Abstract
Drebrin is a filament binding protein involved in organizing the dendritic pool of actin. Previous in vivo studies identified the actin-binding domain of drebrin (DrABD), which causes the same rearrangements in the cytoskeleton as the full length protein. Site directed mutagenesis, electron microscopic (EM) reconstruction and chemical cross-linking combined with mass spectrometry analysis were employed here to map the DrABD binding interface on actin filaments. DrABD could be simultaneously attached to two adjacent actin protomers using the combination of 2-iminothiolane (Traut's reagent) and 1,1-methanediyl bis(methanethiosulfonate) (MTS1). Site directed mutagenesis combined with chemical cross-linking revealed that residue 238 of DrABD is located within 5.4 Å from C374 of actin protomer 1, and drebrin's native cysteine 308 is in close proximity to C374 of actin protomer 2. Mass spectrometry analysis revealed that a zero length cross-linker, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), can link the N-terminal G-S extension of the recombinant DrABD, to E99 and/or E100 on actin. Efficient cross-linking of drebrin residues 238, 248, 252, 270, and 271 to actin residue 51 was achieved with reagents of different length (5.4 – 19 Å). These results suggest that the ‘core’ DrABD is centered on actin's subdomain 2 and may adopt a folded conformation upon binding to F-actin. The results of EM reconstruction, which are in a good agreement with the cross-linking data, revealed polymorphism in DrABD binding to F-actin and suggested the existence of two binding sites. These results provide new structural insight into the previously observed competition between drebrin and several other F-actin binding proteins.
Keywords: actin, drebrin, electron microscopy, mass spectrometry, cross-linking, mapping
Introduction
Dendritic spines are known to be very motile and change their shape during neuronal development and in adult brain in response to various stimuli (1). The actin cytoskeleton is a primary modulator of spine morphology. Drebrin is a filament binding protein which is involved in organizing the dendritic pool of actin (1). It was shown that synaptic deterioration in the brains of Alzheimer's disease patients is accompanied by dramatically decreased levels of drebrin. Reduced drebrin level is observed also in Down's syndrome (2). Biochemical analysis of various tissues revealed that depending on the specific growth state and cell density, drebrin can form higher order oligomers named ‘drebrosomes’ which consist of drebrin and actin alone (3; 4). It was hypothesized that such complexes allow for high local concentrations of drebrin and may play a role in the local regulation of actin assembly as was previously shown for some tropomyosins (5).
Drebrin binds to F-actin with a stoichiometry of one to five protomers (Kd ∼ 0.12 μM) and shows no actin severing, nucleating or bundling activity in vitro (6). It was previously reported that drebrins compete with F-actin-binding proteins such as α-actinin, tropomyosin and fascin (6-8). Drebrin inhibits the actin-activated ATPase activity of myosin, but its effect on actomyosin sliding velocity remains unclear. A three-fold decrease in actin sliding velocity was previously reported, but not confirmed in recent studies (9; 10). According to in vitro studies, drebrin can be displaced from actin filaments by cofilin (11). This observation is consistent with the finding that in the brains of Alzheimer's disease patients drebrin loss is accompanied by increased levels of cofilin (2).
Drebrin shares homology with a mammalian actin-binding protein 1 (mAbp1) through an N-terminal ADF-homology domain (ADFH) and helical/charged motif (HCm), which is specific only to these two proteins (12; 13). However, HCm is more extended in drebrins than in Abp1 and contains a unique sequence (residues 233-300/317) that was identified in previous in vivo studies as drebrin's actin-binding domain (DrABD) (Scheme 1) (14; 15). Interestingly, this 85 amino acid DrABD causes the same rearrangements in the actin cytoskeleton as the full length drebrin, and is highly conserved among mammals (8; 14). Both drebrin and the homologous Abp1 are present in neuronal cells and contain an actin-binding domain within their HC motif (1; 16). Nevertheless, overexpression of DrABD and the helical/charged domain of Abp1 has different effects on the morphology and density of dendritic spines. Similar to full length drebrin, overexpression of DrABD in rat hippocampal neurons transforms mature dendritic spine into immature dendritic filopodia (without changing the overall density of the spines) and causes the loss of synaptic contacts. This destabilizing effect of drebrin on spine morphology appears to be mediated entirely through its actin-binding domain (8). Thus, structurally and functionally DrABD represents a unique motif among the known actin-binding modules.
Scheme 1.
The important role of drebrin in actin regulation calls for structural understanding of the actin-drebrin complex. To date, no structural information on drebrin-actin interaction has been reported. In this study we probed the binding interface of DrABD on actin filaments using site directed mutagenesis, electron microscopic reconstruction, and chemical cross-linking combined with mass spectrometry analysis. Our results revealed that DrABD makes extensive contacts with subdomains 1 and 2 on actin and shows polymorphism in F-actin binding. Our data provide structural insight into the previously observed competition between drebrin and some of the actin side-binding proteins like α-actinin, cofilin and tropomyosin.
Results
Characterization of the drebrin constructs
It was previously documented that only the N-terminal part of the drebrin molecule, and DrABD in particular, show actin binding and remodeling activity in vivo. We compared the actin binding properties of the isolated drebrin ADF homology domain (residues 1-134), DrABD, and drebrin construct 1-300 containing both ADF and DrABD domains. Since it was previously shown that overexpression of both the 233-317 (DrABD) and the 233-300 (DrABD300) drebrin fragments causes in vivo effects similar to those of the full length drebrin (14; 15), the actin binding properties of these constructs were tested under identical experimental conditions and compared with each other.
Drebrin 1-300, binds to F-actin with a high affinity, Kd ∼ 0.2 μM (similar to that of full size drebrin) (6) and a binding stoichiometry of ∼1:3 (Fig. 1(a)). Under the conditions of our experiments the isolated ADF-homology domain of drebrin did not show any binding to F-actin (Fig. 1(c, d)).
Fig. 1. Binding of the drebrin constructs to actin filaments.

Binding affinity of drebrin constructs for F-actin was estimated by pelleting assays (see Materials and Methods). (a) Binding of the 1-300 drebrin construct to actin filaments (10 μM). Solid line corresponds to the best data fit. A Kd of the 1-300 drebrin construct for F-actin (0.17±0.005 μM) was calculated based on two independent experiments. (b) Binding affinities of N-GST fused DrABD constructs for F-actin (10 μM). Solid lines correspond to the best data fit with Kd = 6.6 ± 0.4 μM and 7.6 ± 0.6 μM for DrABD and DrABD300, respectively. Kd is an average value obtained in two independent experiments, as described in Materials and Methods. (c) Co-sedimentation of drebrin's ADF homology domain (drADF) with F-actin (Ac) (10 μM). (d) Control co-sedimentation of yeast cofilin (cof) with F-actin (10 μM) under the same conditions as in (d). Buffer composition: 5 mM MOPS, pH 7.2, 0.2 mM CaCl2, 0.4 mM EGTA, 0.2 mM ATP, 1 mM DTT, 50 mM KCl, 2 mM MgCl2. Lanes 1 – 6, 10 μM F-actin co-sedimented with 1.25, 2.5, 5, 10, 15, 25 μM of drebrin's ADF homology domain; lanes 9 – 14, 10 μM F-actin co-sedimented with 1.25, 2.5, 5, 10, 15, 25 μM of yeast cofilin; lanes 7 and 8, F-actin alone, supernatant and pellet, respectively.
Pelleting experiments have shown that N-GST fused DrABD binds to F-actin with a relatively low affinity compared to the construct 1-300 (Kd = 6.6 ± 0.4 μM) (Fig. 1(b)). The C-terminal truncation of DrABD, to produce DrABD300, does not affect its binding to F-actin significantly (Kd = 7.6 ± 0.6 μM). For both constructs the DrABD - F-actin binding stoichiometry was close to 1:2 DrABD : actin protomers; the actual mole ratio (1 : 1.6) may reflect DrABD oligomerization, partial occupancy of actin filament by DrABD or its multiple binding modes (see Discussion).
The actin binding domain of drebrin has no homology among known proteins and its sequence is abundant in glutamic acid (∼19%) and arginine (∼11%) (Fig. 2(a)). The secondary structure of this actin binding module as revealed by circular dichroism spectroscopy (CD), contains 28% helix, 15% β-sheet, 21% turns and 36% random coil (a total of 57% unstructured) (Fig. 2(b)). The secondary structure of DrABD is invariable over the pH range from 5.0 to 8.6 (data not shown).
Fig. 2. Structure of DrABD.
(a) Sequence of DrABD. Residues predicted to form helical structures are colored in gray (Jpred 3). The C-terminal extension that is truncated in DrABD300 construct is underlined. Two extra amino acids at N-terminus of the recombinant DrABD constructs are marked with asterisks. (b) CD spectrum (average of eight runs) of DrABD. Based on the results of two independent experiments, the secondary structure composition of DrABD is estimated to contain 28% helix, 15% β-sheets, 57% turns and random coil.
Electron microscopic reconstruction of F-actin decorated with the drebrin constructs
Electron microscopic images of F-actin alone and actin decorated with DrABD and 1-300 constructs are shown in Fig. 3(a, b and b*), respectively. Despite affinity differences, the same modes of F-actin binding and binding polymorphism were documented for both DrABD (Fig. 3(d-h)) and drebrin 1-300 constructs (Fig. 3(d*-h*)).
Fig. 3. Electron microscopy and 3D-reconstruction of the drebrin-F-actin complex.


Electron micrographs of F-actin alone (a) and (a*), filaments decorated with the DrABD construct (b) and drebrin 1-300 construct (b*). Three-dimensional reconstructions of pure F-actin (c) and five modes of binding of drebrin to F-actin (d - h). Atomic model of actin filament docked into each map is shown as blue ribbons (c - h). F-actin residues 99 and 100 (red), 51 (yellow), and 374 (green) are shown as spheres (c - h). An electron density envelope that corresponds to a globular protein containing 84 amino acid residues is shown as magenta meshwork (d - h). Comparison of the binding modes of DrABD and drebrin 1-300 construct to F-actin (d*) – (h*). Reconstructions of F-actin decorated with DrABD are shown as transparent surfaces, while volumes resulted from filaments complexed with the 1-300 construct containing both DrABD and AFD-homology domain are shown as blue meshwork. Atomic model of actin filament docked into each map is shown as blue ribbons (d* - h*). F-actin residues 99 and 100 (red), 51 (yellow), and 374 (green) are shown as spheres (a - e). An electron density envelope that corresponds to a globular protein comprised of 84 amino acid residues is shown as magenta solid surface. The modes of binding obtained for drebrin 1-300 construct are similar to the ones observed for isolated DrABD
Extensive decoration of actin filaments was observed in the presence of DrABD (Fig. 3(b)) as well as with the shorter construct DrABD300 and N-GST-fused DrABD (data not shown). We collected 9,749 segments from images of actin filaments decorated with the drebrin fragment. During the first step of sorting, segments were separated by the occupancy (see Materials and Methods and Appendix A) and ‘naked’ F-actin segments were reconstructed separately from occupied ones. Reconstruction of the ‘naked’ F-actin (Fig. 3(c)) was similar to the reconstruction of pure F-actin from our previous studies (17). Five modes of DrABD binding to actin filaments are shown in Fig. 3(d-h). To estimate whether the observed additional mass in the reconstructions was consistent with the molecular weight of the drebrin construct, a portion of the globular CH domain of α-actinin (PDB 1wku) that consists of 84 residues (∼9 kDa) was used. This model protein fragment was filtered to ∼24Å resolution, and its expected molecular volume was docked into the drebrin density in each map (Fig. 3(d-h), magenta mesh). In the first mode (Fig. 3(d)) drebrin bridges to two adjacent actin protomers and makes an extensive contact with subdomains 1 and 2. In the mode shown in Fig. 3(e), drebrin is located in front of actin's subdomain 1. We also observed drebrin in a mode similar to the one shown in Fig. 3(d), but in this mode DrABD interacts with one protomer at a time (Fig. 3(f)). We suggest that all three modes shown in Fig. 3(d, e and f) are variations of a major DrABD binding site (∼40% of segments), which involves subdomains 1 and 2 of actin.
We found that ∼15% of the segments decorated with DrABD reflect a different type of attachment (Fig. 3(g, h)). In the binding mode shown in Fig. 3(g) DrABD is attached to the side of subdomain 1 and 2. In this case, the observed mass was larger than the mass corresponding to a globular protein fragment of 84 amino acids, which suggests that in this mode DrABD binds to filaments as an oligomer. Interestingly, in this mode DrABD makes contact with subdomain 4 of an actin protomer on the opposite strand (Fig. 3(g), red arrow). This contact is more prominent in the mode shown in Fig. 3(h).
The same modes of binding as documented for DrABD were observed also for the 1-300 construct, despite of its much higher affinity for F-actin (∼0.2 μM) (Fig. 1(a) and Fig. 3(d*-h*)). It should be noted that the 1-300 construct may contain weak actin-interacting site/sites other than DrABD, which would explain its higher affinity for the actin filaments. However, the results of EM reconstruction suggest that DrABD contains the strongest binding site within the 1-300 drebrin fragment and probably competes with weaker bound structural elements for the interaction with actin when the 1-300 fragment is added in excess. Based on our EM results and previous in vivo studies, the DrABD construct was chosen for mapping the drebrin binding site on F-actin.
DrABD bridges two actin protomers
To probe DrABD - F-actin binding interface via cross-linking reactions, the modification of DrABD with 2-iminothiolane (Traut's reagent) was employed. This reagent was used to introduce sulfydryl (-SH) groups on the surface of the fragment. Potentially, all five lysines (K238, K248, K252, K270 and K271) and the N-terminal amino group can be modified with 2-iminothiolane. Additionally, DrABD contains native cysteine 308 within its C-terminal unstructured extension (residues 301-317). Considering the relatively low affinity of DrABD to actin, we attached first the cross-linking reagents to F-actin, as was done earlier for the mapping of actin-thymosin β4 complex (18). Cross-linking experiments revealed that DrABD modified with Traut's reagent can be covalently attached to MTS1 pre-modified F-actin yielding two protein populations: actin-DrABD heterodimer, and species that according to their molecular weight correspond to a complex of two actin protomers and one DrABD molecule (Fig. 4(a)). A control experiment was carried out using MTS1-pre-modified F-actin and intact DrABD construct with no modifications. A significant amount of actin-DrABD heterodimer was detected by SDS PAGE under non-reducing conditions indicating that native C308 on DrABD is involved in the cross-linking (Fig. 4(b)).
Fig. 4. DrABD is in close proximity to the C-terminal regions of two adjacent actin protomers.

(a) DrABD treated with 2-iminothiolane (Traut's reagent) can be covalently attached to MTS1 pre-modified skeletal F-actin: lane 1 – MTS1 modified FA (10 μM) (A), lane 2 - 2-iminothiolane-treated DrABD (20 μM) incubated for 5 min. with MTS1 modified actin. Two main cross-linking products were detected: actin-DrABD heterodimer (AD) and the species that according to molecular weight and mass spectrometry analysis corresponds to two actin protomers and one DrABD (ADA). (b) Native C308 of DrABD is within 5.4 Å from C374 on actin: lanes 1 – 3 - skeletal F-actin; lanes 4 - 6 - WT yeast actin; lanes 7 – 9 - yeast actin mutant C374A. Lanes 1, 4, 7 - MTS1 modified actins; lanes 2, 5, 8 - MTS1 modified actins in the presence of DrABD (5 min reaction time); lanes 3, 6, 9 – same as lanes 2, 5, 8 but reaction time is 17 min. The final concentrations of actin and DrABD were 9.5 μM and 28.5 μM, respectively.
C-terminal region of DrABD (residues 301-317) cross-links to C374 on actin
Our experiments revealed that DrABD can be efficiently cross-linked to both skeletal muscle (α-) and yeast WT (cytoplasmic) F-actin pre-modified with MTS1. The similar cross-linking yield (∼ 60%) obtained for both actins may indicate that the DrABD-actin interaction is not isoform specific. The C-terminal cysteine 374 is the most reactive cysteine on actin and is expected to be efficiently modified with MTS1 reagents (19). However, to confirm that the cross-linking on actin indeed involves C374, we used a yeast actin mutant with this residue substituted to alanine (C374A). No cross-linking was detected in the case of C374A actin mutant, which indicates that drebrin's native C308 locates within 5.4 Å from C-terminal cysteine 374 on actin (Fig. 4(b)).
Mass spectrometry analysis of the purified complex of 2-iminothiolane-modified DrABD attached simultaneously to two actin protomers revealed that disulfide (MTS1) cross-linking between native C308 on DrABD and C10 on actin may also occur (Appendix B). However the fact that unmodified DrABD can be efficiently cross-linked to WT yeast actin, which lacks C10, suggests that this type of attachment reflects a minor mode of DrABD-actin binding.
N-terminal part (233-271) of DrABD can be cross-linked to Subdomains 1 and 2 of two adjacent actin protomers
Site directed mutagenesis was employed to locate the N-terminal part of DrABD (sequence 233-271) on actin filaments. Based on the results obtained with 2-iminothiolane-modified DrABD (Fig. 5(a)), all five lysines (K238, K248, K252, K270 and K271) and the N-terminal glycine were potential candidates for cross-linking to the second actin protomer. To identify the residues on DrABD that are in close proximity to actin we created five mutants with single lysine to cysteine replacements in the construct DrABD300. These mutants did not impair the complex formation of DrABD300 with F-actin (see Materials and Methods). The fact that DrABD bridges two actin protomers, making contacts with their C-terminal segments, called for probing the DrABD interaction with actin subdomain 2. Actin mutants D51C/C374S and S60C/C374A were employed for such a mapping. Mutant A144C/C374A was chosen to test for the binding of DrABD to actin's hydrophobic cleft between subdomains 1 and 3, which is known to interact with several actin-binding proteins. All yeast actin mutants employed in this study show normal polymerization properties (20; 21).
Fig. 5. Mapping DrABD binding interface on F-actin.
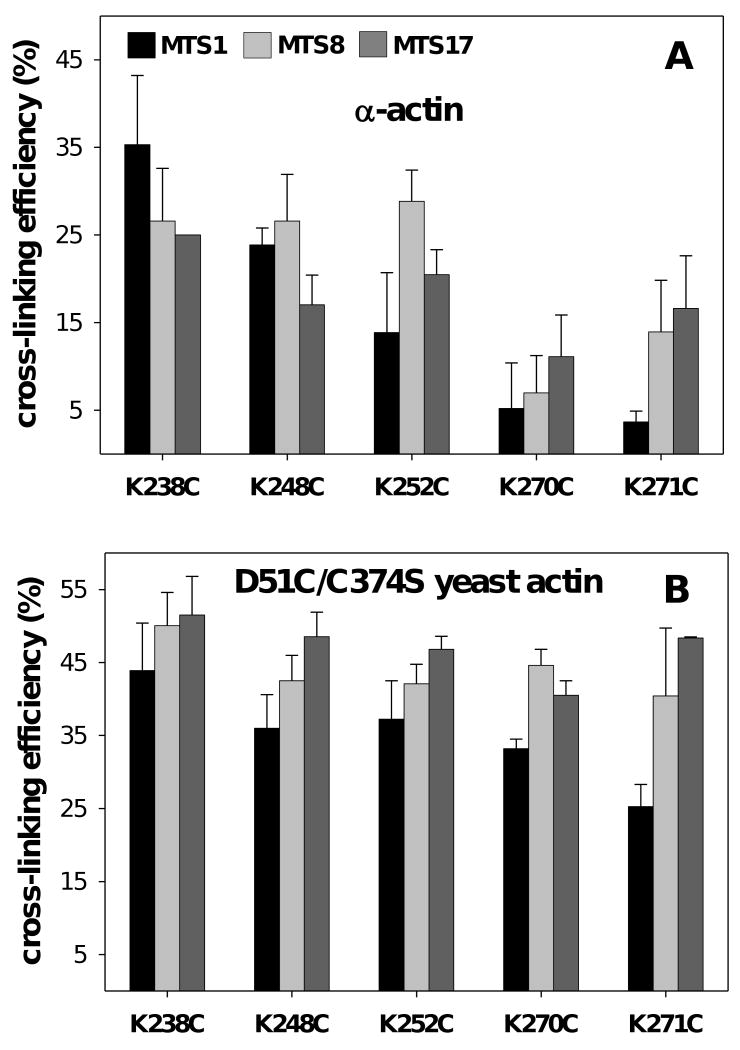
(A) Thiol specific cross-linking of five DrABD mutants to skeletal F-actin (a) or to yeast actin mutant D51C/C374S (b) modified with MTS reagents of different length. Drebrin's residue 238 is within 5.4 Å from the Cys 374 of actin (C-terminus). Drebrin's residues 238, 248, 252, 270 and 271 are within ∼12.1 Å from residue 51 on actin (D-loop, subdomain 2). F-actin was pre-modified with MTS immediately prior to the cross-linking. The final concentrations of actin and DrABD were 10 μM and 30 μM, respectively. The reactions were stopped with NEM after 5 min and the resulting mixtures were analyzed by SDS PAGE. Relative intensities of protein bands were determined by densitometric analysis. Cross-linking efficiencies were estimated as follows: [actin total (before the reaction) – uncross-linked actin monomer left after 5 min]/total actin, %. Black: MTS1 (5.4 Å); light grey: MTS8 (12.1 Å); dark grey: MTS17 (19 Å).
We used a series of MTS reagents as molecular rulers to estimate the distances between single reactive cysteines on actin and on DrABD mutants (21). Experiments with skeletal F-actin pre-modified with MTS1 (5.4 Å) revealed that among the five DrABD300 mutants, K238C is the one cross-linked most efficiently to C374 on actin (Fig 5(a)). The cross-linking yields for three DrABD mutants, K238C, K248C and K252C, and the MTS8 pre-modified F-actin were very similar (27 – 29%). These results suggest that lysines 238, 248 and 252 are in close enough proximity to actin's C374 (∼12 Å) to be involved in the formation of a trimer consisting of 2-iminothiolane-modified DrABD attached simultaneously to two actin protomers (Fig. 4(a)). The low cross-linking efficiency documented for mutants K270C and K271C and actin pre-modified with the MTS reagents of different length precludes their proximity to C374 on actin (Fig 5(a)).
The experiments with MTS-modified yeast actin D51C/C374S revealed that all five DrABD300 mutants could be linked very efficiently to actin with reagents of ∼12.1 (MTS8) – 19 Å (MTS17) length range (Fig 5(b)). These data indicate that the N-terminal part of DrABD is centered on subdomain 2 of actin. However, in the case of MTS1-modified D51C/C374S actin, the cross-linking efficiency of the introduced cysteine residues decreases with increasing distance from the N-terminus of the DrABD construct (Fig 5(b)). The fact that all five DrABD300 mutants can be cross-linked efficiently to residue 51 on actin may indicate that the construct can adopt a folded conformation upon binding to F-actin.
No cross-linking was detected with all five DrABD300 mutants and yeast actins S60C/C374S, with the reactive cysteine in Subdomain 2 facing the nucleotide binding cleft, and A144C/C374A, containing a reactive cysteine in the actin's hydrophobic cleft (Subdomain 3) (data not shown).
Zero length cross-linking of DrABD to Subdomain 1 of actin
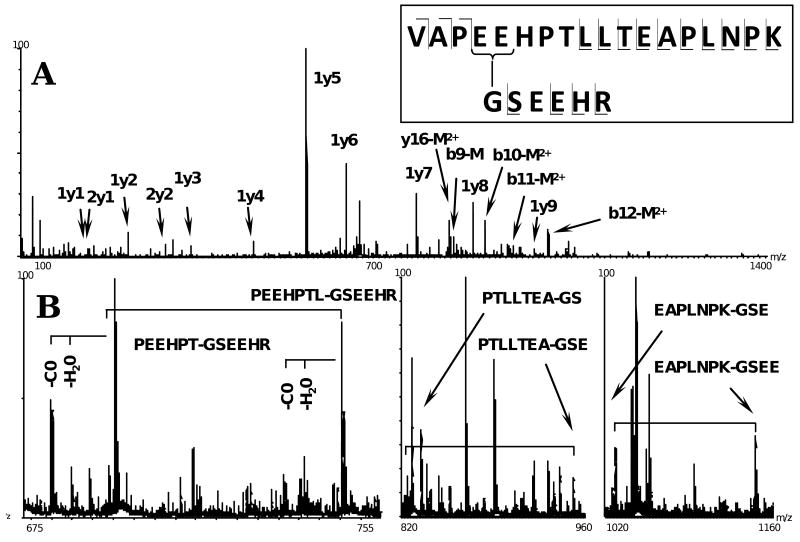
Our experiments showed that DrABD can be efficiently cross-linked to F-actin using the zero length cross-linker, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) (Fig. 6(a), Appendix C). To map the cross-linked sites, bands corresponding to actin, DrABD and actin-DrABD heterodimer were excised from the SDS PAGE gel and subjected to in-gel trypsin digest. Cleavage products were analyzed by electrospray ionization (ESI) tandem mass spectrometry (MS/MS). A peptide ion ([M+4H]4+ = m/z 663.61) unique to the cross-linked heterodimer was fragmented by MS/MS and matched to the two cross-linked peptides: actin 96 – 113 and drebrin 233 – 236, containing extra G-S extension at the N-terminus. To fully assign the spectrum the fragments were deconvoluted to a zero-charge spectrum and matched with theoretical masses predicted for the three cross-linking sites on the actin peptide (E99, E100 and E107) and the single cross-linking site on drebrin (the N-terminus). To ensure high quality of the matches, the data were first filtered using a maximum error of ±100 ppm. In a second filtering step, the average and standard deviation of the errors were calculated and one standard deviation from the average (27 ±37 ppm) was used as a filter. The identified fragments were then matched with the raw spectrum, to increase the confidence by using the multiple charge states of each fragment. Fifty-seven fragments were found with unique interpretations (24 had 2 or more charge states), 11 fragments matched to sequences occurring more than once in the peptide sequence and 16 ions could be matched to more than one fragment (within experimental error). To assign the latter to single fragments, the number of fragmentations necessary to form the fragment and the presence of supporting ions from related fragments were evaluated. The mass-to-charge ratio (m/z) and the charge of each fragment were manually verified from the raw spectrum. The interpretation of the data fits with a cleavage of 24 bonds out of 26. Twenty-one fragment support a cross-link at actin's E99 or E100 located in Subdomain 1 (major population), while 6 fragments i. e., a minor population, supports a cross-link at E107 (Fig. 6(b), Appendix C).
Fig. 6. N-terminus of DrABD constructs can be attached to Subdomain 1 of actin with zero length cross-linking reagent, EDC.
(a) Tryptic peptides of DrABD-actin hetorodimer were analyzed by tandem mass spectrometry. The 663.61 (M +4H)4+ peak in MS spectra, corresponding to cross-linked peptides, was fragmented using ESI-MS/MS (Waters Synapt QTOF mass spectrometer). Some of the identified fragments are indicated in the figure. The first digit indicates the peptide from which the fragment originates (1 = actin and 2 = drebrin), the letter refers to the ion series and the last digit to the ion number. Hyphenated labels indicate cross-linked fragments with the left fragment originating from actin and the right one from drebrin. M represents intact drebrin peptide. The inset shows a schematic representation of the cross-linked peptides. In the scheme, the cross-linked residues are connected with a solid line (major site), a minor cross-linking site on actin peptide is marked with the asterisk. (b) Internal ions of the cross-linked peptides support the attachment of N-terminal Gly of DrABD to E99/E100 (major population) and E107 (minor cross-link) on actin.
Discussion
In order to identify contact sites between actin and drebrin, we employed site directed mutagenesis, electron microscopic reconstruction, chemical cross-linking and mass spectrometry analysis. Using a set of thiol specific reagents of different length as molecular rulers, we were able to assign the distances between selected residues on actin and DrABD in the range from 0 to ∼19 Å. The results of electron microscopic reconstruction revealed polymorphism in DrABD binding to actin filaments and suggested the existence of at least two binding sites for DrABD.
The content of helical and β- structures estimated for DrABD using CD spectroscopy was 28% and 15% respectively. However, secondary structure prediction algorithms did not predict any β-structures in DrABD (Fig. 2). Our current crystallization attempts, if successful, would resolve this contradiction. The level of random coil and turns estimated by CD (57%) was in good agreement with the predictions made by Jpred 3 (∼50%) which suggests proper folding (22). The affinity of DrABD for F-actin determined by pelleting experiments (Kd ∼6.6 – 7.6 μM) was relatively low compared with the affinity reported for the full length drebrin (0.12 μM) (6). Our observation that drebrin construct 1-300 has a significantly higher affinity for F-actin compared to DrABD (∼0.2 μM, Fig. 1) raises the possibility that regions other than DrABD may also contribute to the actin binding. We hypothesize that either ADF homology domain or the linker region (residues 143 - 232) contribute to the stronger binding of drebrin 1-300 to F-actin than that of DrABD. Our EM reconstruction results (see below) suggest that DrABD is the strongest actin binding region within the 1-300 drebrin fragment and identification of the weaker binding site/sites is a subject for a separate study.
The fact that the shorter construct of DrABD300 (sequence 233-300, with deletion of 17 C-terminal amino acids) has approximately the same affinity to F-actin as the longer construct (sequence 233-317) indicates that the unstructured region 301-317 does not contribute significantly to the DrABD-F-actin interaction (Fig. 1(b)).
Chemical cross-linking for mapping the protein binding interface
To probe the actin-DrABD binding interface several DrABD and actin mutants containing single reactive cysteines were reacted with a series of thiol specific bifunctional MTS reagents. In general, the cross-linking efficiency depends on the reactivity and accessibility of the targeted residues, stability of the cross-linking reagents in solution, and experimental conditions (pH, temperature, reaction time).
The results of our cross-linking and pelleting experiments suggest that DrABD bridges two adjacent actin protomers. However, these data are insufficient to determine the orientation of DrABD on actin filaments. Based on our results summarized in Fig. 7, we discuss the interaction of DrABD with Protomers 1 and 2 in the actin filament (23).
Fig. 7. Summary of the cross-linking results.

Holmes model of actin filament structure (36). Actin protomers are marked as 1, 2 and 3. Actin residues involved in cross-linking with DrABD are shown in magenta. Actin peptides involved in interactions with cofilin (17) and α-actinin (32) are shown in yellow and blue (Protomer 1), respectively. The overlap between cofilin and α-actinin binding sites is shown in green (Protomer 1)
Cross-linking of DrABD to actin Subdomain 1 (Protomer 1)
After thrombin cleavage, the recombinant DrABD constructs contain a G-S N-terminal extension. Mass spectrometry analysis identified the N-terminal glycine on DrABD as a residue cross-linked to F-actin with a zero length cross-linking reagent (EDC) (Appendix C). On actin, three residues in subdomain 1 were shown to be involved in EDC cross-linking with DrABD: E99 and/or E100 (major population), and E107 (minor population) (Fig. 6). Although a distinction between cross-linking to E99 and E100 is not possible from the fragmentation pattern of the cross-linked peptides, we conclude based on the abundance of the internal fragments of the cross-linked peptides that DrABD cross-linking to E107 of actin represents a minor population. The analysis performed using GETAREA 1.0 software shows a significant decrease in solvent accessibility for actin residue E107 compared to E99 and 100. Based on that, we can not rule out the possibility that the N-terminus of DrABD is flexible and locates in close proximity to all three residues (E100, E99 and E107) but its coupling to E107 is restricted by the accessibility of that residue. It is also conceivable that minor cross-linking to E107 of actin may reflect an alternative mode of DrABD binding or result from local damage to actin over the course of the reaction. Despite high flexibility of DrABD in solution (our unpublished NMR data) and the predicted disordered state of its N-terminal region, only one cross-linked peptide was identified in the sample containing the DrABD-actin heterodimer, which indicates a specific DrABD interaction with the 99 – 107 actin region.
Cross-linking experiments employing skeletal α-actin pre-modified with MTS reagents and DrABD mutants containing single cysteine substitutions revealed that the N-terminal part of DrABD makes contact with the C-terminus of actin. Based on the highest cross-linking efficiency of the K238C mutant to MTS1-modified actin, we conclude that residue 238 of DrABD locates within ∼5.4 Å from actin's C374 (Fig 5(a)). The fact that longer spanning reagents (MTS8 (12.1 Å) and MTS17 (19 Å)) cross-link the same K238C mutant to actin at lower efficiency may indicate that in the extended conformation these reagents do not fit well into the space between the two cysteine residues in the DrABD-actin complex, while the “gauche” conformations of the reagents may not have the appropriate geometry for bridging residues 374 and 238. Similar amounts of actin-DrABD heterodimer were obtained in the presence of mutants K238C, K248C and K252C with MTS8, which indicates that these three residues are within ∼12 Å from actin's C-terminus. Low cross-linking efficiency between C374 of actin and DrABD mutants C270 and C271 observed with the MTS reagents of different length indicates that those residues are distant from the C-terminus of actin (Fig 5(a)).
Cross-linking of DrABD to actin Subdomains 1 and 2 (Protomer 2)
Experiments with yeast actin mutant D51C/C374S containing a single reactive cysteine in subdomain 2 revealed that DrABD is close to this actin region. Residues 238, 248, 252, 270 and 271 on DrABD can be all efficiently attached to residue 51 on actin with MTS reagents of various length (5.4 – 19 Å) (Fig. 5(b)). The efficiency of these reactions with MTS1 and MTS8 is slightly higher (by ∼8%) for the DrABD mutant K238C (Fig. 5(b)). According to secondary structure predictions lysines 248 and 252 of DrABD are located on a helix and lower cross-linking efficiency for these mutants, compared to K238C may indicate a decreased solvent accessibility of these two residues. Our results indicate that all five lysines on DrABD are located within ∼12 Å or even closer (5.4 Å in the case of K238) from residue 51 on actin.
Our cross-linking experiments revealed also that the C-terminal region of DrABD interacts with actin's subdomain 1 and that drebrin's native cysteine 308 locates within 5.4 Å from C374 on actin (Fig. 4(b)). Minor cross-linking between C10 on actin and C308 on DrABD may reflect the flexibility of the C-terminal region of the DrABD construct, but could indicate also an alternative binding mode (Appendix B).
EM reconstruction of F-actin decorated with DrABD is consistent with cross-linking results
We used a single particle approach for the EM reconstruction of F-actin decorated with DrABD (24). This method allows for sorting relatively short filament segments (∼400 Å long) by occupancy and the mode of drebrin binding to F-actin.
In four modes of binding (Fig. 3 (d-f, and h)), the mass attributed to DrABD is consistent with a globular protein of ∼9 kDa attached to F-actin. The results of EM reconstruction together with the mapped location of residues 238, 248, 252, 270 and 271 on DrABD within ∼12 Å from residue 51 on actin lead to the conclusion that DrABD may adopt globular conformation upon binding to actin. Our data suggest that in modes (d) – (f), and (h) (Fig. 3) DrABD binds to F-actin predominantly as a monomer. However, we can not exclude the possibility that in these four modes actin filaments are decorated by drebrin dimers/oligomers, which are not observed due to low occupancy or disorder.
According to the EM reconstruction and in a good agreement with our cross-linking results DrABD makes extensive contacts with subdomains 1 and 2 of actin (Fig. 3). In the modes (d) and (f) DrABD interacts with subdomains 1 and 2 that involves residues 51 and 99 – 100. In the mode shown in Fig. 3(e), DrABD is located in front of actin's subdomain 1 and the interface involves residues 99 and 100. In the minor binding mode (g) (Fig. 3) residues 51, 99, 100 and 374 are in proximity to DrABD density. Also, in the mode (h) (Fig. 3) residues 51 and 374 are likely to be involved in the interaction with drebrin. Taking together, EM data support the existence of two binding sites for DrABD on actin: a major site (modes (d) – (f), ∼40% segments), and a minor site (modes (g) – (h), ∼15% segments) where DrABD is shifted to the side of actin subdomains 1 and 2 and makes cross-strand contact with another actin protomer.
Our cross-linking experiments revealed that DrABD can be simultaneously attached to two adjacent actin protomers within one helical strand. According to our mapping, C374 residues on two adjacent protomers are involved in such double cross-linking, which appears to be inconsistent with the results of EM reconstruction (Fig. 4 and 5(a)). However, it should be noted that the C-terminal extension of DrABD that cross-links to actin's C374 with MTS1 (Fig. 4(b)) is predicted to be unstructured. Computational analysis performed using INSIGHT II software revealed that the length of this C-terminal extension (a.a. 301 – 317, Cα to Cα) spans between 4.7 and 26.7 Å. If this region is disordered it would not be observed in the EM reconstructions. According to our mass spectrometry analysis, a short N-terminal extension of DrABD makes contact with actin residues 99 – 100. We may speculate that DrABD consists of a helical core with the unstructured extensions docked to the subdomain 1 regions of two adjacent actin protomers. An atomic resolution structure of the DrABD-actin complex will be required to confirm this hypothesis.
Polymorphism of DrABD binding to actin: implications for the competition with other ABP
Along with drebrins, many other actin binding proteins (ABP) are involved in the regulation of the actin cytoskeleton in neuronal cells. At the molecular level, the interplay between ABPs in this system is poorly understood. Our results provide new structural insight into the reciprocal relationship between drebrin and other ABPs in the cell. Multiple binding modes to F-actin documented here for DrABD were reported earlier for other proteins such as cofilin (17), utrophin (25), and tropomyosin (26-29). Thus, binding polymorphism is not an exception among actin interacting proteins and may play an important role in actin cytoskeletal regulation. Because drebrin's construct 1-300 binds to F-actin significantly tighter than DrABD yet shows the same modes of attachment to actin filaments (Fig. 1(a) and Fig. 3(d* - h*)), the in vivo competition of DrABD with other actin-binding proteins would be functionally important.
This study revealed that the main DrABD binding site overlaps with the cofilin binding site on actin filaments, which would explain the competition between these two proteins (11). Cofilin interacts with two actin protomers within the same helical strand: with an upper protomer at the hydrophobic cleft between subdomains 1 and 3, and with a lower protomer at the interface formed by subdomains 1 and 2. On the lower protomer, peptides 44-50, 28-29 and 88-101 appear to be involved in cofilin binding (F-binding site) (17; 30). On the upper protomer, the C-terminus of actin was shown to interact with cofilin (21; 31). Thus, our mapping of DrABD sites on actin to regions proximal to residues D51, E99, E100 and the C-terminus indicates their overlap with the cofilin binding site.
It has been shown that drebrin overexpression causes displacement of α-actinin from dendritic spines, which is consistent with earlier in vitro observations (8). An α-actinin was documented previously to interact with residues 86 - 117 and 350 - 375 on actin (32). We have shown here that actin residues 99 – 107 and its C-terminus are in close proximity to DrABD, explaining the previously observed competition between these two proteins. According to the in vitro studies, α-actinin can interact with both F-actin (through tandem CH domains) and NMDA receptors (through its central ‘rod’ domain), anchoring actin filaments to the membrane (33; 34). It is likely that the competition between drebrin and α-actinin affects the anchoring of actin filaments to the membrane and leads to the formation of the long protrusions observed upon drebrin overexpression. We may hypothesize that the observed polymorphism of DrABD binding to F-actin plays a role in the competition with spectrin family proteins, like utrophin, which have multiple actin-binding modes (25).
Based on in vitro studies it was suggested that drebrin and tropomyosin compete for the same actin binding site. In the absence of atomic resolution structures, the detailed mechanism of such competition remains unclear. Also, we can not exclude that observed inhibition of drebrin binding occurs due to tropomyosin-induced conformational changes in F-actin. Our data suggest that the primary binding site of drebrin (Fig. 3(d - f) is inconsistent with simultaneous interaction of drebrin and myosin with F-actin, because myosin binds to a similar interface on F-actin (35). It is possible that the attachment of F-actin decorated by drebrin A to a glass surface coated with myosin-V occurs because of the shift of DrABD from its primary binding site to the minor one located on the side of the filament (10).
Materials and Methods
Materials
Bis(methanethiosulfonate) (MTS) cross-linking reagents, MTS1 [1,1-methanediyl bis(methanethiosulfonate)], MTS-8 [3,6-dioxaoctane-1,8-diyl bis(methanethiosulfonate)] and MTS-17 [3,6,9,12,15-pentaoxaheptadecane-1,17-diyl bis(methanethiosulfonate)] were purchased from Toronto Research Chemicals Inc. (North York, Ontario, Canada). Millipore-filtered water and analytical grade reagents were used in all experiments.
Molecular cloning and mutagenesis
Mouse (Mus musculus) brain RNA was purified using TRIzol reagent (Invitrogen). Reverse transcriptase (RT)-PCR was performed using SuperScript II (Invitrogen) to generate cDNA. Full-length drebrin A cDNA was cloned into pCR2.1-TOPO vector (Invitrogen) and used as a template for all drebrin constructs. DrABD was cloned into BamH1 and EcoR1 sites of pGEX-4T1expression vector. DrABD300 construct was created by introducing a stop codon after aa 300 (Val) in DrABD with QuikChange kit (Stratagene). DrABD mutants K238C, K248C, K252C, K270C and K271C that contain single lysine to cysteine replacements were created using the same kit. Drebrin construct 1-300 was obtained by introducing a stop codon after residue 300 in full length drebrin A DNA subcloned into pGEX-4T1 expression vector. The primers for cloning and mutagenesis are given in Appendix D.
Protein expression and purification
Rabbit skeletal actin was purified from rabbit back muscle as described in Spudich and Watt (36). Yeast actin was purified as described previously (21). All drebrin ABD constructs were expressed in Rosetta cells. Cells were grown at 37 °C until OD600 = 0.6 - 0.8 following the induction with 0.2 mM IPTG and 2 hours expression. Proteins were purified on glutathione-agarose according to the manufacturer's instructions and as detailed in Appendix A. The single cysteine mutations introduced into DrABD300 construct did not impair its complex formation with F-actin (Fig. 8). Consequently, we employed these mutants for mapping the drebrin interface on actin.
Fig. 8. Single cysteine substitutions introduced into DrABD do not impair its ability to bind F-actin.

Time course of cross-linking reactions (0, 5, 10, 20, 40 and 75 min) between skeletal F-actin (10μM) and DrABD300 mutants (30μM) in the presence of EDC (30μM). The higher mobility bands correspond to actin; the lower mobility bands represent the cross-linked actin-DrABD complex. Reaction conditions: 5 mM MOPS pH 7.2, 0.2 mM CaCl2, 0.2 mM ATP, 0.11 mM TCEP, 100 mM KCl, 2 mM MgCl2.
Actin polymerization and DrABD binding assays
Actin polymerization was monitored via light scattering with the PTI fluorometer set at 350 nm for the excitation and emission wavelengths. For pelleting experiments polymerization of skeletal actin was induced by addition of 2.0 mM MgCl2 and 100 mM KCl to the actin solution in 5 mM Tris, pH 8, 0.2 mM CaCl2, 0.2 mM ATP and 5 mM β-mercaptoethanol. The samples were centrifuged at 312,500 g for 30 min, at 4 °C, in a Beckman TLA-100 rotor. Resulting pellets were solubilized in gel sample buffer and analyzed by SDS PAGE. To quantify the amount of N-GST fused DrABD cosedimented with F-actin, 0.4 – 3.6 μg of purified constructs were loaded on each gel as the standards. Gels were stained with Coomassie Blue. The intensities of the bands were estimated using Scion Image Software. Binding parameters (Kd and Bmax) were obtained by fitting the average data points in SigmaPlot 9.0. The resulting curves (Fig. 1) represent the best fit.
Actin modification and cross-linking
Immediately prior to the reaction, DTT was removed from G-actin samples over a Sephadex G-50 spin column equilibrated with thiol free buffer containing 5 mM MOPS (pH 7.2), 0.2 CaCl2 and 0.2 mM ATP. Drebrin ABD constructs were passed through a Zeba Desalt Spin Column (Pierce) equilibrated with the same buffer. Actin was polymerized in the presence of 2.0 - 3.0 mM MgCl2 and 100 mM KCl. The reactions of F-actin modification with bis(methanethiosulfonate) (MTS) were carried out at room temperature (1 - 10 min), at molar ratios of 0.95:1 of MTS : actin. Cross-linking reaction was started by mixing DrABD constructs with MTS pre-modified F-actin. Aliquots were withdrawn from the reaction mixtures at selected time points and free cysteine residues were blocked with NEM. Cross-linking progress was monitored by SDS-PAGE under non-reducing conditions.
Circular dichroism
Circular dichroism spectra of DrABD (0.5 mg/ml) in 5 mM Tris, pH 7.8 at 25°C were measured using J-700 polarimeter (Jasco). Eight replicates of each spectrum were recorded using scanning speed of 20 nm/min, data pitch of 0.5 nm and 4-s dwell time. Samples were measured in a quartz cuvette with a path length of 0.01 cm. CD spectra were deconvoluted using the Selcon3 algorithm. Secondary structure obtained from the CD spectra was compared with that calculated from the secondary structure prediction algorithm Jpred 3.
Mass spectrometry and data analysis
Actin, DrABD and EDC cross-linked heterodimers were separated using PAGE. The gel bands were digested as described (37). Actin, DrABD and the trimer, consisting of 2-iminothiolane-modified DrABD attached to two adjacent protomers through MTS1, were separated using size exclusion chromatography and digested in-solution as described earlier (38) and detailed in the Appendix A. The peptides were first analyzed by MALDI-TOF MS (Voyager DE-STR, Applied Biosystems, Framingham, MA, USA) using the dried droplet method with dihydroxybenzoic acid (DHB) as matrix, and then by tandem mass spectrometry. The digests was desalted using microcolumns (39) packed with poros R2 beads (Applied Biosytems, Framingham, MA) and eluted directly into glass capillaries (Proxeon Biosystems, Odense, Denmark) to be analyzed manually by MS/MS (Synapt HDMS, Manchester, UK).
Spectra for the unmodified samples were acquired first and then used as controls during the analysis of the cross-linked sample to determine unique peptide ions. In each analysis, a few relevant peptides were analyzed, each up to 25 minutes to ensure high spectrum quality. A small portion of the sample, cross-linked using MTS1 was incubated with 10 mM DTT for 1 h and analyzed by tandem MS to verify the disulfide nature of the cross-link. The cross-linked peptides were identified through manual interpretation of the raw spectra. To unambiguously assign the cross-linking site, the spectra were exhaustively assigned using in-house written Perl scrips. First, theoretical masses were calculated from the cross-linked peptides, allowing for multiple cross-linking sites and modifications, and then the theoretical masses were matched with mass lists generated by processing the raw spectra with Mascot Distiller 2.0 using an error of 0.1 Da or 100 ppm. Assignments were verified in the raw spectra using isotope patterns and charge states. In some cases, more than one peptide assignment was possible within the experimental error, and then the number of cleavages and supporting ions (e.g. water loss or ammonium loss) were used to select the most likely candidate.
Electron microscopic reconstruction
G-actin was purified using Superdex-200 column and frozen in liquid nitrogen. Before each experiment an aliquot of actin was thawed at 4°C, and clarified by centrifugation (100,000 g, 1 hour). Actin (5 μM) was polymerized in 10 mM Tris-HCl buffer, pH 7.8, containing 50 mM KCl, 1 mM MgCl2, 1 mM DTT, 0.2 mM ATP for 1.5 - 2 hours. To obtain the filaments decorated with DrABD, 1 - 2 μM of F-actin was incubated with 10 - 15 μM of DrABD for 12 - 15 min at room temperature. Identical procedure was used for decorating actin with the 1-300 drebrin construct. Samples were applied to glow-discharged carbon-coated grids and stained with 2% uranyl acetate. The grids were examined in a Tecnai-12 electron microscope (FEI) under minimal-dose conditions at an accelerating voltage of 80 keV and a nominal magnification of 30,000.
The SPIDER software package (40) was used for most image processing, but the EMAN package (41) was used to extract filament images from micrographs. A Nikon COOLPIX 8000 scanner was used to digitize micrographs at a raster of 4.16 Å per pixel. IHRSR method (24) was used to generate an overall reconstruction from 9,749 segments (each 416 Å long) of actin filaments decorated with DrABD (Fig. 3(b)). Comparison of the overall reconstruction with the reconstruction of pure F-actin (17) revealed an additional mass attached to the front and side parts of SD1 and SD2 of actin protomers (see Appendix A for the details). To evaluate whether that density was arising from a single drebrin molecule or it was rather a superposition of multiple states of binding, a set of models was designed to decompose the additional density into several classes based on the possible location of drebrin on F-actin. The size of the additional mass along with the quality of the actin portion of the map was used as a guideline in the sorting process. The position of drebrin in its five modes of binding to F-actin relative to the additional density in the overall reconstruction is shown in Supplemental Fig.1. Detailed description of the sorting procedures has been published earlier (25; 42).
UCSF Chimera software (43) was used to fit the crystal structure of actin (44) into the experimental maps. Atomic coordinates from crystal structures were converted to density maps, and these were filtered to the resolution of the experimental map and docked manually.
Supplementary Material
Acknowledgments
We would like to thank Courtney White for the help with isolating drebrin encoding DNA. We are grateful to Dora Warshaviak for the assistance with computational analysis. This work was supported by USPHS, U.S. Public Health Service Grants GM 077190 (to E. R.) and RR 20004 (to J. A. L.), NIH, the National Institute of Health Grant GM081303 (to E. H. E.), and NSF, the National Science Foundation Grant MCB 0316269 (to E. R.).
Abbreviation used
- DrABD
drebrin's actin-binding domain (seq 233-317)
- DrABD300
drebrin's sequence 233-300
- drADF
ADF-homology domain of drebrin (seq 1-134)
- HCm
helical charged motif
- mAbp1
mammalian actin-binding protein 1
- EDC
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide
- MTS
bis(methanethiosulfonate) cross-linking reagents
- SD1 – SD4
actin subdomains 1 – 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int. 2007;51:92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 2.Kojima N, Shirao T. Synaptic dysfunction and disruption of postsynaptic drebrin-actin complex: A study of neurological disorders accompanied by cognitive deficits. Neurosci Res. 2007;58:1–5. doi: 10.1016/j.neures.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Peitsch WK, Hofmann I, Pratzel S, Grund C, Kuhn C, Moll I, Langbein L, Franke WW. Drebrin particles: components in the ensemble of proteins regulating actin dynamics of lamellipodia and filopodia. Eur J Cell Biol. 2001;80:567–579. doi: 10.1078/0171-9335-00194. [DOI] [PubMed] [Google Scholar]
- 4.Peitsch WK, Hofmann I, Endlich N, Pratzel S, Kuhn C, Spring H, Grone HJ, Kriz W, Franke WW. Cell biological and biochemical characterization of drebrin complexes in mesangial cells and podocytes of renal glomeruli. J Am Soc Nephrol. 2003;14:1452–1463. doi: 10.1097/01.asn.0000069222.63700.de. [DOI] [PubMed] [Google Scholar]
- 5.Grenklo S, Hillberg L, Zhao Rathje LS, Pinaev G, Schutt C, Lindberg U. Tropomyosin assembly intermediates in the control of microfilament system turnover. Eur J Cell Biol. 2008;87:905–920. doi: 10.1016/j.ejcb.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa R, Hayashi K, Shirao T, Xue Y, Takagi T, Sasaki Y, Kohama K. Drebrin, a development-associated brain protein from rat embryo, causes the dissociation of tropomyosin from actin filaments. J Biol Chem. 1994;269:29928–29933. [PubMed] [Google Scholar]
- 7.Sasaki Y, Hayashi K, Shirao T, Ishikawa R, Kohama K. Inhibition by drebrin of the actin-bundling activity of brain fascin, a protein localized in filopodia of growth cones. J Neurochem. 1996;66:980–988. doi: 10.1046/j.1471-4159.1996.66030980.x. [DOI] [PubMed] [Google Scholar]
- 8.Biou V, Brinkhaus H, Malenka RC, Matus A. Interactions between drebrin and Ras regulate dendritic spine plasticity. Eur J Neurosci. 2008;27:2847–2859. doi: 10.1111/j.1460-9568.2008.06269.x. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi K, Ishikawa R, Ye LH, He XL, Takata K, Kohama K, Shirao T. Modulatory role of drebrin on the cytoskeleton within dendritic spines in the rat cerebral cortex. J Neurosci. 1996;16:7161–7170. doi: 10.1523/JNEUROSCI.16-22-07161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa R, Katoh K, Takahashi A, Xie C, Oseki K, Watanabe M, Igarashi M, Nakamura A, Kohama K. Drebrin attenuates the interaction between actin and myosin-V. Biochem Biophys Res Commun. 2007;359:398–401. doi: 10.1016/j.bbrc.2007.05.123. [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, Weisbart RH, Teter B, Frautschy SA, Cole GM. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- 12.Kessels MM, Engqvist-Goldstein AEY, Drubin DG. Association of Mouse Actin-binding Protein 1 (mAbp1/SH3P7), an Src Kinase Target, with Dynamic Regions of the Cortical Actin Cytoskeleton in Response to Rac1 Activation. Mol Biol Cell. 2000;11:393–412. doi: 10.1091/mbc.11.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu W, Stamnes M. The actin-depolymerizing factor homology and charged/helical domains of drebrin and mAbp1 direct membrane binding and localization via distinct interactions with actin. J Biol Chem. 2006;281:11826–11833. doi: 10.1074/jbc.M510141200. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi K, Ishikawa R, Kawai-Hirai R, Takagi T, Taketomi A, Shirao T. Domain analysis of the actin-binding and actin-remodeling activities of drebrin. Exp Cell Res. 1999;253:673–680. doi: 10.1006/excr.1999.4663. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi K, Shirao T. Change in the shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons. J Neurosci. 1999;19:3918–3925. doi: 10.1523/JNEUROSCI.19-10-03918.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haeckel A, Ahuja R, Gundelfinger ED, Qualmann B, Kessels MM. The actin-binding protein Abp1 controls dendritic spine morphology and is important for spine head and synapse formation. J Neurosci. 2008;28:10031–10044. doi: 10.1523/JNEUROSCI.0336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galkin VE, Orlova A, Lukoyanova N, Wriggers W, Egelman EH. Actin depolymerizing factor stabilizes an existing state of F-Actin and can change the tilt of F-actin subunits. J Cell Biol. 2001;153:75–86. doi: 10.1083/jcb.153.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichert A, Heintz D, Echner H, Voelter W, Faulstich H. Identification of contact sites in the actin-thymosin beta4 complex by distance-dependent thiol cross-linking. J Biol Chem. 1996;271:1301–1308. doi: 10.1074/jbc.271.3.1301. [DOI] [PubMed] [Google Scholar]
- 19.Kim E, Miller C, Reisler E. Polymerization and in vitro motility properties of yeast actin: a comparison with rabbit skeletal a-actin. Biochemistry. 1996;35:16566–16572. doi: 10.1021/bi9623892. [DOI] [PubMed] [Google Scholar]
- 20.Gerson JH, Kim E, Muhlrad A, Reisler E. Tropomyosin-troponin regulation of actin does not involve subdomain 2 motions. J Biol Chem. 2001;276:18442–18449. doi: 10.1074/jbc.M011070200. [DOI] [PubMed] [Google Scholar]
- 21.Grintsevich EE, Benchaar SA, Warshaviak D, Boontheung P, Halgand F, Whitelegge JP, Faull KF, Ogorzalek Loo RR, Sept D, Loo JA, Reisler E. Mapping the cofilin binding site on yeast G-actin by chemical cross-linking. J Mol Biol. 2008;377:395–409. doi: 10.1016/j.jmb.2007.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole C, Barber JD, Barton GJ. The Jpred 3 secondary structure prediction server. Nucl Acids Res. 2008;36:W197–W201. doi: 10.1093/nar/gkn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- 24.Egelman EH. A robust algorithm for the reconstruction of helical filaments using single-particle methods. Ultramicroscopy. 2000;85:225–234. doi: 10.1016/s0304-3991(00)00062-0. [DOI] [PubMed] [Google Scholar]
- 25.Galkin VE, Orlova A, VanLoock MS, Rybakova IN, Ervasti JM, Egelman EH. The utrophin actin-binding domain binds F-actin in two different modes: implications for the spectrin superfamily of proteins. J Cell Biol. 2002;157:243–251. doi: 10.1083/jcb.200111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehman W, Galinska-Rakoczy A, Hatch V, Tobacman LS, Craig R. Structural basis for the activation of muscle contraction by troponin and tropomyosin. J Mol Biol. 2009;388:673–681. doi: 10.1016/j.jmb.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacchiocchi C, Graceffa P, Lehrer SS. Myosin-induced movement of [alpha][alpha], [alpha][beta], and [beta][beta] smooth muscle tropomyosin on actin observed by multisite FRET. Biophys J. 2004;86:2295–2307. doi: 10.1016/S0006-3495(04)74287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehman W, Craig R, Vibert P. Ca2+-induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstruction. Nature. 1994;368:65–67. doi: 10.1038/368065a0. [DOI] [PubMed] [Google Scholar]
- 29.Xu C, Craig R, Tobacman L, Horowitz R, Lehman W. Tropomyosin Positions in Regulated Thin Filaments Revealed by Cryoelectron Microscopy. Biophys J. 77:985–992. doi: 10.1016/S0006-3495(99)76949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGough A, Pope B, Chiu W, Weeds A. Cofilin Changes the Twist of F-Actin: Implications for Actin Filament Dynamics and Cellular Function. J Cell Biol. 1997;138:771–781. doi: 10.1083/jcb.138.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paavilainen VO, Oksanen E, Goldman A, Lappalainen P. Structure of the actin-depolymerizing factor homology domain in complex with actin. J Cell Biol. 2008;182:51–59. doi: 10.1083/jcb.200803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGough A, Way M, DeRosier D. Determination of the alpha-actinin-binding site on actin filaments by cryoelectron microscopy and image analysis. J Cell Biol. 1994;126:433–443. doi: 10.1083/jcb.126.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M. Competitive binding of [alpha]-actinin and calmodulin to the NMDA receptor. Nature. 1997;385:439–442. doi: 10.1038/385439a0. [DOI] [PubMed] [Google Scholar]
- 34.Shirao T, Sekino Y. Clustering and anchoring mechanisms of molecular constituents of postsynaptic scaffolds in dendritic spines. Neurosci Res. 2001;40:1–7. doi: 10.1016/s0168-0102(01)00209-7. [DOI] [PubMed] [Google Scholar]
- 35.Holmes KC, Angert I, Jon Kull F, Jahn W, Schroder RR. Electron cryo-microscopy shows how strong binding of myosin to actin releases nucleotide. Nature. 2003;425:423–427. doi: 10.1038/nature02005. [DOI] [PubMed] [Google Scholar]
- 36.Spudich JA, Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- 37.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 38.Ytterberg AJ, Peltier JB, van Wijk KJ. Protein profiling of plastoglobules in chloroplasts and chromoplasts. A surprising site for differential accumulation of metabolic enzymes. Plant Physiol. 2006;140:984–997. doi: 10.1104/pp.105.076083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gobom J, Nordhoff E, Mirgorodskaya E, Ekman R, Roepstorff P. Sample purification and preparation technique based on nano-scale reversed-phase columns for the sensitive analysis of complex peptide mixtures by matrix-assisted laser desorption/ionization mass spectrometry. J Mass Spectrom. 1999;34:105–116. doi: 10.1002/(SICI)1096-9888(199902)34:2<105::AID-JMS768>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Frank J, Shimkin B, Dowse H. Spider - a modular software system for electron image processing. Ultramicroscopy. 1981;6:343–357. [Google Scholar]
- 41.Ludtke SJ, Baldwin PR, Chiu W. EMAN: Semiautomated Software for High-Resolution Single-Particle Reconstructions. J Struct Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 42.Galkin VE, Orlova A, Lukoyanova N, VanLoock MS, Haag P, Bullard B, Egelman EH. The Location of Ubiquitin in Lethocerus Arthrin. J Mol Biol. 2003;325:623–628. doi: 10.1016/s0022-2836(02)01309-8. [DOI] [PubMed] [Google Scholar]
- 43.Pettersen EE, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera - visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 44.Schutt CE, Myslik JC, Rozycki MD, Goonesekere NCW, Lindberg U. The structure of crystalline profilin-[beta]-actin. Nature. 1993;365:810–816. doi: 10.1038/365810a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.