Abstract
Objective
The aim of this study was to develop a clinically applicable non-invasive method to quantify changes in androgen receptor (AR) levels based on 18F-FDHT PET in prostate cancer patients undergoing therapy.
Methods
Thirteen patients underwent dynamic 18F-FDHT PET scans over a selected tumor. Concurrent venous blood samples were acquired for blood metabolite analysis. A second cohort of 25 patients, injected with 18F-FDHT underwent dynamic PET imaging of the heart. These data were used to generate a population-based input function, essential for pharmacokinetic modeling. Linear compartmental pharmacokinetic models of increasing complexity were tested on the tumor tissue data. Four suitable models were applied and compared using the Bayesian Information Criterion (BIC). Model 1 consisted of an instantaneously equilibrating space followed by a unidirectional trap. Models 2a and 2b contained a reversible space between the instantaneously equilibrating space and the trap, into which metabolites were excluded (2a) or allowed (2b). Model 3 built upon Model 2b with the addition of a second reversible space preceding the unidirectional trap and from which metabolites were excluded.
Results
The half-life of the 18F-FDHT in blood was determined to be between 6-7 minutes. As a consequence, the uptake of 18F-FDHT in prostate cancer lesions reached a plateau within 20-minutes as the blood-borne activity was consumed. Radiolabeled metabolites were shown not to bind to AR in in-vitro studies with CWR22 cells. Model 1 produced reasonable and robust fits for all datasets and was judged best by the BIC for 16 out of 26 tumor scans. Models 2a, 2b and 3 were judged best in seven, two and one case, respectively.
Conclusion
Our study explores the clinical potential of using 18F-FDHT PET to make estimates of free AR concentration. This process involved the estimation of a net-uptake parameter such as Model 1’s ktrap that could serve as a surrogate measure of AR expression in metastatic prostate cancer. Our initial studies suggest a simple body-mass normalized standard uptake value (SUV) is reasonably well correlated to model based ktrap estimates, which we surmise may be proportional to AR expression. Validation studies to test this hypothesis are underway.
The androgen receptor (AR) is known to be important in the development and progression of prostate cancer. Castration resistant prostate cancers in particular harbor a series of oncogenic alterations in AR including overexpression, increased copy number, mutations that affect ligand specificity, and an increase in the enzyme levels responsible for antigen synthesis (1). It is for this reason, that there is increasing interest in the development of therapies directed at these alterations.
Currently, a direct biopsy of a metastatic lesion is required to assess the AR status in tumor when treatment is being considered. While technically feasible this procedure is invasive, costly, not a part of routine practice and difficult to repeat. Moreover, the AR status determined histopathologically in one metastasis may not be representative of all metastatic lesions.
A PET ligand that could provide a signal which is predictive of AR expression levels in prostate cancer would not only have great potential in the diagnosis of this disease, but also could have implications in determining the appropriate therapy and in assessing its efficacy. This is especially pertinent in an era when targeted therapies are becoming available clinically, as molecular imaging approaches can potentially be used to select patients likely to respond to such therapies and to monitor the therapeutic effectiveness of these treatments.
16β-Fluoro-5α-dihyrotestosterone (FDHT) is a structural analog of 5α-dihydrotestosterone (DHT), the principal intra-prostatic form of androgen (2). Among fluorinated androgen analogs studied in animals, 18F-FDHT uptake in the prostate was blocked (reduced ~10-fold) by co-administration of cold testosterone and yielded the highest levels of unmetabolized radioligand in blood up to 45 min post-injection (pi) and the highest prostate-to-bone and prostate-to-muscle activity concentration ratios up to 4 hr pi. Thus, 18F-FDHT appears to bind specifically to androgen receptors in vivo and to have the most favorable targeting properties for non-invasive imaging among androgen receptor binding radiotracers studied to date. In addition, like androgens generally, most of the 18F-FDHT in circulation is bound to sex-hormone binding globulin (3). Such plasma-protein binding presumably serves to retard degradation of endogenous androgens and to facilitate their transport into cells. These considerations led to the selection of 16β-[18F]-fluoro-5α dihydrotestosterone or 18F-FDHT as the lead radiopharmaceutical for further evaluation in clinical studies. Two clinical studies subsequently demonstrated successful PET imaging of prostate cancer using 18F-FDHT (4,5,6). These studies showed rapid tumor uptake and systemic metabolism of 18F-FDHT and provided some evidence that the foci of activity seen on 18F-FDHT PET images correlates with AR-expressing tissue as demonstrated by immunohistochemical staining. In this paper we describe our initial investigations seeking to use pharmacokinetic modeling of 18F-FDHT time-activity data in prostate tumors, as measured by dynamic PET, to assess relative levels of AR in such tumors.
Materials and Methods
Patients
Patients were selected prospectively under the auspices of the Memorial Sloan-Kettering Cancer Center Institutional Review Board (IRB), IRB protocol 00-095. All who agreed to participate in the study signed informed consent forms. The study cohort consisted of 13 patients who then underwent dynamic 18F-FDHT PET scans over a selected metastatic tumor site, located in either the pelvis or abdomen. Since satisfactory region-of-interest (ROI) based blood time-activity data (i.e. input functions) could not, in general, be obtained from these images, arterial input data was extracted from a larger cohort of 25 patients, who underwent dynamic 18F-FDHT PET scanning of the heart. Data from ROI’s placed over the aorta in these patients were averaged together to generate a population-based input function used in the pharmacokinetic modeling.
For the 13 patients recruited into the first cohort, there were a total of 30 18F-FDHT scans conducted. This consisted of three patients who underwent a single 18F-FDHT baseline scan only, three patients who received one pre- and one post-therapy 18F-FDHT scan, and seven who received a baseline scan followed by two post-therapy scans.
Radiochemical Synthesis of 18F-FDHT
18F-FDHT was synthesized as previously described (5). The total radiochemistry synthesis time was approximately 100 min, and the radiochemical yield to end of bombardment was nearly 30%. Chemical and radiochemical quality assurance (QA) was performed by radio-TLC and reverse phase HPLC by co-elution with a fully characterized non-radioactive standard. Additional QA included confirmation of color, appearance, radioactive half-life, pH, sterility and apyrogenicity. All QA results were in full accordance to the approved specifications. The radiochemical purity was >99%.
To maintain chemical stability of the 18F-FDHT compound, the final product was formulated in a 5% ethanol solution. This precluded the use of a bolus injection because of the burning sensation at the injection site associated with the i.v. administration of alcohol. As a consequence, injections were manually performed from a lead shielded syringe over a duration of between 40 seconds and 2 minutes where the rate was reduced based upon patient feedback.
Binding studies with 18F-FDHT
Displacement studies were performed with 18F-FDHT and CWR22-rv1 cells with FDHT and DHT as competitors. Briefly, triplicate samples of cells were mixed with 20,000 cpm 18F-FDHT and increasing amounts of cold competitor (1 pM to 1μM). The solutions were then shaken on an orbital shaker at ambient temperature and after 60 minutes the cells were isolated and washed with ice cold Tris buffered saline using a μ-24T cell harvester (Brandel). All of the isolated cell samples were counted, with appropriate standards of total activity and blank controls, and the specific uptake of 18F-FDHT determined. These data were plotted against the concentration of the cold competitor to give sigmoidal displacement curves (Fig. 1). The IC50 values were determined using a one site model and a least squares curve fitting routine (Origin, OriginLab, Northampton, MA). The r2 of the curve-fit was 0.99.
FIGURE 1.
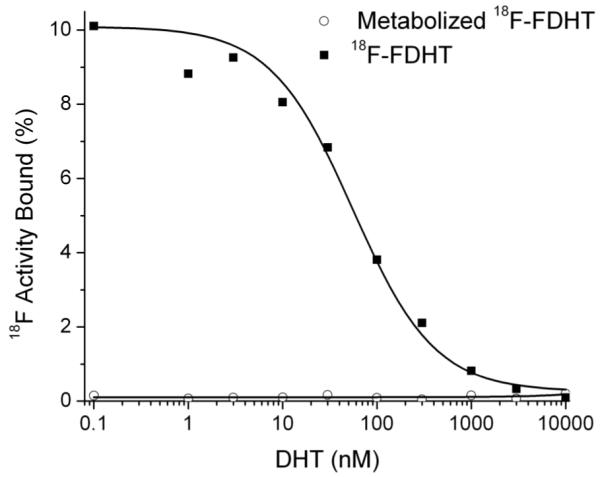
Displacement binding of 18F-FDHT and final 18F-FDHT metabolite to CWR22-rv1 cells by DHT.
A sample of 18F-FDHT drug product was evaporated to remove the ethanol and mixed with a 10% suspension of rat liver homogenate in PMS for 30 minutes at 37° C. At the end of this period, the solution was diluted to 40% acetonitrile and the precipitated proteins pelleted by centrifugation. The metabolized 18F-FDHTsupernatant was then purified by reversed-phase HPLC (C18 Ultrasphere column Beckman; 5 μm, 250 × 4.6 mm) using an gradient elution of 40% acetonitrile/10 mM phosphoric acid up to 90% acetonitrile/10 mM phosphoric acid over 10 minutes at a flow rate of 1 mL/min. Under these conditions, 18F-FDHT elutes at around 8 minutes and the main metabolite elutes at 4 minutes. The purified metabolite was then used in a displacement binding study as described above.
PET Imaging
Each patient underwent at least one whole-body 18F-FDG PET/CT scan that was followed within 1 to 7 days by dynamic and whole-body 18F-FDHT PET scans. The initial 18F-FDG PET/CT scan was used to identify one or more lesions to be followed in the subsequent dynamic 18F-FDHT scans. A subset of the patients received up to two additional sets of scans, approximately four and twelve weeks later during their course of treatment.
All studies were performed on either a GE Discovery LS or GE Advance PET scanner (General Electric Medical Systems, Milwaukee, WI). Prior to the 18F-FDG scans patients were required to fast for at least 6 h and a blood sample was obtained to measure the serum glucose level. No fasting was required for the 18F-FDHT scan. For all 18F-FDHT scans, each patient had one peripheral IV line placed into each arm, one for injection and one for blood sampling. Scanning in all cases was performed in 2-dimensional mode (septa-in).
The dynamic 18F-FDHT PET emission scan was initiated coincident with the start of the injection. The first two patients underwent a dynamic scan of 55 minutes in duration. Analysis of this dynamic imaging data along with plasma metabolite analysis showed little to no change in activity levels in tumor occurring beyond 20-minutes post injection, and that metabolism of the compound was almost complete. The protocol was therefore modified for patients 3-13 reducing the duration of the dynamic PET scan to 30-minutes. After the dynamic scan, patients were allowed to dismount from the table to rest for approximately 10-minutes. Patients were encouraged to urinate prior to the acquisition of a whole-body PET scan which was used to determine overall biodistribution and to explore other potential metastatic sites. The whole-body scan was performed from the skull base to the pelvic floor. All images were reconstructed using both filtered back projection and iterative reconstruction.
The 25 patients from the second cohort underwent an almost identical scan procedure, except that the 30-minute dynamic scan was performed over the chest to include the heart rather than over a metastatic index lesion. Region of interest data were derived from the aorta of these patients was used to generate a population average input function for 18F-FDHT.
Radiometabolite Analysis of 18F-FDHT
Patients who underwent 18F-FDHT scans had blood samples drawn to determine the clearance of 18F-FDHT and the rate of metabolite formation. Blood samples were drawn at all or some of the following time points after injection with 18F-FDHT: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 40, 50 minutes. Patients who underwent 30-minute dynamic scans did not have the 40- and 50-minute blood samples, but a late time point following the whole body PET scan was obtained whenever possible. The activities in whole blood, plasma, 30-kDa filtered plasma and acetonitrile-precipitated plasma were determined. In addition, the relative amounts of 18F-FDHT and 18F-FDHT metabolites in the plasma were determined by HPLC.
Aliquots of whole blood were transferred to pre-weighed tubes. Portions of the blood samples were centrifuged and aliquots of plasma transferred into pre-weighed tubes. The remaining plasma samples underwent ultra-filtration using 30-kDa spin filters (Centricon YM-30; Millipore) and aliquots of the filtrate transferred to pre-weighed tubes. 18F activity in all samples was assayed in a gamma counter (LKB Wallac 1282 Compugamma) calibrated for 18F, corrected for decay to the time of injection, and expressed as a percent of the injected dose per gram (%ID/gm).
The counted plasma samples were mixed with 0.45 mL of acetonitrile containing unlabeled FDHT (0.05 mg/mL) as a reference compound. After centrifugation at 2,000g for 5 min, the precipitated proteins were also counted in a gamma counter. The protein-free supernatant was analyzed by RP C18 HPLC. The Shimadzu HPLC system consisted of two LC10AT pumps and a SCL10A controller coupled to a SPD-10A ultraviolet detector and a Packard Radiometric 625TR flow detector (500 μL cell with BGO crystals) in series. The reverse-phase C18 column (Beckman Ultrasphere; 5 μm, 250 × 4.6 mm) was eluted applying a gradient from 40% acetonitrile/10 mM phosphoric acid to 90% acetonitrile/10 mM phosphoric acid over 10 minutes at a flow rate of 1 mL/min. Under these conditions, 18F-FDHT elutes at around 8 minutes and the main metabolized product at 4 minutes. The data were expressed as a percentage of the total activity in plasma.
ROI Analysis
One Nuclear Medicine reader performed the region of interest (ROI) analysis in this investigation. The tumor was identified based upon the 18F-FDG PET/CT scan displayed alongside the whole-body 18F-FDHT and regional dynamic 18F-FDHT scans. Separate ROIs circumscribing a homogeneous region well within the borders of the index tumor were drawn on images from a summed frame, iteratively reconstructed, covering the last 15-minutes of the 18F-FDHT PET emission data. ROIs were also generated for the descending aorta (3 studies in 2 patients) or iliac arteries (20 studies in 9 patients) using summed frames covering the first two minutes post-18F-FDHT injection. All ROIs were applied to filtered back projection (FBP) reconstructed images to generate curves of mean activity concentration versus time. FBP images were used here in order to avoid problems associated with spatially variant convergence rates encountered with iterative reconstruction methods. Multiple ROIs were weighted (by their fractional volume) and summed for structures extending over several adjacent images.
Because of the relatively large diameter of the descending aorta, we chose the three curves, so derived, as the reference against which to judge the accuracy of the iliac artery-derived curves and the venous blood samples. A comparison of the blood time-activity curves both within and among studies suggested that data from the venous blood samples and from ROIs drawn over the iliac arteries likely do not accurately represent the true blood-activity time course at early times post 18F-FDHT injection. Only time-activity curves generated from ROIs drawn over the descending aorta showed the expected first-pass peak (Fig. 2). At late times (> 15 minutes) all curves tended to plateau, with the aorta derived and venous blood derived data reaching approximately the same activity concentration level.
FIGURE 2.
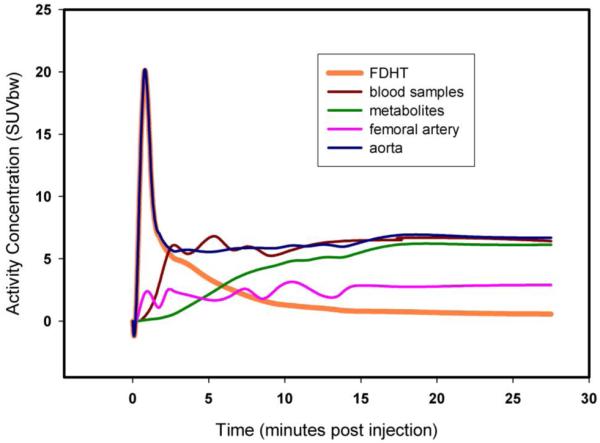
Time activity curves in SUV units for the blood samples (total, 18F-FDHT and metabolites) and the PET derived data from iliac artery and aorta. SUVbw = SUV normalized by body weight.
The descending aorta was within the field of view for only two patients in the first cohort, therefore it was decided to use data from a second separate cohort of patients for which dynamic PET scans of 18F-FDHT were obtained over the heart. This data was used to establish a population-based blood time-activity curve. Curves derived from ROIs drawn over the aorta from this cohort of 25 patients were converted to standard uptake value units (SUV = tissue activity per kg / injected activity per kg of body weight) and averaged together. Individual radiolabeled metabolite and parent 18F-FDHT input functions were calculated for each patient by first scaling the population-based blood time-activity curve so that it matched the venous blood sample activity level at late times and then multiplying the result by a population-based metabolite-fraction time-activity curve derived from the original cohort. The parent 18F-FDHT input function could then be calculated by subtracting this metabolite input function from the scaled population-based blood time-activity curve (Fig. 2).
Pharmacokinetic Modeling
A series of linear compartmental pharmacokinetic models of increasing complexity were tested on the tumor tissue data and compared using the Bayesian Information Criteria (BIC) as formulated by Schwartz (7). The definition of BIC is given as
where n is the number of data points, RSS the residual sum of squares from the estimated model and k, the number of free parameters used in the model fit.
The BIC provides a statistical metric with which to compare the results of different model fits to the data. The model yielding the lowest BIC value is judged to be of sufficient dimension (i.e. have enough parameters) to describe the data. The BIC increases as a function of the residual sum of squares of the fitted data, but it also increases as the number of model parameters used to fit the data are increased. In this way the introduction of more than a requisite number of compartments is penalized. Hence, lower BIC implies either fewer explanatory variables, better fit, or both.
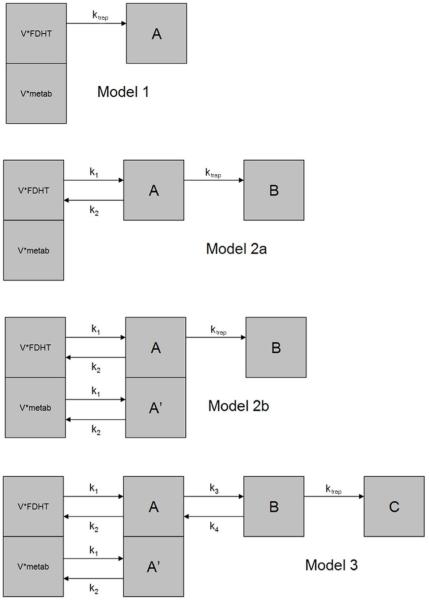
The patient data were fitted to four different compartmental models which were then compared using the BIC metric. These models are shown in diagrammatic form in Figure 3. The first and simplest of these models, Model 1, consisted of an instantaneously equilibrating space (often also referred to as a “fractional blood volume”) followed by a unidirectional trap, thus requiring just two parameters. The instantaneously equilibrating space was assumed to contain both parent 18F-FDHT and its metabolites whereas the trap was assumed to contain only parent 18F-FDHT. The exclusion of the metabolites from the final compartment was justified based on our in vitro studies demonstrating that 18F-FDHT’s radiolabeled metabolites do not bind to CWR22-rv1 cells.
FIGURE 3.
Four compartmental models used to fit 18F-FDHT data to the tumor uptake profiles. V*FDHT = volume times FDHT concentration; V*metab = volume times metabolite concentration.
The next two models, Model 2a and 2b, are similar to the first except for the addition of a reversible space between the instantaneously equilibrating space and the trap. The two variants differed in their exclusion (2a) and allowance (2b) of metabolites into this intermediate space. When allowed, the metabolites were forced to enter and exit this space using rate constants constrained to equal that of the parent 18F-FDHT, thus each of the two variants required four parameters. We chose to equate these rate-constants under the assumption that the associated space represents a region of disrupted vasculature into which radiolabeled molecules bound to plasma proteins were leaking. For the most complex model tried, Model 3, an additional reversible space preceding the unidirectional trap was added, requiring a total of six parameters. In this model, metabolites were allowed to enter the first reversible space (again using rate constants constrained to equal that of 18F-FDHT) but not allowed to enter the second.
In each of these models we have assumed that the entirety of the parent FDHT in the blood is equally available for uptake into the tumor, whether it is bound to plasma proteins or to steroid hormone-binding globulin (SHBG) or other constituents of the blood, or free in the plasma. Therefore, our input functions are based on whole-blood activity concentrations.
The 18F time-activity data for each lesion in each patient PET study was independently fitted by each of the described model variants using the SAAM II software package (University of Washington, Seattle, WA). Parameter values were adjusted so as to minimize the sum of the weighted squared differences between the model estimate and the corresponding measured values. The weight for each PET time-point was chosen to be the inverse of the duration of its frame. The parent 18F-FDHT and metabolite input functions applied to the models were the individually scaled population-based curves as described above. BIC values for each of the fits were calculated by SAAM II.
RESULTS
Clinical Image Data
There were 13 patients studied in this protocol. Most of the metastatic sites were in bone. A summary of the patient scans and treatment regimens is provided in Table 1. A detailed description of these scans is beyond the scope of this manuscript. However in brief, several of these patients exhibited concordant PET lesion detection by 18F-FDHT and 18F-FDG PET, with one patient (#12) showing 18F-FDG positivity and 18F-FDHT negativity and patient (#4) exhibiting only abnormal 18F-FDHT uptake. Two of the patients (#12 and #13) did not demonstrate 18F-FDHT uptake in any tumors and were thus excluded from further analysis. Some of the patients demonstrated mixed findings between the SUV’s measured for corresponding lesions, with high SUV on 18F-FDG and low SUV on 18F-FDHT scans., or vice versa. Most patients with positive 18F-FDHT baseline scans exhibited uptake on post therapy scans at reduced levels. However, there were instances of increased uptake on the follow-up 18F-FDHT PET scans e.g. patient #1. One patient (#10) had multiple positive lesions on the baseline 18F-FDG scan and a negative baseline 18F-FDHT scan, but new abnormal 18F-FDHT uptake in these regions on the later PET scans. Although several of the patients had some soft tissue areas of abnormal 18F-FDG uptake without corresponding 18F-FDHT uptake, one patient (#7), showed abnormal 18F-FDG and 18F-FDHT uptake in lymph nodes in the left neck and retroperitoneum, with increasing 18F-FDHT uptake on subsequent scans.
Table 1.
| Patient no. |
Age (y) |
PSA at baseline |
Gleason Score |
KPS | No. of PET Scans |
Site of Disease | Therapeutic Regimen |
|---|---|---|---|---|---|---|---|
| 1 | 65 | 885.05 | 9 | 80 | 3 | Bone | Docetaxel (Sanofi-Aventis) |
| 2 | 68 | 96.62 | 10 | 80 | 3 | Bone, Lymph Node, Prostatic Mass, Soft Tissue |
Docetaxel (Sanofi-Aventis) |
| 3 | 75 | 10.44 | 5 | 90 | 1 | Bone | 17AAG (AG Scientific), Docetaxel (Sanofi-Aventis) |
| 4 | 73 | 24.90 | 7 | 90 | 3 | Lymph Node | Abiraterone (Cougar Biotechnology) |
| 5 | 59 | 7.52 | 9 | 90 | 3 | Bone, Lymph Node | Abiraterone (Cougar Biotechnology) |
| 6 | 49 | 27.04 | 7 | 90 | 3 | Lymph Node | MDV3100 (Medivation, Inc) |
| 7 | 53 | 96.45 | 9 | 90 | 3 | Bone, Lymph Node | 17AAG (AG Scientific), Docetaxel (Sanofi-Aventis) |
| 8 | 74 | 47.22 | 7 | 80 | 2 | Bone | Docetaxel (Sanofi-Aventis) |
| 9 | 70 | 0.49 | 7 | 90 | 2 | Bone | 17AAG (AG Scientific), Docetaxel (Sanofi-Aventis) |
| 10 | 59 | 5.28 | 9 | 80 | 3 | Liver | Docetaxel (Sanofi-Aventis) |
| 11 | 76 | 10.42 | 6 | 90 | 2 | Bone | Palliative RT to Sacrum |
| 12 | 67 | 17.51 | 10 | 80 | 1 | Bone, Lymph Node, Liver | 5-Fluorouracil (Pharmacia and Upjohn), oxaliplatin (Sanofi-Aventis) |
| 13 | 79 | 1.52 | 7 | 80 | 1 | NA | Bicalutamide (AstraZeneca) |
Reading for patient 13 had a confidence of metastases of equivocal, therefore sites of disease were not identified.
From this patient cohort in table 1, the following values are observed: The average age of the patients is 66.7 years with a range of 49 – 79 years. The median prostate specific antigen (PSA) value is 94.65, with a minimum of 0.49 and a maximum of 885.05. The average Gleason Score for these patients was 7, with a range of 5 to 10. The Karnofsky Performance Status (KPS) values were either 80 (6 patients) or 90 (7 patients), which an average value of 90. Nine patients had bone disease, eight had visceral/lymph node lesions, and there were four patients with both bone and visceral/lymph node disease.
Metabolite Analysis
There was no free 18F-FDHT as determined by size exclusion filtration of serum samples. Recovery of 18F activity from the acetonitrile precipitated serum samples was around 89% and showed no trend with increasing metabolite levels. A series of HPLC chromatograms for one patient study is shown in Figure 4. The earliest samples, obtained at one minute post injection, show the activity eluting at 7.6 minutes as 18F-FDHT. Later serum samples show increasing amounts of a metabolite eluting at 4 minutes. The serum count data and HPLC analysis data were combined to give a time activity curve for total blood activity as well as 18F-FDHT and radiolabeled metabolite levels (Fig. 5)
FIGURE 4.
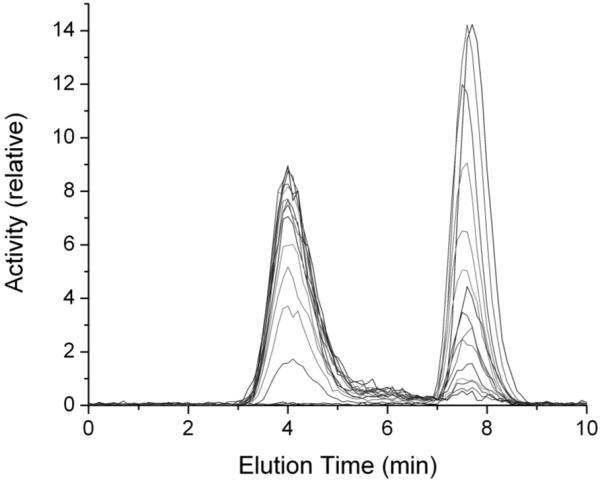
Series of HPLC elution profiles showing progressive decrease in 18F-FDHT (retention time, 6.7 minutes) and increase in metabolites (retention time, 4.0 minutes)
FIGURE 5.
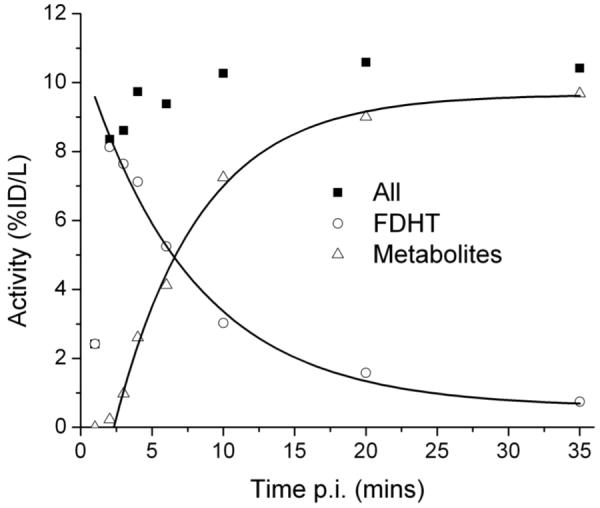
Time course of 18F-FDHT and metabolite levels in serial patient sera.
Compartmental Analysis
A total of 26 tumor time-activity datasets were fitted by each of the four compartmental models proposed. All datasets fit well to Model 1; for 16 of the datasets, the BIC found Model 1 to be of the most appropriate dimension. By these criteria, model 2a was most appropriate for 7 cases and model 2b was best in 2 cases. However, in both of these models the distribution volumes for the metabolites was found to be quite high (28% and 67%), possibly inconsistent with our in vitro findings that the metabolites do not enter or bind to the cell membrane. The most complex Model 3 was only best by the BIC for one dataset. In this case, the unidirectional rate-constant, ktrap, went to zero.
As can be seen from the results shown in Table 2, the pattern of the model selection does not appear to correlate with individual tumors i.e. repeat studies of the same tumor yielded different models judged as the most appropriate. In the few cases where either Model 2b or Model 3 was judged best, the BIC value was not substantially better (i.e. lower) than its values for Models 1 and 2a, suggesting perhaps that their seeming superiority might be due to random correlations in the noise. In any case, in the interest of clarity and brevity of exposition, we present in Table 2 parameter values only for Models 1 and 2a, leaving description of Model 2b and 3 results to the Discussion. Also in Table 2 are Model 1 and 2a’s estimation of the fraction of the measured tumor tissue activity that is bound to AR at 30 minutes post-injection and the SUV value at that time. The correlation between Model 1’s ktrap term and the 30 minute SUV value is shown in Figure 6, suggesting the possibility of using a simplified imaging protocol that does not involve blood sampling or modeling.
Table 2.
| M1 | M2a | M2b | M3 | Model 1 parameter values | Model 2a parameter values | SUVbw max |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pat # | scan | tumor | BIC | BIC | BIC | BIC | V | ktrap | bound fraction |
V | k1 | k2 | ktrap | Ki | bound fraction |
30 min pi |
||||||
| 1 | pre | 1 | 1.20 | 1.27 | 1.29 | 1.29 | 0.050 | (0.031) | 0.115 | (0.004) | 0.95 | 0.033 | (0.032) | 0.126 | (0.008) | 0.004 | (0.003) | 0.000 | (0.000) | 0.000 | 0.00 | 8.0 |
| 1 | post1 | 1 | 0.94 | 1.03 | 1.02 | 1.17 | 0.027 | (0.029) | 0.074 | (0.004) | 0.96 | 0.000 | (0.000) | 0.199 | (0.237) | 1.507 | (4.246) | 0.952 | (0.994) | 0.077 | 0.99 | 4.5 |
| 1 | post 2 | 1 | 0.32 | 0.42 | 0.51 | 0.65 | 0.001 | (0.015) | 0.037 | (0.002) | 1.00 | 0.000 | (0.000) | 0.042 | (0.007) | 0.015 | (0.041) | 0.078 | (0.211) | 0.035 | 0.78 | 2.3 |
| 2 | pre | 1 | 2.16 | 2.29 | 2.28 | 2.43 | 0.042 | (0.066) | 0.120 | (0.009) | 0.96 | 0.000 | (0.000) | 0.237 | (0.347) | 0.763 | (3.779) | 0.829 | (1.783) | 0.124 | 0.99 | 11.2 |
| 2 | post | 1 | 1.35 | 1.28 | 1.37 | 1.52 | 0.010 | (0.035) | 0.096 | (0.005) | 0.99 | 0.000 | (0.000) | 0.138 | (0.023) | 0.073 | (0.074) | 0.154 | (0.102) | 0.093 | 0.93 | 6.8 |
| 3 | pre | 1 | 2.09 | 1.29 | 1.86 | 2.00 | 0.185 | (0.090) | 0.036 | (0.011) | 0.63 | 0.000 | (0.000) | 0.166 | (0.017) | 0.080 | (0.016) | 0.018 | (0.006) | 0.031 | 0.47 | 3.3 |
| 4 | pre | 1 | 0.88 | 0.91 | 0.77 | 0.83 | 0.027 | (0.006) | 0.090 | (0.002) | 0.97 | 0.017 | (0.010) | 0.139 | (0.051) | 0.259 | (0.507) | 0.467 | (0.506) | 0.089 | 0.96 | 6.6 |
| 4 | pre | 2 | 1.78 | 1.87 | 1.83 | 1.80 | 0.054 | (0.022) | 0.063 | (0.005) | 0.91 | 0.025 | (0.047) | 0.210 | (0.292) | 0.828 | (2.228) | 0.364 | (0.382) | 0.064 | 0.93 | 5.5 |
| 4 | post | 1 | 1.10 | 1.25 | 1.22 | 1.33 | 0.045 | (0.018) | 0.103 | (0.004) | 0.95 | collapsed to model 1 | 5.5 | |||||||||
| 4 | post | 2 | 1.04 | 1.15 | 1.21 | 1.31 | 0.138 | (0.032) | 0.070 | (0.005) | 0.81 | 0.125 | (0.037) | 0.083 | (0.022) | 0.013 | (0.044) | 0.032 | (0.216) | 0.058 | 0.41 | 4.5 |
| 5 | post | 1 | 0.71 | 0.79 | 0.84 | 0.98 | 0.054 | (0.014) | 0.054 | (0.003) | 0.90 | 0.040 | (0.020) | 0.100 | (0.068) | 0.304 | (0.775) | 0.360 | (0.478) | 0.054 | 0.90 | 3.1 |
| 5 | post | 2 | 0.60 | 0.74 | 0.64 | 0.81 | 0.111 | (0.020) | 0.055 | (0.003) | 0.81 | collapsed to model 1 | 3.3 | |||||||||
| 5 | post | 1 | 0.61 | 0.67 | 0.78 | 1.20 | 0.000 | (0.019) | 0.078 | (0.003) | 0.81 | 0.000 | (0.000) | 0.09 | (0.003) | 0.01 | (0.002) | 0.00 | (0.000) | 0.000 | 0.90 | 4.5 |
| 5 | post | 2 | 0.90 | 0.94 | 1.10 | 1.80 | 0.005 | (0.005) | 0.026 | (0.002) | 0.81 | 0.000 | (0.000) | 0.05 | (0.011) | 0.11 | (0.014) | 0.12 | (0.014) | 0.024 | 0.90 | 1.6 |
| 6 | pre | 1 | 1.55 | 1.70 | 1.77 | 1.92 | 0.267 | (0.032) | 0.074 | (0.005) | 0.70 | collapsed to model 1 | 10.1 | |||||||||
| 6 | pre | 2 | 1.25 | 1.28 | 1.44 | 1.58 | 0.200 | (0.017) | 0.094 | (0.003) | 0.80 | 0.189 | (0.018) | 0.106 | (0.010) | 0.008 | (0.015) | 0.012 | (0.129) | 0.062 | 0.19 | 11.0 |
| 6 | pre | 3 | 1.67 | 1.81 | 1.76 | 1.89 | 0.227 | (0.041) | 0.059 | (0.006) | 0.69 | collapsed to model 1 | 8.1 | |||||||||
| 7 | post 2 | 1 | 2.28 | 1.64 | 1.73 | 2.18 | 0.179 | (0.101) | 0.120 | (0.014) | 0.85 | 0.002 | (0.055) | 0.276 | (0.032) | 0.073 | (0.022) | 0.045 | (0.016) | 0.106 | 0.71 | 8.2 |
| 8 | pre | 1 | 1.13 | 0.17 | 0.80 | 0.94 | 0.057 | (0.031) | 0.054 | (0.004) | 0.89 | 0.006 | (0.012) | 0.092 | (0.006) | 0.035 | (0.009) | 0.022 | (0.014) | 0.036 | 0.43 | 3.5 |
| 8 | post | 1 | 1.78 | 1.62 | 1.79 | 1.94 | 0.000 | (0.000) | 0.183 | (0.003) | 1.00 | 0.000 | (0.000) | 0.241 | (0.026) | 0.040 | (0.032) | 0.097 | (0.062) | 0.170 | 0.86 | 11.5 |
| 9 | pre | 1 | 1.71 | 1.83 | 1.78 | 1.92 | 0.080 | (0.042) | 0.032 | (0.006) | 0.77 | 0.065 | (0.046) | 0.041 | (0.012) | 0.013 | (0.013) | 0.000 | (0.000) | 0.000 | 0.00 | 3.1 |
| 9 | post | 1 | 1.79 | 1.17 | 1.45 | 1.59 | 0.093 | (0.050) | 0.041 | (0.007) | 0.79 | 0.000 | (0.000) | 0.209 | (0.037) | 0.381 | (0.129) | 0.109 | (0.021) | 0.046 | 0.91 | 4.1 |
| 10 | pre | 1 | 1.74 | 1.88 | 1.85 | 1.99 | 0.009 | (0.043) | 0.036 | (0.006) | 0.97 | 0.004 | (0.048) | 0.039 | (0.012) | 0.005 | (0.014) | 0.000 | (0.000) | 0.000 | 0.00 | 3.1 |
| 10 | post | 1 | 1.99 | 1.98 | 2.04 | 1.96 | 0.119 | (0.054) | 0.088 | (0.007) | 0.86 | 0.077 | (0.052) | 0.115 | (0.014) | 0.014 | (0.005) | 0.000 | (0.000) | 0.000 | 0.00 | 7.9 |
| 11 | pre | 1 | 1.49 | 1.03 | 1.60 | 1.19 | 0.063 | (0.029) | 0.070 | (0.004) | 0.90 | 0.022 | (0.019) | 0.100 | (0.009) | 0.022 | (0.012) | 0.021 | (0.031) | 0.048 | 0.38 | 7.2 |
| 11 | post | 1 | 1.18 | 1.09 | 1.05 | 1.19 | 0.153 | (0.032) | 0.031 | (0.004) | 0.63 | 0.053 | (0.066) | 0.290 | (0.306) | 1.444 | (1.695) | 0.236 | (0.066) | 0.041 | 0.84 | 3.2 |
| 12 | pre | 1 | no FDHT uptake therefore excluded from further analysis | |||||||||||||||||||
| 13 | pre | 1 | no FDHT uptake therefore excluded from further analysis | |||||||||||||||||||
Units for ktrap, k1, k2 and Ki are all 1/minutes. The V parameters are unit-less, as are the bound-fraction and SUVbw. Bolded and italicized BIC values are the minimums for the corresponding. The values in (parenthesis) are standard deviations.
FIGURE 6.
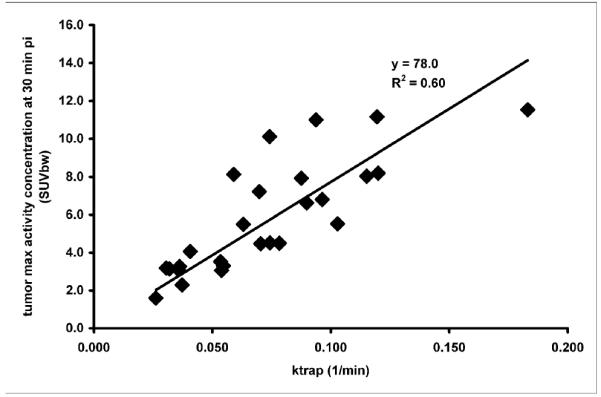
Scattergram showing relationship between the ktrap parameter values calculated in Model 1 with the 30 minute SUV value. These data are fitted with a line forced through the origin.
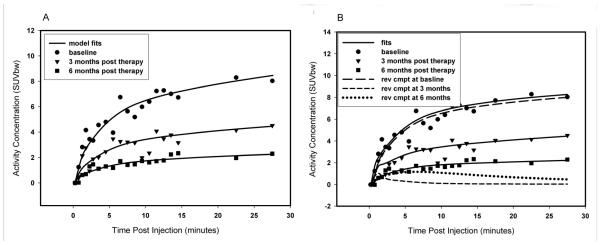
Sample results of the compartmental modeling are presented in Figure 7A, which shows the fits for Model 1 to the measured tumor time-activity data for 18F-FDHT in patient #1. This patient underwent one pre- and two post-therapy 18F-FDHT scans and for all three of these scans Model 1 was selected by the BIC. The results consistently show what might be interpreted as a progressive reduction in AR concentration (or a reduction in AR-expressing viable cells) in response to therapy, but as will be detailed in the Discussion, alternative interpretations cannot be ruled out on the basis of these data alone.
FIGURE 7.
(A) Tumor time versus activity concentration data for the three studies (1 pre-therapy and two post-therapy) of patient 1 fitted with model 1. (B) The same 3 datasets as in A, this time fitted by model 2a. Time-course of modeled reversible compartment for each of these is also shown. SUVbw = SUV normalized by body weight; Rev cmpt = reversible compartment.
DISCUSSION
The objectives of this study were: (a) to define the tracer’s pharmacokinetic properties, (b) to determine the metabolism of 18F-FDHT in blood and (c) to determine whether the data could support a model having parameters that might prove to be correlated with AR concentration. The prospects for measuring a parameter correlated to AR concentration are founded on the assumption that the rate of 18F-FDHT to AR binding is proportional to the product of the unbound concentrations of 18F-FDHT and AR, where the proportionality constant is the bimolecular association rate constant, kon. In compartmental modeling terms, this means identifying a rate constant that is equal to (or at least consistently proportional to) the product of kon and the concentration of unbound AR (the latter of which is essentially a constant given tracer levels of 18F-FDHT). It was our goal in this study to ascertain the limits of our dynamic PET measurements in resolving model parameters that may prove to be so correlated.
Of the compartmental models tested, two were frequently chosen by the BIC as being most consistent with the available data. The first of these, Model 1, fits reasonably well to all datasets. Model 1 is a simple two-parameter model with one parameter being the rate constant associated with a uni-directional trap (at least over the time period of these experiments) of 18F-FDHT into tumor. Thus in model 1, the resultant ktrap term comprises of a combination of intra-cellular transport and association with the binding domain. As a consequence, tumor-to-tumor (or tumor response) differences may be the result of changes in intra-cellular transport in addition to any change in AR concentration. For example, P-glycoprotein-mediated differences in the efflux of DHT between various tumor cell lines has been reported (8), differences that could be reflected in this rate constant.
The results for Model 2a suggest that in some cases it may be possible to derive a parameter which is more directly related to the free AR concentration, but our results also suggest that the determination of this parameter is less robust. More robust was the determination of the composite parameter describing the steady-state net influx rate constant, Ki, which in the case of Model 2a is equal to k1ktrap⧸(k2 + ktrap). The value for Ki was in general found to be close to the ktrap value of Model 1. However, there were some cases where the final trapping term and thus Ki went to zero. Although infrequent, this emphasizes that over the time frame of the current datasets (30 minutes) a significant portion of the measured PET signal might be due to the distribution of 18F-FDHT into reversible precursor compartments, i.e. upstream transport events rather than binding to AR.
Similar quality fits to the data can be obtained with models 1 and 2a (see Figs. 7A and 7B corresponding to different model fits to the same patient data) with associated differences in interpretation. Lower residuals are obtained with model 2a than for Model 1, but according to the BIC, the degree of improvement in the fit does not justify the additional parameters. This does not in and of itself mean that the model 2a interpretation is wrong but rather its compartments merely cannot be reliably discriminated by this data. With this in mind, one would have to accept the possibility of the model 2a interpretation where there is little to no binding of FDHT to AR in the tumor (described in Figure 7B) at baseline and only moderate binding at 3 and 6 months post therapy; compared with the perhaps more plausible interpretation of model 1, that of progressive response to therapy.
In order to distinguish which of the foregoing scenarios is most likely, it is necessary to consider other relevant data. Evidence that 18F-FDHT uptake is indeed measuring the binding of 18F-FDHT to AR is provided by in vitro and in vivo blocking studies, the latter conducted in baboons (2). In the baboon study, 18F-FDHT was co-administered with relatively high levels of non-radiolabeled testosterone, which was shown to reduce the amount of 18F-FDHT uptake into the prostate. If we assume that the transport of 18F-FDHT is not itself saturable, then the reduced uptake is most likely explained by a reduction in free AR owing to its binding to testosterone derived DHT. This same study also showed a loss of 18F-FDHT from baboon prostate after about three hours, perhaps from a slowly equilibrating compartment but also possibly due to reversible binding of 18F-FDHT to AR. Furthermore, biopsy samples from lesions positive on 18F-FDHT also stained positive for AR by immunohistochemistry (5).
Although significant questions remain regarding the potential for 18F-FDHT to directly measure free AR concentration, taken together the results of this and other studies suggests the potential that clinically useful information may be provided from a net-uptake parameters such as Model 1′s ktrap or Model 2′s Ki. Indeed, this parameter may be more useful clinically, since it is the binding of the AR-testosterone complex to androgen response elements on the DNA that impacts gene expression (8), not AR expression levels per se.
Caveats of our analysis included our inability to obtain input functions from arterial blood sampling (which would impede patient accrual and limit protocol utility). Since most patients did not have lesions close to the heart, we for the time being, overcame this limitation through the use of a population-derived input function (derived from the aorta ROIs of 25 patients) for modeling. The parent 18F-FDHT input function was then calculated by subtracting the metabolite input function (determined from venous blood samples) from the scaled population-based blood time-activity curve as shown in Figure 2. In general, the high rate of 18F-FDHT metabolism is a difficulty in that it gives rise to what is in effect a second input function resulting from the recirculation of the radiolabeled metabolites into the blood. However our metabolite analysis showed the 18F species appears to be tightly bound to plasma proteins and thus remain in the blood compartment. Whereas egress of these metabolites into the interstitium cannot be ruled out, we have demonstrated by in-vitro studies with CWR-22 cell lines that these metabolites do not bind to AR.
CONCLUSION
Independent of these difficulties, the success of Model 1 in fitting the tumor time-activity curves suggests the potential for simpler acquisition protocols involving a static PET measurement with and without a simultaneous venous blood sample. As described here and in a previous publication from our group (5), the metabolism of 18F-FDHT is such that the parent compound is almost entirely eliminated from the blood by 15 minutes. After this time the area under the input function curve increases by less than 1% per minute. This coupled with an assumption of rapid equilibration of any reversible space(s) within the model gives rise to a system in which the PET tumor activity measurement rapidly reaches a plateau confounded primarily by metabolites whose concentration is proportional to the metabolite level in the blood. If we assume a reasonable value for this proportionality constant, then an offset correcting the measured tumor activity concentration for metabolites can be estimated. The activity in the blood sample would also be used to scale the area under the population-based 18F-FDHT input curve. The metabolite-corrected tumor tissue value divided by the scaled area under the curve is then equal to ktrap. If the contribution of the metabolites to the tumor tissue measurement is small, then a simple SUV type measure may suffice.
In the future, we plan further validation of our simplified approach by testing the correlation of 18F-FDHT SUV (>15-minutes post injection) with histological androgen receptor staining intensity and by testing the correlation of changes in SUV with measures of clinical outcome. We also hope to modify the formulation of the injectate so as to avoid the problems with injection and therefore input function variability.
ACKNOWLEDGEMENTS
This work was supported by the Memorial Sloan-Kettering Center grants P50-CA92629 “SPORE in Prostate Cancer”, P50 CA086438 “In Vivo Center for Molecular and Cellular Imaging” and K23: CA102544.
This work was supported by the Memorial Sloan-Kettering Center grants P50-CA92629 “SPORE in Prostate Cancer” (P.I. H. Scher) and P50 CA086438 “In Vivo Center for Molecular and Cellular Imaging” (P.I. R. Blasberg & S.M. Larson).
Contributor Information
Bradley J. Beattie, Neurology, MSKCC.
Peter M. Smith-Jones, Radiology, MSKCC.
Yuliya S. Jhanwar, Dept. of Radiology, St. Luke’s-Roosevelt Hospital, New York.
Heiko Schöder, Radiology, MSKCC.
Ross Schmidtlein, Medical Physics, MSKCC
Michael Morris, Genitourinary Oncology Service, Dept. of Medicine, MSKCC.
Pat Zanzonico, Medical Physics, MSKCC
Olivia Squire, Radiology, MSKCC.
Gustavo S. P. Meirelles, Department of Radiology,Federal University of São Paulo,Brazil.
Ron Finn, Radiology, MSKCC
Mohammad Namavari, Department of Radiology and Bioengineering, Bio-X Program, Stanford University, Stanford, CA
Shangde Cai, Radiology, MSKCC
Howard I. Scher, Genitourinary Oncology Service, Dept. of Medicine, MSKCC.
Steven M. Larson, Radiology, MSKCC.
John L. Humm, Medical Physics, MSKCC
REFERENCES
- 1.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacology. 2008;8:440–448. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choe YS, Lidström PJ, Chi DY, Bonasera TA, Welch MJ, Katzenellenbogen JA. Synthesis of 11 beta-[18F]fluoro-5 alpha-dihydrotestosterone and 11 beta-[18F]fluoro-19-nor-5 alpha-dihydrotestosterone: preparation via halofluorination-reduction, receptor binding, and tissue distribution. J Med Chem. 1995;38:816–825. doi: 10.1021/jm00005a009. [DOI] [PubMed] [Google Scholar]
- 3.Bonasera TA, Oneil JP, Xu M, et al. Preclinical evaluation of fluorine-18-labeled androgen receptor ligands in baboons. J Nucl Med. 1996;37:1009–1015. [PubMed] [Google Scholar]
- 4.Dehdashti F, Picus J, Michalski JM, et al. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur J Nucl Med Mol Imaging. 2005;32:344–350. doi: 10.1007/s00259-005-1764-5. [DOI] [PubMed] [Google Scholar]
- 5.Larson SM, Morris M, Gunther I, et al. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366–373. [PubMed] [Google Scholar]
- 6.Zanzonico PB, Finn R, Pentlow KS, et al. PET-based radiation dosimetry in man of 18F-fluorodihydrotestosterone, a new radiotracer for imaging prostate cancer. J Nucl Med. 2004;45:1966–1971. [PubMed] [Google Scholar]
- 7.Schwartz G. Estimating Dimension of a Model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 8.Fedoruk MN, Gimenez-Bonafe P, Guns ES, Mayer LD, Nelson CC. P-glycoprotein increases the efflux of the androgen dihydrotestosterone and reduces androgen responsive gene activity in prostate tumor cells. Prostate. 2004;59:77–90. doi: 10.1002/pros.10354. [DOI] [PubMed] [Google Scholar]