Abstract
Background
Clinical treatment goals of type 1 diabetes mellitus (T1DM) have changed since the Diabetes Control and Complications Trial (DCCT) demonstrated reduced long-term complications with intensive diabetes therapy. There have been few longitudinal studies to describe the clinical course of T1DM in the age of intensive therapy. Our objective was to describe the current-day clinical course of T1DM.
Methods
An analysis of the cumulative incidence of long-term complications was performed. The DCCT (1983-1993) assigned patients to conventional or intensive therapy. Since 1993, the DCCT has been observational, and intensive therapy was recommended for all patients. The Pittsburgh Epidemiology of Diabetes Complications (EDC) study is an observational study of patients with T1DM from Allegheny County, Pennsylvania. The study population comprised the DCCT T1DM cohort (N=1441) and a subset of the EDC cohort (n=161) selected to match DCCT entry criteria. In the DCCT, intensive therapy aimed for a near-normal glycemic level with 3 or more daily insulin injections or an insulin pump. Conventional therapy, with 1 to 2 daily insulin injections, was not designed to achieve specific glycemic targets. Main outcome measures included the incidences of proliferative retinopathy, nephropathy (albumin excretion rate >300 mg/24 h, creatinine level ≥2 mg/dL [to convert to micromoles per liter, multiply by 88.4], or renal replacement), and cardiovascular disease.
Results
After 30 years of diabetes, the cumulative incidences of proliferative retinopathy, nephropathy, and cardiovascular disease were 50%, 25%, and 14%, respectively, in the DCCT conventional treatment group, and 47%, 17%, and 14%, respectively, in the EDC cohort. The DCCT intensive therapy group had substantially lower cumulative incidences (21%, 9%, and 9%) and fewer than 1% became blind, required kidney replacement, or had an amputation because of diabetes during that time.
Conclusion
The frequencies of serious complications in patients with T1DM, especially when treated intensively, are lower than that reported historically.
The clinical course of type 1 diabetes mellitus (T1DM), including its treatment, metabolic outcomes, and long-term clinical complications, has changed dramatically in the past 20 years. Treatment innovations, including multiple daily injection regimens, continuous subcutaneous insulin infusion with external pumps, new insulin analogues with more physiologic pharmacokinetic characteristics, and wide-spread self-monitoring, and improved treatment of comorbidities such as hypertension and dyslipidemia, have all contributed to changes in the management of T1DM. Moreover, the Diabetes Control and Complications Trial (DCCT)1 and its long-term observational follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) study,2 and other clinical trials3 have demonstrated the powerful effects of more physiologic control of glycemia on microvascular and macrovascular disease.3-7 All of these changes are projected to change the clinical course of T1DM. Unfortunately, most of the published descriptions of the clinical course of T1DM are either outdated, based on observations in small-sized populations, or rely onself-reported complications.8-12 Although several recent studies have reported declines in complications over time, they represent single-center experience and/or do not fully reflect the potential impact of intensive management.13-16
The DCCT, a multicenter, controlled clinical trial, recruited its population from 29 centers across Canada and the United States between 1983 and 1989 and thus offers a broad perspective.1,4 The detailed collection of clinical data including treatments and outcomes, using uniform, standardized methods over the past 25 years in the DCCT and then the EDIC, provides the opportunity to describe the clinical course of T1DM in the latter part of the 20th and early 21st century. Although the DCCT/EDIC cohort is not a population-based sample, it has the advantage of being concurrent, widely distributed in North America, and virtually complete in its follow-up, with 92% of the baseline cohort (96% of the surviving cohort) followed up for the entire mean DCCT/EDIC period of 19 years (range, 16-22 years), and having objective measures of outcomes. The interventions applied during the mean 6.5-year course of the controlled clinical trial17 and subsequently, since 1993, when the volunteers’ clinical care was turned over to their own health care providers, are well documented.7 The therapy applied in the conventional treatment group was designed to represent the standard of clinical care at the time.1 Subsequently, based on the results of the DCCT, subjects in the conventional treatment group were offered training in intensive therapy, and all subjects were advised to perform intensive therapy, which became the new standard of care for the treatment of T1DM.18
We describe the clinical care, metabolic results, and outcomes of the DCCT/EDIC conventional and intensive treatment groups over a diabetes duration of 30 years and compare these results with those of the Pittsburgh Epidemiology of Diabetes Complications (EDC) study,13,19 a more population-based observational study20 with clinical data collected with methods similar to the DCCT and EDIC study during an overlapping period. The results reported are framed over duration of diabetes, rather than in study time, to provide a measure of long-term complications that can be translated into clinical care and communicated to patients as the anticipated outcomes for diabetes at present and in the future.
METHODS
THE DCCT/EDIC STUDY
Subjects
The inclusion and exclusion criteria for the DCCT and the treatment protocol have been described in detail.1,4 Briefly, 1441 subjects with T1DM aged between 13 and 39 years were recruited into the DCCT between 1983 and 1989 (53% were male). The primary prevention cohort consisted of 726 subjects with no retinopathy, a urinary albumin excretion rate (AER) of less than 40 mg/24 h, and a diabetes duration of 1 to 5 years. The secondary intervention cohort consisted of 715 subjects who had nonproliferative retinopathy, urinary AER of less than 200 mg/24 h, and a diabetes duration of 1 to 15 years. As part of the screening for the DCCT, individuals were excluded if they had hypertension (defined as systolic blood pressure ≥140 or diastolic blood pressure ≥90 mm Hg), a history of symptomatic is chemicheart disease, the presence of major electrocardiographic abnormalities, or severe hypercholesterolemia. Subjects were randomly assigned to either intensive or conventional treatment arms and were assessed for complications at frequent follow-up visits. Conventional therapy patients (n=730) were treated with methods consistent with the standard therapy of the time, consisting of 1 or 2 daily insulin injections and daily urine or blood glucose testing; the goal was freedom from symptoms of hyperglycemia and frequent or severe hypoglycemia. The conventional treatment group maintained a median hemoglobin A1c (HbA1c) level of approximately 9.0% (to convert to proportion of 1, multiply by 0.01) during the 6.5 mean years of DCCT follow-up (Figure 1A). The patients randomly assigned to intensive treatment were treated with multiple (at least 3) daily injections or with continuous subcutaneous insulin infusion with external insulin pumps with the goal of achieving glycemic control as close to the nondiabetic range as safely possible. Insulin dose selection was guided by frequent (at least 4 daily) self-monitoring of blood glucose test results, taking into account meal size and composition and activity levels. Ninety-nine percent of the patients completed the study. Baseline characteristics of participants have been provided elsewhere.4
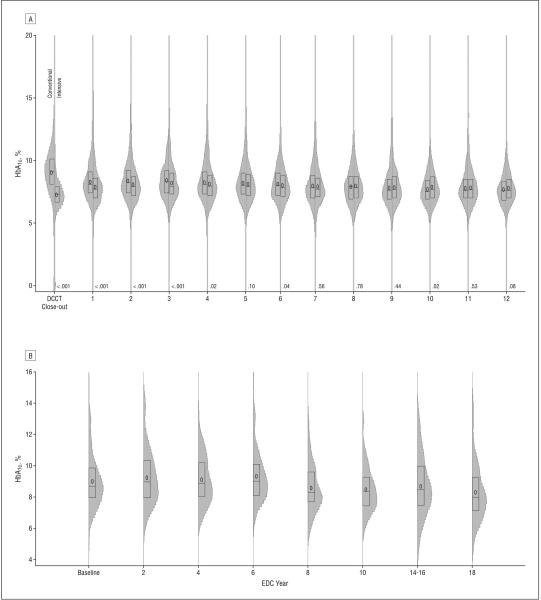
Figure 1.
Distribution of hemoglobin A1c (HbA1c) values over time in the DCCT/EDIC cohort, with intensive and conventional treatment groups shown separately (A), and the EDC cohort (B). The plot summarizes the distribution of HbA1c by study period as box plots (showing the 25th to 75th percentiles) superimposed on vertical density histograms (“violin” plots). The median of the distribution is indicated by a horizontal line and the mean by “0.” The P values on the x-axis compare the DCCT intensive and conventional treatment groups. DCCT/EDIC indicates Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT 1983-1993 and EDIC 1994-2005); EDC, Pittsburgh Epidemiology of Diabetes Complications (1986-2006). To convert HbA1c to proportion of 1, multiply by 0.01.
In 1994, after completion of the DCCT, 1375 subjects (96% of the surviving cohort; 688 from the conventional arm and 687 from the intensive arm) agreed to participate in the EDIC follow-up study, which included annual examinations measuring diabetic complications.2 With the initiation of EDIC, the conventional treatment participants were offered instruction in intensive therapy reflecting the current recommendations for the management of TIDM.21
Clinical Outcomes
Demographic data and health history were self-reported, and an annual, standardized physical examination measured clinical status. Body mass index, calculated as weight in kilograms divided by height in meters squared, was measured every 3 months during the DCCT and yearly during the EDIC study. All laboratory measurements were performed at the DCCT/EDIC Central Biochemistry Laboratory at the University of Minnesota, Minneapolis, as previously described.1 Hemoglobin A1c was measured every 3 months during the DCCT and yearly during the EDIC study.1,2 Long-term stability of the HbA1c assay has been described.22 Albumin excretion rate was measured annually during the DCCT and on alternate years during the EDIC study using a timed 4-hour urine collection and expressed as milligrams per 24 hours.1,2 Serum lipid levels were measured using conventional enzymatic methods from fasting serum samples yearly during the DCCT and on alternative years during the EDIC study.
Retinopathy was measured by standardized 7-field fundus photography biannually during the DCCT. During the EDIC study, it was assessed with identical methods but in approximately one-quarter of the cohort each year and in the entire cohort at EDIC years 4 and 10. All photographs were graded centrally using the final Early Therapy Diabetic Retinopathy Study (ETDRS) grading scale23 and DCCT methods,24 with the graders masked to DCCT therapy assignment. Visual acuity was assessed by ETDRS methods.23
The severe hypoglycemic events reported herein are limited to those leading to coma and/or seizure. During the DCCT quarterly visits, study coordinators asked about the occurrence of hypoglycemia since the previous visit. During the EDIC study, the severe hypoglycemic events that occurred in the 3 months prior to the annual visit were documented on the annual history form, and further details surrounding these events were recorded. Comparable recording of metabolic outcomes was not available in the EDC study.
The primary diabetes complications reported in this article are retinopathy, nephropathy, and cardiovascular disease (CVD). For the present analyses, the levels of retinopathy included those that are clinically most important, ie, proliferative diabetic retinopathy or worse, clinically significant macular edema (CSME), photocoagulation therapy (focal or scatter), and blindness. Patients who received pan-retinal scatter photocoagulation (laser) therapy in either eye were counted as having the most severe level of retinopathy thereafter, and patients who received focal photocoagulation for macular edema were counted as having CSME thereafter. Nephropathy was defined as an AER of 300 mg/24 h or higher, a serum creatinine level of 2 mg/dL or higher (to convert to micromoles per liter, multiply by 88.4), or dialysis or renal transplant. A CVD event, as described in previous publications, was any of the following events: nonfatal myocardial infarction or stroke, death judged to be secondary to CVD, subclinical myocardial infarction, angina confirmed by ischemic changes with exercise tolerance testing or by clinically significant obstruction on coronary angiography, or revascularization with angioplasty or coronary artery bypass.7 Subclinical (“silent”) myocardial infarctions were determined on the annual electrocardiograms. Neuropathy was not included in these analyses primarily because the methods used in the DCCT/EDIC and EDC studies were not comparable. We, however, do report the occurrence of amputations as a measure of severe consequence of neuropathy.
THE EDC STUDY
The EDC study is an observational study of T1DM and its complications that has collected data on patients who received a diagnosis, or who were seen within 1 year of diagnosis, at the Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania, between 1950 and 1980.19,20,25 Despite being clinic based, this population has been shown to be representative of the T1DM population of Allegheny County, Pennsylvania.19 It was selected for comparison with the DCCT/EDIC cohort since it is contemporaneous and has used methods that are similar to those used in the DCCT/EDIC. A subset of the EDC population (n=161) that was similar to the DCCT cohort (age at baseline, 13-39 years; diabetes duration <15 years; and retinopathy grade <30) was selected for these analyses.
The EDC population and methods have been described in detail in previous publications.13,19,25 Participants were recruited between 1986 and 1988 and seen biennially until between 1996 and 1998 and selectively thereafter until between 2004 and 2007, when an 18-year follow-up examination was performed. Retinopathy was determined by 3-field fundus photography that was graded by the same reading center and in the same manner as for the DCCT/EDIC study. Albuminuria was determined immunonephelometrically on multiple-timed urine specimens at each visit, with the clinic specimen used in these analyses. The CVD end point was restricted to the events noted for the DCCT/EDIC study, except that ischemic electrocardiographic readings (Minnesota Codes 1.3, 4.1-4.3, 5.1-5.3, and 7.1) were included and angina confirmed only by exercise tolerance testing was excluded. The reported HbA1c values have been converted using a DCCT-aligned method.26
STATISTICAL ANALYSIS
Clinical characteristics were compared between sexes using Wilcoxon rank sum tests for quantitative variables and χ2 tests for categorical variables.27 The event rates of hypoglycemia and diabetic ketoacidosis are presented as number per 100 patient-years. The cumulative incidence of a CVD, retinopathy, or nephropathy event was estimated by Weibull regression model for interval-censored data.28 The Weibull assumption was verified by an empirical survival estimation for interval-censored data.
RESULTS
COHORTS
The DCCT/EDIC cohort had been followed up continuously for a mean of 18.5 years as of their 12th annual EDIC visit that took place between 2004 and 2005. Their baseline characteristics, collected between 1983 and 1989 and listed separately for the groups assigned to conventional and intensive therapy, are given in Table 1. There were no significant differences in the baseline characteristics for the 92% of the original cohort who were followed up at EDIC year 12 compared with the total cohort (data not shown). The mean (SD) diabetes duration for the entire cohort (no difference between intensive and conventional therapy at the DCCT baseline) at baseline was 5.6 (4.2) years (range, 1-15 years); at EDIC year 12, the mean (SD) diabetes duration was 24.3 (4.9) years (range, 17-37 years). Mortality accounted for the majority of the subjects lost to follow-up, with 53 having died. The baseline characteristics for the EDC cohort (n=161) are also given in Table 1. They differed from the DCCT/EDIC cohort by having a slightly longer diabetes duration (approximately 5 years), despite a lower mean age (approximately 6 years).
Table 1. Clinical Characteristics of the DCCT/EDIC and EDC Cohorts.
| Conventional |
Intensive |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| DCCT |
EDIC |
EDC |
DCCT |
EDIC |
|||||
| Characteristic | Baseline (1983-1989) (n=730) |
Closeout (1993) (n=723) |
Year 12 (2005) (n=606) |
Baseline (1986-1988) (n=161) |
Year 10 (1996) (n=105) |
Year 18 (2006) (n=88) |
Baseline (1983-1989) (n=711) |
Closeout (1993) (n=698) |
Year 12 (2005) (n=620) |
| Age, mean (SD), y | 27 (7) | 33 (7) | 46 (7) | 20 (4) | 31 (4) | 40 (4) | 27 (7) | 34 (7) | 46 (7) |
| Duration, mean (SD), y | 5 (4) | 12 (5) | 24 (5) | 11 (2) | 21 (2) | 30 (2) | 6 (4) | 12 (5) | 25 (5) |
| BMI, mean (SD) | 24 (3) | 25 (3) | 28 (5) | 24 (3) | 26 (4) | 28 (5) | 23 (3) | 27 (4) | 28 (5) |
| BMI ≥30, % | 2 | 6 | 28 | 3 | 1 | 27 | 1 | 19 | 31 |
| Current smoker, % | 18 | 20 | 12 | 20 | 17 | 15 | 19 | 20 | 15 |
| HbA1c, % (SD) | 8.9 (1.6) | 9.1 (1.5) | 7.7 (1.2) | 9.0 (1.7) | 8.5 (1.4) | 8.3 (1.8) | 8.9 (1.6) | 7.4 (1.1) | 7.8 (1.2) |
| Proliferative retinopathy, %a | 0b | 24b | 40b | ||||||
| 1 Prevention | 0 | 0.6 | 12.7 | NA | NA | NA | 0 | 0.6 | 4.4 |
| 2 Intervention | 0 | 12.7 | 37.3 | NA | NA | NA | 0 | 4.2 | 13.2 |
| Renal | |||||||||
| AER, mg/24 hc | 12 (7-19) | 10 (6-20) | 10 (6-20) | 14 (9-26) | 14 (7-51) | 11 (7-34) | 12 (7-17) | 9 (6-14) | 10 (6-17) |
| ≥40 mg/24 h, % | 5 | 13 | 15 | 19 | 29 | 25 | 5 | 7 | 11 |
| ≥300 mg/24 h, % | 0 | 3 | 6 | 4 | 15 | 13 | 0 | 1 | 3 |
| Serum creatinine ≥2 mg/dL, % | 0 | 0.3 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| Dialysis/transplant, % | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0.5 |
| Medications, % | |||||||||
| ACE inhibitors or ARBs | NA | NA | 45 | 1 | 15 | 42 | NA | NA | 42 |
| >14 Aspirin per mo | NA | NA | 43 | 0 | 0 | 25 | NA | NA | 40 |
| β-Blocker | NA | NA | 7 | 1 | 0 | 5 | NA | NA | 4 |
| Statin | NA | NA | 36 | 1 | 2 | 32 | NA | NA | 38 |
Abbreviations: ACE, angiotensin-converting enzyme; AER, albumin excretion rate; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DCCT, Diabetes Control and Complications Trial; EDC, Pittsburgh Epidemiology of Diabetes Complications Experience; EDIC, Epidemiology of Diabetes Interventions and Complications; HbA1c, hemoglobin A1c; NA, not applicable.
SI conversion factors: To convert creatinine to micromoles per liter, multiply by 88.4; HbA1c to a proportion of 1, multiply by 0.01.
Proliferative diabetic retinopathy or worse.
The EDC participants were not stratified by prevention cohort.
Median (first quartile–third quartile).
DIABETES TREATMENT AND GLYCEMIA
Treatment of the DCCT/EDIC conventional and intensive treatment groups is summarized in Table 2. The differences in therapy between the treatment groups during the DCCT reflect the protocol-directed interventions; during the DCCT, both treatment groups were highly adherent to their assigned therapies, with 97% of DCCT study time spent on assigned therapy. After the end of the DCCT, the care of all subjects was returned to their own physicians, and intensive therapy was recommended for all. During the post-DCCT/EDIC follow-up, the original conventional treatment group differed from the original intensive treatment group only with regard to a somewhat lower use of insulin pumps. The EDC population also had a change in interventions over time that followed, with a 2- to 4-year time lag, the changes in interventions of the DCCT conventional treatment group, suggesting that the clinical trial evidence generated by the DCCT was adopted and translated in the T1DM population in the United States over time (Table 2).
Table 2. Diabetes Management by Treatment Group During the DCCT/EDIC and EDC Studies.
| Conventional |
Intensive |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DCCT |
EDIC |
EDC |
DCCT |
EDIC |
|||||||
| Diabetes Management |
Year 1 (n=730) |
Closeout (n=723) |
Year 6 (n=660) |
Year 12 (n=606) |
Year 1 (n=161) |
Year 10 (n=105) |
Year 18 (n=88) |
Year 1 (n=711) |
Closeout (n=698) |
Year 6 (n=654) |
Year 12 (n=620) |
| Insulin delivery, % | |||||||||||
| CSII | 0.1 | 1.5 | 22.6 | 45.2 | 2.5 | 6.7 | 38.6 | 29.8 | 41.4 | 37.9 | 48.4 |
| MDI | 1.0 | 3.6 | 57.4 | 49.3 | 4.3 | 36.2 | 37.5 | 70.0 | 56.0 | 56.8 | 48.9 |
| 1-2 Injections | 98.9 | 94.9 | 19.4 | 5.1 | 89.4 | 53.4 | 15.9 | 0.3 | 2.6 | 5.1 | 2.1 |
| Insulin dose, mean (SD), U/kg/d |
0.68 (0.25) | 0.66 (0.20) | 0.67 (0.21) | 0.64 (0.25) | 0.90 (0.25) | 0.75 (0.22) | 0.72 (0.22) | 0.73 (0.26) | 0.71 (0.24) | 0.73 (0.25) | 0.68 (0.27) |
| Self-monitoring blood glucose, % (≥4 times/d) |
1.7 | 3.7 | 43.5 | 63.7 | NA | 23.8 | 46.6 | 69.1 | 52.9 | 47.1 | 56.1 |
Abbreviations: CSII, continuous subcutaneous insulin infusion (external insulin pump); DCCT, Diabetes Control and Complications Trial; EDC, Pittsburgh Epidemiology of Diabetes Complications Experience; EDIC, Epidemiology of Diabetes Interventions and Complications; MDI, multiple (≥3) daily injection therapy; NA, not applicable.
The distribution of HbA1c values over time in the DCCT/EDIC conventional and intensive treatment groups reflects the different study goals during the DCCT and subsequently the universal recommendation for intensive therapy during the EDIC follow-up (Figure 1A). The HbA1c values over time also presumably reflect the change in the intensity of diabetes care from the clinical trial period, when a dedicated team of physicians, nurses, dietitians, and behaviorists provided frequent oversight of the patients and all care and diabetes equipment and supplies were given free of charge, to the post-DCCT follow-up, when all care was returned to the patients’ own physicians and only a modest number of monitoring strips and limited amount of insulin were given to the patients. The mean HbA1c value in the conventional treatment group was 9.1% averaged over the entire DCCT period, with only 4.3% with HbA1c values of 7% or lower. During the EDIC follow-up, the mean HbA1c fell to approximately 8%, with 13.1% with HbA1c values of 7% or lower. In the intensive treatment group, mean HbA1c values were 7.1% averaged over the 6.5 years of DCCT, and 44.3% had mean HbA1c values of 7% or lower. During the EDIC follow-up, the HbA1c values in the intensive treatment group ranged from 7.8% to 8.1%, such that the mean HbA1c levels were not significantly different from those in the conventional treatment group over the 12 years of EDIC follow-up. During the EDIC study, 18.8% of the intensive treatment group maintained an HbA1c level of 7.0% or lower. The HbA1c levels in the EDC cohort over time resemble the values in the conventional treatment group, with mean values of 9.0% to 9.3% (median values, 8.7%-9.0%) until approximately EDC year 8 (1994-1996), when the means and medians fell by approximately 0.5 HbA1c percentage points (Figure 1B). The percentage of the EDC cohort with an HbA1c of 7% or lower during the most recent visit was 16.8%.
LONG-TERM COMPLICATIONS
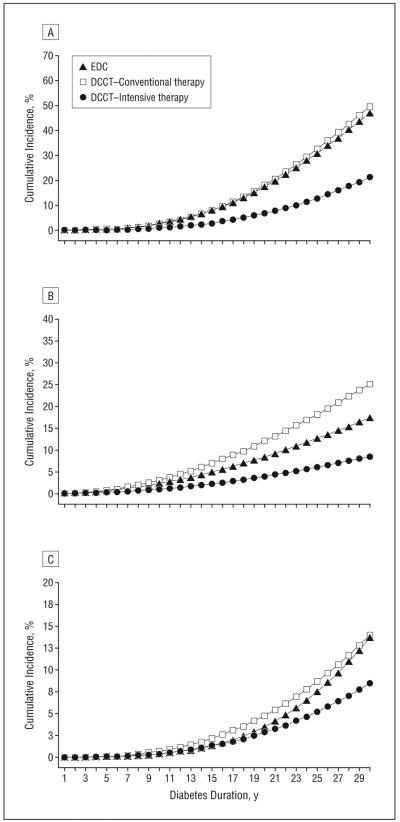
The development of complications for the DCCT/EDIC intensive and conventional treatment cohorts and the EDC study, based on duration of diabetes, are shown in Figure 2. After a diabetes duration of 30 years, the cumulative incidences of proliferative retinopathy, nephropathy, and CVD, defined in the “Methods” section, were 50%, 25%, and 14%, respectively, in the DCCT/EDIC conventional treatment group, which was similar to the EDC cohort with 47%, 17%, and 14% cumulative incidences, respectively. By contrast, the DCCT intensive treatment had cumulative incidences of 21%, 9%, and 9% for proliferative retinopathy, nephropathy, and CVD, respectively, reflecting the powerful effect of intensive therapy over time.
Figure 2.
Estimated cumulative incidences of proliferative retinopathy or worse (A), nephropathy (B), and cardiovascular disease (C) over time. Nephropathy was defined as an albumin excretion rate of 300 mg/24 h or higher, a serum creatinine level of 2 mg/dL or higher, or dialysis or renal transplant. Cardiovascular disease was defined as described in the “Clinical Outcomes” subsection of the “Methods” section. DCCT/EDIC indicates Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications; EDC, Pittsburgh Epidemiology of Diabetes Complications.
Other complications of clinical importance are given in Table 3 by DCCT treatment group. Of note, only 5 of 1441 DCCT participants had a loss in visual acuity worse than 20/100 in either eye (from diabetic retinopathy in 1 conventional and 1 intensive treatment subject and from non-diabetic causes in 3 intensive treatment group subjects), and only 3 subjects ever became legally blind in both eyes. Only 36 subjects (26 from the conventional and 10 from the intensive treatment group) developed renal insufficiency, including those with a serum creatinine level of 2.0 mg/dL or higher or receiving renal replacement therapy with dialysis or transplantation (n=18 [14 conventional and 4 intensive treatment group subjects]). Fifteen persons (8 conventional and 7 intensive treatment group subjects) had amputations (1 below the knee and the remainder were all toes). In the EDC study, 2 persons (1%) developed vision loss in both eyes, 3 (2%) developed renal insufficiency, and 3 (2%) had amputations.
Table 3. Complications That Occurred During the DCCT/EDIC and EDC Studies by Treatment Groupa.
| No. (%) |
|||
|---|---|---|---|
| Complicationb | DCCT/EDIC Conventional (n=730) |
EDC (n=161) |
DCCT/EDIC Intensive (n=711) |
| CVD | 61 (8) | 18 (11) | 38 (5) |
| Retinopathy | |||
| PDR | 173 (25) | 70 (43) | 71 (10) |
| CSME | 183 (25) | 33 (21) | 93 (13) |
| Scatter laser | 129 (18) | NAc | 42 (6) |
| Focal laser | 80 (11) | NAc | 30 (4) |
| Blind (<20/200 in either eye), % |
1 (0.1) | 7 (4) | 4 (1) |
| Nephropathy | 118 (16) | 22 (14) | 41 (6) |
| Renal insufficiency | 26 (4) | 3 (2) | 10 (1) |
| Renal replacement | 14 (2) | 2 (1) | 4 (1) |
| Neuropathy: amputations | 8 (1) | 3 (2) | 7 (1) |
Abbreviations: CSME, clinically significant macular edema; CVD, cardiovascular disease; DCCT, Diabetes Control and Complications Trial; DKA, diabetic ketoacidosis; EDC, Pittsburgh Epidemiology of Diabetes Complications Experience; EDIC, Epidemiology of Diabetes Interventions and Complications; NA, not available; PDR, proliferative diabetic retinopathy or worse.
Through EDIC year 12 or EDC year 18.
Cardiovascular disease was defined as cardiovascular death, nonfatal acute myocardial infarction, silent myocardial infarction, revascularization, confirmed angina, and nonfatal cerebrovascular event. Retinopathy was defined as PDR or CSME. Nephropathy was defined as an albumin excretion rate higher than 300 mg/24 h or renal insufficiency (serum creatinine level of 2 mg/dL or higher [to convert to micromoles per liter, multiply by 88.4] or dialysis or renal transplant). Renal insufficiency was defined as a serum creatinine level of 2.0 mg/dL or higher or renal replacement. Renal replacement was defined as dialysis or transplant. In the DCCT/EDIC study, all amputations were of toes, except for 1 amputation below the knee.
The EDC participants are not routinely photographed if laser therapy is reported.
ADVERSE METABOLIC EVENTS: DIABETIC KETOACIDOSIS, HYPOGLYCEMIA, AND WEIGHT GAIN
The adverse consequences of diabetes therapy, including the annual incidence of severe hypoglycemia resulting in loss of consciousness or seizure, which is recognized to increase with intensive therapy, and of diabetic ketoacidosis are given in Table 4. The mean weight of the subjects increased over time (Table 1), with intensive therapy having an association with increasing prevalence of obesity (body mass index ≥30), from 1% of subjects at the DCCT baseline (secondary to eligibility criteria) to 31% at EDIC year 12.
Table 4. Hypoglycemia and DKA Event Ratea by Treatment Group During the DCCT, EDIC, and EDC Studies.
| Conventional |
EDC |
Intensive |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| DCCT | EDIC Year 6 |
EDIC Year 12 |
Baseline | Year 10 |
Year 18 |
DCCT | EDIC Year 6 |
EDIC Year 12 |
|
| Hypoglycemia, coma/seizure | 5.4 | 16.4 | 9.2 | 19.0 | 14.6 | 10.4 | 16.3 | 6.7 | 13.6 |
| Requiring assistance | 18.7 | 47.3 | 39.6 | NA | NA | NA | 61.2 | 38.5 | 48.4 |
| DKA | 1.8 | 2.4 | 0 | 3.1 | 1.3 | 0.9 | 2.0 | 1.2 | 0 |
Abbreviations: DCCT, Diabetes Control and Complications Trial; DKA, diabetic ketoacidosis; EDC, Pittsburgh Epidemiology of Diabetes Complications Experience; EDIC, Epidemiology of Diabetes Interventions and Complications; NA, not applicable.
Per 100 patient-years.
DCCT/EDIC Research Group.
Study Chairs
S. Genuth, D. M. Nathan, B. Zinman (vice chair), O. Crofford (past)
Group Participants
Albert Einstein College of Medicine: J. Crandall, M. Reid, J. Brown-Friday, S. Engel, J. Sheindlin, H. Martinez (past), H. Shamoon (past), H. Engel (past), M. Phillips (past); Case Western Reserve University: W. Dahms (deceased), R. Gubitosi-Klug, L. Mayer, S. Pendegras, H. Zegarra, D. Miller, L. Singerman, S. Smith-Brewer, M. Novak, P. Gaston, J. Quin, Saul Genuth (past), M. Palmert (past); Cornell University Medical Center: D. Brillon, M. E. Lackaye, V. Reppucci, T. Lee, M. Heinemann (past); Henry Ford Health System: F. Whitehouse, M. McLellan, D. Kruger, J. D. Carey, E. Angus, A. Thomas, M. Croswell (past), A. Galprin (past), D. Kahkonen (past); International Diabetes Center: R. Bergenstal, R. Cuddihy, M. Johnson, D. Etzwiler (deceased), D. Kendall, D. Walk, D. Freking, D. Noller (past), M. Spencer (past); Joslin Diabetes Center: A. Jacobson, E. Golden, R. Beaser, O. Ganda, O. Hamdy, H. Wolpert, P. G. Sharuk, P. Arrigg, A. Burwood (past), J. Rosenzweig (past), L. Rand (past); Massachusetts General Hospital: D. M. Nathan, M. Larkin, J. Godine, E. Cagliero, P. Lou, S. Fritz (past); Mayo Foundation: J. Service, G. Ziegler, J. Pach, P. Dyck, J. Daube; Medical University of South Carolina: M. Lopes-Virella, J. Soule, S. Caulder, K. Hermayer, M. Brabham, A. Blevins (past), J. Parker (past), D. Lee, P. Lindsey, M. Bracey, K. Lee, M. Nutaitis, A. Farr (past), S. Elsing (past), T. Thompson (past), J. Selby (past), T. Lyons (past), S. Yacoub-Wasef (past), M. Szpiech (past), D. Wood (past), R. Mayfield (past), J. Colwell; Northwestern University: M. Molitch, B. Schaefer, L. Jampol, A. Lyon, M. Gill, Z. Strugula, L. Kaminski, J. Shankle, P. Astlesford, D. Blackburn, S. Ajroud-Driss, O. Stone, C. West, I. Burnett-Zeigler; University of California, San Diego: O. Kolterman, G. Lorenzi, M. Goldbaum; University of Iowa: W. Sivitz, M. Bayless, T. Weingeist, E. Stone, H. Culver Boldt, K. Gehres, S. Russell, J. Bayless, J. Kramer, J. Long, R. L. Larson, R. Zeitler (past); University of Maryland School of Medicine: D. Counts, T. Donner, S. Johnsonbaugh, J. Gordon, R. Hemady, A. Kowarski (past), D. Ostrowski (past), M. Hebdon (past), S. Steidl (past), B. Jones (past); University of Michigan: W. Herman, C. Martin, R. Pop-Busui, S. Elner, E. Feldman, J. Albers, D. Greene (past), M. J. Stevens (past), A. K. Vine (past); University of Minnesota: J. Bantle, N. Wimmergren, A. Cochrane, T. Olsen, E. Steuer (past), P. Rath (past), B. Rogness; University of Missouri: D. Hainsworth, S. Hitt, J. Giangiacomo, D. Goldstein (past); University of New Mexico: D. Schade, J. Canady, J. Chapin, C. Johannes (past), D. Hornbeck (past); University of Pennsylvania: S. Schwartz, P. A. Bourne, B. J. Maschak-Carey (past), L. Baker (deceased), S. Braunstein, A. Brucker; University of Pittsburgh: T. Orchard, N. Silvers, C. Ryan, T. Songer, B. Doft, S. Olson, R. L. Bergren, L. Lobes, P. Paczan Rath, D. Becker, D. Rubinstein, A. Drash (past); University of South Florida: A. Morrison, M. L. Bernal, J. Vaccaro-Kish, J. Malone, P. R. Pavan, N. Grove, M. N. Iyer, A. F. Burrows, E. A. Tanaka (past), R. Gstalder (past); University of Tennessee: S. Dagogo-Jack, C. Wigley, H. Ricks, A. Kitabchi, M. B. Murphy, S. Moser, D. Meyer, A. Iannacone, E. Chaum, S. Yoser, M. Bryer-Ash (past), S. Schussler (past), H. Lambeth (past); University of Texas Southwestern University Medical Center: P. Raskin, S. Strowig, S. Vernino; University of Toronto: B. Zinman, A. Barnie, R. Devenyi, M. Mandelcorn, M. Brent, S. Rogers, A. Gordon; University of Washington: J. Palmer, S. Catton, J. Brunzell, I. deBoer, J. Purnell, J. Ginsberg, J. Kinyoun, M. Weiss, G. Meekins, J. Distad, L. Van Ottingham (past); University of Western Ontario: J. Dupre, J. L. Mahon, J. Harth, J. McCallum, D. Nicolle, N. W. Rodger (past), M. Jenner (past), I. Hramiak (past), C. Canny (past); Vanderbilt University: M. May, J. Lipps, A. Agarwal, T. Adkins, L. Survant, R. Lorenz (past), S. Feman (past); Washington University, St Louis: N. White, L. Levandoski, I. G. Grand, M. Thomas, D. D. Joseph, K. Blinder, G. Shah, N. Englebrecht, I. Boniuk (past), D. Burgess (past), J. Santiago (deceased); Yale University School of Medicine: W. Tamborlane, P. Gatcomb, K. Stoessel, J. Goldstein, S. Novella
Clinical Coordinating Center
Case Western Reserve University: W. Dahms (deceased), R. Gubitosi-Klug, J. Quin, P. Gaston, M. Palmert (past), R. Trail (past)
Data Coordinating Center
The George Washington University, Biostatistics Center: J. Lachin, P. Cleary, D. Kenny (past), J. Backlund, W. Sun, B. Rutledge (past), B. Waberski, N. Younes, K. Klumpp, K. Chan (past), L. Diminick, D. Rosenberg (past), B. Petty (past), A. Determan (past), C. Williams (past), L. Dews, M. Hawkins
National Institute of Diabetes and Digestive and Kidney Disease Program Office
C. Cowie, J. Fradkin, C. Siebert (past), R. Eastman (past)
Central Fundus Photograph Reading Center
University of Wisconsin: R. Danis, M. Davis, L. Hubbard, H. Wabers, M. Burger, J. Dingledine, V. Gama, R. Sussman
Central Biochemistry Laboratory
University of Minnesota: M. Steffes, J. Bucksa, B. Chavers
Central Carotid Ultrasound Unit
New England Medical Center: D. O’Leary, L. Funk, J. Polak, A. Harrington
Central ECG Reading Unit
University of Minnesota: R. Crow (past), B. Gloeb (past), S. Thomas (past), C. O’Donnell (past)
Central ECG Reading Unit
Wake Forest University: R. Prineas, C. Campbell
Central Neuropsychological Coding Unit
C. Ryan, D. Sandstrom, T. Williams, M. Geckle, E. Cupelli, F. Thoma, B. Burzuk, T. Woodfill
Central ANS Reading Unit
Mayo Clinic: P. Low, C. Sommer, K. Nickander
Computed Tomography Reading Center
Harbor UCLA Research and Education Institute: R. Detrano, N. Wong, M. Fox, L. Kim, R. Oudiz
External Advisory Committee
G. Weir (Chairman), C. Clark (past), R. D’Agostino (past), M. Espeland, B. Klein, T. Manolio, L. Rand, D. Singer, M. Stern, A. Boulton, C. Hsu
Molecular Risk Factors Program Project
Medical University of South Carolina: M. Lopes-Virella, W. T. Garvey (past), T. J. Lyons, A. Jenkins, R. Klein, G. Virella, A. A. Jaffa, R. Carter, D. Lackland (past), M. Brabham (past), D. McGee (past), D. Zheng (past), R. K. Mayfield (past)
Genetic Studies Group
Hospital for Sick Children: A. Paterson, A. Boright, S. Bull, L. Sun, S. Scherer (past), B. Zinman (past)
Lipoprotein Distribution/Obesity Group
University of Washington: J. Brunzell, I. deBoer, S. Marcovina, J. Purnell, S. Deeb
Editor, EDIC Publications
D. M. Nathan
COMMENT
Four recent reports have suggested substantial improvements in the rate of complications in T1DM; however, these data are based on single hospital experiences and have not consistently shown improvements in all complications.13-16 The long-term clinical outcome results in the DCCT conventional treatment group, confirmed by the EDC study data, provide a reliable sense of the clinical course that can be expected with modern-day therapy during the past 25 years. With the demonstration by the DCCT in 1993 of the beneficial effects of intensive therapy, largely attributable to the lowering of the level of chronic glycemia, intensive therapy has been universally recommended. While the adoption of intensive treatment by the DCCT conventional treatment group during the EDIC observational follow-up may, arguably, not be representative of the general T1DM population, the similarity in metabolic control and outcomes with the more population-based EDC population supports the generalizability of these data. The decrease in HbA1c levels over time in the DCCT conventional treatment and EDC groups (Table 1), in concert with the reported increase in use of insulin pumps and multiple daily injections (Table 2), are the most objective evidence that intensive treatments have been adopted and are succeeding. The development of complications over a diabetes duration of 30 years reported herein support a long-term outcome of T1DM that is improved compared with the results reported in cohorts with the onset of diabetes in the 1950s to 1970s.14,15,29,30 Different methods of ascertaining and defining long-term complications may make such historical comparisons problematic; however, the 30% and 12% cumulative incidences of proliferative retinopathy and nephropathy, respectively, in the DCCT/EDIC study after a diabetes duration of 25 years (the period selected to match older literature) (Figure 2A and B) are lower than the 40% to 53% cumulative incidences of proliferative retinopathy14,15,29 and the approximately 35% cumulative incidence of nephropathy14 in studies of cohorts that developed their diabetes 10 to 20 years prior to that of subjects in the DCCT and that used comparable study methods and definitions. The absolute rates of functional impairment, such as loss of vision, renal failure requiring replacement therapy, CVD events, and amputations, are also difficult to compare with historical data; nevertheless, the absolute risks of such events in the DCCT/EDIC study are low, with only 3 of 1441 subjects having become legally blind (20/200 or worse in both eyes) and 18 subjects requiring renal replacement therapy, after a mean diabetes duration of 25 years (Table 3). The 30-year cumulative incidences of renal replacement in the DCCT/EDIC conventional (4%) and intensive treatment (1%) groups compare favorably with the 8% recently reported in all Finnish patients who received a diagnosis between 1965 and 1999.16 Of course, other interventions, such as more attentive surveillance and more aggressive application of blood pressure and lipid control and laser therapy, may also have contributed to the improved outcomes presented herein. The increased use of aspirin, angiotensin-converting enzyme inhibitors, and statins, some of which were not available or widely recommended during the DCCT period, have clearly increased during the last decade (Table 1). However, the increased frequency of overweight and obesity, first seen in the intensive DCCT treatment group but subsequently in the total cohort and paralleling the epidemic of obesity in the nondiabetic population, may have had a negative impact on the long-term outcomes, especially CVD.
While the results of the DCCT/EDIC conventional therapy and of the EDC study supply clinicians with a realistic description of the clinical outcomes that they can discuss with their patients who have had their T1DM in the past 25 years, the intensive treatment group results provide a view of what patients with T1DM can expect in the future. Intensive therapy, now the standard of care, should result in more than 50% reduction in the rates of complications over time, with implementation early in the course of diabetes providing the most powerful salutary effect.4 Moreover, the durable effect of such intervention, termed metabolic memory, expands the benefits of intensive therapy.5,6
The limitations of these analyses are largely owing to the selection of the DCCT population. Despite matching the 2 cohorts based on major DCCT eligibility criteria, the EDC population had an earlier onset of diabetes that may have had an effect on the development of complications compared with the DCCT cohort. However, the similar results in the EDC population suggest that the DCCT conventional treatment results are generalizable. There were also minor differences in the methods used. The DCCT used 7-field photography, which almost certainly detected more retinopathy than the 3-field photography in the EDC study. However, previous studies have shown that 2- or 3-field fundus photography has 80% to more than 90% sensitivity for proliferative retinopathy compared with 7-field photography.31
Overall, the prospects for patients with T1DM are far better than they were in the past. The future of T1DM care will need to address improved implementation of intensive care to reduce patient burden and the risk of hypoglycemia; however, until prevention or cures are developed, intensive therapy must be implemented universally and as early as is practical and safe to ensure the health of persons with T1DM.
Acknowledgments
Funding/Support: The DCCT/EDIC project is supported by contracts with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases; the National Eye Institute; the National Institute of Neurological Disorders and Stroke; the General Clinical Research Centers Program; and the Clinical and Translation Science Centers Program, National Center for Research Resources; and by Genentech through a Cooperative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases. Contributors of free or discounted supplies and/or equipment include Lifescan, Roche, Aventis, Eli Lilly, OmniPod, Can-Am, B-D, Animas, Medtronic, Medtronic Minimed, Bayer (donation one time in 2008), and Omron. The EDC is supported by grant DK 034818 from the National Institute of Diabetes and Kidney Diseases.
Footnotes
Trial Registration: clinicaltrials.gov Identifiers: NCT00360815 and NCT00360893
Financial Disclosure: None reported.
REFERENCES
- 1.The DCCT Research Group The Diabetes Control and Complications Trial (DCCT): design and methodologic considerations for the feasibility phase. Diabetes. 1986;35(5):530–545. [PubMed] [Google Scholar]
- 2.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group Epidemiology of Diabetes Interventions and Complications (EDIC): design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22(1):99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reichard P, Nilsson B-Y, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329(5):304–309. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342(6):381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290(16):2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in type 1 diabetes mellitus. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type 1 diabetes. Am J Med. 1985;78(5):785–794. doi: 10.1016/0002-9343(85)90284-0. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy, IX: four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1989;107(2):237–243. doi: 10.1001/archopht.1989.01070010243030. [DOI] [PubMed] [Google Scholar]
- 10.Lee ET, Keen H, Bennett PH, Fuller JH, Lu M. Follow-up of the WHO multinational study of vascular disease in diabetes: general description and morbidity. Diabetologia. 2001;44(suppl 2):S3–S13. doi: 10.1007/pl00002936. [DOI] [PubMed] [Google Scholar]
- 11.EURODIAB IDDM Complications Study Group Microvascular and acute complications in IDDM patients: the EURODIAB IDDM Complications Study. Diabetologia. 1994;37(3):278–285. doi: 10.1007/BF00398055. [DOI] [PubMed] [Google Scholar]
- 12.Walsh MG, Zgibor J, Borch-Johnsen K, Orchard TJ, DiaComp Investigators A multinational assessment of complications in type 1 diabetes: the DiaMond substudy of complications (DiaComp) level 1. Diab Vasc Dis Res. 2006;3(2):84–92. doi: 10.3132/dvdr.2006.018. [DOI] [PubMed] [Google Scholar]
- 13.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55(5):1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 14.Hovind P, Tarnow L, Rossing K, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26(4):1258–1264. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 15.Nordwall M, Bojestig M, Arnqvist HJ, Ludvigsson J. Declining incidence of severe retinopathy and persisting decrease of nephropathy in an unselected population of type 1 diabetes: the Linköping Diabetes Complications Study. Diabetologia. 2004;47(7):1266–1272. doi: 10.1007/s00125-004-1431-6. [DOI] [PubMed] [Google Scholar]
- 16.Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA. 2005;294(14):1782–1787. doi: 10.1001/jama.294.14.1782. [DOI] [PubMed] [Google Scholar]
- 17.DCCT Research Group Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care. 1995;18(3):361–376. doi: 10.2337/diacare.18.3.361. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association Implications of the Diabetes Control and Complications Trial. Diabetes. 1993;42(11):1555–1558. doi: 10.2337/diab.42.11.1555. [DOI] [PubMed] [Google Scholar]
- 19.Orchard TJ, Dorman JS, Maser RE, et al. Factors associated with avoidance of severe complications after 25 yr of IDDM: Pittsburgh Epidemiology of Diabetes Complications Study I. Diabetes Care. 1990;13(7):741–747. doi: 10.2337/diacare.13.7.741. [DOI] [PubMed] [Google Scholar]
- 20.Wagener DK, Sacks JM, LaPorte RE, Macgregor JM. The Pittsburgh Study of insulin-dependent diabetes mellitus: risk for diabetes among relatives of IDDM. Diabetes. 1982;31(2):136–144. doi: 10.2337/diab.31.2.136. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(suppl 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 22.Steffes M, Cleary P, Goldstein D, et al. The DCCT/EDIC Research Group Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications Study. Clin Chem. 2005;51(4):753–758. doi: 10.1373/clinchem.2004.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Early Therapy Diabetic Retinopathy Study (ETDRS) Manual of Operations. National Technical Information Service; Spring-field, VA: 1985. NTIS accession No. PB85223006. [Google Scholar]
- 24.The Diabetes Control and Complications Trial Research Group The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus: the Diabetes Control and Complications Trial. Arch Ophthalmol. 1995;113(1):36–51. doi: 10.1001/archopht.1995.01100010038019. [DOI] [PubMed] [Google Scholar]
- 25.Orchard TJ, Dorman JS, Maser RE, et al. Prevalence of complications in IDDM by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39(9):1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 26.Prince CT, Becker DJ, Costacou T, Miller RG, Orchard TJ. Changes in glycaemic control and risk of coronary artery disease in type 1 diabetes mellitus: findings from the Pittsburgh Epidemiology of Diabetes Complications Study (EDC) Diabetologia. 2007;50(11):2280–2288. doi: 10.1007/s00125-007-0797-7. [DOI] [PubMed] [Google Scholar]
- 27.Fleiss JL. Statistical Methods for Rates and Proportions. 2nd ed. John Wiley; New York, NY: 1981. pp. 38–46. [Google Scholar]
- 28.Odell PM, Anderson KM, D’Agostino RB. Maximum likelihood estimation for interval-censored data using a Weibull-based accelerated failure time model. Biometrics. 1992;48(3):951–959. [PubMed] [Google Scholar]
- 29.Klein R, Klein BE, Moss SE. Epidemiology of proliferative diabetic retinopathy. Diabetes Care. 1992;15(12):1875–1891. doi: 10.2337/diacare.15.12.1875. [DOI] [PubMed] [Google Scholar]
- 30.Rossing P, Rossing K, Jacobsen P, Parving H-H. Unchanged incidence of diabetic nephropathy in IDDM patients. Diabetes. 1995;44(7):739–743. doi: 10.2337/diab.44.7.739. [DOI] [PubMed] [Google Scholar]
- 31.Moss SE, Meuer SM, Klein R, Hubbard LD, Brothers RJ, Klein BE. Are seven standard photographic fields necessary for classification of diabetic retinopathy. Invest Ophthalmol Vis Sci. 1989;30(5):823–828. [PubMed] [Google Scholar]