Abstract
Objective
While detection of pituitary tumors with magnetic resonance imaging (MRI) may reduce diagnostic costs and improve surgical outcomes for patients with Cushing's disease, the optimal T1-weighted spin echo MRI protocol remains unknown. We hypothesized that specific MR scanning parameters influence detection of corticotropinomas.
Design and patients
Between December 1997 and November 2004, 21 of 84 consecutive patients with Cushing's disease had a falsely negative initial pituitary MRI study and a lesion identified subsequently at the National Institutes of Health Clinical Center. This study retrospectively reviewed and compared technical parameters used for the two pituitary T1-weighted spin echo MRIs in 18 patients with available scans.
Measurements
Repetition time (TR)/echo times (TE), field of view (FOV), matrix size, magnetic field strength, slice thickness, use of Gadolinium contrast and the time interval between studies were recorded.
Results
The MRI inter-scan interval was 5.4 ± 1.1 months. All scans used gadolinium, matrix sizes were similar and nearly all had 3 mm slice thickness. Parameters that differed between the NIH and outside scans were: TR (400 ms vs. 492±19 ms, P = 0.0002); TE (10.3 ± 0.5 vs. 17.2 ms ± 1.2 ms, P = 0.0003); FOV (12×12 cm vs.17±0.6 × 18±0.7 cm, P<0.0001). Immunohistochemistry of tumors resected at transsphenoidal surgery confirmed all tob be corticotropinomas.
Conclusions
Not all “T1-weighted spin echo” scans are equally accurate. MRI technique, particularly FOV and TR/TE value, influences results. We recommend that endocrinologists consider pituitary MRI parameters when interpreting the results.
Keywords: Magnetic resonance imaging, Cushing's disease, diagnosis
INTRODUCTION
Cushing's syndrome is most often caused by excessive ACTH production from a pituitary corticotrope tumor (so-called Cushing's disease). Better imaging techniques to identify these tumors potentially would improve diagnostic accuracy and facilitate their surgical removal. 1, 2
Magnetic resonance imaging (MRI) has supplanted computerized tomography as the method of choice for the evaluation of patients with pituitary lesions. However, MRI performed by the standard T1-weighted spin echo (SE) technique only detects up to 60% of corticotrope tumors, perhaps because they tend to be microadenomas with signal and enhancing characteristics similar to normal pituitary tissue3. A more recent MRI technique of spoiled gradient recalled acquisition in the steady state (SPGR) improved the tumor detection rate.3, 4 However, this method is not widely used in the general community, and standard T1-weighted spin echo MR studies continue to be more common.
A number of parameters influence the final T1-weighted spin echo MR image. T1 weighting requires a short repetition time (TR) of 500–700 ms and short echo time (TE) of 15–25 ms.4 Other variables that affect the MR image include magnetic field strength and field of view (FOV), which optimally focuses on the pituitary gland (12×12 cm) rather than the entire brain. The use of a contrast agent also enhances detection of pituitary adenomas, which take up contrast slower than surrounding normal tissue 4. Additionally, thin interleaved slice images of 3 mm or less improve resolution4, 5.
We present a series of patients with surgically proven Cushing's disease who had a reportedly negative T1-weighted SE MRI at an outside institution but a positive study at our institution. We hypothesized that this discrepancy was caused by differences in MRI technical parameters, and that understanding these differences would suggest an optimal imaging protocol. To test this hypothesis, we compared the imaging results and the technical features of the two studies.
SUBJECTS AND METHODS
Subjects
The patients reported here represent a subset of 84 consecutive individuals with Cushing's disease recruited by a single investigator (LN) between December 1997 and November 2004 for study at the National Institutes of Health Clinical Center (NIH). All were studied under Investigational Review Board-approved protocols after giving oral and written informed consent. Most patients participated in a study comparing jugular venous sampling and inferior petrosal sinus sampling (Clinical Trials Identifier NCT 00001453) 6. We do not perform inferior petrosal sinus sampling in patients with a lesion on pituitary MRI and responses to provocative tests suggestive of Cushing's disease. As a result, the protocol stipulated that patients provide a report documenting a negative MRI study before admission. After a few patients were found to have an abnormal MRI at the NIH, the criterion was changed to require review of the outside films before inclusion in the NIH study. Twenty-one of the 84 patients had an initially negative spin echo MRI result at an outside institution and subsequently had a visible tumor on repeat MRI at the NIH. No lesion was seen on either the outside or NIH MRI in the remaining 63 patients. The spin echo MRI of some of these patients was reported previously3. We were able to obtain 18 of the 21 outside coronal MR images and reports to compare with those obtained at the NIH.
Imaging techniques
At the NIH, MR images were obtained using a 1.5 Tesla magnet strength scanner (Signa, General Electric, Milwaukee, WI). Coronal pre-contrast T1-weighted spin echo (SE) scans were obtained using the following protocol: repetition time/echo time, 400/9 msec; 192 × 256 matrix; two excitations; 12-cm field of view; and interleaved sections, 3 mm in thickness without intersection gap. Scan time was 5.10 min. The study was repeated immediately after IV administration of gadolinium contrast material [0.01 mmol/kg gadopentetate dimeglumine (Magnevist, Berlex Laboratories, Inc., Montville, NJ)].
Data capture and analysis
The MRI parameters actually used for each individual NIH study and the outside studies were obtained based on information on the films. We noted the date, repetition time (TR), echo times (TE), field of view (FOV), matrix size, magnetic field strength, slice thickness and use of gadolinium contrast for each study and calculated the time interval between them. An experienced neuroradiologist (NP) reviewed the non-NIH and NIH studies for presence of a pituitary lesion without knowledge of the surgical findings, radiology reports, or pathology results. The presence or absence of an abnormality was determined initially for all outside scans and then for the NIH scans, to prevent bias based on the positive NIH studies or comparison of each patient's images. Subsequently the images were reviewed in pairs to determine if the lesion seen on the NIH study was present on the outside study. The presence, size, and position (right, left and central) of any lesion was recorded and compared to the surgical findings by the neurosurgeon (EHO). Histopathological examination of the surgical specimen and postoperative hypocortisolism were used to confirm the diagnosis of an ACTH-secreting adenoma.
For continuous endpoints the mean, median, and standard error of the mean were calculated. Paired t-tests were used to compare technical parameters. Two comparisons were performed: one included all patients and one included only the 15 patients who had negative outside scans according to the NIH review. A P value of < 0.05 was considered statistically significant.
RESULTS
The patients included 16 women and 2 men with a mean age of 36 years (range, 21–63 years). The surgeon located and removed an adenoma at transsphenoidal surgery in all patients. The tumor size estimated at surgery was 6.3 mm ± 0.6 mm (mean ± SE, range 3–15mm) (Table 1). Immunohistochemistry confirmed an ACTH-containing adenoma in all cases and each patient became hypocortisolemic after surgery.
Table 1.
Patients' characteristics, parameters of T1-weighted SE MRIs obtained at the non-NIH institution, size of lesions on NIH MRI and measured tumor size at transsphenoidal surgery1
| No. | Age (yrs) | Sex | Inter-scan Interval (mo) | FOV (cm)2 | TR/TE3 | Magnet (Tesla)4 | NIH MRI Findings (size in mm, location) | Tumor size at TSS (mm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 36 | F | 2 | 18×18 | 500/20 | 1.5 | lesion R | 5 |
| 2 | 39 | F | 6 | 19×19 | 500/14 | 1.5 | 5×8, R | 5 |
| 3 | 33 | F | 4 | 20×20 | 350/19 | 1.5 | 6, L | 7 |
| 4 | 34 | F | 6 | 15.8×18 | 422/26 | 0.35 | 5, L | 6 |
| 5 | 31 | F | 6 | 20×20 | 450/25 | 0.5 | 4×7, C | 6 |
| 6 | 45 | F | 5 | 16.5×22 | 600/7 | 3.0 | 3, L | 10 |
| 7 | 41 | M | 5 | 16×20 | 450/15 | 0.35 | 4×4, L | 4 |
| 8 | 39 | F | 1 | 20×15 | 600/19 | 1.0 | 6, R | 5 |
| 9 | 30 | F | 3 | 18×18 | 450/15 | 1.5 | 5×6, L | 5 |
| 10 | 21 | F | 3 | 16×16 | 416/18 | 1.5 | 5×5, L | 4 |
| 11 | 26 | F | 5 | 14×16 | 635/17 | 1.5 | 9, C | 15 |
| 12 | 41 | F | 3 | 24×28 | 600/20 | N/A | 5×5, | 8 |
| 13 | 63 | M | 1 | 16×16 | 466/14 | 1.5 | 10×8, L | 35 |
| 14 | 32 | F | 2 | 18×18 | N/A6 | 0.5 | 4, R | 3 |
| 15 | 27 | F | 8 | 16×16 | 500/24 | 1.5 | 7×3.5, R | 7 |
| 16 | 37 | F | 7 | 14×14 | 550/15 | 1.5 | lesion, C | 6 |
| 17 | 41 | F | 3 | 17.5×20 | 420/15 | 1.5 | 7, R | 7 |
| 18 | 38 | F | 12 | 18×18 | 450/9 | N/A | 4–5, L | 5 |
M = male, F = female, R = right, L = left, C = center, mo = month, magnet = magnetic field strength of MRI machine, TSS = transsphenoidal surgery. Patient numbers in bold font are those whose non-NIH studies were found to be positive when reviewed by the NIH neuroradiologist. The results of the statistical comparisons were not changed when these three patients were excluded from analysis.
P = 0.001 compared to NIH values of 12 × 12 cm
Compared to NIH values, TR: 401 ± 0.9 ms; P = 0.0002; TE: 10.3 + 0.5 ms, P = 0.0003
Compared to NIH magnetic field strength of 1.5 Tesla P = 0.228
At surgery, an ill-defined 3mm area was measured; however, biopsies throughout the soft gland revealed tumor.
Gradient echo technique used7
Interpretation of scans
The neuroradiologist's interpretation of the scans is shown in the Table 1. A representative pair of scans is shown in Figure 1. All of the MRI findings corresponded to the location of tumor at surgery. In addition, our neuroradiologist detected a tumor on three MR scans from outside institutions for which the outside interpretation was negative (Table 1). When scans were re-evaluated as pairs, no new lesions were detected on the non-NIH films.
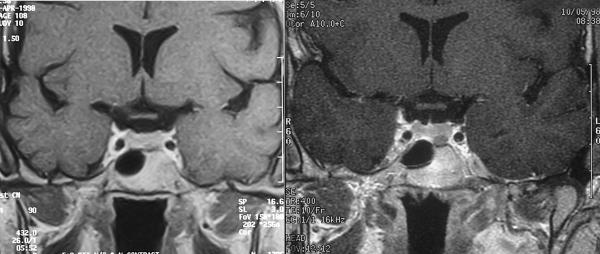
Figure 1.
Left: Initial MRI of patient 4, read as negative: TR/ TE 422/26 ms; FOV 15.8×18.0 cm. Right: Subsequent NIH MRI of the same patient showing tumor on left: TR/TE 400/10 ms; FOV 12×12 cm.
Time interval between the two MRI studies
The average time interval between the two scans was 5.4 ± 1.1 months, with a range of 24 days to 12 months, and median of 4.5 months. When the three patients with visible tumor on the initial study were excluded, the time interval was 4.4 ± 0.7 months, with a median of 4.0 months. The range was unchanged.
TR/ TE values
The TR and TE were highly variable at outside institutions (Table 1). Sixteen of the seventeen evaluable spin echo MRI scans used a TR greater than 400ms, with a mean TR value of 492 ms ± 19.4 ms (NIH value 401 ms; P = 0.0002). One patient was studied with a gradient echo T1-weighted rather than a spin echo technique.
Sixteen of the seventeen available TE values from other institutions (range 7 ms to 26 ms) were higher than those used at our institution (range 9 – 10ms) (Table 1). That mean outside TE value (17.2 ± 1.2 ms) was significantly different than the NIH value (10.3 ± 0.5 ms, P = 0.0003). These differences remained significant when the patients with visible lesions on the initial study were excluded (TR: P = 0.002; TE: P = 0.003).
Field of view (FOV)
At our institution the FOV in all coronal images was standard at 12 × 12 cm. The FOV at other imaging centers was larger, ranging from 14 × 14 cm to 24 × 28 cm (Table 1). The mean FOV at other institutions was 18 ± 0.6 × 19 ± 0.7 cm (P<0.0001 vs. NIH). The mean FOV excluding the three patients with visible lesions on the initial study was similar (18 ± 0.07 × 19 ± 0.09, (P<0.0001 vs. NIH).
Magnetic field strength
The magnetic field strength used at other institutions was similar to that used at the NIH, 1.5 Tesla. Ten of the outside scans used a 1.5 Tesla field strength. Of the remaining scans, five used magnet strength less than 1.5 Tesla, one used a 3 Tesla machine, and two studies did not note magnet strength (Table 1). Compared to the NIH magnet strength, these were not statistically different (including or excluding three patients with initially visible tumor, P = 0.23).
Slice thickness
To detect pathology, thin slice images of 3 mm or less are best for pituitary imaging.4, 5 All NIH studies used 3 mm slice thickness. At the other institutions, 3 mm slices were used in fourteen of eighteen scans. Three studies used slices less than 2.5 mm and one used a 4 mm slice thickness.
Matrix size
A fine matrix size helps to achieve a high degree of spatial resolution.7 Matrix size was not different between outside institutions and NIH. The NIH used a matrix size of 256 × 192; matrix size at other institutions ranged from 256 × 122 to 256 × 300.
Contrast material
The use of gadolinium contrast material is mandatory for the evaluation of pituitary abnormalities.8 All MRI scans in this study were performed before and after intravenous administration of contrast. The standard dose of 0.01 mmol/kg was used at the NIH (and presumably also at the outside centers).
DISCUSSION
In Cushing's syndrome, MRI identification of a pituitary mass in conjunction with positive non-invasive endocrine tests (such as CRH stimulation) obviates the need for invasive diagnostic tests such as inferior petrosal sinus sampling. Localization of the tumor by MRI also allows the neurosurgeon to target a specific area during TSS and so may improve the success of surgical treatment.1, 2 Thus, efforts to improve MRI detection of these tumors may reduce diagnostic costs and improve treatment outcomes for patients with Cushing's disease. To that end, we sought to identify MR protocol parameters associated with improved detection of an adenoma.
Spin echo (SE) MRI protocols were the first to gain widespread popularity and continue to be the most commonly used pulse sequence in the evaluation of the pituitary gland.7 The SE sequence is made up of two radiofrequency pulses - one pulse that excites the spins in the tissue and a subsequent 180 degree pulse that refocuses a resultant `echo.' T1-weighted images use a short TR and TE. As a result, tissues that relax more quickly (such as fat) present as bright signal. Tumors have longer T1 relaxation times and show as a dark signal. 5 Based on this, T1-weighted spin echo MRI has been recommended for the routine evaluation of pituitary adenomas. 8–10
Besides the spin echo technique, other MRI protocols have been used for the detection of corticotropinomas. In one study, dynamic MRI, in which a gradient echo technique was obtained within seconds of contrast administration, identified tumor in 11 out of 14 patients with microadenoma, compared to 8 positive studies with conventional spin echo imaging. However, three falsely positive tumors were identified, suggesting in this report, an important loss of specificity.11 We previously compared the performance of a spoiled gradient recalled acquisition in the steady state (SPGR) protocol with conventional spin echo MR where SPGR had superior sensitivity (80% vs. 49%) but a higher false positive rate (2% vs. 4%). 3
The current study demonstrated that not all T1-weighted SE protocols are alike, and suggests specific MRI parameters that may influence results. The two most important factors were the field of view and TR/TE. Other parameters such as the use of thin (3 mm or less) slices, use of contrast, and a fine matrix size are known to influence resolution. However, these were not different in the non-NIH and NIH studies, so their relative contribution could not be evaluated.
The field of view appeared to be crucial for tumor detection, probably because a large field of view has less resolution for a given matrix.12 Thus the resolution is inherently superior in a pituitary study performed with a FOV of 12 ×12 cm as compared to a FOV of 18 ×18 cm for the same matrix of 192 × 256.
A second influential MRI parameter was the TR/ TE value. Previous publications recommend TR/TE values in the range of 500–700/15–25 ms.7, 9, 13, 14 Since the contrast resolution of the soft tissues is strongly influenced by short TR/TE values, we have adopted the shortest possible values for the available gradient strength of our magnet. In this study, 86% of outside scans had TR values greater than 416 ms, while 90% of TE values were greater than 10 ms.
Magnetic field strength also may affect the detection of corticotropinomas. We used a 1.5 Tesla magnet, as described in a number of previous studies. 4, 9, 11. Twenty-five percent of the outside scans used a magnet field strength less than 1.5 Tesla, suggesting that a lower magnetic field strength may decrease the ability to detect these tumors. While it is possible that this parameter was important, it was not statistically different between the two sets of studies.
Another important MRI parameter is the use of a contrast agent, which improves the detection of ACTH-secreting adenomas by taking advantage of the different signal dynamics of contrast enhancement between the normal tissue and tumor.11 The T1-weighted images of the normal pituitary gland, stalk, and cavernous sinus all increase in signal rapidly following administration of gadolinium.15 A pituitary adenoma enhances less intensely than the adjacent normal tissue and appears as a focal hypointense area. Steiner et al. demonstrated that the detection of pituitary adenomas on T1-weighted SE images improved from 47% to 91% after administration of gadolinium. 16 In the current study, all scans, both at NIH and other institutions, used contrast material.
Other MRI parameters such as thin sections (3 mm or less) and a fine matrix are important for high resolution. 15 However, these two parameters were similar between the studies performed at the NIH and the studies done at other institutions. Thus, it seems unlikely that contrast administration, section thickness, or matrix size contributed to the differences in detection of the pituitary adenoma in our patients.
One explanation for the ability to detect tumor at the (later) NIH study might be that the tumors grew sufficiently in the interval between the studies to allow detection. We doubt that this factor influenced the difference in detection rate of these 3 – 15 mm adenomas. The median interval of 4.0 months was almost certainly not sufficient for dramatic growth, as ACTH-secreting tumors typically grow extremely slowly. Another explanation is that of a referral bias in favor of the NIH technique, since tumors seen on MRI at another institution were not included in the study.
Observer error also may explain differences in tumor identification. Our neuroradiologist identified three pituitary lesions on outside films that were initially read as negative at another institution. These were not small tumors, ranging from 5 to 7 mm in size. Thus, optimizing MRI techniques is essential, but an experienced reader of the MRI films also is required.
In conclusion, we have identified MR protocol parameters associated with improved identification of a pituitary tumor on a T1-weighted spin echo study. Preoperative localization of the pituitary lesion by MRI has been associated with a better outcome, presumably because it facilitates operative identification of the pathological tissue.1, 2 We recommend that endocrinologists understand and discuss optimal parameters on pituitary MR scans with their radiology colleagues, to increase their diagnostic utility and to potentially improve surgical outcomes.
Acknowledgements
This study was supported in part by the intramural programs of the National Institute of Child Health and Human Development, the National Institute of Neurologic Disease and Stroke, and the Warren Grant Magnuson Clinical Center, National Institutes of Health. The authors have nothing to declare.
REFERENCES
- 1.Bochicchio D, Losa M, Buchfelder M. Factors influencing the immediate and late outcome of Cushing's disease treated by transsphenoidal surgery: a retrospective study by the European Cushing's Disease Survey Group. J Clin Endocrinol Metab. 1995;80:3114–3120. doi: 10.1210/jcem.80.11.7593411. [DOI] [PubMed] [Google Scholar]
- 2.Salenave S, Gatta B, Pecheur S, San-Galli F, Visot A, Lasjaunias P, Roger P, Berge J, Young J, Tabarin A, Chanson P. Pituitary magnetic resonance imaging findings do not influence surgical outcome in adrenocorticotropin-secreting microadenomas. J Clin Endocrinol Metab. 2004;89:3371–3376. doi: 10.1210/jc.2003-031908. [DOI] [PubMed] [Google Scholar]
- 3.Patronas N, Bulakbasi N, Stratakis CA, Lafferty A, Oldfield EH, Doppman J, Nieman LK. Spoiled gradient recalled acquisition in the steady state technique is superior to conventional postcontrast spin echo technique for magnetic resonance imaging detection of adrenocorticotropin-secreting pituitary tumors. J Clin Endocrinol Metab. 2003;88:1565–1569. doi: 10.1210/jc.2002-021438. [DOI] [PubMed] [Google Scholar]
- 4.Stadnik T, Stevenaert A, Beckers A, Luypaert R, Buisseret T, Osteaux M. Pituitary microadenomas: diagnosis with two-and three-dimensional MR imaging at 1.5 T before and after injection of gadolinium. Radiology. 1990;176:419–428. doi: 10.1148/radiology.176.2.2164234. [DOI] [PubMed] [Google Scholar]
- 5.Rajan S. MRI: A conceptual overview. Springer-Verlag New York Inc.; 1998. [Google Scholar]
- 6.Ilias I, Chang R, Pacak K, Oldfield EH, Wesley R, Doppman J, Nieman LK. Jugular venous sampling: an alternative to petrosal sinus sampling for the diagnostic evaluation of adrenocorticotropic hormone-dependent Cushing's syndrome. J Clin Endocrinol Metab. 2004;89:3795–3800. doi: 10.1210/jc.2003-032014. [DOI] [PubMed] [Google Scholar]
- 7.Atlas S. Magnetic Resonance Imaging of the Brain and Spine. 3rd Edition Williams and Wilkins; Lippincott: 2002. [Google Scholar]
- 8.Dwyer AJ, Frank JA, Doppman JL, Oldfield EH, Hickey AM, Cutler GB, Loriaux DL, Schiable TF. Pituitary adenomas in patients with Cushing disease: initial experience with Gd-DTPA-enhanced MR imaging. Radiology. 1987;163:421–426. doi: 10.1148/radiology.163.2.3562821. [DOI] [PubMed] [Google Scholar]
- 9.Peck WW, Dillon WP, Norman D, Newton TH, Wilson CB. High-resolution MR imaging of pituitary microadenomas at 1.5 T: experience with Cushing disease. AJR Am J Roentgenol. 1989;152:145–151. doi: 10.2214/ajr.152.1.145. [DOI] [PubMed] [Google Scholar]
- 10.Doppman JL, Frank JA, Dwyer AJ, Oldfield EH, Miller DL, Nieman LK, Chrousos GP, Cutler GB, Jr., Loriaux DL. Gadolinium DTPA enhanced MR imaging of ACTH-secreting microadenomas of the pituitary gland. J Comput Assist Tomogr. 1988;12:728–735. doi: 10.1097/00004728-198809010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Tabarin A, Laurent F, Catargi B, Olivier-Puel F, Lescene R, Berge J, Galli FS, Drouillard J, Roger P, Guerin J. Comparative evaluation of conventional and dynamic magnetic resonance imaging of the pituitary gland for the diagnosis of Cushing's disease. Clin Endocrinol (Oxf) 1998;49:293–300. doi: 10.1046/j.1365-2265.1998.00541.x. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni MV, Lee KF, McArdle CB, Yeakley JW, Haar FL. 1.5-T MR imaging of pituitary microadenomas: technical considerations and CT correlation. AJNR Am J Neuroradiol. 1988;9:5–11. [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols DA, Laws ER, Jr., Houser OW, Abboud CF. Comparison of magnetic resonance imaging and computed tomography in the preoperative evaluation of pituitary adenomas. Neurosurgery. 1988;22:380–385. doi: 10.1227/00006123-198802000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Kucharczyk W, Davis DO, Kelly WM, Sze G, Norman D, Newton TH. Pituitary adenomas: high-resolution MR imaging at 1.5 T. Radiology. 1986;161:761–765. doi: 10.1148/radiology.161.3.3786729. [DOI] [PubMed] [Google Scholar]
- 15.Chakeres D, Curtin A, Ford G. Magnetic Resonance imaging of Pituitary and Parasellar Abnormalities. Radiologic Clinics of North America. 1989;27:265–281. [PubMed] [Google Scholar]
- 16.Steiner E, Imhof H, Knosp E. Gd-DTPA enhanced high resolution MR imaging of pituitary adenomas. Radiographics. 1989;9:587–598. doi: 10.1148/radiographics.9.4.2756189. [DOI] [PubMed] [Google Scholar]