Abstract
Alzheimer’s disease and other tauopathies are characterized by the intracellular accumulation of insoluble filaments of the microtubule-associated protein tau. The six canonical tau isoforms in the adult brain consist of an N-terminal “projection” domain followed by a proline-rich region, a microtubule-binding repeat region, and a C-terminal tail. However, alternative splicing in exon 6 produces an additional set of tau isoforms, termed 6D and 6P, which contain only the N-terminus and part of the proline-rich region. We have previously shown that constructs representing N-terminal fragments of tau, which resemble the naturally occurring 6P and 6D isoforms, inhibit polymerization of the full-length protein in an in vitro filament formation assay, and traced the inhibitory activity to amino acids 18-42. Here we report that 6P and 6D tau isoforms inhibit polymerization of full-length tau (hTau40) in a similar manner, likely by stabilizing full-length tau in a soluble conformation. The absence of exons 2 and 3 decreased the effectiveness of the 6D isoforms, but not the 6P variants or the N-terminal tau fragments from our previous study, indicating that the 18-42 region is not the sole determinant of inhibitory ability. Finally, this paper demonstrates that inhibition is blocked by pseudophosphorylation of tyrosines 18 and 29, providing a potential link between tyrosine phosphorylation and disease progression. Taken together, these results indicate that the 6P/6D isoforms are potential endogenous inhibitors of tau filament formation, and suggest a mechanism by which this ability may be disrupted in disease.
The microtubule-associated protein tau forms intracellular, filamentous aggregates in Alzheimer’s disease (AD) and other tauopathies. The formation of tau pathology is thought to be intimately linked to neurodegeneration, in part because the appearance of tau pathology in AD follows a spatial and temporal progression through the anatomical regions that underlie the clinical symptoms. Filamentous tau deposition is first found in areas associated with learning and memory, and later spreads through much of the cerebral cortex (reviewed in (1)). Yet even in severe AD some areas, including the cerebellum and primary sensory-motor areas, remain relatively free of tau pathology (2-4). Identifying the differences between susceptible and protected neuronal populations, including endogenous triggers and inhibitors of tau aggregation, may be crucial to understanding selective cellular vulnerability in AD.
Although tau was historically considered to be an unstructured protein in solution, recent evidence indicates that soluble tau occupies a number of folded states, and that these conformations influence tau’s propensity to form filaments. The amino terminus of tau has emerged as an important regulator of tau folding. The association of the extreme amino terminus (5-15) with the microtubule binding repeat region (MTBR), recognized by the conformational antibody Alz50, is thought to underlie the transition from soluble to filamentous tau (reviewed in (5)). The amino terminus is also an important negative regulator of tau aggregation. When present in molar excess, constructs containing only the N-terminus inhibit polymerization of the full-length protein in an in vitro filament formation assay. This inhibitory effect requires amino acids 18-42 in the N-terminal fragments and residues 392-421 in the C-terminus of full-length tau (5). The N-terminal fragments may act by stabilizing a “paperclip” conformation of tau (5), in which the C-terminus associates with the MTBR region and the N-terminus comes into close proximity to the C-terminus. This conformation is observed when tau is a soluble monomer, and may discourage polymerization (6-8).
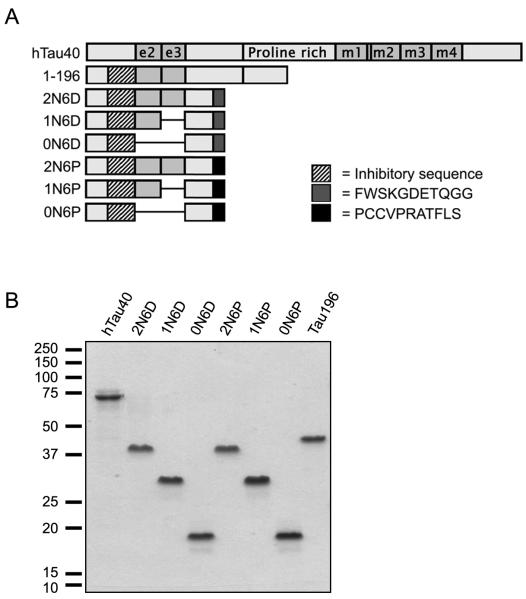
The majority of tau studies to date focus on six canonical isoforms in the adult CNS produced by alternative splicing in the amino terminus (exons 2 and 3) and the MTBR region (exon 10) of the protein (reviewed in (9)). This picture grew more complicated with the discovery of additional tau isoforms that contain all or part of the sequence encoded by exon 6, which is absent from the canonical isoforms. Inclusion of the entire exon (6+) extends the proline-rich region of tau, and results in isoforms that are otherwise identical to their canonical counterparts. The 6+ isoforms may play a regulatory role in neurite elongation (10). In addition to the 3′ splice site at the end of exon 6, there are two alternate 3′ splice sites within exon 6 itself, termed “6P” and “6D” according to location (Fig. 1). Use of either of these splice sites results in a frame shift that introduces a unique amino acid sequence, PCCVPRATFLS (6P) or FWSKGDETQGG (6D), followed by a stop codon. As a result, tau 6P and 6D isoforms are truncated, lacking part of the proline-rich region, the MTBR region, and C-terminus (11, 12). Of these, the MTBR region is absolutely essential to tau-microtubule binding (13-15) and tau aggregation (16, 17).
Figure 1.
Schematic illustrating the relative positions of the 3′ splice sites in exon 6, with the amino acid sequences resulting from use of each site indicated below. Flanking areas of exons 5 and 7 are also shown. Use of either splice site internal to exon 6 (6p or 6d) results in a frame shift in the message that introduces a unique 11-amino acid sequence followed by a stop codon (denoted by an asterisk).
The expression of 6P/6D tau isoforms is spatially and developmentally regulated. At the mRNA level, 6P levels are similar in fetal and adult brain, while 6D is more prominent in fetal brain (18). In adult brain, 6P and 6D mRNA levels are highest in cerebellum and spinal cord, but are detectable in all CNS tissues examined, including the cerebral cortex and the hippocampus (11). Using an antibody to the C-terminus of 6D, Luo et al. compared the expression of 6D to and full-length isoforms in various human tissues. 6D levels were comparable to full-length isoforms in cerebellum, and levels were reduced but detectable in the cerebral cortex, hippocampus. It is notable that 6D protein expression is particularly high in the cerebellum, which is not affected by tau lesions in AD, and lowest in tangle-prone areas (hippocampus, cerebral cortex). Even within AD hippocampus, anti-6D labeling does not colocalize with an antibody that recognizes neurofibrillary tangles (Tau-5; (19)).
In light of the polymerization suppressive properties of N-terminal tau fragments and the expression pattern in human brain, we asked whether 6P/6D isoforms might act as endogenous inhibitors of tau aggregation. To begin to address this question, we assessed the effects of 6P and 6D isoforms on the polymerization of full-length tau in an in vitro filament formation assay (20, 21). We report that 6P and 6D isoforms inhibit polymerization of full-length tau, and that the efficacy of each isoform depends on whether it is of the 6D or 6P variety, and on the presence or absence of N-terminal exons 2 and 3. We also demonstrate that inhibition is abolished by mutations in the crucial 18-42 region that mimic phosphorylation. Collectively, our results indicate that the 6P/6D isoforms have the potential to act as endogenous regulators of tau filament assembly, and suggest a basis for the disruption of this regulatory ability by disease-related phosphorylation events.
EXPERIMENTAL PROCEDURES
Recombinant proteins
The six canonical tau isoforms contain zero, one, or two alternatively spliced N-terminal inserts (designated 0N, 1N, and 2N, respectively), and either three or four MTBRs (3R or 4R). The full-length tau used in this study (hTau40) is the longest canonical isoform in the human central nervous system, containing 441 amino acids, including both alternatively spliced N-terminal exons (e2 and e3) and four microtubule binding repeats (m1-m4; Fig. 2A). The various 6D and 6P isoforms were generated by restriction digestion and ligation of cDNA constructs described previously (19) and hTau40 (2N4R) (22), hTau23 (0N3R) and hTau37 (1N3R) (23). 6DY18/29F and 6DY18/29E were created by site-directed mutagenesis (Stratagene) on the 2N6D background. The constructs 1-196 has been described previously (5). All constructs were verified by sequencing prior to protein purification. Proteins were expressed in E. coli and purified by means of an N-terminal poly-histidine tag (16, 22). Protein concentrations were determined by the Lowry assay (24).
Figure 2.
Schematic of the tau constructs used in this study (A). A tau construct containing a stop codon at Y197 (1-196) has been described elsewhere (5). Constructs containing 0, 1 or 2 alternately spliced N-terminal exons (e2 and e3) were created on the background of the 6P and 6D isoforms. The key indicates the specific N-terminal sequence required to inhibit polymerization of full-length tau (residues 18-42), as well as sequences unique to 6P and 6D isoforms. (B) Depiction of all purified proteins used in this study separated by SDS-PAGE electrophoresis and stained with Coomassie Brilliant Blue R.
Polymerization
Arachidonic acid (AA) was obtained from Cayman Chemical (Ann Arbor, MI) and stored at −20°C. Working solutions were prepared in 100% ethanol immediately prior to use. Tau polymerization was induced by arachidonic acid as previously described (21). Briefly, tau protein (4 μM) was incubated at room temperature in reaction buffer (10 mM HEPES, pH 7.6, 100 mM NaCl, 0.1 mM EGTA, 5 mM DTT) in the presence of 75 μM AA The final volume of ethanol in these reactions was 3.8%, and this volume was added to control reactions in the absence of AA. Unless otherwise noted, 6P/6D constructs and 1-196 were added at a concentration of 8 μM to the polymerization reaction mixture prior to the addition of arachidonic acid. Polymerization was monitored at room temperature by the intensity of right angle laser-light scattering (is; (21)). End-point (t = 300 min) laser light scatter data from at least three independent experiments were analyzed. Statistical significance was determined by comparing polymerization in the presence and absence of N-terminal constructs by a student’s two tailed t-test. Time course data were fit with curves using GraphPad Prism 3.0 software. Error bars in all figures represent plus and minus one standard error of the mean.
Electron Microscopy
Polymerization reactions were allowed to proceed at least five hours, fixed with 2% glutaraldehyde (Electron Microscopy Sciences, EMS, Hatfield, PA), spotted onto 300 mesh formvar/carbon coated copper grids (EMS), and negatively stained with 2% uranyl acetate (EMS) as previously described (20). Grids were examined using a JEOL JEM-1220 electron microscope at 60kV and 12,000x magnification, and photographed using a MegaScan 794/20 digital camera and DigitalMicrograph software version 3.9.3 (Gatan). Optimas 6.0 imaging software (Media Cybernetics) was used to automatically identify and measure filaments (defined as objects > 20 nm in length). At least five separate fields from each grid were randomly chosen under low illumination to prevent bias. In experiments where different constructs were analyzed for effects on hTau40, data from each experiment were normalized to control reactions containing hTau40 alone and expressed as percent of the hTau40 value. Results from at least three independent experiments were analyzed by student’s two-tailed t tests to determine if polymerization was significantly different from controls (GraphPad Prism 3.0 software).
Filament Sedimentation
Polymerization reaction mixtures were incubated for 5 h in the presence of arachidonic acid. Following assembly, a pretreatment sample was removed and the remainder of the reaction mixture was centrifuged at 100,000 x g for 20 minutes at 25° C over a 40% glycerol cushion. Samples of the starting material and supernatants were diluted in 2X Laemmli buffer and boiled. Pellets were resuspended in an amount of polymerization buffer equal to the starting volume, prior to addition of 2X Laemmli buffer. Proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with the amino-terminal antibody Tau-12 (4 ng/mL) (25) to visualize all tau constructs, and the carboxy-terminal antibody Tau-7 (40 ng/mL) (5), which recognizes full-length hTau40 but not the 6D/6P isoforms. HRP-conjugated goat anti-mouse secondary antibody (Vector Laboratories, Burlingame, CA) and ECL (GE Healthcare, Amersham, UK) were used to detect proteins.
RESULTS
6D and 6P isoforms share common features with N-terminal fragments that inhibit the polymerization of full-length tau
To examine the effects of the 6D and 6P isoforms on the polymerization of full-length tau (hTau40), several protein constructs were created and purified (Fig. 2A-B). The 6P and 6D constructs, like the previously described N-terminal tau construct 1-196, lack the MTBR region of tau known to be necessary for polymerization (16, 17). Alternative splicing of exons 2 and 3 produces canonical tau isoforms containing zero, one, or two N-terminal inserts (designated 0N, 1N, and 2N, respectively). Hence, constructs containing zero, one or two alternately spliced N-terminal exons were created on the background of the 6P and 6D isoforms. An additional tau protein construct, 1-196, that was previously shown to inhibit hTau40 polymerization (5) was included as a positive control. All constructs contain the specific amino acid region (18-42) previously identified as crucial for the inhibition of hTau40 polymerization (5).
2N6D and 2N6P inhibit the polymerization of full-length hTau40
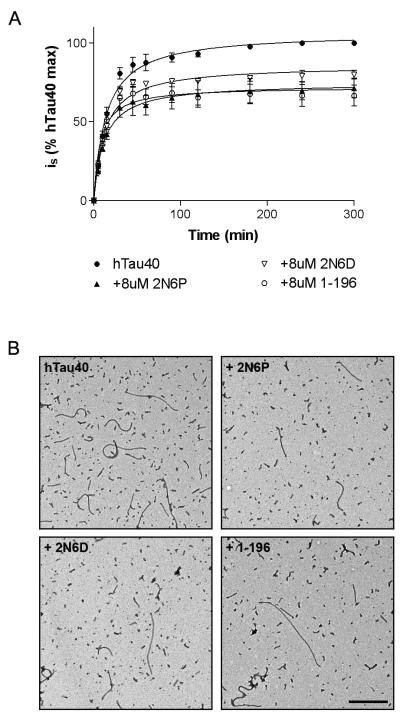
To determine whether 6P and 6D isoforms influence tau polymerization, hTau40 (4 μM) was incubated in the presence or absence of a twice-molar excess of 2N6D, 2N6P, or 1-196. Polymerization was induced by the addition of arachidonic acid, and right angle laser-light scattering (LLS) was used to monitor filament formation (Fig. 3A). After five hours of polymerization, a similar degree of inhibition was apparent in the presence of 2N6P (28.63 ± 6.27%, P < 0.05) and 1-196 (33.40 ± 6.59%, P < 0.05). 2N6D also significantly inhibited hTau40 polymerization, albeit to a lesser extent (20.20 ± 2.85%, P < 0.05).
Figure 3.
6D and 6P isoforms inhibit the polymerization of hTau40, as measured by laser-light scattering (LLS). (A) LLS was used to monitor the polymerization of hTau40 in the absence (●) or presence of a twice-molar excess of 2N6P (▲) and 2N6D (□). An N-terminal construct know to inhibit hTau40 polymerization, Tau1-196 (○), was included as an experimental control. (B) Representative electron micrographs of hTau40 filaments formed alone or in the presence of 8 μM 2N6P, 2N6D, or 1-196. Scale bar represents 500 nm.
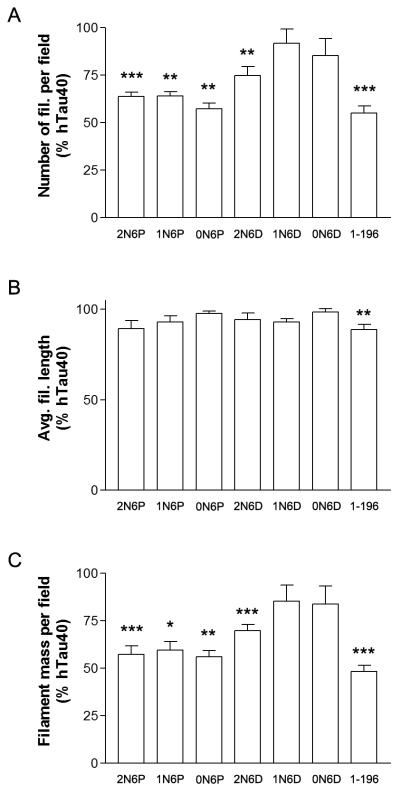
To verify the results of the LLS experiments and to further characterize the effects of 2N6P and 2N6D on hTau40 polymerization, we performed quantitative electron microscopy (EM) on filaments formed under each experimental condition (Fig. 3B). Previous work from our lab demonstrated that incubation with N-terminal tau fragments reduced the overall mass of hTau40 filaments formed, with the primary effect being a reduction in the number of filaments per field (5). Quantitative analysis revealed similar effects in the present study (Fig. 4). Polymerization of hTau40 (4 μM) in the presence of 2N6P (8 μM) caused a significant reduction in the number of filaments per field (36.26 ± 2.36%; P < 0.001), and in the overall mass of polymerized material per field (42.80 ± 4.64%; P < 0.001). Incubation with 2N6D also reduced filament number (25.20 ± 4.62%; p < 0.001), and overall polymer mass (30.12 ± 3.23%; P < 0.001). In agreement with our previous report (5), incubation with 1-196 reduced both the number and overall mass of filaments (45.07 ± 3.86% and 51.63 ± 3.27%, respectively; P < 0.001). These results are consistent with the reduction in polymer mass as observed by LLS. Taken together, the results of the LLS and EM experiments indicate that, like the N-terminal fragments previously examined, 2N6P and 2N6D inhibit polymerization of full-length tau.
Figure 4.
The presence of alternatively-spliced N-terminal exons differentially impacts the effects of 6D and 6P constructs on hTau40 polymerization. Incubation with 2N6P reduced the number (63.74 ± 2.36 % of control) and mass (57.20 ± 4.64 % of control) of hTau40 filaments per field. Filament number and mass were also reduced with 0N6P and 1N6P (see Results section). Although 2N6D significantly reduced filament number (74.80 ± 4.62 % of control) and mass (69.88 ± 3.23 % of control), 6D constructs containing zero or one N-terminal exon (0N6D and 1N6D) failed to significantly inhibit hTau40 polymerization. In agreement with our previous report (5), incubation with 1-196 reduced both the number and overall mass of filaments (* P < 0.05, ** P < 0.01, *** P< 0.001).
The presence of alternatively-spliced N-terminal exons differentially impacts the effects of 6D and 6P constructs on hTau40 polymerization
Alternative splicing of exons 2 and 3 on the 6D/6P background potentially gives rise to three isoforms apiece (see Fig. 2A). To determine whether alternatively spliced 6P and 6D differentially affect hTau40 polymerization, we assayed inhibition by each isoform using quantitative EM (Fig. 4). Filament number was significantly reduced by 0N6P and 1N6P in a manner similar to 2N6P (42.72 ± 2.94% and 36.08 ± 2.45%, respectively; p < 0.01), as was filament mass (43.95 ± 3.22% and 40.38 ± 4.30%, respectively; p < 0.01, p < 0.05). This is in agreement with previous results demonstrating that exons 2 and 3 had no effect on the inhibitory abilities of N-terminal fragments (5). In contrast, while 2N6D significantly reduced filament number and mass, 6D constructs containing zero or one N-terminal exons (0N6D and 1N6D) failed to significantly inhibit hTau40 polymerization. These experiments indicate that although alternative splicing has no effect on the ability of 6P isoforms to inhibit hTau40 polymerization, the absence of exons 2 and/or 3 reduces the effectiveness of 6D isoforms.
6D and 6P isoforms remain in the soluble fraction
In a previous study, we demonstrated that N-terminal tau fragments do not associate with hTau40 filaments in a co-sedimentation assay (5). This result suggested that the fragments act in the soluble fraction of the polymerization mixture to produce their effects. To determine whether 6D and 6P isoforms inhibit polymerization through a similar mechanism, we assembled hTau40 (4 μM) in the presence or absence of 2N6P, 2N6D, or 1-196 (8 μM) and then separated the mixtures into soluble and filamentous fractions through ultracentrifugation. The resulting supernatants and pellets were processed by SDS-PAGE gel electrophoresis, transferred to nitrocellulose membranes, and probed with antibodies against tau (Fig. 5). All three constructs, 2N6P, 2N6D and Tau196, remained in the supernatant fraction and were absent from the filamentous pellet, even at longer exposures (data not shown). These results indicate that 2N6P and 2N6D act in the soluble fraction to inhibit hTau40 polymerization, as did the N-terminal tau fragments previously studied.
Figure 5.

Tau6D and 6P isoforms remain in the soluble fraction of the polymerization reaction. Polymerized samples were subjected to ultracentrifugation over a 40% glycerol cushion and separated by gel electrophoresis. From left to right: hTau40 polymerization; hTau40 polymerization with twice molar 2N6P, 2N6D, or 1-196. Short N-terminal constructs were probed with the amino terminal antibody Tau-12, while full-length tau was probed with the carboxy terminal antibody Tau-7. Like 1-196, 2N6P and 2N6D do not pellet with the mass of polymerized hTau40, but instead remain in the supernatant. St: pre-spin starting material; S: supernatant; P: pellet.
Pseudophosphorylation in a crucial N-terminal sequence modulates the effects of the 6D/6P isoforms
We have previously demonstrated that the inhibitory effect of N-terminal tau fragments requires amino acids 18-42 in the amino terminus (5). Since 6D and 6P isoforms have similar effects on full-length tau polymerization (i.e. reduce filament number, remain in the soluble fraction), it is likely that amino acids 18-42 are crucial for their effect as well. This sequence contains tyrosine residues at positions 18 and 29, which are potential targets for kinase activity in AD (reviewed in (26)). Therefore, we asked whether phosphorylation at these tyrosine residues affects the ability of 6D and 6P isoforms to inhibit polymerization of the full-length protein.
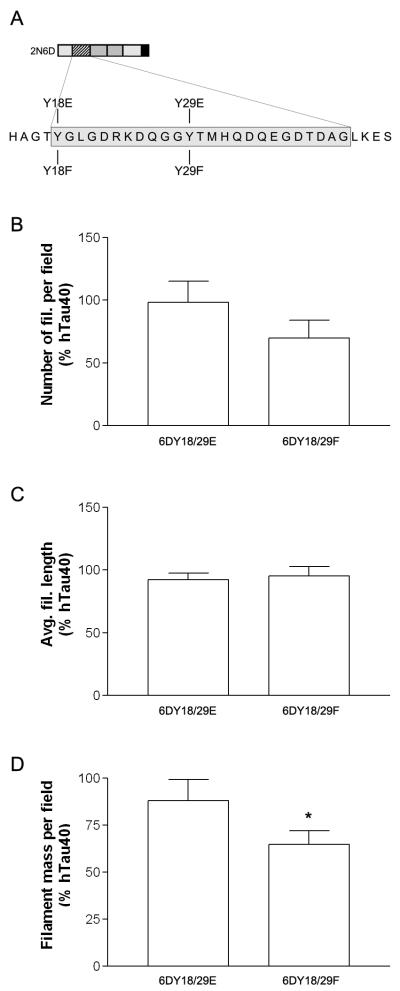
In order to overcome technical problems associated with in vitro phosphorylation reactions (i.e. inefficient phosphorylation, phosphorylation at unintended secondary sites), we used pseudophosphorylation, a well-established strategy that employs site-directed mutagenesis to replace a phosphorylatable residue with glutamic acid (27). We modeled the effects of tyrosine phosphorylation on the ability of 6D and 6P isoforms to inhibit tau polymerization by generating a construct based on 2N6D in which both tyrosine residues were mutated to glutamic acid (Y18/29E). As a control, we generated a construct in which both residues were mutated to phenylalanine (Y18/29F) (see Table 1, Fig 6A).
Table 1.
Tau constructs used in this study
| Name | Description | Purpose |
|---|---|---|
| hTau40 | Full-length tau; 2 N-terminal inserts and 4 MTBRs |
|
| 1-196 | hTau40 truncated at amino acid 196 |
Positive control for inhibition of hTau40 polymerization |
| 2N6D, 1N6D, 0N6D |
6D isoforms containing 0, 1, or 2 N-terminal inserts |
Isoform inhibition studies |
| 2N6P, 1N6P, 0N6P |
6D tau containing 0, 1, or 2 N-terminal inserts |
Isoform inhibition studies |
| 6DY18/29E | 2N6D with two Tyr→Glu mutations |
Mimics phosphorylation at Tyr18 and 29 |
| 6DY18/29F | 2N6D with two Tyr→Phe mutations |
Control construct that does not mimic phosphorylation |
Figure 6.
Mutations that mimic phosphorylation modifications impair the ability of 2N6D to influence hTau40 polymerization. (A) Schematic illustrating the position of modifications created in the N-terminal inhibitory region of 2N6D. (B) We polymerized hTau40 (4 μM) in the presence or absence of 8 μM wild-type 2N6D (data not shown), Y18/29E, or Y18/29F and measured the effects on filament number, average length, and mass by quantitative EM. Pseudophosphorylation at both tyrosine residues (Y18/29E) blocked the ability of 2N6D to inhibit hTau40 polymerization. In contrast, Y18/29F decreased filament mass and number similar to wild-type 2N6D (* P < 0.05).
We polymerized hTau40 (4 μM) in the presence or absence of these constructs (8 μM), and measured the effects on filament number, average length, and mass by quantitative EM. Pseudophosphorylation at both tyrosine residues (Y18/29E) blocked the ability of 2N6D to inhibit hTau40 (Figure 6B-D). In contrast, the effects of the control construct containing mutation to phenanlynine (Y18/29F) were not significantly different than wild-type 2N6D. These results indicate that tyrosine phosphorylation may block the inhibitory effects of the N-terminal isoforms, and that the residues18-24 identified in our earlier work using N-terminal hTau40 fragments (5), is also important for the inhibitory effects of the 6D and 6P isoforms.
DISCUSSION
Tau 6D and 6P isoforms inhibit in vitro polymerization of full-length tau
Alternative splicing of tau exon 6 produces isoforms that contain the amino terminus of the canonical protein, but lack the proline rich region, MTBR region and the C-terminal tail. The 6D/6P isoforms bear a striking resemblance to N-terminal tau fragments previously shown to inhibit in vitro polymerization of full-length hTau40 (5). Based in part on this physical resemblance, we assayed 6D and 6P isoforms for effects on hTau40 filament formation. The results presented here demonstrate that 6P/6D isoforms inhibit in vitro polymerization of full-length tau. Like the N-terminal fragments previously studied (5), the 6D/6P isoforms reduce the number of filaments formed and the overall filament mass, and remain in the soluble fraction of the polymerization mixture, consistent with a model in which these isoforms stabilize a conformation of full-length tau that discourages polymerization (Fig. 7).
Figure 7.
Schematic illustrating a potential mechanism by which 6D/6P isoforms or N-terminal tau fragments might promote the solubility of full-length hTau40. In this model, the N-terminus stabilizes an interaction between the C-terminus and the microtubule binding repeat region.
Surprisingly, although all 6P/6D isoforms contain the region necessary for inhibition (a.a. 18-42), we observe differences in their effectiveness. In general, 6P isoforms are more potent inhibitors than 6D isoforms. Additionally, the absence of exons 2 and 3 decreased the effectiveness of the 6D isoforms, but not the 6P variants or the N-terminal tau fragments previously tested (5). A simple interpretation is that the C-terminal 6D sequence encourages a protein folding event that limits accessibility of the 18-42 sequence, and that this effect is counteracted by the presence of exons 2 and 3. Future insights into the structure of these short isoforms, as well as their interaction with full-length tau, should distinguish between this and other possible explanations.
Our results demonstrate that 6P/6D isoforms are potential endogenous inhibitors of filament formation, but the question of whether they do so in the brain is still unanswered. A twice- molar excess of the 6D/6P isoforms was necessary to inhibit in vitro polymerization, but with the exception of the cerebellum, 6D/6P levels are low relative to canonical tau isoforms in adult brain (11, 18, 19, 28). In this in vitro assay system, however, the 6D/6P isoforms (8 μM) must compete with the arachidonic acid (75 μM) that drives polymerization, presumably by inducing hTau40 to adopt an Alz50-like conformation. Lower levels of the 6D/6P isoforms may be required in the context of a cellular system. It is also possible that enrichment of 6D/6P isoforms in certain parts of the cell results in increased local levels relative to canonical tau. Supporting this idea, anti-6D immunoreactivity in cultured cells and in brain is punctuate, and enriched in the proximal section and distal tips of neuronal processes (19). A candidate site for 6D/6P enrichment is the plasma membrane, which interacts with the amino terminus of tau (29). Because these truncated isoforms lack the MTBR region, they may even be more likely to interact with the plasma membrane than full-length isoforms. Future studies in cellular or animal models are necessary to determine whether 6D/6P isoforms are endogenous inhibitors of tau aggregation.
Future studies of 6P/6D expression levels and patterns in human brain may reveal more about the potential of these isoforms as endogenous regulators of tau polymerization. Although we propose that expression of 6D/6P may protect the cerebellum in AD, this region develops fibrillar pathology in other tauopathies (30, 31). This indicates either that 6D/6P is not protective against tau aggregation, or that this ability is compromised in these diseases. It is not known at present whether 6D/6P expression or phosphorylation (discussed below) is disrupted in these tauopathies, although brain-specific changes in the expression of these isoforms were detected in myotonic dystrophy type 1, a disease marked by tau aggregation (28). Comparative neuropathological studies of 6D/6P expression in various tauopathies may inform this issue.
Posttranslational modifications of 6D/6P isoforms
In the present study, we demonstrate that pseudophosphorylation of tyrosines 18 and 29 blocks the inhibitory ability of the 6D/6P isoforms. Pseudophosphorylation may disrupt the interaction of 18-42 with full-length tau directly or through conformational effects that reduce the availability of this region. Phosphorylation at tyrosine 18 is elevated in AD (32, 33), and may be targeted by Fyn or other members of the src family of tyrosine kinases (reviewed in (26)). Affinity of src tyrosine kinases for the 6D/6P isoforms might be reduced though, because they are truncated prior to an SH3-binding motif (233PKSP236) that binds src family tyrosine kinases (34, 35). In addition to phosphorylation, other factors with the potential to affect the inhibitory abilities of the 6D/6P isoforms include nitration at tyrosines 18 and 29 (36, 37), the mutations R5L (38) and R5H (39), which cause frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17), and removal of the amino terminus, which occurs early in AD progression and may be mediated by caspase 6 (40) or puromycin-sensitive aminopeptidase (41). If the 6D/6P isoforms represent endogenous inhibitors of filament formation, these changes in the amino terminus could contribute to disease progression by removing a barrier to tau aggregation.
Additional cell biological functions for 6P/6D isoforms
Recent studies indicate a role for the N-terminus of tau, and therefore the 6P/6D isoforms, in intracellular signaling. The N-terminus of tau may be part of the cellular response to beta-amyloid (Aβ) (42-45). Expression of N-terminal fragments of tau in cell culture can be neuroprotective (46) or toxic, depending on the length, and can induce cell death through a mechanism involving N-methyl-D-aspartate (NMDA) receptors and calpain (46, 47). In isolated axoplasm, 6P/6D isoforms selectively inhibit kinesin-based axonal transport by activating a signaling cascade that results in phosphorylation of kinesin light chains (48). Finally, the unique C-terminal 6D and 6P sequences may confer isoform-specific cellular roles to these proteins. Further work is necessary to reveal the role of these truncated tau isoforms in human brain.
ABBREVIATIONS
- AA
arachidonic acid
- AD
Alzheimer’s disease
- CNS
central nervous system
- DTT
dithiothreitol
- EGTA
ethylene glycol bis(β-aminoethylether)- N,N,N’,N’-tetraacetic acid
- FTDP-17
frontotemporal dementia and Parkinsonism linked to chromosome 17
- HEPES
N-(2-hydroxyethyl)piperazine-N’-2-ethanesulfonic acid
- hTau40
the 441 amino acid isoform of human tau
- is
intensity of scattered light
- MTBR
microtubule binding repeat
- PHF
paired helical filament
Footnotes
Supported by NIH Awards NS049834 (N.L.), AG09466 (L.I.B).
REFERENCES
- 1.Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW. Tau, tangles, and Alzheimer’sease. Biochim Biophys Acta. 2005;1739:216–223. doi: 10.1016/j.bbadis.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. On areas of transition between entorhinal allocortex and temporal isocortex in the human brain. Normal morphology and lamina-specific pathology in Alzheimer’s disease. Acta Neuropathol. 1985;68:325–332. doi: 10.1007/BF00690836. [DOI] [PubMed] [Google Scholar]
- 3.Mesulam MM. Neuroplasticity failure in Alzheimer’s disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 4.Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60:729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- 5.Horowitz PM, LaPointe N, Guillozet-Bongaarts AL, Berry RW, Binder LI. N-terminal fragments of tau inhibit full-length tau polymerization in vitro. Biochemistry. 2006;45:12859–12866. doi: 10.1021/bi061325g. [DOI] [PubMed] [Google Scholar]
- 6.Jeganathan S, von Bergen M, Brutlach H, Steinhoff HJ, Mandelkow E. Global hairpin folding of tau in solution. Biochemistry. 2006;45:2283–2293. doi: 10.1021/bi0521543. [DOI] [PubMed] [Google Scholar]
- 7.Mukrasch MD, Bibow S, Korukottu J, Jeganathan S, Biernat J, Griesinger C, Mandelkow E, Zweckstetter M. Structural polymorphism of 441-residue tau at single residue resolution. PLoS Biol. 2009;7:e34. doi: 10.1371/journal.pbio.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry RW, Abraha A, Lagalwar S, LaPointe N, Gamblin TC, Cryns VL, Binder LI. Inhibition of tau polymerization by its carboxy-terminal caspase cleavage fragment. Biochemistry. 2003;42:8325–8331. doi: 10.1021/bi027348m. [DOI] [PubMed] [Google Scholar]
- 9.Andreadis A. Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim Biophys Acta. 2005;1739:91–103. doi: 10.1016/j.bbadis.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Luo MH, Leski ML, Andreadis A. Tau isoforms which contain the domain encoded by exon 6 and their role in neurite elongation. Journal of cellular biochemistry. 2004;91:880–895. doi: 10.1002/jcb.20029. [DOI] [PubMed] [Google Scholar]
- 11.Wei ML, Andreadis A. Splicing of a regulated exon reveals additional complexity in the axonal microtubule-associated protein tau. J Neurochem. 1998;70:1346–1356. doi: 10.1046/j.1471-4159.1998.70041346.x. [DOI] [PubMed] [Google Scholar]
- 12.Wei ML, Memmott J, Screaton G, Andreadis A. The splicing determinants of a regulated exon in the axonal MAP tau reside within the exon and in its upstream intron. Brain Res Mol Brain Res. 2000;80:207–218. doi: 10.1016/s0169-328x(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 13.Goode BL, Feinstein SC. Identification of a novel microtubule binding and assembly domain in the developmentally regulated inter-repeat region of tau. J Cell Biol. 1994;124:769–782. doi: 10.1083/jcb.124.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goode BL, Denis PE, Panda D, Radeke MJ, Miller HP, Wilson L, Feinstein SC. Functional interactions between the proline-rich and repeat regions of tau enhance microtubule binding and assembly. Mol Biol Cell. 1997;8:353–365. doi: 10.1091/mbc.8.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukrasch MD, von Bergen M, Biernat J, Fischer D, Griesinger C, Mandelkow E, Zweckstetter M. The “jaws” of the tau-microtubule interaction. J Biol Chem. 2007;282:12230–12239. doi: 10.1074/jbc.M607159200. [DOI] [PubMed] [Google Scholar]
- 16.Abraha A, Ghoshal N, Gamblin TC, Cryns V, Berry RW, Kuret J, Binder LI. C-terminal inhibition of tau assembly in vitro and in Alzheimer’s disease. J Cell Sci. 2000;113(Pt 21):3737–3745. doi: 10.1242/jcs.113.21.3737. [DOI] [PubMed] [Google Scholar]
- 17.von Bergen M, Friedhoff P, Biernat J, Heberle J, Mandelkow EM, Mandelkow E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc Natl Acad Sci U S A. 2000;97:5129–5134. doi: 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Tse SW, Andreadis A. Tau exon 6 is regulated by an intricate interplay of trans factors and cis elements, including multiple branch points. J Neurochem. 2007;100:437–445. doi: 10.1111/j.1471-4159.2006.04252.x. [DOI] [PubMed] [Google Scholar]
- 19.Luo MH, Tse SW, Memmott J, Andreadis A. Novel isoforms of tau that lack the microtubule-binding domain. J Neurochem. 2004;90:340–351. doi: 10.1111/j.1471-4159.2004.02508.x. [DOI] [PubMed] [Google Scholar]
- 20.King ME, Ahuja V, Binder LI, Kuret J. Ligand-dependent tau filament formation: implications for Alzheimer’s disease progression. Biochemistry. 1999;38:14851–14859. doi: 10.1021/bi9911839. [DOI] [PubMed] [Google Scholar]
- 21.Gamblin TC, King ME, Dawson H, Vitek MP, Kuret J, Berry RW, Binder LI. In vitro polymerization of tau protein monitored by laser light scattering: method and application to the study of FTDP-17 mutants. Biochemistry. 2000;39:6136–6144. doi: 10.1021/bi000201f. [DOI] [PubMed] [Google Scholar]
- 22.Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem. 1996;271:32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- 23.King ME, Gamblin TC, Kuret J, Binder LI. Differential assembly of human tau isoforms in the presence of arachidonic acid. J Neurochem. 2000;74:1749–1757. doi: 10.1046/j.1471-4159.2000.0741749.x. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Ghoshal N, Garcia-Sierra F, Wuu J, Leurgans S, Bennett DA, Berry RW, Binder LI. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer’s disease. Exp Neurol. 2002;177:475–493. doi: 10.1006/exnr.2002.8014. [DOI] [PubMed] [Google Scholar]
- 26.Lebouvier T, Scales TM, Williamson R, Noble W, Duyckaerts C, Hanger DP, Reynolds CH, Anderton BH, Derkinderen P. The microtubule-associated protein tau is also phosphorylated on tyrosine. J Alzheimers Dis. 2009;18:1–9. doi: 10.3233/JAD-2009-1116. [DOI] [PubMed] [Google Scholar]
- 27.Huang W, Erikson RL. Constitutive activation of Mek1 by mutation of serine phosphorylation sites. Proc Natl Acad Sci U S A. 1994;91:8960–8963. doi: 10.1073/pnas.91.19.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leroy O, Wang J, Maurage CA, Parent M, Cooper T, Buee L, Sergeant N, Andreadis A, Caillet-Boudin ML. Brain-specific change in alternative splicing of Tau exon 6 in myotonic dystrophy type 1. Biochim Biophys Acta. 2006;1762:460–467. doi: 10.1016/j.bbadis.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Brandt R, Leger J, Lee G. Interaction of tau with the neural plasma membrane mediated by tau’s amino-terminal projection domain. J Cell Biol. 1995;131:1327–1340. doi: 10.1083/jcb.131.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 31.Piao YS, Hayashi S, Wakabayashi K, Kakita A, Aida I, Yamada M, Takahashi H. Cerebellar cortical tau pathology in progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol. 2002;103:469–474. doi: 10.1007/s00401-001-0488-2. [DOI] [PubMed] [Google Scholar]
- 32.Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, Do LH, Andreadis A, Van Hoesen G, Ksiezak-Reding H. Phosphorylation of tau by fyn: implications for Alzheimer’s disease. J Neurosci. 2004;24:2304–2312. doi: 10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vega IE, Cui L, Propst JA, Hutton ML, Lee G, Yen SH. Increase in tau tyrosine phosphorylation correlates with the formation of tau aggregates. Brain Res Mol Brain Res. 2005;138:135–144. doi: 10.1016/j.molbrainres.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhaskar K, Yen SH, Lee G. Disease-related modifications in tau affect the interaction between Fyn and Tau. J Biol Chem. 2005;280:35119–35125. doi: 10.1074/jbc.M505895200. [DOI] [PubMed] [Google Scholar]
- 35.Lee G, Newman ST, Gard DL, Band H, Panchamoorthy G. Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci 111. 1998;(Pt 21):3167–3177. doi: 10.1242/jcs.111.21.3167. [DOI] [PubMed] [Google Scholar]
- 36.Reyes JF, Reynolds MR, Horowitz PM, Fu Y, Guillozet-Bongaarts AL, Berry R, Binder LI. A possible link between astrocyte activation and tau nitration in Alzheimer’s disease. Neurobiol Dis. 2008;31:198–208. doi: 10.1016/j.nbd.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds MR, Reyes JF, Fu Y, Bigio EH, Guillozet-Bongaarts AL, Berry RW, Binder LI. Tau nitration occurs at tyrosine 29 in the fibrillar lesions of Alzheimer’s disease and other tauopathies. J Neurosci. 2006;26:10636–10645. doi: 10.1523/JNEUROSCI.2143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poorkaj P, Muma NA, Zhukareva V, Cochran EJ, Shannon KM, Hurtig H, Koller WC, Bird TD, Trojanowski JQ, Lee VM, Schellenberg GD. An R5L tau mutation in a subject with a progressive supranuclear palsy phenotype. Ann Neurol. 2002;52:511–516. doi: 10.1002/ana.10340. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi S, Toyoshima Y, Hasegawa M, Umeda Y, Wakabayashi K, Tokiguchi S, Iwatsubo T, Takahashi H. Late-onset frontotemporal dementia with a novel exon 1 (Arg5His) tau gene mutation. Ann Neurol. 2002;51:525–530. doi: 10.1002/ana.10163. [DOI] [PubMed] [Google Scholar]
- 40.Horowitz PM, Patterson KR, Guillozet-Bongaarts AL, Reynolds MR, Carroll CA, Weintraub ST, Bennett DA, Cryns VL, Berry RW, Binder LI. Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer’s disease. J Neurosci. 2004;24:7895–7902. doi: 10.1523/JNEUROSCI.1988-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sengupta S, Horowitz PM, Karsten SL, Jackson GR, Geschwind DH, Fu Y, Berry RW, Binder LI. Degradation of tau protein by puromycin-sensitive aminopeptidase in vitro. Biochemistry. 2006;45:15111–15119. doi: 10.1021/bi061830d. [DOI] [PubMed] [Google Scholar]
- 42.King ME, Kan HM, Baas PW, Erisir A, Glabe CG, Bloom GS. Tau-dependent microtubule disassembly initiated by prefibrillar beta-amyloid. J Cell Biol. 2006;175:541–546. doi: 10.1083/jcb.200605187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbato C, Canu N, Zambrano N, Serafino A, Minopoli G, Ciotti MT, Amadoro G, Russo T, Calissano P. Interaction of Tau with Fe65 links tau to APP. Neurobiol Dis. 2005;18:399–408. doi: 10.1016/j.nbd.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Williamson R, Scales T, Clark BR, Gibb G, Reynolds CH, Kellie S, Bird IN, Varndell IM, Sheppard PW, Everall I, Anderton BH. Rapid tyrosine phosphorylation of neuronal proteins including tau and focal adhesion kinase in response to amyloid-beta peptide exposure: involvement of Src family protein kinases. J Neurosci. 2002;22:10–20. doi: 10.1523/JNEUROSCI.22-01-00010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernandez P, Lee G, Sjoberg M, Maccioni RB. Tau phosphorylation by cdk5 and Fyn in response to amyloid peptide Abeta (25-35): involvement of lipid rafts. J Alzheimers Dis. 2009;16:149–156. doi: 10.3233/JAD-2009-0933. [DOI] [PubMed] [Google Scholar]
- 46.Amadoro G, Serafino AL, Barbato C, Ciotti MT, Sacco A, Calissano P, Canu N. Role of N-terminal tau domain integrity on the survival of cerebellar granule neurons. Cell Death Differ. 2004;11:217–230. doi: 10.1038/sj.cdd.4401314. [DOI] [PubMed] [Google Scholar]
- 47.Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci U S A. 2006;103:2892–2897. doi: 10.1073/pnas.0511065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LaPointe NE, Morfini G, Pigino G, Gaisina IN, Kozikowski AP, Binder LI, Brady ST. The amino terminus of tau inhibits kinesin-dependent axonal transport: implications for filament toxicity. J Neurosci Res. 2009;87:440–451. doi: 10.1002/jnr.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]