Abstract
Chuvash polycythemia, the first hereditary disease associated with dysregulated oxygen-sensing to be recognized, is characterized by a homozygous germ-line loss-of-function mutation of the VHL gene (VHLR200W) resulting in elevated hypoxia inducible factor (HIF)-1α and HIF-2α levels, increased red cell mass and propensity to thrombosis. Organ volume is determined by the size and number of cells, and the underlying molecular control mechanisms are not fully elucidated. Work from several groups has demonstrated that the proliferation of cells is regulated in opposite directions by HIF-1α and HIF-2α. HIF-1α inhibits cell proliferation by displacing MYC from the promoter of the gene encoding the cyclin-dependent kinase inhibitor, p21Cip1, thereby inducing its expression. In contrast, HIF-2α promotes MYC activity and cell proliferation. Here we report that the volumes of liver, spleen, and kidneys relative to body mass were larger in 30 individuals with Chuvash polycythemia than in 30 matched Chuvash controls. In Hif1a+/− mice, which are heterozygous for a null (knockout) allele at the locus encoding HIF-1α, hepatic HIF-2α mRNA was increased (2-fold) and the mass of the liver was increased, compared with wild-type littermates, without significant difference in cell volume. Hepatic p21Cip1 mRNA levels were 9.5-fold lower in Hif1a+/− mice compared with wild-type littermates. These data suggest that, in addition to increased red cell mass, the sizes of liver, spleen, and kidneys are increased in Chuvash polycythemia. At least in the liver, this phenotype may result from increased HIF-2α and decreased p21Cip1 levels leading to increased hepatocyte proliferation.
Keywords: Polycythemia, VHL, Hypoxia inducible factor, Organ size, p21Cip1, Genetics, Gene expression, Hematology, HIF, Hypoxia, Mouse models
Introduction
Chuvash polycythemia (familial erythrocytosis, type 2, OMIM #263400), the first hereditary disease associated with augmented hypoxia-sensing to be recognized, is an autosomal recessive disorder with increased red cell mass [1]. While congenital polycythemia is sporadic worldwide, hundreds of patients with Chuvash polycythemia are found in the Chuvash population of central Russia [1, 2] and the condition is also endemic on the Italian island of Ischia [3]. Chuvash polycythemia is due to homozygosity for the 598C> T missense mutation in the von Hippel–Lindau gene (VHL) that results in the substitution of tryptophan for arginine at codon 200 (designated R200W) [4]. The VHL protein, which is the recognition component of an E3 ubiquitin-protein ligase complex, mediates proteasomal degradation of HIF-1α and HIF-2α under normoxic conditions [5]. HIF-1α and HIF-2α are subject to hydroxylation on specific proline residues by prolyl hydroxylase domain protein 2 (PHD2), an enzyme that requires oxygen as a substrate, and proline hydroxylation is required for the interaction of HIF-1α and HIF-2α with VHL [6, 7]. Thus, HIF-1α and HIF-2α levels rise in response to hypoxia and this leads to increased expression of erythropoietin [8] and other hypoxia-inducible genes [7]. Homozygosity for the VHLR200W allele leads to impaired interaction of VHL with both HIF-1α and HIF-2α proteins, increased levels of these HIFs in normoxia, and altered expression of multiple HIF-regulated genes including increased serum levels of erythropoietin [2, 4, 9, 10]. In addition to polycythemia, affected individuals may manifest altered cardiovascular and ventilatory responses and pulmonary hypertension [2, 10, 11].
Organ size is determined by cell growth, proliferation, and survival [12]. The sizes of the spleen [13] and liver [14] appear to be regulated by humoral factors active in embryonic as well as in adult life. The number of hematopoietic stem cells and hepatocytes is determined by both intrinsic and humoral factors [15, 16], and circulating cytokines can induce rapid regeneration of the bone marrow and liver [17]. Humoral factors that regulate organ size include growth hormone, insulin, insulin-like growth factor, thyroid hormone, sex hormones, and cytokines [17–19]. Intrinsic factors that lead to growth restraints on organs and that determine organ size during a set point in embryonic development are largely unknown [14]. Several of the humoral factors that regulate organ size also modulate the expression of HIF-1α and HIF-2α [20, 21].
Hypoxia influences proliferation of mammalian cell cultures as well as in animal models [22]. Under hypoxic conditions, HIF-1α induces cell cycle arrest associated with decreased CDK2 activity and hypo-phosphorylation of the retinoblastoma protein. This arrest is mediated in part by HIF-1α-induced expression of p21Cip1, an inhibitor of cyclin-dependent kinases. Under hypoxic conditions, HIF-1α displaces MYC on the p21Cip1 promoter and activates gene transcription [23]. In contrast, HIF-2α promotes cell proliferation in various cell lines under hypoxic conditions by stimulating MYC binding to its target gene promoters [24]. Mice in which the Vhl gene is conditionally disrupted in hepatocytes have increased levels of HIF-2α, increased target gene expression, hepatomegaly, and reduced body weight [25]. Transgenic mice expressing a mutant HIF-2α protein that is resistant to prolyl hydroxylation and VHL-mediated degradation have hepatomegaly but not decreased body weight [26]. The goal of the present study was to determine if, in addition to increased red blood cell mass, the constitutional up-regulation of HIF-1α and HIF-2α in Chuvash polycythemia is associated with increased size of solid organs.
Materials methods
Study subjects and determination of organ size by computerized tomography
Thirty patients with Chuvash polycythemia and 32 control residents of Chuvashia, Russia were studied at a time when they were ambulatory and in their usual state of health. The research in humans was approved by the IRB of Howard University and by Chuvash Republic Cardiac Center, Cheboksary, Chuvashia, Russia, and written informed consent was obtained from all participants. Thirty of the controls were matched by sex and age to the patients with Chuvash polycythemia. Computerized tomography was performed with 5-mm sections using helical mode. After obtaining preliminary non-enhanced scans, approximately 120 ml of non-ionic iodinated contrast agent was injected and arterial and venous phase images were obtained. The volumes of the spleen, liver and kidneys were measured using the disc summation method with DicomWorks v1.3.5 software. The area of each axial slice of the organ was measured, the resultant areas were then added, and then the sum of the areas was multiplied by the thickness of each image (5 mm).
PCR of the VHLR200W mutation
Genomic DNA was isolated using a QIAGEN column (QIAGEN Inc, Valencia, CA) and PCR reactions were performed in 50 µl containing 20 mM Tris-HCl pH 8.4, 50 mM KCl, 1.5 mM MgCl2, 100 µM dNTP, 300 nM primers, and 2.5 U/reaction Taq DNA polymerase (Life Technologies, Grand Island, NY). The following primers were used for amplification of VHL exon 3: VHL3F, 5′-CCTTGTACTGAGACCCTAG; VHL3R, 5′-GCTGAGATGAAACAGTGTA. Ten microliters of PCR product were incubated with 5 U of Fnu4HI (New England Biolabs Inc, Beverly, MA) for 2 h to detect the VHLR200W mutation. The VHL598C> T (VHLR200W) mutation abolishes a restriction site for Fnu4HI, resulting in an uncut 296-base-pair band detected by 1.2% agarose gel electrophoresis [4]. We confirmed the validity of this methodology on 88 additional genomic DNA samples by also performing allelic specific PCR using an ABI custom designed probe/primer mix (Applied Biosystems, Foster City, CA). Genomic DNA, 50 ng, was used for allele specific PCR and the reaction was carried out at 50°C for 2 min, 95°C for 10 min, followed by 45 cycles at 92°C for 15 s and 60°C for 1 min. There was an absolute concordance of results in 51 VHLR200W homozygotes, 4 heterozygotes and 33 VHL wildtype samples that were examined by both methods.
Hif1a+/− mice and wild-type littermates
Hif1a+/− mice were generated and maintained as previously described [27]. The animal experimentation conformed to protocols approved by the animal care committees of the University of Utah. Hif1a+/− mice and wild-type littermates older than 12 weeks of age were weighed, euthanized, and then dissected to remove the liver, spleen and kidneys. These organs were weighed in 1 ml of PBS and the weight was expressed as percentage of total body weight. One third of one lobe of each liver was fixed overnight, embedded in paraffin, and cut into 6-µm-thick sections and stained with hematoxylin and eosin followed by dewaxing and rehydration. Hepatocyte area was measured using ImageJ software (NIH).
Total RNA was extracted from livers of Hif1a+/− mice and wild-type littermates using Trizol (Molecular Research Center, Cincinnati, OH). Three aliquots of total RNA (300 ng) from each littermate were collected and pooled and analyzed at the University of Utah Microarray Core Facility using the Affymetrix Microarray Platform (Affymetrix, Santa Clara, CA). Results were analyzed by the University of Utah Bioinformatics Core. To quantify HIF-2α and p21cip1 mRNA levels, hepatic RNA (500 ng) was reverse transcribed into cDNA using SuperScript II with oligo dT primer according to the manufacturer's instructions (Invitrogen, Carlsbad, CA) and 2µl of cDNA was used for real-time PCR (ABI 7000, Applied Biosystems, Foster City, CA). For HIF-2α mRNA and 18S rRNA, we used SYBR Green dye with specific primers (Hif-2α: 5′-CTT GTA CCT GAA AGC CTT GG-3′ and 5′-GTC CCA TGA ACT TGC TGA TG-3′; 18S: 5′-TTG ACG GAA GGG CAC CAC CAG-3′ and 5′-GCA CCA CCA CCC ACG GAA TCG-3′) and the reaction was carried out at 50°C for 2 min, 95°C for 10 min, followed by 50 cycles of 92°C for 15 s and 58°C for 1 min. For p21Cip1 we used a Taqman probe (Assay ID, Mn01303209_m1) and followed the manufacturer's suggested protocol (ABI, Carlsbad, CA). ΔCt was expressed after normalization against 18S. Fold change was calculated by the ΔΔCt method [28].
Statistical analysis
Continuous variables were compared between Chuvash polycythemia patients and controls with the paired Student's t test. Organ volumes were also compared between patients and controls in multiple linear regression models. Mouse organ/body mass ratios were compared with the Kruskal–Wallis test.
Results
Organ size in Chuvash polycythemia and unaffected control subjects
Clinical features
VHLR200W homozygosity was confirmed by PCR in 23 of the 30 subjects with the diagnosis of Chuvash polycythemia, whereas in seven polycythemic subjects DNA was not available. VHL wild-type status was confirmed by PCR in 27 of the control subjects, two control subjects were VHLR200W heterozygotes, and DNA was not available for three controls. In addition to higher hemoglobin concentration, the Chuvash polycythemia subjects had lower body weight, body mass index, and systolic blood pressure compared to controls (Table 1) as previously reported [2, 10].
Table 1.
Clinical characteristics and organ volumes of study participants
| Chuvash polycythemia N=30 | Controls N=30 | P | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 47±8 | 47±8 | – |
| Female sex | 21 (70%) | 21 (70%) | – |
| Weight (kg) | 59.7±8.4 | 65.7±12.0 | 0.024 |
| Height (cm) | 162±6 | 163±9 | 0.315 |
| Body mass index (kg/m2) | 22.8±2.9 | 24.6±4.2 | 0.040 |
| Systolic blood pressure (mm Hg) | 116±17 | 127±18 | 0.002 |
| Diastolic blood pressure (mm Hg) | 78±14 | 82±12 | 0.107 |
| Hemoglobin (g/dL) | 16.9±3.5 | 12.4±1.5 | <0.0001 |
| Organ volume | |||
| Liver volume (cm3) | 1520±329 | 1369±303 | 0.041 |
| Spleen volume (cm3) | 363±229 | 171±63 | 0.0002 |
| Kidneys volume (cm3)a | 319±77 | 289±65 | 0.072 |
| Organ volume to body mass ratio | |||
| Liver (100× cm3/kg) | 2.57±0.54 | 2.09±0.29 | 0.0006 |
| Spleen (100×cm3/kg) | 0.59±0.30 | 0.26±0.08 | 0.0001 |
| Kidneysa (100×cm3/kg) | 0.54±0.14 | 0.45±0.09 | 0.005 |
Results in mean±SD or number of subjects (%). Comparison by the paired t test
Combined volume of right and left kidneys
Organ volumes
Despite decreased body weight, the 30 subjects with Chuvash polycythemia had larger volumes of the liver, spleen, and kidneys compared to 30 age- and sex-matched controls, as determined by computerized tomography and the disc summation method (Table 1). Expressed as the ratio of organ volume to body mass, the mean volume of the liver in Chuvash polycythemia patients was greater than in controls by a factor of 1.2 (95% confidence interval of 1.1 to 1.3; P=0.0006), the mean volume of the spleen was greater by a factor of 2.3 (1.8 to 2.8; P=0.0001) and the mean volume of the kidneys was greater by a factor of 1.2 (1.1 to 1.4; P=0.005). The present study was conducted in adults, and therefore reflects the size of the body and selected organs after the development that occurs during the embryonic, fetal and childhood periods of life.
Because of recognized relationships of larger organ size with greater body mass, [29] larger organ size with exposure to androgens [30], and decreasing organ size with advancing age [31], organ volume was examined among patients with Chuvash polycythemia and controls with multiple linear regression analyses that included body mass, sex, and age (Table 2). As expected [29], increased body weight was independently associated with increased volumes of the liver, spleen, and kidneys (P≤0.003) and male sex with increased volumes of the liver and kidneys (P=0.001). Consistent with some [32] but not all studies [31], liver and kidney size did not decrease with more advanced age, and spleen size did not decrease with female sex or age. Chuvash polycythemia was associated with an increase in liver volume comparable to a 13 kg greater body mass (P<0.0005), an increase in spleen volume comparable to a 42 kg greater body mass (P<0.0001), and an increase in kidney volume comparable to a 13 kg greater body mass (P<0.05). In similar analyses that included body mass index rather than body weight as an explanatory variable, the adjusted liver volume (P<0.001), spleen volume (P<0.0005), and total kidney volume (P<0.05) continued to be larger in patients with Chuvash polycythemia compared to controls.
Table 2.
Multiple linear regression analyses of organ volume in Chuvash polycythemia patients and controls
| Body weight increase of 10 kg |
Male vs. female sex | Age increase of 10 years |
Chuvash polycythemia vs. control |
|
|---|---|---|---|---|
| Liver (N=62) | ||||
| Estimated change in volume (cm3) | 166 | 253 | −70 | 215 |
| 95% confidence interval | 107–225 | 131–376 | −145 to 5 | 104–327 |
| P | <0.0001 | 0.001 | 0.068 | 0.0003 |
| Spleen (N=62) | ||||
| Estimated change in volume (cm3) | 41 | 12 | −9 | 172 |
| 95% confidence interval | 15–66 | −67 to 42 | −41 to 23 | 123–221 |
| P | 0.003 | 0.657 | 0.579 | <0.0001 |
| Kidneys (N=62) | ||||
| Estimated change in volume (cm3) | 24 | 62 | −15 | 30 |
| 95% confidence interval | 10–38 | 32–91 | −32 to 3 | 4–56 |
| P | 0.001 | 0.0001 | 0.095 | 0.026 |
Two additional controls added compared to Table 1
Analysis of HIF-1α-deficient mice
Organ size of Hif1a+/− mice and Hif1a+/+ littermates
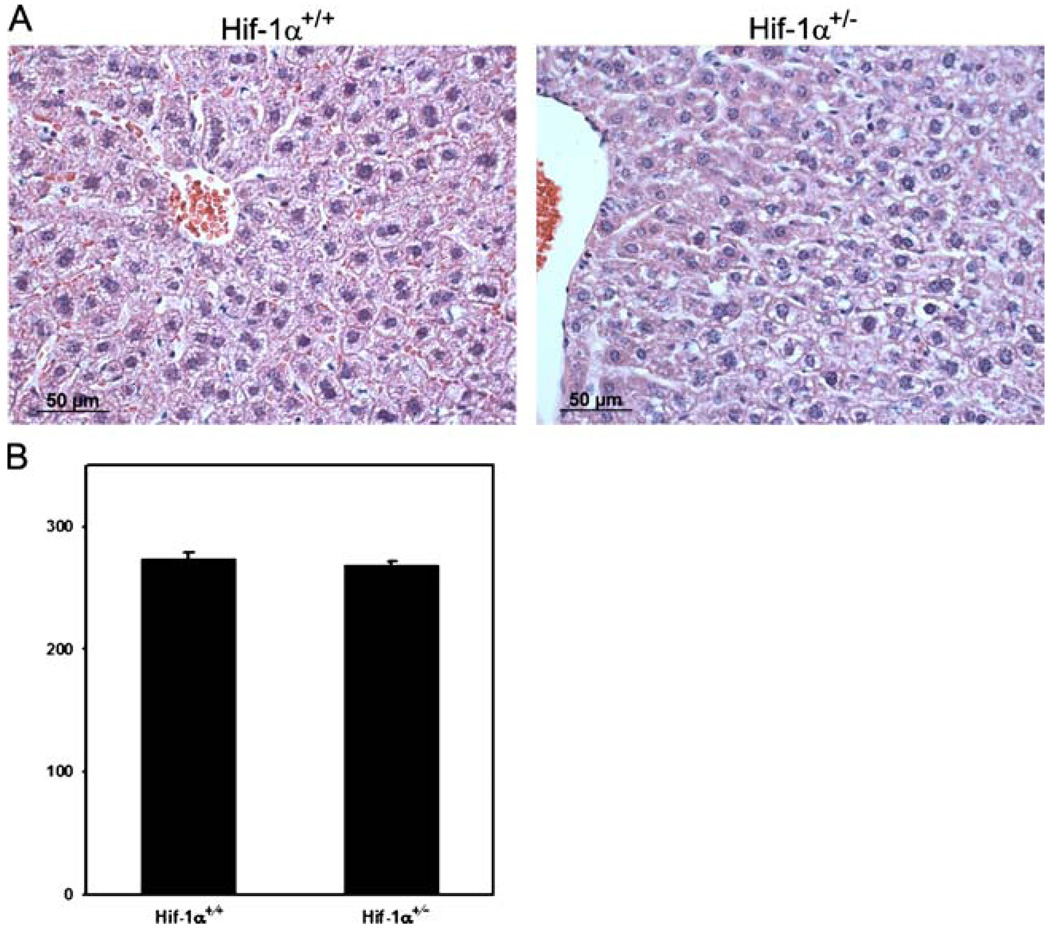
Since we demonstrated increased HIF-1α levels in Chuvash polycythemia patients homozygous for the VHLR200W allele [4] and other investigators demonstrated increased HIF-2α levels in mice carrying this mutation [9], we hypothesized that increased HIF activity may contribute to larger organ volume in VHLR200W homozygotes. To test this hypothesis, the organ to body mass ratio was determined in Hif1a+/− mice, which are heterozygous for a null allele at the locus encoding HIF-1α, [27] and their wild-type littermates. As shown in Table 3, Hif1a+/− mice had increased median organ-to-body mass ratio for the liver (factor of 1.5, P<0.005) relative to wild-type littermates. Hepatocyte area was not significantly different in Hif1a+/− mice and their wild-type littermates (Fig. 1), suggesting that an increase in cell number rather than cell volume was responsible for the increased mass of Hif1a+/− livers.
Table 3.
Organ to body mass ratios of Hif1a+/− and Hif1a+/+ mice
| Hif1a+/− (N=7) | Hif1a+/+ (N=5) | P | |
|---|---|---|---|
| Liver | 5.27 (4.19–5.94) | 3.51 (3.16–4.12) | 0.004 |
| Spleen | 0.43 (0.17–0.79) | 0.24 (0.12–0.34) | 0.167 |
| Kidneysa | 1.69 (1.17–2.18) | 1.20 (1.03–2.29) | 0.372 |
Results shown are median (range). Statistical analysis was performed by the Kruskal–Wallis test
Combined mass of right and left kidney
Fig. 1.
Hematoxylin- and eosin-stained sections of livers from Hif1a+/+ and Hif1a+/− mice. a The light microscope images are representative from five animals in each genotype. b Size of hepatocytes was not significantly different between Hif1a+/+ and Hif1a+/− mice
Gene expression
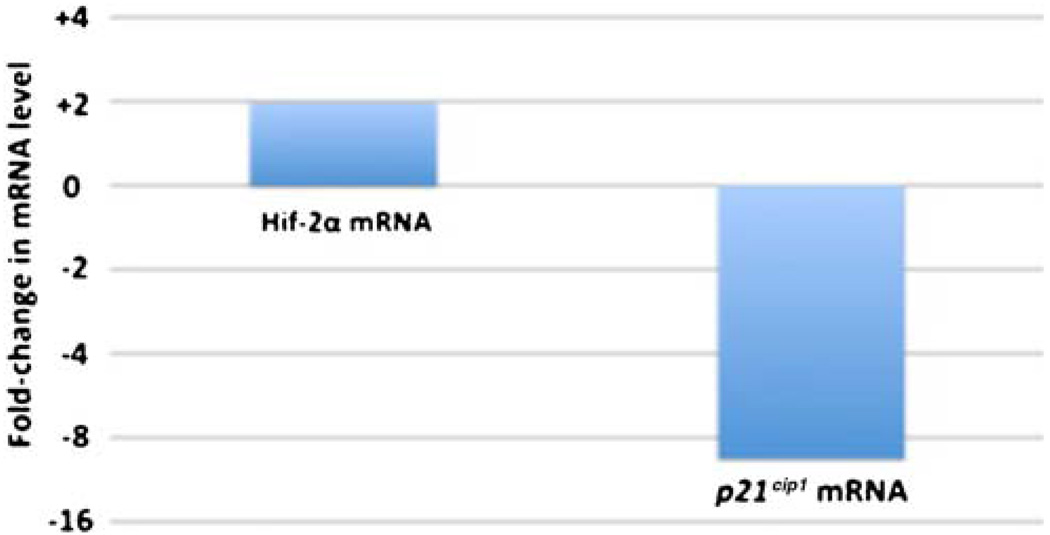
To explore potential molecular mechanisms underlying the increased liver-to-body mass ratio in Hif1a+/− mice, total RNA was extracted from livers of Hif1α+/− mice and wild-type littermates, and gene expression profiles were performed using Affymetrix microarrays. This procedure revealed that 2,167 genes were down-regulated more than two-fold in Hif1a+/− mice compared to wild-type mice genes and 1,628 genes were up-regulated more than two-fold. Among these differentially expressed genes were HIF-2α, which was consistently up-regulated, and p21Cip1, which was consistently down-regulated. The changes of expression of p21Cip1 and HIF-2α were confirmed using quantitative real-time RT-PCR. As shown in Fig. 2, HIF-2α mRNA levels were increased 2-fold (P<0.05) and p21Cip1 mRNA levels were decreased 9.5-fold (P=0.05) in the livers of Hif1α+/− mice compared to wild-type littermates. These findings are consistent with the hypothesis that HIF-regulated pathways are important in determining the volume of body organs [20, 21], specifically by a pathway in which increased HIF-2α expression leads to induction of MYC activity, inhibition of p21Cip1 expression and a resultant increase in cell proliferation [23, 24].
Fig. 2.
Analysis of gene expression in livers of Hif1a+/+ and Hif1a+/− mice using real-time PCR. Quantitative real-time RT-PCR was performed using specific primers for HIF-2α and p21Cip1 mRNA. Fold-change in mRNA levels for HIF-2α and p21Cip1 in Hif1a+/− mice compared to Hif1a+/+ mice is depicted
Discussion
Previous studies indicated that homozygosity for the VHLR200W mutation is associated with elevated hemoglobin concentration, cerebral vascular events, peripheral thrombosis, benign vertebral hemangiomas, varicose veins, heart murmurs, lower systemic blood pressure, elevated pulmonary artery pressure, increased expression of HIF-regulated genes, elevated levels of homocysteine and glutathione, altered inflammatory cytokine profiles, and no increased risk of malignancies associated with the von Hippel–Lindau syndrome [2, 10, 11, 33, 34]. Our present work indicates that volumes of the liver, spleen, and kidneys, as adjusted for body weight, sex, and age, are significantly greater in patients with Chuvash polycythemia compared with control subjects, and that like polycythemia, this may be due to increased cell proliferation.
Past studies demonstrating that HIF-1α decreases cell proliferation by inducing p21Cip1 expression [23] and that HIF-2α induces MYC activity and cell proliferation [24] were carried out in various tumor cells. Our present findings potentially relate these pathways to the larger size of the liver in Hif1a+/− compared with Hif1a+/+ mice. Our previous work showed a higher level of HIF-1α in EBV-transformed lymphocytes of Chuvash polycythemia patients although HIF-2α levels were not tested [4]. Recently, mice bearing the VhlR200W gene were generated by homologous recombination and found to recapitulate many features of human Chuvash polycythemia, including elevated hematocrit and serum erythropoietin levels. Moreover, in the livers of VhlR200W homozygous mice, HIF-2 target gene transcripts were preferentially up regulated compared to HIF-1 target gene transcripts [9]. Taken together with published data, our present observations suggest that, at least in the liver of patients with Chuvash polycythemia, the HIF-1α:HIF-2α ratio may be altered to favor HIF-2α, and that increased HIF-2α levels may lead to suppression of p21Cip1 expression, resulting in increased proliferation of hepatocytes and larger organ volume to body mass ratio.
There are a number of limitations to our study. Firstly, the diagnosis of Chuvash polycythemia was not confirmed by genotyping in seven of 30 patients. However, the clinical diagnosis of Chuvash polycythemia by physicians in Chuvashia is highly accurate in predicting VHLR200W homozygous genotype. Of 150 patients with the diagnosis of Chuvash polycythemia who we have genotyped to date, 142 have been VHLR200W homozygotes, two VHLR200W heterozygotes and six VHL wildtype individuals (Gordeuk 2010, unpublished data). Secondly, the genotype of three of the 32 controls was not determined. In our studies to date, none of 449 persons in Chuvashia without the diagnosis of Chuvash polycythemia have proved to be VHLR200W homozygotes when genotyped (Gordeuk 2010, unpublished data). Thirdly, two of the 29 controls who were genotyped proved to be VHLR200W heterozygotes. The study was designed to select both patients and controls on a clinical basis, before genotyping was performed. In our studies to date, heterozygotes have not had significantly increased hemoglobin concentrations compared to controls [2]. Fourthly, the study did not match cases and controls according to body mass index, and the controls had a slightly but significantly higher body mass index compared to cases. However, in multivariate analysis that were adjusted either for body weight or for body mass index, the least square mean volumes of the liver, spleen and kidneys were significantly greater in patients with Chuvash polycythemia compared to controls. The lower body mass index associated with Chuvash polycythemia observed here is consistent with our previous work [2] and may reflect the altered expression of multiple HIF-regulated genes in the setting of homozygosity for the VHLR200W allele.
Whether there are any selective advantages or disadvantages to larger size of solid organs in association with Chuvash polycythemia is unclear. Amechanismof enhanced cellular proliferation due to HIF-2α-induced suppression of p21Cip1 expression may contribute not only to larger organ size but also other manifestations of Chuvash polycythemia. Erythroid progenitors in Chuvash polycythemia display heightened sensitivity to erythropoietin [4], suggesting that other mechanisms in addition to increased erythropoietin levels contribute to the increased proliferation of erythroid progenitors. Pulmonary hypertension is a complication of Chuvash polycythemia [10, 11], the pathophysiology of which includes increased proliferation and hypertrophy of pulmonary vascular smooth muscle cells [35].
Acknowledgements and Disclosure Statement
This work was supported by the National Heart, Lung and Blood Institute and the Office of Research on Minority Health (UH1-HL03679-05 to V.R.G.); by the National Institute of Research Resources (Howard University General Clinical Research Center Grant No. MO1-RR10284); by the National Heart, Lung and Blood Institute (R01HL079912-01 to V.R. G., R01HL66333-01 to J.T.P. and V.R.G., R01HL50077-14 o J.T.P.); and by the Veterans Administration (merit grant to J.T.P.).We would like to thank the University of Utah Microarray Core Facility and Bioinformatics Core for their services and advice. Dr. Gordeuk has received consulting fees from Amgen and a grant from Merck. Daniel Okhotin has received a grant from Amgen.
Footnotes
Author contributions Donghoon Yoon contributed to study design, conducting the study, analyzing the data and writing the manuscript. David Okhotin contributed to conducting the study, analyzing the data and writing the manuscript. Bumjun Kim contributed to study design, conducting the study, analyzing the data and writing the manuscript. Yulia Okhotina contributed to conducting the study and writing the manuscript. Daniel J. Okhotin contributed to study design, conducting the study and writing the manuscript. Galina Y. Miasnikova contributed to study design and conducting the study. Adelina I. Sergueeva contributed to study design and conducting the study. Lydia A. Polyakova contributed to study design and conducting the study. Alexei Maslow contributed to study design and conducting the study. Yonggu Lee contributed to study design, conducting the study, and analyzing the data. Gregg L. Semenza contributed to study design, analyzing the data and writing the manuscript. Josef T. Prchal contributed to study design, conducting the study, analyzing the data and writing the manuscript. Victor R. Gordeuk contributed to study design, conducting the study, analyzing the data and writing the manuscript.
Contributor Information
Donghoon Yoon, Department of Medicine, University of Utah, Salt Lake, UT 84132, USA.
David V. Okhotin, Center for Sickle Cell Disease, Howard University, Washington, DC 20060, USA
Bumjun Kim, Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX 77030, USA.
Yulia Okhotina, Center for Sickle Cell Disease, Howard University, Washington, DC 20060, USA.
Daniel J. Okhotin, Russian Research Services, Camas, WA 98607, USA
Galina Y. Miasnikova, Chuvash Republic Clinical Hospital No. 1, Cheboksary 428022, Russia
Adelina I. Sergueeva, Cheboksary Children’s Hospital, Cheboksary 428022, Russia
Lydia A. Polyakova, Chuvash Republic Clinical Hospital No. 1, Cheboksary 428022, Russia
Alexei Maslow, President Hospital, Moscow 101000, Russia.
Yonggu Lee, Department of Medicine, University of Utah, Salt Lake, UT 84132, USA.
Gregg L. Semenza, The Johns Hopkins University School of Medicine, Baltimore, MD 21206, USA gsemenza@jhmi.edu
Josef T. Prchal, Department of Medicine, University of Utah, Salt Lake, UT 84132, USA
Victor R. Gordeuk, Center for Sickle Cell Disease, Howard University, Washington, DC 20060, USA Center for Sickle Cell Disease, Howard University, 2041 Georgia Ave. NW, Washington, DC 20060, USA vgordeuk@howard.edu.
References
- 1.Polyakova LA. Familial erythrocytosis among inhabitants of the Chuvash ASSR. Problemi Gematologii I perelivaniya Krovi. 1974;10:30–36. [PubMed] [Google Scholar]
- 2.Gordeuk VR, Sergueeva AI, Miasnikova GY, Okhotin D, Voloshin Y, Choyke PL, Butman JA, Jedlickova K, Prchal JT, Polyakova LA. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood. 2004;103:3924–3932. doi: 10.1182/blood-2003-07-2535. doi:10.1182/blood-2003-07-2535, 2003-07-2535 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Perrotta S, Nobili B, Ferraro M, Migliaccio C, Borriello A, Cucciolla V, Martinelli V, Rossi F, Punzo F, Cirillo P, Parisi G, Zappia V, Rotoli B, Ragione FD. Von Hippel-Lindaudependent polycythemia is endemic on the island of Ischia: identification of a novel cluster. Blood. 2006;107:514–519. doi: 10.1182/blood-2005-06-2422. doi:2005-06-2422 [pii], 10.1182/blood-2005-06-2422. [DOI] [PubMed] [Google Scholar]
- 4.Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, Maxwell PH, Stockton DW, Semenza GL, Prchal JT. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–621. doi: 10.1038/ng1019. doi:10.1038/ng1019, ng1019 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Safran M, Kaelin WG., Jr HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779–783. doi: 10.1172/JCI18181. doi:10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. doi:S1097-2765(08)00292-X [pii], 10.1016/j.molcel. 2008.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. doi:24/2/97 [pii], 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 8.Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, Wiesener M, Eckardt KU, Koch CJ, Ellies LG, Haddad G, Haase VH, Simon MC, Poellinger L, Powell FL, Johnson RS. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell. 2008;133:223–234. doi: 10.1016/j.cell.2008.02.038. doi:S0092-8674(08)00289-4 [pii], 10.1016/j.cell.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickey MM, Lam JC, Bezman NA, Rathmell WK, Simon MC. von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2alpha signaling and splenic erythropoiesis. J Clin Invest. 2007;117:3879–3889. doi: 10.1172/JCI32614. doi:10.1172/JCI32614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bushuev VI, Miasnikova GY, Sergueeva AI, Polyakova LA, Okhotin D, Gaskin PR, Debebe Z, Nekhai S, Castro OL, Prchal JT, Gordeuk VR. Endothelin-1, vascular endothelial growth factor and systolic pulmonary artery pressure in patients with Chuvash polycythemia. Haematologica. 2006;91:744–749. [PubMed] [Google Scholar]
- 11.Smith TG, Brooks JT, Balanos GM, Lappin TR, Layton DM, Leedham DL, Liu C, Maxwell PH, McMullin MF, McNamara CJ, Percy MJ, Pugh CW, Ratcliffe PJ, Talbot NP, Treacy M, Robbins PA. Mutation of von Hippel–Lindau tumour suppressor and human cardiopulmonary physiology. PLoS Med. 2006;3:e290. doi: 10.1371/journal.pmed.0030290. doi:05-PLME-RA-0484R2 [pii], 10.1371/journal.pmed.0030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conlon I, Raff M. Size control in animal development. Cell. 1999;96:235–244. doi: 10.1016/s0092-8674(00)80563-2. doi:S0092-8674(00)80563-2 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Metcalf D. Restricted growth capacity of multiple spleen grafts. Transplantation. 1964;2:387–392. doi: 10.1097/00007890-196405000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. doi:nature05537 [pii], 10.1038/ nature05537. [DOI] [PubMed] [Google Scholar]
- 15.Muller-Sieburg CE, Cho RH, Sieburg HB, Kupriyanov S, Riblet R. Genetic control of hematopoietic stem cell frequency in mice is mostly cell autonomous. Blood. 2000;95:2446–2448. [PubMed] [Google Scholar]
- 16.Robin C, Ottersbach K, Durand C, Peeters M, Vanes L, Tybulewicz V, Dzierzak E. An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Dev Cell. 2006;11:171–180. doi: 10.1016/j.devcel.2006.07.002. doi:S1534-5807(06)00303-0 [pii], 10.1016/j. devcel.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. doi:10.1146/annurev.cellbio.17.1.387, 17/1/387 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Bryant PJ, Simpson P. Intrinsic and extrinsic control of growth in developing organs. Q Rev Biol. 1984;59:387–415. doi: 10.1086/414040. [DOI] [PubMed] [Google Scholar]
- 19.Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, Carre C, Noselli S, Leopold P. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. doi:1119432 [pii], 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- 20.Catrina SB, Botusan IR, Rantanen A, Catrina AI, Pyakurel P, Savu O, Axelson M, Biberfeld P, Poellinger L, Brismar K. Hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha are expressed in kaposi sarcoma and modulated by insulin-like growth factor-I. Clin Cancer Res. 2006;12:4506–4514. doi: 10.1158/1078-0432.CCR-05-2473. doi:12/15/4506 [pii], 10.1158/1078-0432.CCR-05-2473. [DOI] [PubMed] [Google Scholar]
- 21.Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. doi:10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner LB, Corn PG. Hypoxic regulation of mRNA expression. Cell Cycle. 2008;7:1916–1924. doi: 10.4161/cc.7.13.6203. doi:6203 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. doi:10.1038/sj. emboj.7600196, 7600196 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. doi:S1535-6108 (07)00059-1 [pii], 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel–Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;98:1583–1588. doi: 10.1073/pnas.98.4.1583. doi:10.1073/pnas.98.4.1583, 98/4/1583 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim WY, Safran M, Buckley MR, Ebert BL, Glickman J, Bosenberg M, Regan M, Kaelin WG., Jr Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. EMBO J. 2006;25:4650–4662. doi: 10.1038/sj.emboj.7601300. doi:7601300 [pii], 10.1038/sj.emboj.7601300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, Rainey K, Ponka P, Semenza GL, Schumacher A, Prchal JT. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem. 2006;281:25703–25711. doi: 10.1074/jbc.M602329200. doi:M602329200 [pii], 10.1074/jbc. M602329200. [DOI] [PubMed] [Google Scholar]
- 29.Selman C, Lumsden S, Bunger L, Hill WG, Speakman JR. Resting metabolic rate and morphology in mice (Mus musculus) selected for high and low food intake. J Exp Biol. 2001;204:777–784. doi: 10.1242/jeb.204.4.777. [DOI] [PubMed] [Google Scholar]
- 30.Crawford BA, Singh J, Simpson JM, Handelsman DJ. Androgen regulation of circulating insulin-like growth factor-I during puberty in male hypogonadal mice. J Endocrinol. 1993;139:57–65. doi: 10.1677/joe.0.1390057. [DOI] [PubMed] [Google Scholar]
- 31.Serste T, Bourgeois N. Ageing and the liver. Acta Gastroenterol Belg. 2006;69:296–298. [PubMed] [Google Scholar]
- 32.Meier JM, Alavi A, Iruvuri S, Alzeair S, Parker R, Houseni M, Hernandez-Pampaloni M, Mong A, Torigian DA. Assessment of age-related changes in abdominal organ structure and function with computed tomography and positron emission tomography. Semin Nucl Med. 2007;37:154–172. doi: 10.1053/j.semnuclmed.2007.02.001. doi:S0001-2998(07)00022-0 [pii], 10.1053/j.semnuclmed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Niu X, Miasnikova GY, Sergueeva AI, Polyakova LA, Okhotin DJ, Tuktanov NV, Nouraie M, Ammosova T, Nekhai S, Gordeuk VR. Altered cytokine profiles in patients with Chuvash polycythemia. Am J Hematol. 2008 doi: 10.1002/ajh.21327. doi:10.1002/ajh.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sergueeva AI, Miasnikova GY, Okhotin DJ, Levina AA, Debebe Z, Ammosova T, Niu X, Romanova EA, Nekhai S, DiBello PM, Jacobsen DW, Prchal JT, Gordeuk VR. Elevated homo-cysteine, glutathione and cysteinylglycine concentrations in patients homozygous for the Chuvash polycythemia VHL mutation. Haematologica. 2008;93:279–282. doi: 10.3324/haematol.11851. doi:haematol.11851 [pii], 10.3324/haematol.11851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones R, Capen D, Jacobson M. PDGF and microvessel wall remodeling in adult lung: imaging PDGF-Rbeta and PDGFBB molecules in progenitor smooth muscle cells developing in pulmonary hypertension. Ultrastruct Pathol. 2006;30:267–281. doi: 10.1080/01913120600820336. doi:P64676738W8625L3 [pii], 10.1080/01913120600820336. [DOI] [PubMed] [Google Scholar]