Abstract
Background
FoxP3 is the most specific available marker for regulatory T cells (Tregs). Tumor-associated FoxP3-positive Tregs have been identified in various neoplasms, including cutaneous T-cell lymphoma (CTCL). FoxP3 expression in CTCL varies across groups; few studies have compared CTCL with inflammatory conditions.
Methods
Lesional skin biopsies from 20 patients with CTCL (13 mycosis fungoides [MF]; 7 Sézary syndrome) and 22 with inflammatory dermatoses (11 spongiotic; 11 lichenoid or interface) were examined for FoxP3 expression by immunohistochemistry. Epidermal FoxP3-positive lymphocytes were counted as a percentage of the total epidermal CD3-positive T-cell population.
Results
FoxP3-positive T cells composed the minority of infiltrate in all major categories. Lower numbers of epidermal FoxP3-positive T cells were observed in CTCL, particularly MF, than in inflammatory dermatoses (P<.001). CTCL neoplastic T cells did not express FoxP3.
Conclusions
FoxP3-positive T cells are less frequently encountered in MF than in inflammatory dermatoses. FoxP3-positive T cells occur in higher proportions in the dermis than in the epidermis and likely correlate with coexisting inflammatory components. CTCL neoplastic cells do not typically express a Treg phenotype and are associated with low numbers of FoxP3-positive Tregs in the infiltrate. FoxP3 expression by immunohistochemistry may aid histologic evaluation of these conditions.
Keywords: FoxP3 protein, human; lichenoid eruptions; mycosis fungoides; T-lymphocyte, regulatory
Introduction
FoxP3 is regarded as the most reliable marker for regulatory T cells (Tregs). Tregs are a subset of CD4-positive, CD25-positive, naturally occurring lymphocytes that make up 5% to 10% of circulating T cells and play a vital role in self-tolerance by suppressing the immune response (1). Increasing attention has been focused on the relation between Tregs and neoplastic and autoimmune diseases.
Tumor-associated Tregs have been studied in various malignancies (2), including cutaneous T-cell lymphoma (CTCL). Klemke et al (3) identified an increased number of FoxP3-positive T cells in skin biopsies from patients who had mycosis fungoides (MF) but not from patients who had Sézary syndrome. A group of Danish researchers has suggested that higher numbers of FoxP3-positive T cells in CTCL is associated with a favorable disease stage and improved survival (4).
Despite the prognostic implications of Tregs demonstrated in CTCL, an explicit understanding of the relationship between FoxP3-positive Tregs and CTCL has been elusive. Berger et al (5) induced FoxP3 expression in neoplastic T cells isolated or cultured from patients with Sézary syndrome or MF with peripheral blood involvement, leading some investigators to consider Tregs as a putative cell of origin in CTCL. Several groups (4,6,7) have demonstrated Treg expression in rare examples of CTCL; however, to date, there is no convincing evidence from ex vivo studies that CTCL is a neoplasm of regulatory T cells.
FoxP3 expression by tumor cells in an adult T-cell leukemia/lymphoma (ATLL) is well established, making it useful as a diagnostic marker (8,9). Although some studies have previously implicated a Treg phenotype in CTCL, the presence or absence of FoxP3-positive T cells has not been used as a diagnostic marker in CTCL because FoxP3 expression by the neoplastic population has not been consistently confirmed.
The epidermis has been shown to harbor a relatively high proportion of neoplastic cells in lesional biopsies of patch or plaque-stage MF, in contrast to the dermis, which often contains a mixture of tumor cells and reactive inflammatory T cells (10,11). The determination of FoxP3 expression by tumor-associated T cells specifically in the epidermis of CTCL patients has not been well characterized in previous studies. We analyzed lesional skin biopsies of CTCL (MF and Sézary syndrome) and inflammatory (spongiotic and lichenoid or interface) dermatoses to determine the prevalence of epidermal FoxP3-positive T cells.
Materials and Methods
After approval by the institutional review board, 20 cases of CTCL (13 MF and 7 Sézary syndrome) and 22 cases of inflammatory dermatoses (11 cases of spongiotic dermatitis and 11 cases of lichenoid or interface dermatitis) were identified from the institutional medical records database for patients treated at Mayo Clinic between 1993 and 2008. Medical records were examined to confirm clinical and pathologic correlation.
Cases of spongiotic and lichenoid dermatitis were confirmed by correlating the clinical impression (usually irritant, asteatotic, atopic, or contact dermatitis; and fixed drug, graft-vs-host disease, lupus, lichen planus, lichenoid actinic keratosis, or benign lichenoid keratosis, respectively) with the histologic features. Two cases of spongiotic dermatitis demonstrated atypical features (ie, extensive exocytosis with microabscess formation resembling Pautrier microabscesses, cytologic atypia, and bandlike dermal infiltrate) (Figure 1) but were confirmed to represent dermatitis on clinical follow-up with pathologic correlation. Both biopsies were negative for T-cell clonality on lesional biopsy tissue by polymerase chain reaction. Cases of inflammatory (spongiotic or lichenoid or interface) dermatitis were included if there was a quantifiable epidermal lymphocytic infiltrate.
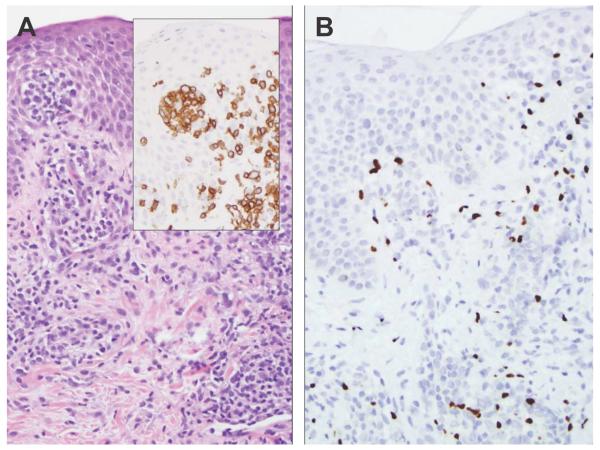
Figure 1.
A, This example of spongiotic dermatitis contains prominent exocytosis and Pautrier-like abscesses containing CD3-positive T cells (inset). B, The FoxP3 immunostain labels approximately 15% of the epidermal and dermal lymphocytes (original magnification, ×200).
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue blocks were stained for antibodies to FoxP3 and interpreted in the context of CD3, CD4, CD5, and CD8 immunostains (Table 1). Immunohistochemical stains for each patient were interpreted semiquantitatively at magnification ×200 by light microscopy of a minimum of 5 fields per case by 2 pathologists (D.A.W. and L.E.G. or R.H.W.). FoxP3-positive lymphocytes were counted as a percentage of the total CD3-positive T-cell population. Epidermal and dermal components of the lymphoid infiltrate were counted separately. Due to the semiquantitative nature of the analysis, the percentages of the infiltrate labeled by the specific antibodies were placed into increments of 5 (range, 0-100). A test for differences was performed using the Wilcoxon rank sum test. FoxP3 expression in the dermis and epidermis was compared using the Wilcoxon signed rank test. The statistical software package JMP version 7.0 (SAS Institute, Inc, Cary, North Carolina) was used to perform statistical analysis.
Table 1.
Immunohistochemical Stain Characteristics
| Antigen | Antibody | Manufacturer | Antigen Retrieval Method |
|---|---|---|---|
| FoxP3 | 236A/E7 | Abcam, Cambridge, Massachusetts |
HIER |
| CD3 | PS1 | Novocastra, Leica Microsystems, Wetzlar, Germany |
HIER |
| CD4 | 4B12 | Novocastra | HEIR |
| CD5 | 4C7 | Novocastra | HIER |
| CD8 | C8144B | Dako Denmark, A/S, Glostrup, Denmark |
HEIR |
Abbreviation: HIER, heat-induced epitope retrieval.
Results
Immunohistochemistry
The CD3 immunostain labeled more than 95% of epidermal lymphocytes, confirming that the epidermal infiltrate was primarily composed of T cells. CD4 and CD8 immunostains interpreted in the context of the histologic findings were found to support the previously rendered diagnoses (data not shown). Immunostaining for FoxP3 was positive on a subset of lymphocytes in a high-intensity nuclear pattern as observed in examples of spongiotic dermatitis (Figures 1 and 2). One case of MF (tumor stage, no epidermal involvement) was not included in the statistical analysis because of the absence of an epidermal infiltrate.
Figure 2.
A, The CD3 immunostain highlights epidermal and dermal T cells in spongiotic dermatitis (original magnification, ×200). B, The FoxP3 immunostain labels 30% of the epidermal infiltrate (original magnification, ×200).
The FoxP3 immunostain labeled the minority (<50%) of epidermal T cells in cases of CTCL and in all but 1 case of inflammatory dermatoses (benign lichenoid keratosis). FoxP3-positive cells composed 5% or less of the intraepidermal infiltrate in 8 of 12 cases of MF in contrast to only 3 of 22 cases of inflammatory disorders. These 3 cases consisted of 2 examples of graft-vs-host disease and 1 case of lupus erythematosus.
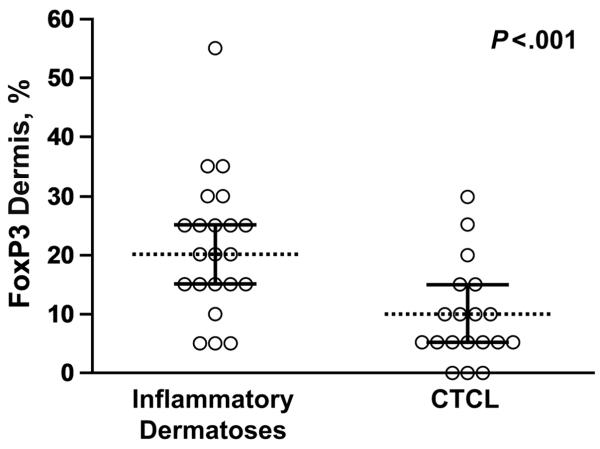
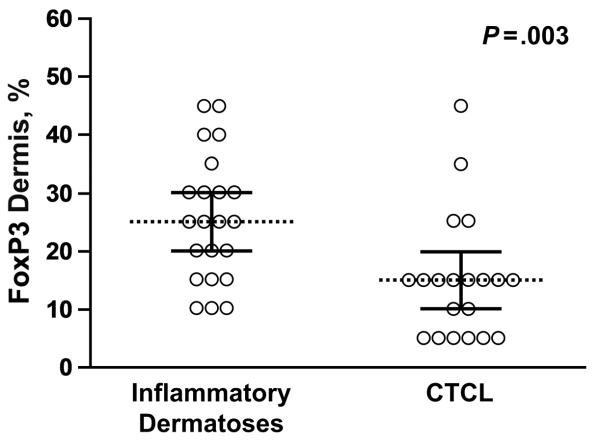
The percentages of intraepidermal FoxP3-positive lymphocytes observed in patients with CTCL (MF and Sézary syndrome) were lower than those observed in patients with spongiotic and lichenoid or interface dermatoses (P<.001; Figure 3). The distinction was not as evident in Sézary syndrome alone, in which percentages of epidermal FoxP3-positive lymphocytes were observed to be 20% or greater in 3 of 7 patients. FoxP3-positive cells made up 15% of the epidermal infiltrate in both cases of spongiotic dermatitis with atypical histologic features. Dermal FoxP3-positive cells were also more common in the inflammatory group than in CTCL (P=.003; Figure 4).
Figure 3.
The proportion of intraepidermal FoxP3-positive cells is greater in the inflammatory group (median, 20%) than in the cutaneous T-cell lymphoma group (median, 5%) (P<.001).
Figure 4.
The proportion of intradermal FoxP3 cells is greater in the inflammatory group (median, 25%) than in the cutaneous T-cell lymphoma group (median, 15%) (P=.003).
Discussion
Tregs are classically described as having a CD4-positive, CD25-positive, FoxP3-positive phenotype, although defining this subset of T cells in humans has been challenging (12). Non-Treg—activated effector T cells have been shown to express CD25, and not all Tregs express CD25 (2). Ex vivo studies on Tregs have demonstrated FoxP3 to be a superior marker for Tregs over CD25 (3).
In a small series of patients, our experience with CD25 stains was consistent with the purported lack of specificity (data not shown). Although the induction of FoxP3 in T cells of non-Treg origin has also been demonstrated, we selected FoxP3 because it is currently accepted as the most reliable marker for Tregs and because it is the most widely used in studies examining the relation between Tregs and human cancer (4,13).
The variable percentages of FoxP3-positive T cells observed in our cases of Sézary syndrome was not surprising, given the high frequency of nonspecific histologic features that have been demonstrated in skin biopsies of these patients (14). Histologic features consistent with chronic dermatitis were previously demonstrated in 33% of the skin biopsies from patients with Sézary syndrome (14). In our series, variable FoxP3 immunopositivity was noted in the epidermal T cells of patients with Sézary syndrome, albeit it was higher than that observed in patients with MF. Cases of CTCL in which FoxP3-positive cells composed 10% or more of the infiltrate (including 5 of the 7 cases of Sézary syndrome and 3 of the 4 cases of MF) also contained higher populations of CD8-positive T cells, which may reflect the involvement of a coexisting reactive or inflammatory T-cell population in these specimens (data not shown). This possibility is supported by the presence of higher proportions of FoxP3-positive T cells in the dermal infiltrate compared with those in the epidermis in cases of CTCL, a finding that was similar in the dermis and epidermis of the inflammatory group. None of the biopsy specimens from our patients contained clearly detectable FoxP3 expression in the neoplastic population.
The characterization of FoxP3 expression by T cells in patients with CTCL in comparison with inflammatory skin lesions has not been performed until recently (15,16). Fujimura et al (16) identified high numbers of FoxP3-positive T cells in the epidermis of patients with psoriasis or MF but lower numbers in patients with eczematous dermatitis. Our distinct results showed a lower percentage (≤10%) of FoxP3-positive T cells in the epidermis of lesions from patients with MF than reported previously (46%) (16). Solomon and Magro (15) have reported findings similar to ours (14% FoxP3-positive Tregs in patients with MF, and 22-25% in patients with eczematous and lichenoid dermatitis). This discrepancy may be explained at least in part by the use of different antibodies to FoxP3 in the 2 studies; we used mouse monoclonal A236/E7 (as did Solomon and Magro), whereas rabbit anti-FoxP3 antibody (clone not specified) was used by Fujimura et al (16).
In a letter to the editor, Banham et al (17) brought attention to the variability of FoxP3 expression across several studies. This group demonstrated immunoreactivity of the hFOXY anti-FoxP3 antibody in transformed CTCL cells in a cytoplasmic pattern, but the cells were not immunoreactive to the 3 other anti-FoxP3 monoclonal antibodies (including 236A/E7), which had been shown to bind to major isoforms of FoxP3. Furthermore, the hFOXY antibody was reported to label a substantial population of CD4-negative T cells by flow cytometry. Hallermann et al (18) subsequently retested a subset of their cases using 236A/E7 and found them to be negative. Banham et al (17) therefore supported the use of 236A/E7 for FoxP3 immunolabeling of T cells for clinical diagnostic purposes.
Controversy has existed regarding the expression of Treg markers in CTCL. In 2006, Berger et al (5) provided data that led to the suggestion that CTCL may represent a proliferation of Tregs. This conclusion was based on the expression of a Treg phenotype by in vitro CTCL cells treated with cell lysates/cytokines derived from preconditioned dendritic cells. FoxP3 expression by the neoplastic cells in CTCL was subsequently demonstrated in a series of 5 patients with MF who had large-cell transformation by immunohistochemistry using the hFOXY antibody, although these findings were later questioned and revised (see above) (6,18), and in a study using target gene expression and flow cytometry on patients with Sézary syndrome and MF with peripheral blood involvement (7). Capriotti et al (7) demonstrated FOXP3 mRNA (messenger ribonucleic acid) expression in the neoplastic population in a minority (5 of 28) of cases of Sézary syndrome, although only 2 patients had a 10-fold or greater expression level compared with that of the control sample.
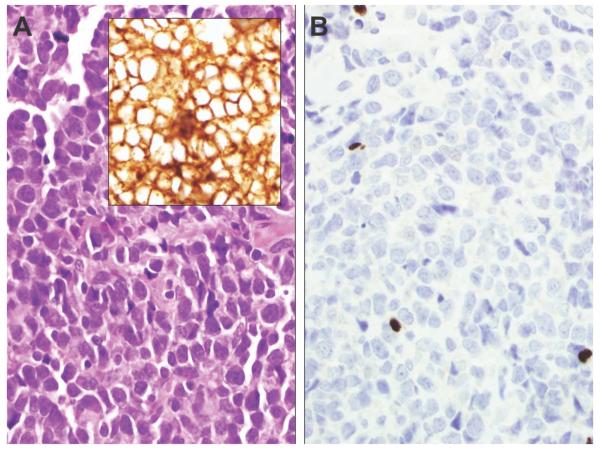
Other groups have provided evidence that FoxP3 expression is unusual in the neoplastic population in CTCL, including Tiemessen et al (19), who conducted ex vivo analysis of CTCL cells to demonstrate the absence of a Treg phenotype in tumor cells, as did Solomon and Magro (15), who recently demonstrated a low frequency of FoxP3-positive Tregs in MF compared with those in inflammatory dermatosis. The paucity of FoxP3-positive T cells in the epidermal infiltrate of MF in our study (Figure 5) supports the latter finding because the malignant epidermotropic population was negative for FoxP3 in all MF lesions, as well as in 2 cases of tumor stage CTCL (Figure 6).
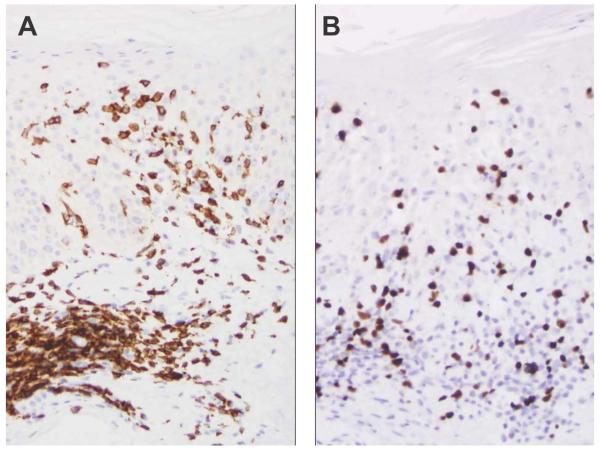
Figure 5.
A, The CD3 immunostain highlights atypical epidermal and dermal T cells in this example of mycosis fungoides (original magnification, ×200). B, The FoxP3 stain is negative in the epidermotropic population but positive in small, scattered dermal lymphocytes (original magnification, ×200).
Figure 6.
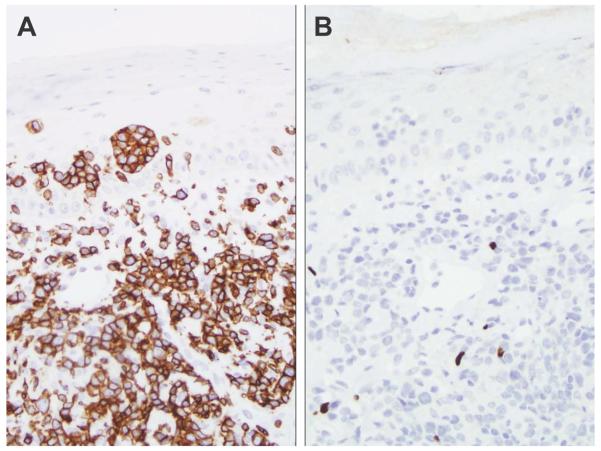
A, Tumor-stage mycosis fungoides (original magnification, ×400) with a sheet of CD4-positive neoplastic cells (inset, original magnification, ×400). B, The large tumor cells are negative for FoxP3, whereas occasional small lymphocytes are positive and make up approximately 5% of the tumor (original magnification, ×400).
Our results are consistent with the prevailing theory that neoplastic cells in CTCL do not typically express detectable FoxP3 ex vivo and are thus not characterized as having a Treg phenotype with rare exception. Rare examples of CTCL with convincing evidence of ex vivo FoxP3 expression have represented Sézary syndrome (7) or aggressive epidermotropic CD8-positive cytotoxic T-cell lymphoma (4) instead of MF.
The paucity of FoxP3-positive epidermal T cells observed in MF is of uncertain significance. Neoplastic T cells have been shown to express the Fas ligand, which may result in a reduction of tumor-associated Tregs and other inflammatory populations (20). Alternatively, it may be related to the unsuppressed proliferation of neoplastic lymphocytes in the epidermis in this disorder, although evidence supporting this hypothesis is lacking. In contrast, neoplasm-associated FoxP3-positive Tregs may attenuate antitumor immunity in other malignancies, particularly solid tumors (2), thus providing a possible explanation for the paradoxical differences in the prognostic significance observed for Tregs in CTCL vs other nonhematologic malignancies.
Although our results demonstrated a significant difference between the CTCL and inflammatory groups, 4 of the 12 biopsies from patients who had MF with an intraepidermal infiltrate were observed to contain FoxP3-positive cells that composed 10% or 15% of the infiltrate, which overlapped the percentages observed in the 2 cases of spongiotic dermatitis with histologic features resembling MF. Futhermore, the low overall percentage of FoxP3-positive cells in both groups compounds the potential challenges inherent in the use of FoxP3 as a diagnostic tool because of the semiquantitative nature of immunohistochemical evaluation. Therefore, we cannot presume that the presence or absence of FoxP3-positive T cells is highly reliable for confirmation or exclusion of a diagnosis of MF, especially when there are limited sample numbers. We nonetheless conclude that more epidermal FoxP3-positive cells are observed in spongiotic and most lichenoid dermatitis than in CTCL.
Table 2.
Percentages of Lymphocytes With Positive Staining for FoxP3 in Mycosis Fungoides and Sézary Syndrome
| FoxP3-Positive T Cells, % |
|||
|---|---|---|---|
| Patient | Diagnosis | Epidermis | Dermis |
| 1 | MF, patch or plaque |
5 | 15 |
| 2 | MF, patch or plaque |
0 | 5 |
| 3 | MF, patch or plaque |
0 | 15 |
| 4 | MF, patch or plaque |
0 | 15 |
| 5 | MF, patch or plaque |
5 | 25 |
| 6 | MF, patch or plaque |
5 | 15 |
| 7 | MF, patch or plaque |
10 | 15 |
| 8 | MF, patch or plaque |
15 | 25 |
| 9 | MF, patch or plaque |
10 | 5 |
| 10 | MF, patch or plaque |
10 | 10 |
| 11 | MF, patch or plaque |
5 | 5 |
| 12 | MF, patch or plaque |
5 | 5 |
| 13 | MF, tumor | NA | 5 |
| 14 | SS, tumor | 5 | 10 |
| 15 | SS, erythroderma | 30 | 15 |
| 16 | SS, erythroderma | 25 | 45 |
| 17 | SS, erythroderma | 20 | 35 |
| 18 | SS, erythroderma | 10 | 5 |
| 19 | SS, erythroderma | 15 | 15 |
| 20 | SS, erythroderma | 5 | 15 |
Abbreviations: MF, mycosis fungoides; NA, not applicable; SS, Sézary syndrome.
Table 3.
Percentages of Lymphocytes With Positive Staining for FoxP3 in Acute or Subacute Spongiotic Dermatitis and Lichenoid or Interface Dermatitis
| FoxP3-Positive T Cells, % |
|||
|---|---|---|---|
| Patient | Diagnosis | Epidermis | Dermis |
| 1 | AD | 15 | 20 |
| 2 | SAD | 20 | 30 |
| 3 | SAD | 25 | 40 |
| 4 | SAD | 35 | 40 |
| 5 | SAD | 20 | 30 |
| 6 | SAD | 25 | 45 |
| 7 | SAD | 30 | 20 |
| 8 | SAD | 15 | 10 |
| 9 | SAD | 20 | 45 |
| 10 | SAD | 15 | 25 |
| 11 | SAD | 15 | 25 |
| 12 | Fixed drug | 10 | 20 |
| 13 | GVHD | 5 | 15 |
| 14 | GVHD | 5 | 10 |
| 15 | LE | 5 | 15 |
| 16 | LE | 35 | 25 |
| 17 | LE | 25 | 30 |
| 18 | LP | 25 | 10 |
| 19 | LP | 25 | 25 |
| 20 | LAK | 30 | 30 |
| 21 | BLK | 15 | 15 |
| 22 | BLK | 55 | 35 |
Abbreviations: AD, acute dermatitis, acute spongiotic dermatitis; BLK, benign lichenoid keratosis; GVHD, graft- vs-host disease; LAK, lichenoid actinic keratosis; LE, lupus erythematosus; LP, lichen planus; NA, not applicable; SAD, subacute dermatitis, subacute spongiotic dermatitis.
Acknowledgments
We wish to express special thanks to the biostatisticians in the Center for Translational Science Activities at Mayo Clinic whose work is supported in part by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official view of NCRR or NIH. We also wish to thank Matthew Broeren for assistance with image editing.
Abbreviations
- ATLL
adult T-cell leukemia/lymphoma
- CTCL
cutaneous T-cell lymphoma
- MF
mycosis fungoides
- mRNA
messenger ribonucleic acid
- Tregs
regulatory T cells
Footnotes
Conflict of Interest: None.
References
- 1.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006 Apr;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 2.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006 Aug 1;108(3):804–11. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 3.Klemke CD, Fritzsching B, Franz B, Kleinmann EV, Oberle N, Poenitz N, et al. Paucity of FOXP3+ cells in skin and peripheral blood distinguishes Sezary syndrome from other cutaneous T-cell lymphomas. Leukemia. 2006 Jun;20(6):1123–9. doi: 10.1038/sj.leu.2404182. [DOI] [PubMed] [Google Scholar]
- 4.Gjerdrum LM, Woetmann A, Odum N, Burton CM, Rossen K, Skovgaard GL, et al. FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: association with disease stage and survival. Leukemia. 2007 Dec;21(12):2512–8. doi: 10.1038/sj.leu.2404913. [DOI] [PubMed] [Google Scholar]
- 5.Berger CL, Tigelaar R, Cohen J, Mariwalla K, Trinh J, Wang N, et al. Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory cells. Blood. 2005 Feb 15;105(4):1640–7. doi: 10.1182/blood-2004-06-2181. [DOI] [PubMed] [Google Scholar]
- 6.Hallermann C, Niermann C, Schulze HJ. Regulatory T-cell phenotype in association with large cell transformation of mycosis fungoides. Eur J Haematol. 2007 Mar;78(3):260–3. doi: 10.1111/j.1600-0609.2006.00809.x. [DOI] [PubMed] [Google Scholar]
- 7.Capriotti E, Vonderheid EC, Thoburn CJ, Wasik MA, Bahler DW, Hess AD. Expression of T-plastin, FoxP3 and other tumor-associated markers by leukemic T-cells of cutaneous T-cell lymphoma. Leuk Lymphoma. 2008 Jun;49(6):1190–201. doi: 10.1080/10428190802064917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karube K, Ohshima K, Tsuchiya T, Yamaguchi T, Kawano R, Suzumiya J, et al. Expression of FoxP3, a key molecule in CD4CD25 regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br J Haematol. 2004 Jul;126(1):81–4. doi: 10.1111/j.1365-2141.2004.04999.x. [DOI] [PubMed] [Google Scholar]
- 9.Roncador G, Garcia JF, Garcia JF, Maestre L, Lucas E, Menarguez J, et al. FOXP3: a selective marker for a subset of adult T-cell leukaemia/lymphoma. Leukemia. 2005 Dec;19(12):2247–53. doi: 10.1038/sj.leu.2403965. [DOI] [PubMed] [Google Scholar]
- 10.Cerroni L, Arzberger E, Ardigo M, Putz B, Kerl H. Monoclonality of intraepidermal T lymphocytes in early mycosis fungoides detected by molecular analysis after laser-beam-based microdissection. J Invest Dermatol. 2000 Jun;114(6):1154–7. doi: 10.1046/j.1523-1747.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- 11.Ortonne N, Buyukbabani N, Delfau-Larue MH, Bagot M, Wechsler J. Value of the CD8-CD3 ratio for the diagnosis of mycosis fungoides. Mod Pathol. 2003 Sep;16(9):857–62. doi: 10.1097/01.MP.0000084112.81779.BB. [DOI] [PubMed] [Google Scholar]
- 12.Banham AH, Powrie FM, Suri-Payer E. FOXP3+ regulatory T cells: current controversies and future perspectives. Eur J Immunol. 2006 Nov;36(11):2832–6. doi: 10.1002/eji.200636459. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber TH. The use of FoxP3 as a biomarker and prognostic factor for malignant human tumors. Cancer Epidemiol Biomarkers Prev. 2007 Oct;16(10):1931–4. doi: 10.1158/1055-9965.EPI-07-0396. [DOI] [PubMed] [Google Scholar]
- 14.Trotter MJ, Whittaker SJ, Orchard GE, Smith NP. Cutaneous histopathology of Sezary syndrome: a study of 41 cases with a proven circulating T-cell clone. J Cutan Pathol. 1997 May;24(5):286–91. doi: 10.1111/j.1600-0560.1997.tb00793.x. [DOI] [PubMed] [Google Scholar]
- 15.Solomon GJ, Magro CM. Foxp3 expression in cutaneous T-cell lymphocytic infiltrates. J Cutan Pathol. 2008 Nov;35(11):1032–9. doi: 10.1111/j.1600-0560.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- 16.Fujimura T, Okuyama R, Ito Y, Aiba S. Profiles of Foxp3+ regulatory T cells in eczematous dermatitis, psoriasis vulgaris and mycosis fungoides. Br J Dermatol. 2008 Jun;158(6):1256–63. doi: 10.1111/j.1365-2133.2008.08504.x. [DOI] [PubMed] [Google Scholar]
- 17.Banham AH, Brown PJ, Lyne L, Schulze HJ, Hallermann C. Is FOXP3 expressed in cutaneous T-cell lymphomas [letter]? Eur J Haematol. 2008 Jan;80(1):90–1. doi: 10.1111/j.1600-0609.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 18.Hallermann C, Schulze HJ, Neumann C, Klemke CD. The regulatory T-cell phenotype in progressive mycosis fungoides. G Ital Dermatol Venereol. 2008 Feb;143(1):15–9. [PubMed] [Google Scholar]
- 19.Tiemessen MM, Mitchell TJ, Hendry L, Whittaker SJ, Taams LS, John S. Lack of suppressive CD4+CD25+FOXP3+ T cells in advanced stages of primary cutaneous T-cell lymphoma. J Invest Dermatol. 2006 Oct;126(10):2217–23. doi: 10.1038/sj.jid.5700371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni X, Hazarika P, Zhang C, Talpur R, Duvic M. Fas ligand expression by neoplastic T lymphocytes mediates elimination of CD8+ cytotoxic T lymphocytes in mycosis fungoides: a potential mechanism of tumor immune escape? Clin Cancer Res. 2001 Sep;7(9):2682–92. [PubMed] [Google Scholar]