Africa, the Middle East and Asia since 1937. The virus abruptly appeared in New York City in 1999 and has rapidly spread across the U.S [11]. Over 24,000 cases have been reported by the CDC since 1999 [51]. The rapid spread across the U.S. and Canada has been attributed to the capacity of WNV to infect multiple strains of mosquitoes and many species of birds.
Virus appears in blood of infected patients within 1 to 2 days following a mosquito bite and persists for about one week [19]. Over 89% of patients develop an asymptomatic infection. Over 1820 asymptomatic viremic blood donors have been reported to the CDC since 2002 with virus titers ranging from 25 to 200 RNA viral copies/mL [51]. Fifty-eight percent of cases reported to the CDC are classified as West Nile fever and 42% as West Nile neuroinvasive disease [51]. The incidence of WNV acute flaccid paralysis is about 5–10% of the neuroinvasive disease cases.
Clinical features
Patients with West Nile fever experience an abrupt onset of fever, headache, and fatigue. A maculopapular generalized rash occurs with variably frequency. Patients may also develop anorexia, nausea, myalgia, and lymphadenopathy. West Nile neuroinvasive disease (WNND) includes patients with meningitis, encephalitis, and acute flaccid paralysis [11,53–56]. These patients often have a flu-like prodrome that is quickly followed by development of neurological signs and symptoms. It has been suggested that approximately 35–40% of patients with WNV neuroinvasive disease have meningitis, 55–60% have encephalitis, and 5–10% acute flaccid paralysis [56] although these numbers are quite variable in different case series. Patients with encephalitis typically have fever (85–100%), headache (47–90%), and an altered mental status (46–74%). Following infection, many patients can develop movement disorders which include tremors of various types, myoclonus, and Parkinsonism. Cerebellar signs and symptoms occur in a variable number. Weakness is also common and may be generalized and non-specific or a lower motor neuron pattern of flaccid paralysis associated with absent or reduced reflexes and preserved sensation. Cranial nerve palsies have been reported in 10% or more of cases and most commonly involve the ophthalmic (2nd) and facial (7th) nerves. Visual problems in patients with WNND have become increasingly recognized. Patients often complain of blurry vision, trouble seeing, and photophobia. Clinically, reduced vision, active non-granulomatous uveitis, vitreitis, multifocal chorioretinitis and optic neuritis have been described [34,57 ]. Immunosuppressed patients, such as patients with transplants or AIDS, often develop a prolonged serious encephalitis with a higher mortality [54,58].
Laboratory features
Patients have a normal CBC or mild leukocytosis. Hyponatremia is seen in 1/3rd of patients and abnormal liver functions tests occur in 1/4th [59]. About 40% of patients have an initial CSF that shows a pleocytosis with a neutrophil predominance that then becomes predominately lymphocytes [36]. Cranial CT scans are usually normal but MRI scans may show subtle abnormalities on diffusion-weighted images [60]. A few patients show T2-weighted hyperintense abnormalities in the basal ganglia, thalami, brainstem and cerebellum, often appearing at about one week after onset of illness [61]. MRI imaging of spinal cord in patients with acute flaccid paralysis may be normal or show abnormalities involving the anterior horn area.
In fatal cases, WNV antigen was detected by immunohistochemistry 60% of the time in the brainstem and 13% in the cortex [20]. When acute flaccid paralysis was present, viral antigen was found in 40% of spinal cords. At postmortem viral antigen may also be detected with variable frequency in other organs including kidney, lung, pancreas, and gastrointestinal tract [62].
Diagnosis
The diagnosis of WNV infection is usually made by detection of WNV IgM antibody in serum or CSF [39]. WNV enzyme-linked immunosorbent assays detect cross-reacting antibodies from other flaviviruses infections including St. Louis encephalitis, Japanese encephalitis, dengue, yellow fever, and Powassan viruses requiring titer comparisons with appropriate other arboviruses to confirm the diagnosis [39]. Prior vaccination with yellow fever or Japanese encephalitis viruses may produce cross-reacting antibodies. WNV IgM antibody first appears in serum 6–9 days after infection, which is typically within a day or two of the onset of neuroinvasive illness [39]. Initial false negative IgM antibody tests can occur if serum is taken very early in the illness [39]. Thus, an initial negative serum WNV IgM antibody test in a patient with suspected WNV disease should be repeated three to seven days later. Most immunosuppressed transplant patients with WNND do develop IgM antibody in serum and CSF but may exhibit a prolonged time to seroconversion [58]. IgM antibody persists in blood or CSF for more than 2 months in 50% of patients and for a year or more in 10% [40].
Virus can be isolated from brain of fatal patients and rarely from CSF, but this is rarely attempted except at the CDC or in reference laboratories due to the requirement for appropriate biosafety containment procedures. PCR amplification of WNV nucleic acid from CSF is positive in 57–70% of patients but is less sensitive than detection of WNV specific CSF IgM antibody [63]. Less than 15% of patients with neuroinvasive disease have blood positive for virus by PCR assay [63].
Therapy and prognosis
There is no treatment of proven efficacy for WNV infection. Uncontrolled studies of individual or small groups of cases have reported both possible beneficial effects and no utility for treatment with IFN-alpha. A randomized controlled multicenter trial of an Israeli IVIG preparation (Omr-IgG-am) with high titer anti-WNV antibodies was recently completed, but the results are not yet available. The study was initiated on the basis of reports of possible efficacy in isolated cases. Several effective and safe equine vaccines utilized either formalin inactivated or chimeric recombinant viruses are licensed for veterinary use. Phase I/II trials of a human chimeric vaccine ChimeriVax-West Nile, Acambis) containing WNV envelope and PrM proteins on a yellow fever 17D vaccine strain virus background are currently in process. In initial studies the vaccine was found to be safe and immunogenic in humans [64].
Mortality from WNV is confined almost exclusively to patients with encephalitis and/or AFP and approaches 20% in severely ill patients. The nature, frequency and severity of neurological sequelae are poorly understood and comprehensive studies particularly of cognitive impairment are still very limited [56]. About 40% of patients with movement disorders including tremors, myoclonus and Parkinsonism have persisting symptoms at 6 months post illness, and approximately 20% have residual symptoms at 18 months post illness [56]. Recovery from AFP is variable. It has been estimated that 1/3 of patients return to baseline, 1/3rd have no significant recovery of strength and that 1/3 have partial recovery, with the brunt of improvement occurring within 4 months of onset of illness [56]. Recent studies suggest that many patients with encephalitis may suffer from residual cognitive problems. In one survey 50% of encephalitis patients reported such symptoms at 3 months post illness. Deficits can also involve motor speed and dexterity [56], as might be expected in patients with striatal-thalamic pathology.
Saint Louis encephalitis virus (SLEV) (Family: Flaviviridae)
Epidemiology
Until West Nile virus arrived, SLEV was the most important arbovirus in North America. Cases of encephalitis due to SLEV were first recognized in the 1930’s. SLEV is now widespread in North America ranging from Canada through the United States (except the New England coast) to Central and South America. Strains of virus circulating in the U.S. differ from those in Central and South America. In the eastern U. S., strains are transmitted by Culex pipiens/Culex quinquefasciatus and Culex nigrapalpus mosquitoes and have a greater epidemic potential [65]. In western U.S., the virus is primarily transmitted by Culex tarsalis mosquitoes and is endemic causing sporadic cases. The highest incidence rates have been in Ohio and Mississippi River basins and the Gulf Coast states. Over 4605 cases of SLE have been reported to the CDC since 1964 with most years ranging from 20–50 cases [51]. In 2005, the most recent year for which complete data is available, only 7 cases were reported in the U.S. Outbreaks occurred in 1975 with an estimated 2,800 cases in 31 states [1], in 1990 with 222 cases reported from Florida [66], in 1995 in Texas [67] and in 2001 with 70 cases in Louisiana [68]. Like other arboviruses in the U.S., cases have typically occurred between July and September.
Clinical features
In children the rate of asymptomatic to symptomatic infections is about 800:1, in adults it is 300:1 to 85:1 and in older adults may be only 16:1 [65,69]. The incubation period is from 4–21 days. Patients with the flu-like syndrome often have fever, myalgias, and headaches. Rashes are uncommon [68]. In young adults, meningitis occurs in 40% and encephalitis in 60% while in the those over the age of 60, 90% develop encephalitis [70]. Common signs of encephalitis include depressed consciousness from lethargy and confusion to coma and tremors of eyelids, lips, and limbs [71]. Some patients develop myoclonic jerks, opsoclonus, nystagmus, lower facial weakness, and cerebellar ataxia. Seizures were reported in 4/11 cases (36%) in one recent series with 1 patient developing status epilepticus [67]. Acute flaccid paralysis has been reported in 6% of the encephalitis cases [72] and transverse myelitis may also occur [69]. The acute illness lasts 1–2 weeks but recovery from such symptoms as forgetfulness, tremors, unsteadiness, weakness, and headaches may take months to years.
Laboratory studies
In one series of 11 cases CSF lymphocytic pleocytosis occurred in all (mean 107 cells/mm3, range 5–446), protein elevation in 64% (mean 67 mg/dL, range 39–143), and all had a normal CSF glucose concentration [67]. EEG is usually abnormal with the most common finding being diffuse slowing, although rarely seizures and periodic lateralizing epileptiform discharges have been reported [67]. MRI is more sensitive than CT in detecting abnormalities but is often normal. In one series of six patients with MRIs performed, none had abnormalities on T1 sequences and only two showed T2 hyperintensities, both involving the substantia nigra [67]. EMGs may demonstrate evidence of denervation in limb muscles suggesting viral involvement of anterior horn neurons in the spinal cord. Patients usually have a peripheral leukocytosis and occasionally sterile pyuria [71]. Hyponatremia from SIADH occurs in about 1/3rd of cases. Mild elevations in liver function tests and muscle enzymes can occur.
Diagnosis
Diagnosis is typically based on the demonstration of IgM in serum or CSF by capture ELISA [73]. An increase between acute and convalescent neutralizing serum antibody titer to SLEV also confirm the diagnosis. CSF cultures are usually negative, although virus can be isolated from brain tissue by biopsy or at autopsy.
Therapy and prognosis
No specific therapy of proven efficacy is available. In one non-randomized unblinded study, IFN-α2b 3 million units IV × 1 followed by the same dose subcutaneously 12 hrs later and then daily for 14 days was reported to improve outcome compared to non-treated controls [74]. Mortality in recent studies has varied from 4%–27% [67–68].
Eastern equine encephalitis virus (EEEV) (Family: Togaviridae)
Epidemiology
Of the four lineages of EEEV, group I causes the majority of disease in humans while groups IIA, IIB, and III cause primarily equine disease in Central and South America [75]. In the United States, the majority of cases are located along the eastern seaboard and sporadically located along the Gulf coast, typically within 5 miles of swamp or marsh lands that help to maintain the enzootic cycle. EEEV causes sporadic infections in human populations during the summer months and occasional epidemic outbreaks. The last U.S. outbreak occurred in September, 2005 in Massachusetts and New Hampshire and involved 11 patients with four deaths (36%) [75]. The primary enzootic mosquito vector associated with EEEV transmission is the mosquito Culiseta melanura [76]. Birds serve as the primary reservoir host as well as amplifying hosts, and humans are incidentally infected by a variety of mosquito bridging vectors including Culex and Aedes species [77].
Clinical Features
The clinical features of EEEV are nonspecific. Patients initially develop a prodrome of fever, chills, malaise, and myalgias lasting for 1–2 weeks [78]. The prodrome is followed by recovery or onset of encephalitis characterized by severe headache, confusion, nausea, and vomiting. Seizures, focal neurologic deficits such as cranial nerve palsies or focal weakness, and meningismus are common findings in patients that develop encephalitis [78]. Severely affected cases often progress to coma and death.
Laboratory Studies
Basic serum laboratory studies are remarkable for a leukocytosis and hyponatremia in as many as 60% of patients [78]. Cerebral spinal fluid analysis is abnormal in patients with EEEV with a median pleocytosis of 370 leukocytes/mm3 (range 0–2400) and a median neutrophil predominance of 70% [78]. The protein is elevated with a median value of 97 mg/dL and the glucose ratio may be normal to decreased [78,79].
As for many cases of arbovirus-induced encephalitis, MRI is typically more sensitive than CT for abnormalities associated with infection. While imaging may be normal, EEEV will often show increased signal intensity on T2-weighted images of the basal ganglia and thalami [78]. Noncontiguous lesions may also be seen in the brain stem, cortex, and periventricular white matter.
Diagnosis
In most cases, diagnosis is made with serology. The most common method is the IgM capture ELISA which detects antibody in a single blood or CSF sample, thus providing earlier diagnosis. Detection of IgM against EEEV antigen in the CSF is diagnostic of CSF infection and encephalitis when associated with the appropriate clinical syndrome.
Therapy and Prognosis
No proven antiviral therapy exists for EEEV. Treatment is focused on supportive care and managing complications such as seizures and increased ICP. There is no commercial vaccine for EEEV. In evaluating laboratory and imaging studies for prognostic value, one study found that CSF leukocytosis >500 cells/mm3 and hyponatremia <130 meq/L were predictive of a poor outcome [78]. Typically, the mortality rate represents about one-third of infections [78].
Western Equine encephalitis virus (WEEV) (Family: Togaviridae)
Epidemiology
Although WEEV was the first arbovirus isolated in America (1930), cases of human disease are now exceedingly rare. WEEV activity occurs mainly in western and Midwestern U. S. and western Canada. There have been 639 human cases reported by the CDC since 1964 but only 5 confirmed cases in the U.S. in the last 20 years and none since a single case in Minnesota in 1999 [51]. Major epidemics occurred in Minnesota in 1941 (>3000 human cases and hundreds of thousands of equine cases) [1,80] and in Saskatchewan in 1965 [81]. Reasons for the decline in both horse and human infections are unclear.
Clinical Features
The human incubation period ranges from 2–10 days. Very young and old individuals are at highest risk for severe encephalitis. However, most children and young adults develop a flu-like syndrome with fever and malaise lasting about a week rather than neuroinvasive disease. Meningismus is common. In those developing encephalitis, hemiparesis, stupor, cranial nerve palsies, generalized rigidity, transient tremors, Parkinsonism, and occasional opisthotonus develop [1,65]. Fewer than 10% develop coma which is usually transient. The clinical course usually runs two weeks and convalescence occurs over several weeks.
Laboratory studies
A leukocytosis and hyponatremia due to SIADH may develop. CSF findings are typical for arbovirus encephalitis. There are few reports of neuroimaging studies. EEG typically shows diffuse slowing although rare cases with focal temporal slowing that can mimic that seen in herpes simplex encephalitis have been reported [46].
Diagnosis
Diagnosis is usually made by detection of IgM antibody to WEEV in serum or CSF [1] but virus can be occasionally isolated from brain and CSF [82].
Therapy and prognosis
No specific therapy is available. There is a single case report over half a century ago describing the successful use of equine immune serum in an infected laboratory technician [83]. The overall case mortality is 3–8% but is higher in the elderly and infants [81,84]. Survivors typically recover fully, except for infants and elderly who may be left with neurologic sequelae including mental retardation, dementia, and Parkinsonism [85].
Venezuelan equine encephalitis virus (VEEV) (Family: Togaviridae)
Epidemiology
VEEV is roughly divided into enzootic and epizootic strains. Enzootic strains occur mainly in Venezuela, Central America, and Equator but an Everglade strain exists in Southern Florida that rarely causes human disease [1,86]. Enzootic viral strains cycle between mosquitoes and small rodents such as spiny or cotton rats, causing low viremias and mild disease in horses, and occasionally illness in humans. A recent focus in southern Mexico demonstrated over 60% of older adults had VEEV antibodies but the virus appeared to cause only rare cases of encephalitis [87].
Epizootic strains have a wider range of mosquito hosts, causing high viremias and serious illness in horses, and frequent flu-like illness in humans. These strains are capable of becoming epidemics that spread outside their normal viral territory. From 1969 to 1972, an outbreak in Guatemala spread as far north as southern Texas causing several hundred human cases and over 1,500 horse deaths in Texas [1,88]. Currently, the CDC reports only rare human cases occurring within the U.S.
Viremia in patients with VEE viremia can be as high as 108 × the intracranial LD50 for suckling mice, which is of sufficient magnitude to infect biting mosquitoes. However, human to mosquito to human transmission does not appear to play a significant role in disease spread or pathogenesis [89].
Clinical features
Over 90% of infected individuals develop an inapparent infection [89]. The incubation period in humans is usually 1–5 days, and patients with clinical symptoms usually develop the typical arbovirus flu-like syndrome often with pharyngitis and cervical adenopathy [65,86]. Neurologic features from encephalitis occur in 4–25% of symptomatic patients, mainly in the young and old. Clinical features include confusion, somnolence and seizures, cerebellar ataxia, and cranial nerve palsies [65,89]. Seizures, which are often focal, can be common.
Laboratory features
CSF shows a lymphocytic pleocytosis with a normal or elevated protein level and a normal glucose. Leukopenia and elevated liver transaminase levels are commonly seen.
Diagnosis
VEE can be isolated from blood, CSF and pharyngeal secretions, and viral shedding is often prolonged for a week or more. Detection of VEE nucleic acid in CSF or blood by or PCR is diagnostic [89]. Diagnosis can also be made by detection of VEE IgM antibody in CSF or serum by IgM capture ELISA assay [90].
Therapy and prognosis
There is no specific therapy for VEE. The U.S. Army developed a live attenuated VEE vaccine in 1961(“TC-83”) and a formalin inactivated derivative in 1974 (“C-84”). Vaccination of humans with the live attenuated vaccine followed by a booster dose of inactivated virus results is immunogenicity and is well tolerated [91]. Because of ongoing concerns about the potential use of VEE as a biowarfare weapon, impetus to develop a safe and effective VEE vaccine has intensified and several candidate vaccines are in active development [92]. Mortality in patients with VEE is typically reported as 10–25%, by contrast in symptomatic patients without encephalitis mortality is less than 1%. The prognosis for full recovery in survivors is excellent.
Powassan encephalitis virus (POWV) (Family: Flaviviridae)
Epidemiology
This virus is part of the tick-borne encephalitis group which has been renamed the mammalian group of tick-borne flaviviruses. Most of these viruses are endemic in Europe and Asia and often cause viral hemorrhagic fever or encephalitis.
POWV was first isolated form the brain of a 5 year-old child dying of encephalitis in Ontario, Canada in 1958. Since that time isolated additional cases of encephalitis have occurred both in Canada, and in the northern U.S. [93]. Less than 10 cases of POWV encephalitis have been reported to the CDC since 2001 [51]. Between September 1999 and July 2001 a small outbreak of four cases was reported from Maine and Vermont [94], the first reported U.S. cases since 1994.
The POWV life cycle is mainly between small rodents (squirrels, groundhogs, mice, voles) and hard Ixodes ticks, although POWV can also infect mosquitoes. Infected ticks live for months to over a year, and virus can persist in the tick population by transtatial transmission and in the offspring by transovarial transmission [14]. The principle cause of human disease results from the adult ticks taking a blood meal from humans walking outdoors in forests during the spring to fall months. Infections typically occur between June and September when ticks are actively feeding. Despite the importance of ticks in pathogenesis, patients typically do not recall a bite or finding a tick.
Clinical features
The incubation period ranges from 8 to 34 days. Patients with encephalitis typically have fever, headache, retro-orbital pain and photophobia associated with seizures, focal weakness, and depressed level of consciousness. In the most recent series of four cases the patients ranged in age from 25–70 with three being age 53 years or older. Additional features reported in individual patients in this small series included ophthalmoplegia, gaze paresis, dysarthria, ataxia, muscle twitching, and bilateral proximal leg weakness.
Laboratory studies
In the four cases from the most recent outbreak, CSF cell counts ranged from 47–920 cells/mm3, with three of four patients having >74% lymphocytes. Protein was elevated in the three cases in which data was available (range 67–96 mg/dL). Two patients had areas of increased T2 signal consistent with microvascular ischemia or demyelination, but it was not clear these were related to the acute infection. EEG was diffusely slow in the three patients in which it was performed [94].
Diagnosis
Virus has been isolated from brain at autopsy but rarely from other sites [93]. Diagnosis is made by detection of CSF or serum IgM antibodies or by a 4-fold rise in IgG antibody between acute and convalescent sera. These tests are not available commercially, but can be requested through state public health laboratories and are performed at the CDC.
Therapy and prognosis
There is no specific therapy for POWV. The mortality in patients with encephalitis is 10%–15%, and sequelae in survivors include hemiparesis, aphasia, and flaccid limb paralysis [95,96]. At autopsy the brain shows multifocal areas of infection in the gray matter of the cerebral cortex, brainstem, and spinal cord [93].
California encephalitis group (Lacrosse virus) (Family: Bunyaviridae)
Within the family of Bunyaviridae and genus Bunyavirus; California encephalitis virus, La Crosse virus, Jamestown Canyon virus, and Tahyna virus comprise the major causes of encephalitis in the California encephalitis group. Of these viruses, La Crosse virus, California encephalitis virus, and Jamestown Canyon virus are causes of disease in the United States and Tahyna virus is predominantly a cause of encephalitis in Russia.
Epidemiology
La Crosse virus is the most common cause of disease in the California encephalitis group. It was originally described in 1965 following a postmortem exam of a child that died due to encephalitis in La Crosse, Wisconsin [97]. La Crosse virus is transmitted in an enzootic pattern between squirrels and chipmunks by the mosquito Aedes triseriatus in areas of the Mississippi and Ohio river basins [98]. The Ae. triseriatus vector is a forest dwelling mosquito that inhabits tree-holes and will overwinter by transovarian transmission into Aedes eggs [98]. Like the other viruses in this group, infection is related to contact with wooded forests during warm summer months. In cases of La Crosse virus infection, the ratio of asymptomatic to symptomatic infections is approximately 1000:1 [99]. California encephalitis virus was originally isolated in 1941 [100], and while the group of diseases are named after this virus, it is relatively rare with disease occurrence mainly located in the western United States and Canada. While La Crosse and California encephalitis viruses cause the vast majority of disease in children, Jamestown Canyon virus affects predominantly the elderly in regions of the northern United States with seroprevalence in some areas reaching 10% [101].
Clinical Features
In children infected with La Crosse virus, the mean age is 7.5 years and symptoms typically consist of fever, headache, vomiting in 70% , seizures in 46%, and altered mental status in 42% [99]. Additionally, focal neurologic signs such as hemiparesis, aphasia, dysarthria, and chorea occur. About 10% of patients develop increased ICP and rarely cerebral herniation can occur [99]. Jamestown Canyon virus has similar clinical features.
Laboratory Diagnosis
Peripheral leukocytosis is common and patients can develop severe hyponatremia due to the SIADH syndrome. CSF often reveals a lymphocytic pleocytosis of 600 cells/mm3, with a normal glucose, and an increase protein in 30% of patients [99]. As with other arboviruses, diagnosis is made with serologic techniques as viral isolation is not sensitive. Indirect immunofluorescence is the method of choice to detect IgG and IgM. IgM detection in the CSF or a four-fold rise in paired sera for IgG is considered diagnostic for infection.
Prognosis and Therapy
In general, children infected with La Crosse virus recover with a case fatality rate <1% [99]. While about 6–15% of patients that recover have recurrent seizures, the majority return to normal function with approximately 10% of patients suffering from sequelae following discharge from the hospital. Predictors of a poor outcome include hyponatremia, persistently elevated body temperature, and a Glascow Coma Score of <13 [100,102]. No antiviral therapy currently exists for the California encephalitis group of viruses and no vaccine is available. Physicians are relegated to treating the complications such as seizures, elevated ICP, and other complications.
Colorado tick fever (Family: Reoviridae)
All Colorado tick fever (CTF) viruses have common structural features consisting of segmented, double-stranded RNA surrounded by double-capsid proteins that form a non-enveloped viral particle. While viruses in this family cause a variety of diseases, CTF virus causes an acute febrile syndrome that is occasionally recurrent and can be complicated by encephalitis.
Epidemiology
CTF is transmitted by the wood tick (Dermacentor andersoni) mainly in the western mountain regions of the United States and Canada above 4000 feet in elevation [103]. The enzootic pattern of infection exists between small mammals such as ground squirrels, marmots, or chipmunks and D. andersoni. Once infected, the tick is infected for life (transtadially), throughout the three main stages of development; laval, nymphal, and adult. There is no transovarial (vertical) transmission but sustained viremias in the amplifying, vertebrate hosts allow for successful viral overwintering [103]. The younger stages of the tick (larval and nymphal) typically feed on small mammals; however, the adult tick will often feed on larger mammals including humans. Humans are typically exposed to the habitat of D. andersoni during summer months while hiking, fishing, or camping and are infected as dead-end hosts following a blood meal from an infected tick. Occasional cases of CTF have occurred in patients exposed to the tick from a traveling family member via clothing or equipment [104]. Lastly, occasional cases of CTF in California, outside the D. andersoni range, have been reported and attributed to infection of a different tick vector, D. variabilis [105].
Clinical features
A history of tick bite is obtained in approximately 90% of patients, and the incubation period is 0–14 days, mean 3 days [103]. Patients develop abrupt onset of fever, chills, generalized myalgias, severe headache, and hyperesthetic skin leading to severe malaise that often confines patients to bedrest. Gastrointestinal symptoms such as nausea, vomiting, or diarrhea may be present as well but are not prominent. About 15% of patients will develop a maculopapular or petechial rash. Otherwise, physical findings are elusive and include occasional pharyngitis, mild lymphadenopathy, or mild splenomegaly. In 50% of patients, the fever will resolve after two days and then recur in the biphasic or ‘saddleback’ pattern [103]. Following convalescence, patients over the age of 30 may have persistent fatigue for as long as 3 weeks. Complications such as encephalitis or meningitis predominantly occur in children in about 5–10% of the cases; yet, CTF is rarely fatal [106]. In these patients, neurologic signs and symptoms include nuchal rigidity, photophobia, and mild altered mental status.
Laboratory Studies
CTF virus infects hematopoietic cells resulting in a persistent infection and subsequent neutropenia, thrombocytopenia, and mild anemia [107]. Occasionally, other laboratory abnormalities are found such as elevated liver transaminases and elevated creatinine phosphokinase levels. Analysis of CSF may exhibit a mild lymphocytic pleocytosis with a normal glucose and normal or slightly elevated protein. While virus can be isolated from peripheral blood or by blood smear using indirect immunofluorescence of RBCs [108], the diagnosis of CTF is usually made using IgM capture ELISA, neutralization or complement fixation [109].
Prognosis and Therapy
There is no established treatment for CTF. The vast majority of patients recover following infection, and only three deaths have been reported due to intravascular coagulopathy following CTF infection [106]. Therapy is largely supportive consisting of bed rest, fluid maintenance, and antipyretics with particular avoidance of aspirin and nonsteroidal anti-inflammatory drugs due to their exacerbation of platelet dysfunction and theoretical increase risk of coagulopathy.
Dengue virus (Family: Flaviviridae)
Epidemiology
Dengue virus is widely endemic in tropics around the world. Parts of Mexico have dengue and indigenous dengue infections have occurred in Texas, Hawaii, and Puerto Rico [110,111]. In addition, over 100 American foreign travelers return to the U.S. each year with dengue [112].
Clinical features
Most patients infected with one of the four dengue viruses develop a fever-arthralgia-rash syndrome or viral hemorrhagic fever. However, some patients present with a decreased level of consciousness mainly due to encephalopathy but occasionally encephalitis [113,114]. Patients with encephalitis may develop coma, meningismus, seizures, and focal neurologic signs such as hemiparesis [113,115].
Laboratory studies
The blood typically shows leukopenia and thrombocytopenia. CSF has a mild lymphocytic pleocytosis in 30% of patients with diminished mental status.
Diagnosis
Dengue virus has been isolated or detected by PCR in CSF and IgM antibody to dengue has also been found in CSF [113,114]. For the diagnosis of dengue, one should obtain acute and convalescent serum for antibody titers plus an acute serum for virus isolation [112].
Prognosis and therapy
The overall prognosis is good unless hemorrhagic fever develops.
Conclusions
Arboviruses continue to be a major cause of encephalitis in North America. With the appearance of West Nile virus in 1999, West Nile neuroinvasive disease has become the dominant cause of encephalitis. All North American arbovirus are transmitted to humans by infected mosquitoes or ticks. Most infections are asymptomatic or produce a flu-like illness. Elderly, immunosuppressed individuals, and infants for some arboviruses have the most severe encephalitis. Rapid serum or CSF IgM antibody capture ELISA assays are now available to diagnosis the acute infection for all North American arboviruses. Unfortunately, no antiviral drugs are available for treatment of the encephalitis so therapy is symptomatic.
Acknowledgments
KLT is supported by R01 grants (NS051403, NS050138) from the NIH and a MERIT Grant from the Department of Veterans Affairs and by the Reuler-Lewin Family Professorship of Neurology. JDB is supported by a Department of Veterans Affairs Research Career Development Award (RCDA) and has been supported by an NIH postdoctoral fellowship training grant (T32AI07537).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calisher CH. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev. 1994;7:89–116. doi: 10.1128/cmr.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Condit RC. Fields Virology. 5th edition. Philadelphia: Wolters Kluwer-Lippincott William & Wilkins; 2007. Principles of virology; pp. 25–57. [Google Scholar]
- 3.Roehrig JT, Staudinger LA, Hunt AR, Mathews JH, Blair CD. Antibody prophylaxis and therapy for flavivirus encephalitis infections. Ann NY Acad Sci. 2001;951:286–297. doi: 10.1111/j.1749-6632.2001.tb02704.x. [DOI] [PubMed] [Google Scholar]
- 4.Day JF. Predicting St. Louis encephalitis virus epidemics: lessons from recent, and not so recent, outbreaks. Annu Rev Entomol. 2001;46:111–138. doi: 10.1146/annurev.ento.46.1.111. [DOI] [PubMed] [Google Scholar]
- 5.Pealer L, Marfin A, Petersen L, et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Eng J Med. 2003;349:1236–1246. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto M, Jernigan D, Guasch A, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Eng J Med. 2003;348:2196–2203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 7.CDC. Possible West Nile virus transmission to an infant through breast-feeding Michigan 2002. MMWR Morb Mortal Wkly Rep. 2002;51:877–878. [PubMed] [Google Scholar]
- 8.CDC. Intrauterine West Nile virus infection-New York, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:1135–1136. [PubMed] [Google Scholar]
- 9.Girard YA, Klingeler KA, Higgs S. West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector-borne and zoonotic diseases. 2004;4:109–122. doi: 10.1089/1530366041210729. [DOI] [PubMed] [Google Scholar]
- 10.van Landingham DL, Schneider BS, Klinger K, et al. Real-time reverse transcriptase-polymerase chain reaction quantification of West Nile virus transmitted by Culex Pipiens Quinquefasciatus. Am J Trop Med Hyg. 2004;71:120–123. [PubMed] [Google Scholar]
- 11.Davis LE, DeBiasi R, Goade DE, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- 12.Nacsi R, Savage H, White D, et al. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis. 2001;7:1–3. doi: 10.3201/eid0704.010426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller B, Nasci R, Godsey M, et al. First field evidence for natural vertical transmission of West Nile virus in Culex Univittatus Complex mosquitoes from Rift Valley Province, Kenya. Am J Trop Med Hyg. 2000;62:240–246. doi: 10.4269/ajtmh.2000.62.240. [DOI] [PubMed] [Google Scholar]
- 14.Costero A, Grayson MA. Experimental transmission of Powassan virus (Flaviviridae) by Ixodes scapularis ticks (Acari-ixodidae) Am J Trop Med Hyg. 1996;55:536–548. doi: 10.4269/ajtmh.1996.55.536. [DOI] [PubMed] [Google Scholar]
- 15.Turell M, O’Guinn M, Oliver J. Potential for New York Mosquitoes to transmit West Nile virus. Am J Trop Med Hyg. 2000;62:413–414. doi: 10.4269/ajtmh.2000.62.413. [DOI] [PubMed] [Google Scholar]
- 16.Komar N, Langevin S, Hinten S, et al. Experimental infection of North American birds with New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komar N, Lanciotti R, Bowen R, Langevin S, Bunning M. Detection of West Nile virus in oral and cloacal swabs collected from bird carcasses. Emerg Infect Dis. 2002;8:741–742. doi: 10.3201/eid0807.020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steele KE, Linn MJ, Schoepp RJ, et al. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet Pathol. 2000;37:208–224. doi: 10.1354/vp.37-3-208. [DOI] [PubMed] [Google Scholar]
- 19.Chambers TJ, Diamond MS. Pathogenesis of flavivirus encephalitis. Adv Virus Res. 2003;60:273–342. doi: 10.1016/S0065-3527(03)60008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guarner J, Shieh W, Hunter St, et al. Clinicopathologic study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Hum Pathol. 2004;35:983–990. doi: 10.1016/j.humpath.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Solomon T. Flavivirus encephalitis. N Engl J Med. 2004;351:370–378. doi: 10.1056/NEJMra030476. [DOI] [PubMed] [Google Scholar]
- 22.Samuel MA, Morrey JD, Diamond MS. Caspase 3-dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J Virol. 2007;81:2614–2623. doi: 10.1128/JVI.02311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohtaki E, Matsuishi T, Hirano Y, Maekawa K. Acute disseminated encephalomyelitis after treatment with Japanese B encephalitis vaccine. J Neurol Neurosurg Psychiatry. 1995;59:316–317. doi: 10.1136/jnnp.59.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sejvar JJ, Bode AV, Curiel M, Marfin AA. Post-infectious encephalomyelitis associated with St. Louis encephalitis virus infection. Neurology. 2004;63:1719–1721. doi: 10.1212/01.wnl.0000143061.63041.07. [DOI] [PubMed] [Google Scholar]
- 25.Fratkin J, Leis A, Stokic D, et al. Spinal cord neuropathology in human West Nile virus infection. Arch Pathol Lab Med. 2004;128:533–537. doi: 10.5858/2004-128-533-SCNIHW. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Lobigs M, Lee E, Mullbacher A. CD8 T cells mediate recovery and immunopathology in West Nile virus encephalitis. J Virol. 2003;77:13323–13334. doi: 10.1128/JVI.77.24.13323-13334.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shrestha B, Diamond MS. Role of CD8+ T cells in control of West Nile virus infection. J Virol. 2004;78:8312–8321. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shrestha B, Wang T, Samuel MA, et al. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J Virol. 2006;80:5338–5348. doi: 10.1128/JVI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shrestha B, Samuel MA, Diamond MS. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J Virol. 2006;80:119–129. doi: 10.1128/JVI.80.1.119-129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sitati EM, Diamond MS. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J Virol. 2006;80:12060–12069. doi: 10.1128/JVI.01650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purtha WE, Myers N, Mitaksov V, et al. Antigen-specific cytotoxic T lymphocytes protect against lethal West Nile virus encephalitis. Eur J Immunol. 2007;37:1845–1854. doi: 10.1002/eji.200737192. [DOI] [PubMed] [Google Scholar]
- 32.Kleinman S, Glynn SA, Busch M, et al. The 2003 West Nile virus United States epidemic: the America’s Blood Centers experience. Transfusion. 2005;45:469–479. doi: 10.1111/j.0041-1132.2005.04315.x. [DOI] [PubMed] [Google Scholar]
- 33.Sejvar J, Haddad M, Tierney B, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290:511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 34.Khairallah M, Yahia SB, Ladjimi A, et al. Chorioretinal involvement in patients with West Nile virus infection. Ophthalmology. 2004;111:2065–2070. doi: 10.1016/j.ophtha.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 35.Sejvar JJ, Bode AV, Marfin AA, et al. West Nile virus-associated flaccid paralysis. Emerg Infect Dis. 2005;11:1021–1027. doi: 10.3201/eid1107.040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyler KL, Pape J, Goody RJ, Corkill M. Kleinschmidt-DeMasters BK. CSF findings in 250 patients with serologically confirmed West Nile virus meningitis and encephalitis. Neurology. 2006;66:361–365. doi: 10.1212/01.wnl.0000195890.70898.1f. [DOI] [PubMed] [Google Scholar]
- 37.Gendelman-Marton R, Kimiagar I, Itzhaki A, Klein C, Theitler J, Rabey JM. Electroencephalography findings in adult patients with west Nile virus-associated meningitis and meningoencephalitis. Clin Infect Dis. 2003;37:1573–1578. doi: 10.1086/379516. [DOI] [PubMed] [Google Scholar]
- 38.Al-Shekhlee A, Katirji B. Electrodiagnostic features of acute paralytic poliomyelitis associated with West Nile virus infection. Muscle Nerve. 2004;29:376–380. doi: 10.1002/mus.10557. [DOI] [PubMed] [Google Scholar]
- 39.Shi P-Y, Wong SJ. Serologic diagnosis of West Nile virus infection. Exp Rev of Mol Diagn. 2003;3:733–741. doi: 10.1586/14737159.3.6.733. [DOI] [PubMed] [Google Scholar]
- 40.Roehrig JT, Nash D, Maidin B, et al. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis. 2003;9:376–379. doi: 10.3201/eid0903.020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brooks TJ, Phillpotts RJ. Interferon-alpha protects mice against lethal infection with St Louis encephalitis virus delivered by the aerosol and subcutaneous routes. Antiviral Res. 1999;41:57–64. doi: 10.1016/s0166-3542(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 42.Jordan I, Briese T, Fischer N, et al. Ribavirin inhibits West Nile virus replication and cytopathic effect in neural cells. J Infect Dis. 2000;182:1214–1217. doi: 10.1086/315847. [DOI] [PubMed] [Google Scholar]
- 43.Kimura-Kuroda J, Yasui K. Protection of mice against Japanese encephalitis virus by passive administration with monoclonal antibodies. J Immunol. 1988;141:3606–3610. [PubMed] [Google Scholar]
- 44.Morrey JD, Siddharthan V, Olsen AL, et al. Defining limits of treatment with humanized neutralizing monoclonal antibody for West Nile virus neurological infection in a hamster model. Antimicrob Agents Chemother. 2007;51:2396–2402. doi: 10.1128/AAC.00147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ben-Nathan D, Lustig S, Tam G, et al. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J Infect Dis. 2003;188:5–12. doi: 10.1086/376870. [DOI] [PubMed] [Google Scholar]
- 46.Bia FJ, Thornton GF, Main AJ, Fong CK, Hsiung GD. Western equine encephalitis mimicking herpes simplex encephalitis. JAMA. 1980;244:367–369. [PubMed] [Google Scholar]
- 47.Deas TS, Binduga-Gajewska I, Tilgner M, et al. Inhibition of flavivirus infections by antisense oligomers specifically suppressing viral translation and RNA replication. J Virol. 2005;79:4599–4609. doi: 10.1128/JVI.79.8.4599-4609.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tesh RB, Amelia PA, Travassos dR, Guzman H, Araujo TP, Xiao S-Y. Immunization with heterologous flaviviruses protective against fatal West Nile encephalitis. Emerg Infect Dis. 2002;8:245–251. doi: 10.3201/eid0803.010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanesa-Thasan N, Putnak JR, Mangiafico JA, Saluzzo J-F, Ludwig GV. Absence of protective neutralizing antibodies to west nile virus in subjects following vaccination with Japanese encephalitis or dengue vaccines. Am J Trop Med Hyg. 2002;66:115–116. doi: 10.4269/ajtmh.2002.66.115. [DOI] [PubMed] [Google Scholar]
- 50.Levy C. [assessed August 20, 2007];West Nile virus in Arizona. doi: 10.4269/ajtmh.13-0061. www.cdc.gov/ncidod/dvbid/westnile/conf/2005pdf/AZWNVUpdateCraigLevyVersion.pdf. [DOI] [PMC free article] [PubMed]
- 51.CDC. [assessed August 21, 2007];Arboviral encephalitis. http://www.cdc.gov/ncidod/dvbid/arbor/
- 52.Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13–18. doi: 10.1056/NEJMoa011699. [DOI] [PubMed] [Google Scholar]
- 53.DeBiasi RL, Tyler KL. West Nile virus meningoencephalitis. Nat Clin Pract Neurol. 2006;2:264–275. doi: 10.1038/ncpneuro0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bode V, Sejvar JJ, Pape WJ, et al. West Nile virus disease: a descriptive study of 228 patients hospitalized in a 4-county region of Colorado in 2003. Clin Infect Dis. 2006;42:1234–1240. doi: 10.1086/503038. [DOI] [PubMed] [Google Scholar]
- 55.Kramer LD, Li J, Shi PY. West Nile virus. Lancet Neurol. 2007;6:171–181. doi: 10.1016/S1474-4422(07)70030-3. [DOI] [PubMed] [Google Scholar]
- 56.Sejvar JJ. The long-term outcomes of human West Nile virus infection. Clin Infect Dis. 2007;44:1617–1624. doi: 10.1086/518281. [DOI] [PubMed] [Google Scholar]
- 57.Bakri SJ, Kaiser PK. Ocular manifestations of West Nile virus. Curr Opin Ophthalmol. 2004;15:537–540. doi: 10.1097/01.icu.0000143687.45232.f1. [DOI] [PubMed] [Google Scholar]
- 58.Kleinschmidt-DeMasters BK, Marder BA, Levi ME, et al. Naturally acquired West Nile virus encephalomyelitis in transplant recipients: Clinical, laboratory, diagnostic, and neuropathological features. Arch Neurol. 2004;61:1210–1220. doi: 10.1001/archneur.61.8.1210. [DOI] [PubMed] [Google Scholar]
- 59.Weiss D, Carr D, Kellachan J, et al. Clinical findings of West Nile virus infection in hospitalized patients, New York and New Jersey, 2000. Emerg Inf Dis. 2001;7:654–658. doi: 10.3201/eid0704.010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petropoulou KA, Gordon SM, Prayson RA, Ruggierri PM. West Nile virus meningoencephalitis: MR imaging findings. AJNR Am J Neuroradiol. 2005;26:1986–1995. [PMC free article] [PubMed] [Google Scholar]
- 61.Ali M, Sagriel Y, Sohi J, LLave A, Weather S. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am J Neuroradiol. 2005;26:289–297. [PMC free article] [PubMed] [Google Scholar]
- 62.Armah HB, Wang G, Omalu BI, et al. Systemic distribution of West Nile virus infection: Postmortem immunohistochemical study of six cases. Brain Pathol. 2007;17:345–362. doi: 10.1111/j.1750-3639.2007.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lanciotti RS, Kerst AJ, Nasci RS, et al. Rapid detection of West Nile virus from human clinical specimens, field collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Monath TP, Liu J, Kanesa-Thasan N, et al. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci USA. 2006;103:6694–6699. doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solomon T, Whitley RJ. Arthropod-borne viral encephalitides. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. 3rd edition. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 205–230. [Google Scholar]
- 66.Meehan PJ, Wells DL, Buff PE, et al. Epidemiological features and public health response to a St. Louis encephalitis epidemic in Florida, 1990-1. Epidemiol Infect. 2000;125:181–188. doi: 10.1017/s0950268899004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wasay M, Diaz-Arrastia R, Suss RA, et al. St. Louis encephalitis. A review of 11 cases in a 1995 Dallas, Tex, epidemic. Arch Neurol. 2000;57:114–118. doi: 10.1001/archneur.57.1.114. [DOI] [PubMed] [Google Scholar]
- 68.Jones SC, Morris J, Hill G, Alderman M, Ratard RC. St. Louis encephalitis outbreak in Louisiana in 2001. J La State Med Soc. 2002;154:303–306. [PubMed] [Google Scholar]
- 69.Monath TP. Epidemiology. In: Monath TP, editor. St. Louis Encephalitis. Washington DC: Am Publ Health Assoc; 1980. pp. 239–312. [Google Scholar]
- 70.Brinker KR, Monath TP. The acute disease. In: Monath TP, editor. St. Louis Encephalitis. Washington DC: Am Publ Health Assoc; 1980. pp. 503–534. [Google Scholar]
- 71.Southern PM, Smith JW, Luby JP, Parnett JA, Sanford JP. Clinical and laboratory features of epidemic St. Louis encephalitis. Ann Intern Med. 1969;71:681–689. doi: 10.7326/0003-4819-71-4-681. [DOI] [PubMed] [Google Scholar]
- 72.Quick DT, Thompson JM, Bond JO. The 1962 epidemic of St. Louis encephalitis in Florida. IV. Clinical features of cases occurring in the Tampa Bay area. Am J Epidemiol. 1965;81:415–427. doi: 10.1093/oxfordjournals.aje.a120527. [DOI] [PubMed] [Google Scholar]
- 73.Monath TP, Nystrom RR, Bailey RE, Calisher CH, Muth DJ. Immunoglobulin M antibody capture enzyme-linked immunosorbent assay for diagnosis of St. Louis encephalitis. J Clin Microbiol. 1984;20:784–790. doi: 10.1128/jcm.20.4.784-790.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rahal JJ, Anderson J, Rosenberg C, Reagan T, Thompson LL. Effect of interferon-alpha2b therapy on St. Louis viral meningoencephalitis: Clinical and laboratory results of a pilot study. J Infect Dis. 2004;190:1084–1087. doi: 10.1086/423325. [DOI] [PubMed] [Google Scholar]
- 75.CDC. Eastern equine encephalitis--New Hampshire and Massachusetts, August-September 2005. MMWR Morb Mortal Wkly Rep. 2006;55:697–700. [PubMed] [Google Scholar]
- 76.Loftin KC, Diallo AA, Herbert MW, et al. Five-year surveillance of West Nile and eastern equine encephalitis viruses in Southeastern Virginia. J Environ Health. 2006;68:33–40. [PubMed] [Google Scholar]
- 77.Mitchell CJ, Niebylski ML, Smith GC, et al. Isolation of eastern equine encephalitis virus from Aedes albopictus in Florida. Science. 1992;257:526–527. doi: 10.1126/science.1321985. [DOI] [PubMed] [Google Scholar]
- 78.Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med. 1997;336:1867–1874. doi: 10.1056/NEJM199706263362604. [DOI] [PubMed] [Google Scholar]
- 79.Przelomski MM, O'Rourken E, Grady GF, Berardi VP, Markley HG. Eastern equine encephalitis in Massachusetts: a report of 16 cases, 1970–1984. Neurology. 1988;38:736–739. doi: 10.1212/wnl.38.5.736. [DOI] [PubMed] [Google Scholar]
- 80.Eklund CM. Human encephalitis of the western equine type in Minnesota in 1941. Clinical and epidemiological study of serologically proven cases. Am J Hyg. 1946;43:171–193. doi: 10.1093/oxfordjournals.aje.a119060. [DOI] [PubMed] [Google Scholar]
- 81.Rozdilsky B, Robertson HE, Chorney J. Western encephalitis: report of eight fatal cases. Saskatchewan epidemic, 1965. Can Med Assoc J. 1968;98:79–86. [PMC free article] [PubMed] [Google Scholar]
- 82.Sciple GW, Ray G, La Motte LC, et al. Western encephalitis with recovery of virus from cerebrospinal fluid. Neurology. 1967;17:169–171. doi: 10.1212/wnl.17.2.169. [DOI] [PubMed] [Google Scholar]
- 83.Gold H, Hampil B. Equine encephalomyelitis in a laboratory technician with recovery. Ann Intern Med. 1942;16:556–569. [Google Scholar]
- 84.Longshore WA, Stevens JM, Hollister AV, Gittelsohn A, Lennette EH. Epidemicologic observations on acute infectious encephalitis in California, with special reference to the 1952 outbreak. Am J Hyg. 1956;63:69–86. doi: 10.1093/oxfordjournals.aje.a119793. [DOI] [PubMed] [Google Scholar]
- 85.Earnest MP, Goolishian HA, Calverley JR, Hayes RO, Hill Hr. Neurologic, intellectual and psychologic sequelae following western encephalitis. A follow-up study of 35 cases. Neurology. 1971;21:969–974. doi: 10.1212/wnl.21.9.969. [DOI] [PubMed] [Google Scholar]
- 86.Weaver SC, Ferro C, Barrera R, Boshell J, Navarro J-C. Venezuelan equine encephalitis. Annu Rev Entomol. 2004;49:141–174. doi: 10.1146/annurev.ento.49.061802.123422. [DOI] [PubMed] [Google Scholar]
- 87.Estrada-Franco JG, Navarro-Lopez R, Freier JE, et al. Venezuelan equine encephalitis virus, Southern Mexico. Emerg Infect Dis. 2004;10:2113–2121. doi: 10.3201/eid1012.040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walton TE, Grayson MA. Venezuelan equine encephalomyelitis. In: Monath TP, editor. The Arboviruses: epidemiology and ecology. vol IV. Boca Raton Florida: CRC Press; 1988. pp. 203–231. [Google Scholar]
- 89.Bowen GS, Calisher CH. Virological and serological studies of Venezuelan equine encephalomyelitis in humans. J Clin Microbiol. 1976;4:22–27. doi: 10.1128/jcm.4.1.22-27.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watts DM, Callahan J, Rossi C, et al. Venezuelan equine encephalitis febrile cases among humans in the Peruvian Amazon River region. Am J Trop Med Hyg. 1998;58:35–40. doi: 10.4269/ajtmh.1998.58.35. [DOI] [PubMed] [Google Scholar]
- 91.Pittman PR, Makuch RS, Mangiafico JA, et al. Long-term duration of detectable neutralizing antibodies after administration of live-attenuated VEE vaccine and following booster vaccination with inactivated VEE vaccine. Vaccine. 1996;14:337–343. doi: 10.1016/0264-410x(95)00168-z. [DOI] [PubMed] [Google Scholar]
- 92.Rao V, Hinz ME, Roberts BA, Fine D. Toxicity assessment of Venezuelan Equine encephalitis virus vaccine candidate strain V3526. Vaccine. 2006;24:1710–1715. doi: 10.1016/j.vaccine.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 93.McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Canad Med Ass J. 1959;80:708–711. [PMC free article] [PubMed] [Google Scholar]
- 94.CDC. Outbreak of Powassan encephalitis-Maine and Vermont, 1999–2001. MMWR Morbid Mortal Wkly Rep. 2001;50:761–764. [PubMed] [Google Scholar]
- 95.Smith R, Woodall JP, Whitney E, et al. Powassan virus infection. A report of three human cases of encephalitis. Am J Dis Child. 1974;127:691–693. doi: 10.1001/archpedi.1974.02110240077010. [DOI] [PubMed] [Google Scholar]
- 96.Rossier E, Harrison RJ, Lemieux B. A case of Powassan virus encephalitis. Canad Med Ass J. 1974;110:1173–1180. [PMC free article] [PubMed] [Google Scholar]
- 97.Thompson WH, Kalfayan B, Anslow RO. Isolation of California Encephalitis Group Virus from a fatal human illness. Am J Epidemiol. 1965;81:245–253. doi: 10.1093/oxfordjournals.aje.a120512. [DOI] [PubMed] [Google Scholar]
- 98.Watts DM, Pantuwatana S, DeFoliart GR, Yuill TM, Thompson WH. Transovarial transmission of LaCrosse virus (California encephalitis group) in the mosquito, Aedes triseriatus. Science. 1973;182:1140–1141. doi: 10.1126/science.182.4117.1140. [DOI] [PubMed] [Google Scholar]
- 99.McJunkin JE, los Reyes EC, Irazuzta JE, et al. La Crosse encephalitis in children. N Engl J Med. 2001;344:801–807. doi: 10.1056/NEJM200103153441103. [DOI] [PubMed] [Google Scholar]
- 100.Cramblett HG, Stegmiller H, Spencer C. California encephalitis virus infections in children. Clinical and laboratory studies. JAMA. 1966;198:108–112. [PubMed] [Google Scholar]
- 101.Mayo D, Karabatsos N, Scarano FJ, et al. Jamestown Canyon virus: seroprevalence in Connecticut. Emerg Infect Dis. 2001;7:911–912. doi: 10.3201/eid0705.017529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hilty MD, Haynes RE, Azimi PH, Cramblett HG. California encephalitis in children. Am J Dis Child. 1972;124:530–533. doi: 10.1001/archpedi.1972.02110160068006. [DOI] [PubMed] [Google Scholar]
- 103.Goodpasture HC, Poland JD, Francy DB, Bowen GS, Horn KA. Colorado tick fever: clinical, epidemiologic, and laboratory aspects of 228 cases in Colorado in 1973–1974. Ann Intern Med. 1978;88:303–310. doi: 10.7326/0003-4819-88-3-303. [DOI] [PubMed] [Google Scholar]
- 104.Midoneck SR, Richard J, Murray HW. Colorado tick fever in a resident of New York City. Arch Fam Med. 1994;3:731–732. doi: 10.1001/archfami.3.8.731. [DOI] [PubMed] [Google Scholar]
- 105.Attoui H, Mohd JF, Biagini P, et al. Genus Coltivirus (family Reoviridae):genomic and morphologic characterization of Old World and New World viruses. Arch Virol. 2002;147:533–561. doi: 10.1007/s007050200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spruance SL, Bailey A. Colorado Tick Fever. A review of 115 laboratory confirmed cases. Arch.Intern.Med. 1973;131:288–293. [PubMed] [Google Scholar]
- 107.Philipp CS, Callaway C, Chu MC, et al. Replication of Colorado tick fever virus within human hematopoietic progenitor cells. J Virol. 1993;67:2389–2395. doi: 10.1128/jvi.67.4.2389-2395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Emmons RW, Lennette EH. Immunofluorescent staining in the laboratory diagnosis of Colorado tick fever. J Lab Clin Med. 1966;68:923–929. [PubMed] [Google Scholar]
- 109.Mohd JF, Attoui H, Gallian P, et al. Recombinant VP7-based enzyme-linked immunosorbent assay for detection of immunoglobulin G antibodies to Colorado tick fever virus. J.Clin.Microbiol. 2003;41:2102–2105. doi: 10.1128/JCM.41.5.2102-2105.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.CDC. Dengue hemorrhagic fever—U.S.-Mexico border, 2005. MMWR Morb Mortal Wkly Rep. 2007;56:785–789. [PubMed] [Google Scholar]
- 111.Effler PV, Pang L, Kitsutani P, et al. Dengue fever, Hawaii, 2001–2002. Emerg Infect Dis. 2005;11:742–749. doi: 10.3201/eid1105.041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.CDC. Travel-associated dengue—United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:700–702. [PubMed] [Google Scholar]
- 113.Solomon T, Dung NM, Vaughn DW, et al. Neurological manifestations of dengue infection. Lancet. 2000;355:1053–1059. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 114.Lum LC, Lam SK, Choy YS, George R, Harun F. Dengue encephalitis: a true entity? Am J Trop Med Hyg. 1996;54:256–259. doi: 10.4269/ajtmh.1996.54.256. [DOI] [PubMed] [Google Scholar]
- 115.Pancharoen C, Thisyakorn U. Neurological manifestations of dengue patients. Southeast Asian J Trop Med Public Health. 2001;32:341–345. [PubMed] [Google Scholar]