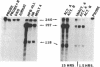Abstract
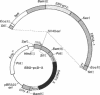
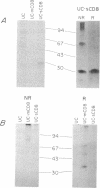
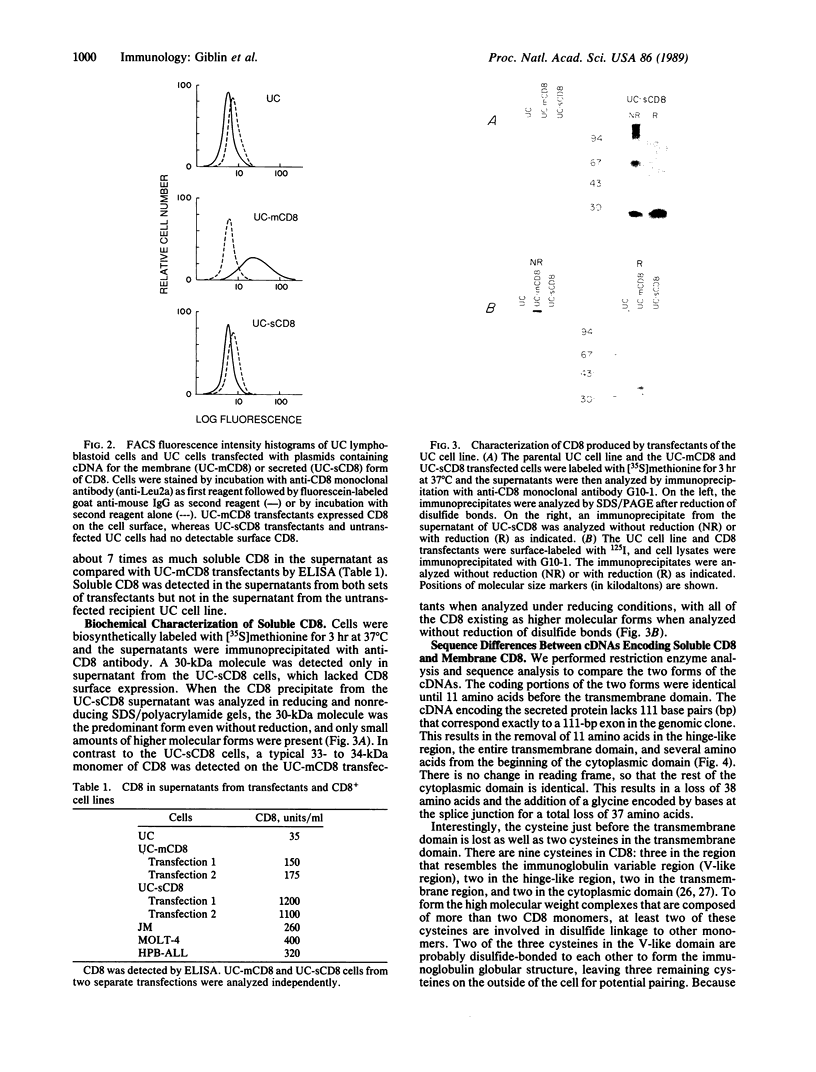
The human lymphocyte differentiation antigen CD8 is encoded by a single gene that gives rise to a 33- to 34-kDa glycoprotein expressed on the cell surface as a dimer and in higher molecular mass forms. We demonstrate that the mRNA is alternatively spliced so that an exon encoding a transmembrane domain is deleted. This gives rise to a 30-kDa molecule that is secreted and exists primarily as a monomer. mRNA corresponding to both forms is present in peripheral blood lymphocytes, Con A-activated peripheral blood lymphocytes, and three CD8+ T-cell lines, with the membrane form being the major species. However, differences in the ratio of mRNA for membrane CD8 and secreted CD8 exist. In addition, the splicing pattern we observe differs from the pattern found for the mouse CD8 gene. This mRNA is also alternatively spliced, but an exon encoding a cytoplasmic region is deleted, giving rise to a cell surface molecule that differs in its cytoplasmic tail from the protein encoded by the longer mRNA. Neither protein is secreted. This is one of the first examples of a different splicing pattern between two homologous mouse and human genes giving rise to very different proteins. This represents one mechanism of generating diversity during speciation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acres R. B., Conlon P. J., Mochizuki D. Y., Gallis B. Phosphorylation of the CD8 antigen on cytotoxic human T cells in response to phorbol myristate acetate or antigen-presenting B cells. J Immunol. 1987 Oct 1;139(7):2268–2274. [PubMed] [Google Scholar]
- Amiot M., Dastot H., Fabbi M., Degos L., Bernard A., Boumsell L. Intermolecular complexes between three human CD1 molecules on normal thymus cells. Immunogenetics. 1988;27(3):187–195. doi: 10.1007/BF00346585. [DOI] [PubMed] [Google Scholar]
- Bernabeu C., van de Rijn M., Lerch P. G., Terhorst C. P. Beta 2-microglobulin from serum associates with MHC class I antigens on the surface of cultured cells. Nature. 1984 Apr 12;308(5960):642–645. doi: 10.1038/308642a0. [DOI] [PubMed] [Google Scholar]
- Blue M. L., Hafler D. A., Craig K. A., Levine H., Schlossman S. F. Phosphorylation of CD4 and CD8 molecules following T cell triggering. J Immunol. 1987 Dec 15;139(12):3949–3954. [PubMed] [Google Scholar]
- Bushkin Y., Demaria S., Le J. M., Schwab R. Physical association between the CD8 and HLA class I molecules on the surface of activated human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3985–3989. doi: 10.1073/pnas.85.11.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmrich F., Strittmatter U., Eichmann K. Synergism in the activation of human CD8 T cells by cross-linking the T-cell receptor complex with the CD8 differentiation antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8298–8302. doi: 10.1073/pnas.83.21.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto J., Levy S., Levy R. Spontaneous release of the Leu-2 (T8) molecule from human T cells. J Exp Med. 1983 Sep 1;158(3):752–766. doi: 10.1084/jem.158.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto J., Stewart S. J., Levy R. Immunochemical analysis of the released Leu-2 (T8) molecule. J Exp Med. 1984 Jul 1;160(1):116–124. doi: 10.1084/jem.160.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
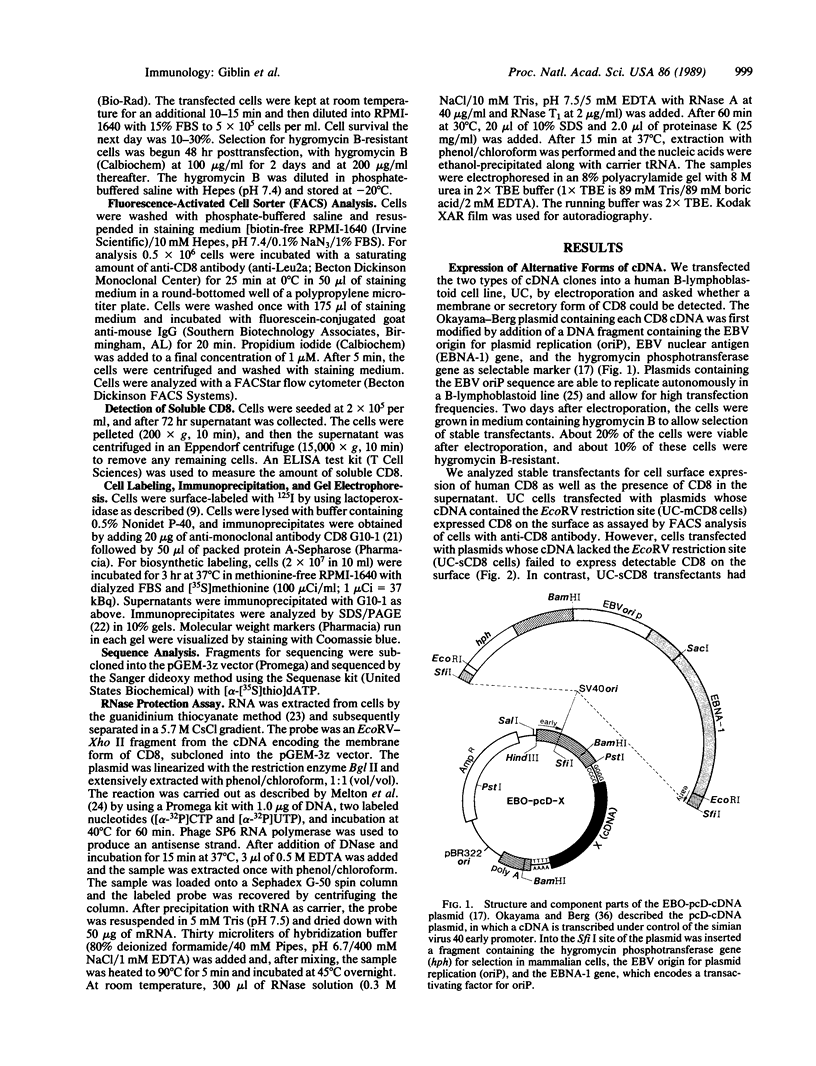
- Gabert J., Langlet C., Zamoyska R., Parnes J. R., Schmitt-Verhulst A. M., Malissen B. Reconstitution of MHC class I specificity by transfer of the T cell receptor and Lyt-2 genes. Cell. 1987 Aug 14;50(4):545–554. doi: 10.1016/0092-8674(87)90027-4. [DOI] [PubMed] [Google Scholar]
- Glassy M. C., Handley H. H., Hagiwara H., Royston I. UC 729-6, a human lymphoblastoid B-cell line useful for generating antibody-secreting human-human hybridomas. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6327–6331. doi: 10.1073/pnas.80.20.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga T. W., van der Schoot C. E., Jost C., Klaassen R., Kleijer M., von dem Borne A. E., Roos D., Tetteroo P. A. The PI-linked receptor FcRIII is released on stimulation of neutrophils. Nature. 1988 Jun 16;333(6174):667–669. doi: 10.1038/333667a0. [DOI] [PubMed] [Google Scholar]
- Johnson P. A human homolog of the mouse CD8 molecule, Lyt-3: genomic sequence and expression. Immunogenetics. 1987;26(3):174–177. doi: 10.1007/BF00365908. [DOI] [PubMed] [Google Scholar]
- Kavathas P., Herzenberg L. A. Stable transformation of mouse L cells for human membrane T-cell differentiation antigens, HLA and beta 2-microglobulin: selection by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1983 Jan;80(2):524–528. doi: 10.1073/pnas.80.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavathas P., Sukhatme V. P., Herzenberg L. A., Parnes J. R. Isolation of the gene encoding the human T-lymphocyte differentiation antigen Leu-2 (T8) by gene transfer and cDNA subtraction. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7688–7692. doi: 10.1073/pnas.81.24.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krangel M. S. Secretion of HLA-A and -B antigens via an alternative RNA splicing pathway. J Exp Med. 1986 May 1;163(5):1173–1190. doi: 10.1084/jem.163.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Rose L. M., Spooner C. E., Beatty P. G., Martin P. J., Clark E. A. Antibodies to common leukocyte antigen p220 influence human T cell proliferation by modifying IL 2 receptor expression. J Immunol. 1985 Sep;135(3):1819–1825. [PubMed] [Google Scholar]
- Ledbetter J. A., Tsu T. T., Clark E. A. Covalent association between human thymus leukemia-like antigens and CD8(Tp32) molecules. J Immunol. 1985 Jun;134(6):4250–4254. [PubMed] [Google Scholar]
- Littman D. R., Thomas Y., Maddon P. J., Chess L., Axel R. The isolation and sequence of the gene encoding T8: a molecule defining functional classes of T lymphocytes. Cell. 1985 Feb;40(2):237–246. doi: 10.1016/0092-8674(85)90138-2. [DOI] [PubMed] [Google Scholar]
- Margolskee R. F., Kavathas P., Berg P. Epstein-Barr virus shuttle vector for stable episomal replication of cDNA expression libraries in human cells. Mol Cell Biol. 1988 Jul;8(7):2837–2847. doi: 10.1128/mcb.8.7.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakauchi H., Nolan G. P., Hsu C., Huang H. S., Kavathas P., Herzenberg L. A. Molecular cloning of Lyt-2, a membrane glycoprotein marking a subset of mouse T lymphocytes: molecular homology to its human counterpart, Leu-2/T8, and to immunoglobulin variable regions. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5126–5130. doi: 10.1073/pnas.82.15.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–3177. [PubMed] [Google Scholar]
- Sitia R., Neuberger M. S., Milstein C. Regulation of membrane IgM expression in secretory B cells: translational and post-translational events. EMBO J. 1987 Dec 20;6(13):3969–3977. doi: 10.1002/j.1460-2075.1987.tb02739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow P. M., Van de Rijn M., Terhorst C. Association between the human thymic differentiation antigens T6 and T8. Eur J Immunol. 1985 May;15(5):529–532. doi: 10.1002/eji.1830150520. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Sizer K. C., Vollmer A. C., Hunkapiller T., Parnes J. R. The T cell differentiation antigen Leu-2/T8 is homologous to immunoglobulin and T cell receptor variable regions. Cell. 1985 Mar;40(3):591–597. doi: 10.1016/0092-8674(85)90207-7. [DOI] [PubMed] [Google Scholar]
- Tagawa M., Nakauchi H., Herzenberg L. A., Nolan G. P. Formal proof that different-size Lyt-2 polypeptides arise from differential splicing and post-transcriptional regulation. Proc Natl Acad Sci U S A. 1986 May;83(10):3422–3426. doi: 10.1073/pnas.83.10.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S., Engleman E. G. Evidence for an association between CD8 molecules and the T cell receptor complex on cytotoxic T cells. J Immunol. 1987 Nov 15;139(10):3231–3235. [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- Zamoyska R., Vollmer A. C., Sizer K. C., Liaw C. W., Parnes J. R. Two Lyt-2 polypeptides arise from a single gene by alternative splicing patterns of mRNA. Cell. 1985 Nov;43(1):153–163. doi: 10.1016/0092-8674(85)90020-0. [DOI] [PubMed] [Google Scholar]