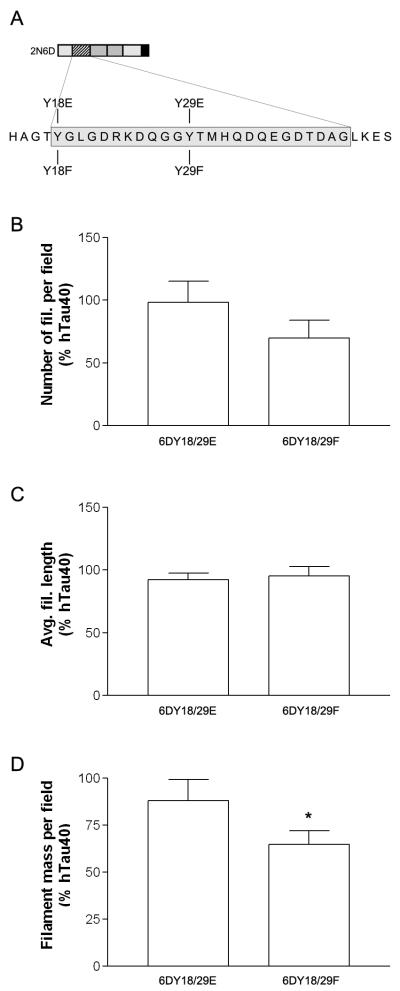
Figure 6.
Mutations that mimic phosphorylation modifications impair the ability of 2N6D to influence hTau40 polymerization. (A) Schematic illustrating the position of modifications created in the N-terminal inhibitory region of 2N6D. (B) We polymerized hTau40 (4 μM) in the presence or absence of 8 μM wild-type 2N6D (data not shown), Y18/29E, or Y18/29F and measured the effects on filament number, average length, and mass by quantitative EM. Pseudophosphorylation at both tyrosine residues (Y18/29E) blocked the ability of 2N6D to inhibit hTau40 polymerization. In contrast, Y18/29F decreased filament mass and number similar to wild-type 2N6D (* P < 0.05).