Abstract
Although the glaucoma-associated protein myocilin has been the focus of intensive research, its biological function is still unknown. One of the limiting factors has been the lack of well characterized antibodies, particularly monoclonal antibodies. We describe the development of six monoclonal antibodies specific to myocilin and characterize their suitability in Western blot and immunohistochemical applications. Three of the six monoclonal antibodies recognize the N-terminus of myocilin (amino acids 33–214), two antibodies recognize the middle third of the protein (amino acids 215–368), and one antibody recognizes the C-terminus (amino acids 369–504). Isotyping revealed all antibodies are of the IgG1κ class except one, which is IgG2bκ. Purified myocilin monoclonal antibodies were able to recognize myocilin in human aqueous humor separated on denatured/reduced and native gels, and human trabecular meshwork lysate by Western blot. Myocilin was also detected by immunohistochemistry in trabecular meshwork, ciliary body, iris, cornea, sclera, choroid, and retinal pigment epithelial cells.
Keywords: myocilin, monoclonal antibodies, TIGR, glaucoma, aqueous humor, trabecular meshwork
INTRODUCTION
Myocilin is a glaucoma-associated protein having been linked to several forms of glaucoma including steroid glaucoma, juvenile open-angle glaucoma, and primary open-angle glaucoma (Clark et al., 2001b; Fingert et al., 1999; Nguyen et al., 1998; Nguyen et al., 1993; Stone et al., 1997). The protein is 504 amino acids in length and contains an N-terminal hydrophobic signal peptide sequence, a coiled-coiled region containing a leucine zipper domain, and a globular C-terminal domain that contains homology to olfactomedins, a family of proteins with function related to neural development (Nguyen et al., 1998). Myocilin is a glycoprotein with commonly identified sizes of 53–57 kDa (doublet) however, some antibodies also recognize a 66 kDa form of the protein. Cleavage of myocilin, resulting in a 20 kDa N-terminal fragment and a 35 kDa fragment has also been reported in human aqueous and select monolayer cells such as 293T cells (Aroca-Aguilar et al., 2005; Goldwich et al., 2003; Russell et al., 2001).
Expression of myocilin has been found in several tissues of the body including areas of the brain, skeletal muscle, kidney and heart (Fingert et al., 1998; Goldwich et al., 2005; Kubota et al., 1997; Li et al., 2006; Ohlmann et al., 2003; Ortego et al., 1997). Ocular expression of myocilin is widespread, having been identified in the iris, trabecular meshwork, ciliary body, sclera, cornea, choroid, photoreceptors, and astrocytes of the optic nerve head (Clark et al., 2001a; Huang et al., 2000; Karali et al., 2000; Kubota et al., 1997; Noda et al., 2000; O’Brien et al., 2000; Ortego et al., 1997; Swiderski et al., 1999). The distribution of myocilin inside and outside cells is less certain, since various locations have been reported. Myocilin has a functional N-terminal signal peptide sequence and has been localized intracellularly to the endoplasmic reticulum and Golgi apparatus (Rao et al., 2000; Russell et al., 2001). Myocilin also appears to associate with mitochondria (Sakai et al., 2007; Ueda et al., 2000; Wentz-Hunter et al., 2002a), cytoplasmic filaments (Fautsch et al., 2006b; Sohn et al., 2002), and intracellular membranes (Stamer et al., 2006). It is also found in aqueous humor, suggesting an extracellular role as well (Rao et al., 2000; Russell et al., 2001). Many of the myocilin mutants that have been identified in juvenile or primary open-angle glaucoma are not secreted but are found sequestered within the endoplasmic reticulum and Golgi apparatus, suggesting that proper folding and export may be required for normal function or that intracellular aggregates may be physiologically disruptive (Caballero, Borras, 2001; Gobeil et al., 2004; Jacobson et al., 2001).
The function of myocilin, particularly regarding its role in normal and glaucomatous aqueous humor drainage through the trabecular meshwork, is unknown. One approach to understanding myocilin’s function is to identify proteins that interact with myocilin in vivo. Myocilin binds to itself through interactions of the N-terminal coiled-coiled region forming dimeric and possibly multimeric complexes (Fautsch, Johnson, 2001; Fautsch et al., 2004; Russell et al., 2001). Several other proteins have been identified as myocilin binding partners including the extracellular proteins fibronectin (Filla et al., 2002; Ueda et al., 2002), optimedin (Torrado et al., 2002), hevin (Li et al., 2006) and collagen type IV (Wentz-Hunter et al., 2002b), and the intracellular proteins myosin regulatory light chain (Wentz-Hunter et al., 2002b), flotillin-1 (Joe et al., 2005) and gamma-synuclein (Surgucheva et al., 2005). Reproducibility of the binding interactions has been inconsistent, partially due to limitations with the specificity of available myocilin antibodies.
Specific, well-characterized antibodies are essential tools for deciphering the biological roles of proteins. The development of myocilin monoclonal antibodies would serve a similar purpose, helping to clarify myocilin properties and determine its biological function. We have established six hybridoma clones secreting mouse monoclonal antibodies against human myocilin. In this report, we introduce the six monoclonal antibodies, characterize their antigenic recognition region, and evaluate their use in immunoblotting and immunohistochemistry.
MATERIALS AND METHODS
Purification of recombinant myocilin
Conditioned media (360 ml) from transformed trabecular meshwork cells (TM5 cells were a gift from Dr. Abe Clark, Alcon Labs, Fort Worth, TX) overexpressing full-length myocilin tagged with C-terminal V5/6x histidine epitopes (Fautsch et al., 2006a) was mixed with 40 ml of 10X preparation buffer [500 mM NaH2PO4 (pH 8.0), 1.5 M NaCl, 10 mM imidizole], 2.5 ml of Ni-NTA resin (4 ml; Qiagen, Valencia, CA), and separated into ten 50-ml conical tubes. Following overnight incubation, Ni-NTA resin was pelleted by centrifugation at 2,500 × g for 5 minutes, combined into one 50-ml conical tube, and resuspended in 40 ml of wash buffer [50 mM NaH2PO4 (pH 8.0), 300 mM NaCl, 20 mM imidizole]. Resin was incubated with shaking at room temperature for 10 minutes, centrifuged at 2,500 × g for 5 minutes, and supernate was removed. Washing of Ni-NTA resin was repeated five times. Two additional washes were performed with 50 mM NaH2 PO4 (pH 8.0), 300 mM NaCl, and 40 mM imidizole. For elution of myocilin from the nickel, the resin was resuspended in 2 ml of 50 mM NaH2 PO4 (pH 8.0), 300 mM NaCl, 250 mM imidizole and rocked at 4°C for 10 minutes. Nickel-resin was centrifuged at 2,500 × g for 5 minutes and the supernate (containing eluted protein) was isolated. The elution step was repeated. Elutions were combined and dialyzed overnight (minimum of 16 hours) against buffer containing 50 mM NaH2 PO4 (pH 8.0), 300 mM NaCl and decreasing amounts of imidizole (100 mM→50 mM→0 mM). Two different purification batches were combined and used in this study.
Immunization of mice
Five adult female Balb/c mice, 8–10 weeks old, were subcutaneously injected with 200 µl of purified recombinant myocilin (approximately 25 µg per mouse) emulsified in a 1:1 ratio with complete Freund’s adjuvant (Becton Dickinson, Franklin Lakes, NJ). Twenty-eight days after immunization, mice were screened for immune responsiveness to purified recombinant myocilin by Western blot. Briefly, purified recombinant myocilin was separated on a one lane 12.5% SDS-polyacrylamide gel and transferred to polyvinylidene difluoride (PVDF) membrane in 49.6 mM Tris, 384 mM glycine, and 0.01% SDS. Membrane was blocked in 20 mM Tris (pH 7.5), 150 mM NaCl, 0.05% Tween, and 2% non-fat evaporated milk overnight. The blot was placed in a Mini Protean II multiscreen apparatus (Bio-Rad, Hercules, CA) and 550 µl of sera from immunized mice was added to independent wells. Rabbit polyclonal anti-myocilin antibodies (Fautsch et al., 2000) and V5 epitope tag-specific antibodies (Invitrogen, Carlsbad, CA) were added to separate wells as controls. After 2 hour incubation, blots were washed three times for 15 minutes each with 20 mM Tris (pH 7.5), 150 mM NaCl, and 0.05% Tween. Horseradish peroxidase–linked anti-mouse Ig (GE Healthcare, Piscataway, NJ) or anti-rabbit Ig (GE Healthcare) were used as secondary antibodies. Antibody:antigen complex was detected using ECL Western blotting signal detection reagent (GE Healthcare). Myocilin immune responsive mice were injected with an additional antigen boost (same as initial immunization) and housed for 72 hours.
Cell fusion and hybridoma preparation
Spleens were removed from myocilin immune responsive mice and single-cell suspensions were prepared. Red blood cells were removed by lysis with ammonium chloride potassium buffer. Lymphocytes and F/O myeloma cells (non-secreting myeloma derived from SP2/0 Balb/c myeloma cells) were mixed at a 2:1 ratio and centrifuged to form a cell pellet. The cell pellet was incubated at 37°C for 90 seconds in 50% polyethylene glycol 1540/RPMI. Following incubation, cells were pelleted by centrifugation at 3000 × g for 10 minutes, washed in 25 ml of PBS, recentrifuged, and cell pellet was resuspended in 100 ml of fresh Iscove’s Modified Dulbecco’s Medium containing 10% fetal bovine serum (Hyclone, Pittsburgh, PA). Aliquots of 100 µl were added to each well of ten 96-well microtiter plates (Corning, Lowell, MA). Twenty four hours later, 100 µl IMDM culture medium supplemented with 1M hypoxanthine (HT), 4 mM aminopterin and 160 mM thymidine (HAT) were added to each microtiter well. Media was replaced after 4 days with complete media (containing HAT and HT). Over the following 10 days, media was removed and replaced with fresh media with reduced or no HAT and HT added. Upon reaching 75% cell confluence, culture supernates were isolated and tested for presence of myocilin immune responsiveness by Western blot (see above for description).
Screening hybridoma supernates
Myocilin immune responsive cell cultures of interest were cloned in limiting dilution cultures at 1 cell per microtiter well (100 cells in 10 ml of media aliquoted 100 µl per well in a 96-well plate). Following cell expansion, supernates were screened by Western blot as described above with the following modification. A control protein lysate containing Posi-Tag protein (5 µg per gel; contains commonly used epitope tags including V5 and 6× His; Covance, Inc, Princeton, NJ) was added to purified recombinant myocilin protein prior to separation on 4–15% SDS-PAGE gradient gels. Inclusion of the Posi-Tag protein enabled simultaneous screening of positive antibodies only to myocilin and not to either the V5 or 6× histidine epitope tags that are present on the C-terminus of recombinant myocilin. Of the nearly 3000 supernates screened, six positive clones were identified. The positive clones were subcloned on 96-well microtiter plates at 0.3 cells per microtiter well. Supernates from subclones were also screened for verification of myocilin immune response. Positive subclones were expanded and cryopreserved. Balb/c spleen cells served as a feeder layer for fusions (5 × 104 per well), cloning and subcloning (3 × 106 per well).
Isotype of monoclonal antibodies
The six positive myocilin monoclonal antibodies were isotyped using IsoStrip, a mouse monoclonal antibody isotyping kit (Roche, Indianapolis, IN). Briefly, 150 µl of supernate from each of the six myocilin mouse monoclonal antibodies was added to the bottom of individual test-tubes containing latex beads coated with anti-mouse kappa or lambda antibodies. An IsoStrip containing bands of goat anti-mouse antibodies representing the various mouse antibody isotypes was lowered into the supernate/latex bead mix and incubated for 10 minutes at room temperature. Presence of a blue band on the IsoStrip at a specific subclass and light chain indicated antibody isotype.
Monoclonal antibody purification from hybridoma media
Chromatography was performed on a Biologic DuoFlow FPLC system (Bio-Rad). Hybridoma supernatants were filtered (0.2 µm filter apparatus, Nalgene, Rochester, NY) and loaded on 5 ml protein G and 5 ml protein A Sepharose FF HiTrap columns (GE Healthscience) connected in series, and equilibrated in 20 mM phosphate buffer, pH 7.0 (loading buffer). The flow-through media was saved and the columns were washed with loading buffer until absorbance at 280 nm reached baseline. IgG was eluted with 100 mM glycine, pH 2.7 into tubes containing 1M Tris pH 9 (10% of total fraction volume) for pH neutralization. The IgG fractions were buffer exchanged into phosphate buffered saline, pH 7.4 on a HiPrep desalting column 26/10 (GE Healthscience). IgG concentration was measured spectrophotometrically using the extinction coefficient for human IgG of 1.4 (0.1% at 280nm)
Stable Cell Line Development
Generation of TM5 cells overexpressing myocilin was previously described (Fautsch et al., 2006a). Human embryonic kidney 293T cells (293T) stably expressing full-length human myocilin was generated with feline immunodeficiency viral (FIV) vector TiPWF2 (Saenz et al., 2005) which express myocilin from an internal CMV promoter and puromycin resistance (pac) from an immediately downstream internal ribosomal entry site (IRES). 293T cells were transiently transfected with our tripartide vector system (Loewen et al., 2003) consisting of VSV-G expressing envelope plasmid MD-G, packaging plasmid CF1Δenv and FIV transfer vector plasmid pTiPWF2. Briefly, 3 × 106 293T cells were incubated overnight with a calcium phosphate transfection mix of 3 µg of MD-G, 3 µg of CF1Δenv, and 1 µg pTiPWF2. Following transfection, media was removed and cells were left exposed to the emerging vector supernatants for several days. Puromycin was added at a concentration of 2 µg/ml, and cells were passaged in the presence of puromycin for approximately 1 month resulting in selection of lentivirally transduced cells that stably expressed resistance.
Similarly, 293T cells stably expressing the premature human myocilin stop codon mutant Gln368Stop were generated by cotransfecting MD-G (3 µg), CF1Δenv (3 µg), and pTiZWF2 coding for Gln368Stop and zeocin resistance (1 µg). The myocilin stop mutant was generated by PCR by introducing a premature stop codon at amino acid position 368 and a downstream EcoR1 site using sense primer 5’-ATATCCTGAGGCGGGAGCG-3’ and antisense primer 5’-ATATGAATTCTATCCGTGGTAGCCAG-3’. The PCR product was subsequently purified, Bsu36I and EcoR1 digested, and cloned into the Bsu36I and EcoR1 sites of pTIPWF2. The puromycin resistance cassette was swapped for zeocin resistance. Zeocin resistant cells were selected for 1 month at a concentration of 300 µg/ml.
Antibody specificity analysis
All six purified myocilin monoclonal antibodies were analyzed by Western blots for the ability to recognize myocilin protein in various conditioned media and cell lysates.
Conditioned media from confluent TM5 cells or 293T cells overexpressing full-length normal myocilin was isolated, centrifuged at 1000 × g for 5 minutes to remove any cellular debris, and collected.
TM5 cells overexpressing normal myocilin or 293T cells overexpressing myocilin mutant Gln368Stop was rinsed in PBS, trypsinized, and centrifuged. TM5 cell pellet was lysed in cell lysis buffer [50 mM Tris pH 8.0, 0.5% sodium dodecyl sulfate, 0.5% Triton, 137 mM NaCl, 3 mM KCl, 8 mM Na2HPO4-7H2O, 1mM KH2PO4, protease inhibitors (Roche)] at 4°C for 30 minutes with occasional vortexing, followed by sonication.
Aqueous humor was isolated from human donor eyes within 12 hours of death. Two independent aqueous samples (60 year old male and 70 year old male) were mixed and separated on a 12.5% SDS-PAGE gel (denatured and reduced). Three additional aqueous humor samples (42, 60 and 70 year old males) were mixed and separated on a 12.5 % gel under native conditions (non-denatured and non-reduced). Proteins were transferred to PVDF membrane and Western blotting performed as described above. Aqueous humor protein concentration was within the reported normal concentration [12–50 mg/dL; (Cole, 1974; Tripathi et al., 1989)].
Trabecular meshworks were dissected from human donor eyes. Meshworks were incubated in cell lysis buffer, crushed with a pestle, and centrifuged at 10,000 × g for 5 minutes.
Conditioned media from TM5 or 293T cells overexpressing myocilin (300 µl), cell lysate from TM5 cells overexpressing myocilin (150 µg), 293T cell lysate from cells overexpressing myocilin mutant Gln368Stop (80 µg), trabecular meshwork lysate (150 µg), and aqueous humor (300 µl) were incubated in Lammeli sample buffer, boiled and layered over a one well 12.5% SDS-PAGE gel. Proteins were transferred to PVDF membrane, blocked in 2% non-fat milk, and placed on a Mini Protean II multiscreen apparatus (Bio-Rad). Purified monoclonal antibodies 1.1 (1:12000), 5.1 (1:80), 7.1 (1:4200), 11.1 (1:75), 12.1 (1:6), 20.1 (1:75), and either control monoclonal antibody V5 (1:12000; Invitrogen, Carlsbad, CA) or polyclonal myocilin antibody Myoc-N (1:10000) and Myoc-C (1:1000) were added to individual wells. Myoc-N was generated against N-terminal peptide spanning amino acids 108–131 as previously described (Fautsch et al., 2000). Myoc-C was generated against C-terminal peptide spanning amino acids 403–426. Washes, secondary antibody incubation, and antibody:antigen detection were performed as described above.
Immunohistochemistry
Monolayer TM5 cells
Sparsely growing TM5 cells overexpressing myocilin were fixed in 4% paraformaldehyde/0.1M PO4 for 10 minutes at room temperature followed by an additional incubation for two hours at 4°C with 4% paraformaldehyde/0.1M PO4 containing 0.1% Triton. Cells were incubated with 3% bovine serum albumin in PBS for 20 minutes and followed with incubation with each of the six monoclonal antibodies at room temperature for 2 hours. An additional reaction with no primary antibody served as a control. Cells were washed with PBS three times for five minutes each and probed with goat anti-mouse Ig Alexa Fluor 488 (Molecular Probes, Eugene, OR). Labeled cells were viewed and photographed with an Olympus DP71 digital camera attached to an Olympus BX60 fluorescence microscope (Olympus; Center Valley, PA).
Trabecular meshwork tissue and whole eye mount
Normal human donor eye tissue (81 year old male and an 84 year-old female) were fixed in 4% paraformaldehyde, dehydrated in a graded series of ethanol (75%, 85%, 95%, 100%) and embedded in paraffin. Sections 5 µm thick were mounted on Superfrost/Plus glass slides (Fisher, Pittsburgh, PA), baked at 60°C for 2 hours, de-paraffinized using xylene, and rehydrated using ethanol (100%, 95%, 80%, 70%, and PBS). Tissue was incubated at 95°C for 30 minutes (1 mM EDTA, pH 8.0), blocked with 3% BSA, and incubated with individual myocilin monoclonal antibodies (1:5). A control slide was performed with no primary antibody. Alexa Fluor 488 conjugated anti-mouse Ig was used as secondary antibody on all slides (Sigma, St. Louis, MO). Nuclei were stained with DAPI in Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). Tissue sections were viewed and photographed as described above.
Results
Production and screening of antibodies
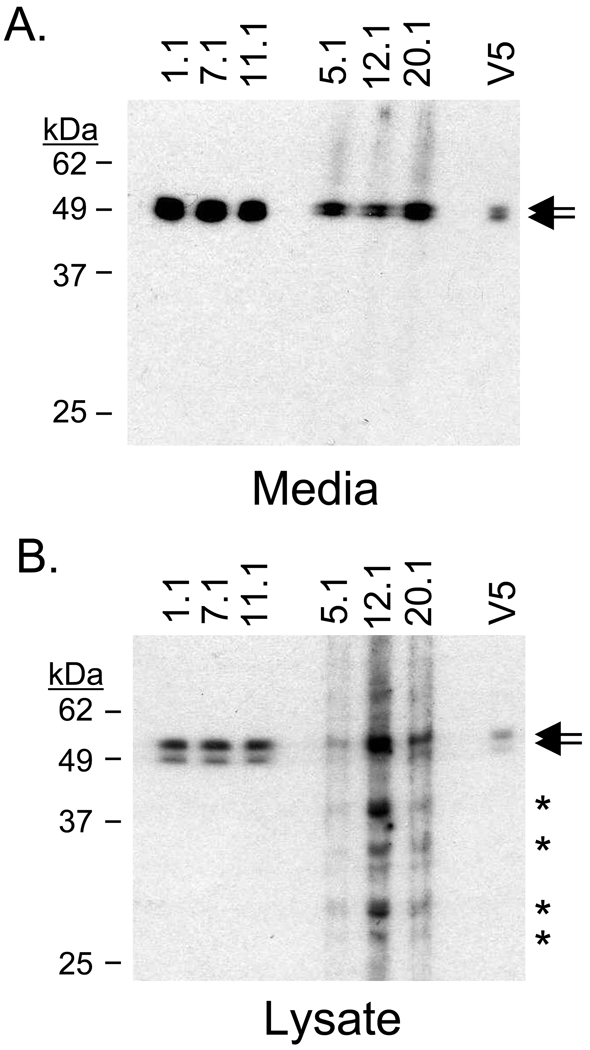
Full length myocilin fused to a C-terminal tag containing V5 and 6× histidine epitopes was purified from TM5 cells and used to immunize Balb/c mice. After screening over 3000 single-cell supernates, six hybridomas were selected, cloned, and purified based on the secreted antibody recognition of myocilin (53–57 kDa; doublet) found in conditioned media and cell lysate isolated from TM5 cells overexpressing myocilin (Figure 1). Several smaller myocilin-related bands were detected in TM5 lysate with antibodies 5.1, 12.1, and 20.1 (Figure 1B). Five of the monoclonal antibodies (1.1, 5.1, 7.1, 12.1, and 20.1) were isotyped as IgG1κ while the sixth, 11.1, was IgG2bκ.
Figure 1. Myocilin monoclonal antibodies recognize myocilin in trabecular meshwork cells.
(A) All six myocilin monoclonal antibodies identified myocilin in conditioned media isolated from TM5 cells overexpressing myocilin. The antibodies detected the myocilin doublet at 53–57 kDa. Mouse molyclonal V5 antibody verified myocilin size. (B) Myocilin monoclonal antibodies detected a myocilin doublet (53–57 kDa) in lysate from trabecular meshwork cells overexpressing myocilin. Arrows indicate myocilin protein. Asterisks indicate myocilin-related protein.
Characterization of antibody binding sites
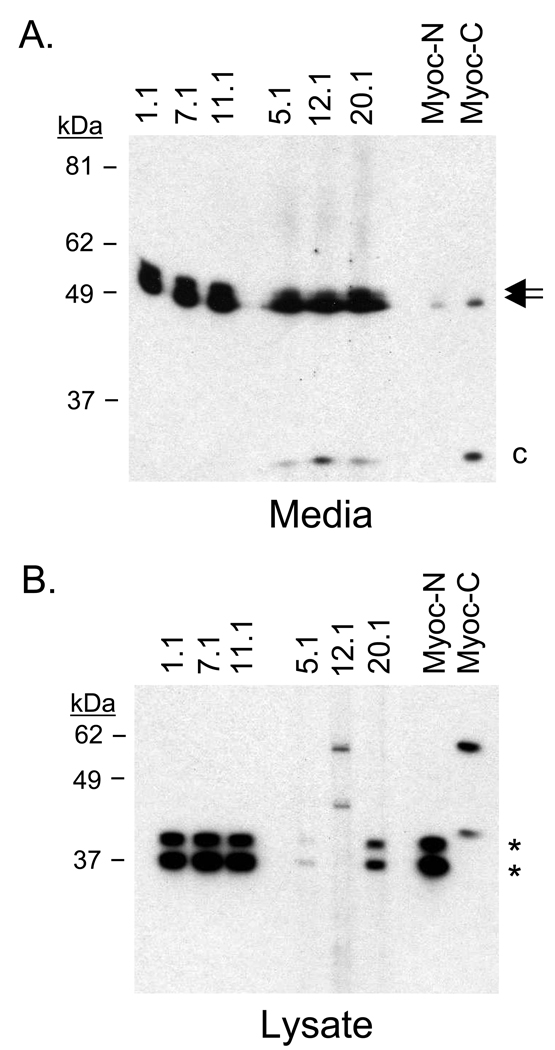
To identify the relative binding sites of the monoclonal antibodies along the myocilin protein, we probed media from 293T cells engineered to express myocilin. Expression of myocilin in 293T cells results in full-length myocilin and a cleaved C-terminal product representing a ~35 kDa fragment containing amino acids 215–504. Analysis of the six monoclonal antibodies identified three antibodies that recognize the N-terminal region of myocilin (1.1, 7.1, and 11.1) and three antibodies that recognize the C-terminal region (5.1, 12.1, and 20.1; Figure 2A). Polyclonal Myoc-N (developed against N-terminus peptide) and Myoc-C (developed against C-terminus peptide) confirmed myocilin size and C-terminal cleavage product.
Figure 2. Partial mapping of myocilin monoclonal antibody epitopes.
(A) Myocilin is cleaved at amino acid 214 in conditioned media isolated from 293T cells overexpressing myocilin. Antibodies 1.1, 7.1, and 11.1 recognize full-length myocilin (amino acids 33–504). Antibodies 5.1, 12.1, and 20.1 recognize full-length myocilin and a 35 kDa C-terminal fragment (amino acids 215–504). (B) Identification of myocilin mutant Gln368Stop by C-terminal antibodies 5.1 and 20.1 suggests the epitope for those two myocilin monoclonal antibodies is found between amino acids 215–368. The inability of myocilin monoclonal antibody 12.1 to recognize Gln368Stop suggests its epitope is found between amino acids 369 and 504. Arrows indicate full-length myocilin. Asterisks indicate Gln368Stop product. Lowercase C indicates C-terminal fragment. Polyclonal antibodies Myoc-N (N-terminus) and Myoc-C (C-terminus) served as controls.
To further characterize the monoclonal antibodies binding regions, cell lysates from 293T cells expressing myocilin mutant Gln368Stop (amino acids 1–368) were probed with the antibody panel (Figure 2B). Gln368Stop is a common myocilin mutation associated with juvenile and primary open-angle glaucoma, but like many of the myocilin mutants, is not secreted. Because of its truncated C-terminus, use of this protein enabled characterization of the myocilin monoclonal antibodies that recognize the C-terminus (5.1, 12.1, and 20.1). N-terminal antibodies 1.1, 7.1, 11.1, C-terminal antibodies 5.1 and 20.1, and polyclonal antibody Myoc-N all recognized the Gln368Stop mutant product (doublet at ~35 kDa). This suggests that C-terminal antibodies 5.1 and 20.1 recognize myocilin between amino acids 214 and 368. Myocilin antibody 12.1, which does not recognize Gln368Stop, has an epitope for myocilin between amino acids 369–504. This was confirmed by polyclonal antibody Myoc-C which does not recognize myocilin mutant Gln368Stop.
Identification of myocilin in human trabecular meshwork and aqueous humor
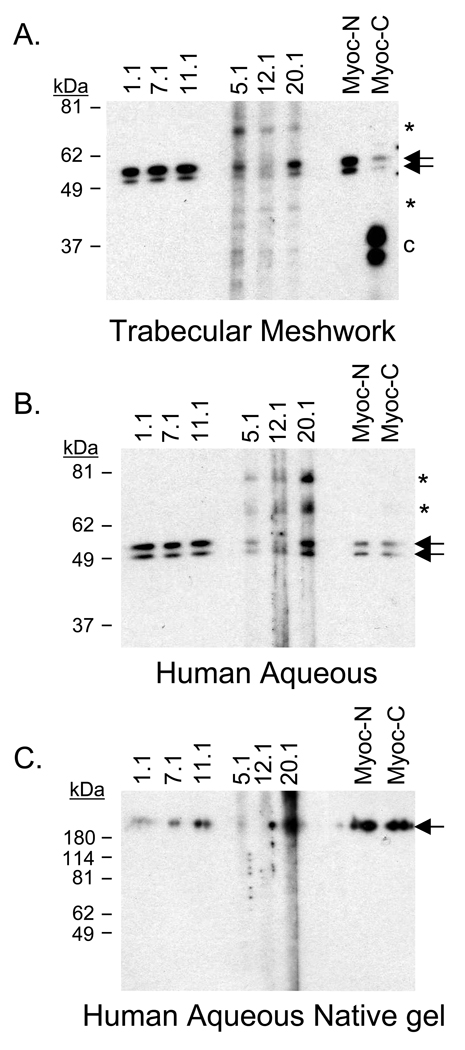
To verify detection of myocilin other than in cells overexpressing the protein, the six myocilin monoclonal antibodies were used to screen human aqueous humor and human trabecular meshwork. Western blot analysis with all six myocilin monoclonal antibodies detected the myocilin doublet (53–57 kDa) in human trabecular meshwork (Figure 3A). Several additional hybridizing bands were identified with monoclonal antibodies 5.1, 12.1, and 20.1 (~65 kDa, 45 kDa, and 35 kDa doublet). In aqueous humor, myocilin was also identified by all six myocilin monoclonal antibodies. N-terminal myocilin antibodies 1.1, 7.1, and 11.1 specifically identified myocilin in denatured/reduced and native gels (Figure 3B and 3C). C-terminal monoclonal antibodies 5.1, 12.1, and 20.1 were less specific, identifying the myocilin doublet as well as a 65 kDa and 80 kDa band (Figure 3B). The C-terminal antibodies failed to specifically identify myocilin under native conditions (Figure 3C).
Figure 3. Myocilin detected in human trabecular meshwork tissue and aqueous humor.
Myocilin monoclonal antibodies identified myocilin in (A) human trabecular meshwork tissue, (B) denatured and reduced human aqueous humor, and (C) native human aqueous humor (nondenatured and nonreduced). All antibodies detected myocilin doublet at 53–57 kDa (arrows) in denatured and reduced gels while the N-terminal antibodies showed greater specificity for myocilin in native gels, identifying myocilin above 180 kDa marker (arrow). Asterisks indicate additional myocilin related bands recognized by C-terminal antibodies 5.1, 12.1, and 20.1. Lowercase C indicates C-terminal fragment. [Note: Protein marker in (C) is denatured and reduced therefore reflects accurate size].
Immunohistochemical analysis of myocilin
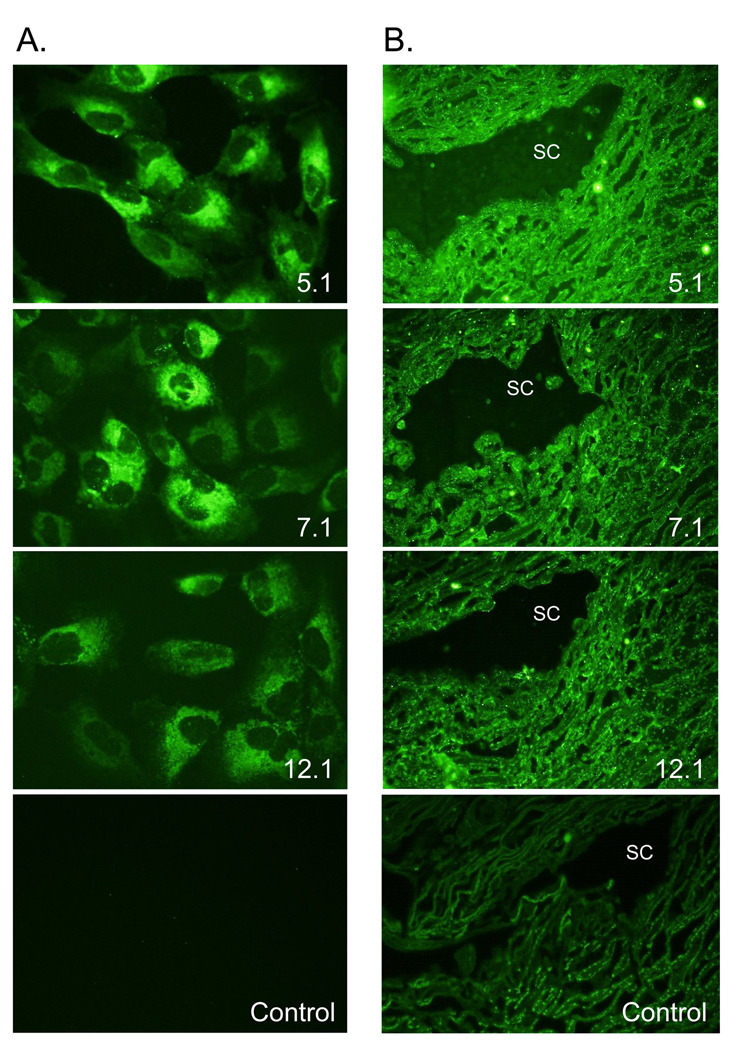
The myocilin monoclonal antibody panel was used to detect myocilin in TM5 cells overexpressing myocilin. All the myocilin monoclonal antibodies detected myocilin perinuclear suggestive of an endoplasmic reticulum/Golgi apparatus localization (Figure 4A; representative images of myocilin monoclonal antibodies 5.1, 7.1, and 12.1). TM5 cells overexpressing myocilin incubated with only secondary antibody showed no background staining (control).
Figure 4. Immunohistochemistry of myocilin monoclonal antibodies.
(A) Myocilin was localized perinuclear with an endoplasmic reticulum/Golgi Apparatus distribution. Representative images from monoclonal antibodies 5.1, 7.1, and 12.1 are shown. All six myocilin monoclonal antibodies showed a similar distribution. 400× magnification. (B) Representative images of myocilin monoclonal antibodies 5.1, 7.1, and 12.1 showing myocilin associated with trabecular cells throughout the meshwork and with Schlemm’s canal cells in paraffin-embedded tissue. 600× magnification.
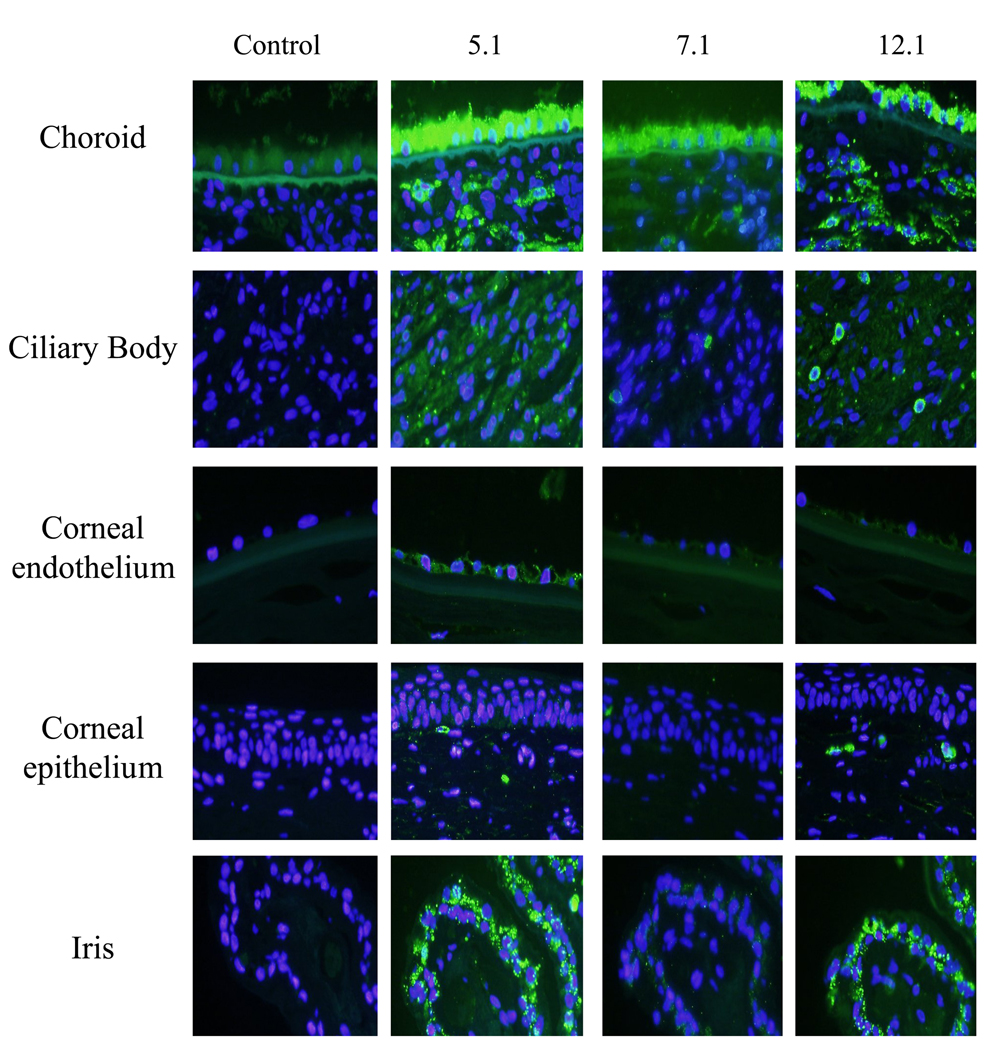
To verify the use of the myocilin monoclonal antibodies for immunohistochemical studies, all the monoclonal antibodies were used on paraffin embedded human trabecular meshwork tissue and whole eye sections. In the trabecular meshwork, myocilin was found associated with trabecular cells lining the elastin beams within the uveal, corneoscleral and juxtacanalicular region of the trabecular meshwork (Figure 4B). Schlemm’s canal cells were also positive for myocilin (Figure 4B). In whole eye sections, antibodies 5.1, 7.1 and 12.1 identified myocilin in retinal pigment epithelium, choroid, and sclera of the posterior segment of the eye; while sparse and granular staining was observed in the ciliary body (Figure 5). C-terminal antibodies 5.1 and 12.1 also showed positive staining for myocilin in the iris and in some stromal cells underneath the corneal epithelium. C-terminal antibody 5.1 was the only antibody to show positive staining in corneal endothelial cells. Incubation with secondary antibody alone showed minimal autofluorescence in retinal pigment epithelium (control).
Figure 5. Recognition of myocilin in various tissues of the human eye.
Images of choroid, ciliary body, corneal endothelium, corneal epithelium and iris show identification of myocilin with selective myocilin monoclonal antibodies. From left to right: Control (secondary antibody alone), 5.1 (middle left column), 7.1(middle right column) and 12.1 (right column). Each column represents cross-sections of a single eye. Images were photographed at 40× magnification.
DISCUSSION
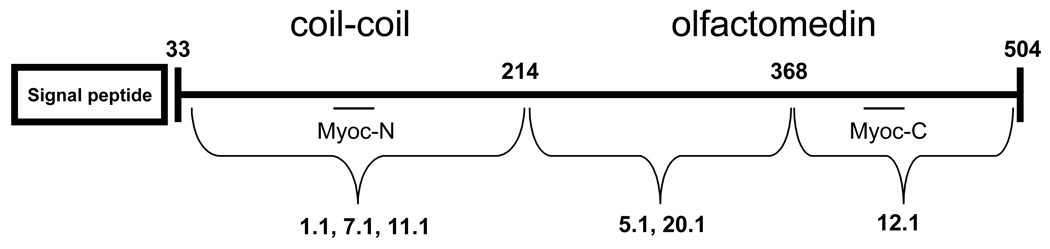
We have described the generation and cloning of six monoclonal hybridomas that secrete antibodies against the glaucoma-associated protein myocilin. Using full-length human recombinant protein as the antigen, three of the antibodies recognize the N-terminus (amino acids 33–214), two others identify the middle portion of myocilin (amino acids 215–368), and one antibody recognizes the C-terminal region between amino acids 369–504 (Figure 6). These antibodies will be useful for clarification of myocilin localization, verification of myocilin binding partners, and use in future experimentation in determining the biological function of myocilin.
Figure 6. Epitope locations for the myocilin monoclonal antibodies.
Myocilin monoclonal antibodies 1.1, 7.1, and 11.1 recognize myocilin between amino acids 33–214. Myocilin monoclonal antibodies 5.1 and 20.1 recognize myocilin between amino acids 215–368. Antibody 12.1 recognizes myocilin between amino acids 369–504. Amino acid 214 represents cleaved myocilin in 293T cells. Amino acid 368 represents the termination site of myocilin mutant Gln368Stop. Myoc-N and Myoc-C are polyclonal antibodies.
The N-terminal myocilin monoclonal antibodies appear to be specific for the 53–57 kDa doublet form of myocilin since no other hybridizing bands were identified in conditioned media and cell lysate from cells overexpressing myocilin, in human trabecular meshwork, and in human aqueous humor. The C-terminal antibodies also identify the 53–57 kDa myocilin doublet but also several additional bands in human trabecular meshwork and aqueous humor. Large myocilin related complexes have been reported even under denatured/reduced SDS-PAGE conditions (Nguyen et al., 1998; Russell et al., 2001). The ~65 kDa band seen in human trabecular meshwork and aqueous humor by our monoclonal antibodies may be the same protein product reported as a 66 kDa form of myocilin (Nguyen et al., 1998). An alternative explanation may be that the C-terminal monoclonal antibodies (5.1, 12.1, and 20.1) are identifying sequence related proteins. Myocilin contains significant C-terminal homology to olfactomedins. The olfactomedin family consists of over 100 proteins from various species (Zeng et al., 2005), with some of the members having molecular weights similar to the protein sizes identified by the C-terminal myocilin monoclonal antibodies in human trabecular meshwork and aqueous humor. Considering that the C-terminal antibodies generated against myocilin map near or within the olfactomedin homology domain, the additional bands seen with the C-terminal myocilin monoclonal antibodies may be identifying olfactomedin related proteins. Taking into account that the N-terminal binding myocilin monoclonal antibodies are specific for the 53–57 kDa myocilin doublet, this scenario seems plausible. Further investigation will be necessary to determine if the additional bands identified by the C-terminal monoclonal antibodies are myocilin related or are related proteins with sequence similarity to myocilin.
Several smaller bands are also detected by the C-terminal myocilin monoclonal antibodies in lysates from monolayer trabecular meshwork cells and in trabecular meshwork tissue lysate. Partial cleavage of normal myocilin has been seen in 293T cells (Aroca-Aguilar et al., 2005; Goldwich et al., 2003). We have observed partial cleavage of normal myocilin in cells overexpressing myocilin mainly in 293T cells but to a lesser extent in trabecular meshwork cells (Fautsch et al., 2006a). Cleavage of myocilin by the endopeptidase calpain II has been reported in aqueous humor (Sanchez-Sanchez et al., 2007). It is worth noting that none of our myocilin monoclonal antibodies detected smaller myocilin related products (<53 kDa) in aqueous humor. However, we have only analyzed a few aqueous humor samples collected from human donor eyes. It will be important to test additional aqueous humor samples collected from both human donor eyes and surgical samples to determine if myocilin cleaved products can be detected with our monoclonal antibodies.
The panel of myocilin monoclonal antibodies also recognized myocilin by immunohistochemistry. Localization of myocilin confirmed an endoplasmic reticulum/Golgi apparatus cell distribution in human trabecular meshwork cells overexpressing the protein. The monoclonal antibodies also recognized myocilin in human trabecular meshwork, Schlemm’s canal cells, retinal pigment epithelium, choroid, sclera, ciliary body, iris, corneal stroma cells, and corneal endothelium. By light microscopy, it was not possible to determine if myocilin was found intracellular or extracellular. Since myocilin has also been localized to both intracellular and extracellular regions, it will be important to see if any of the myocilin monoclonal antibodies will detect myocilin using immunolabeling and transmission electron microscopy. Intracellular and extracellular localization of myocilin has been antibody dependent due mainly to the use of polyclonal antibodies. The use of monoclonal antibodies that recognize different regions of the molecule may serve to verify myocilin’s normal cellular and extracellular distribution.
In summary, we have developed and characterized a panel of monoclonal antibodies against the glaucoma-associated protein myocilin. These antibodies can detect myocilin in human trabecular meshwork monolayer cells, trabecular meshwork tissue, and in aqueous humor. They may serve as useful tools in delineating the biological role and the molecular function of myocilin in both normal and glaucomatous eyes.
ACKNOWLEDGEMENTS
Work was supported in part by National Institutes of Health research grants EY07065, EY15736 and EY14411; Research to Prevent Blindness, Inc., New York, NY; and the Mayo Foundation, Rochester, MN. The authors would like to thank Penny Kirgis for formatting and submitting the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aroca-Aguilar JD, Sanchez-Sanchez F, Ghosh S, Coca-Prados M, Escribano J. Myocilin mutations causing glaucoma inhibit the intracellular endoproteolytic cleavage of myocilin between amino acids Arg226 and Ile227. J. Biol. Chem. 2005;280:21043–21051. doi: 10.1074/jbc.M501340200. [DOI] [PubMed] [Google Scholar]
- Caballero M, Borras T. Inefficient processing of an olfactomedin-deficient myocilin mutant: Potential physiological relevance to glaucoma. Biochem Biophys Res Comm. 2001;282:662–670. doi: 10.1006/bbrc.2001.4624. [DOI] [PubMed] [Google Scholar]
- Clark AF, Kawase K, English-Wright S, Lane D, Steely HT, Yamamoto T, Kitazawa Y, Kwon YH, Fingert JH, Swiderski RE, Mullins RF, Hageman GS, Alward WL, Sheffield VC, Stone EM. Expression of the glaucoma gene myocilin (MYOC) in the human optic nerve head. FASEB J. 2001a;15:1251–1253. doi: 10.1096/fj.00-0663fje. [DOI] [PubMed] [Google Scholar]
- Clark AF, Steely HT, Dickerson JE, Jr, English-Wright S, Stropki K, McCartney MD, Jacobson N, Shepard AR, Clark JI, Matsushima H, Peskind ER, Leverenz JB, Wilkinson CW, Swiderski RE, Fingert JH, Sheffield VC, Stone EM. Glucocorticoid induction of the glaucoma gene MYOC in human and monkey trabecular meshwork cells and tissues. Invest. Ophthalmol. Vis. Sci. 2001b;42:1769–1780. [PubMed] [Google Scholar]
- Cole DF. Comparative aspects of the intraocular fluids. In: Davson H, Graham LT Jr, editors. The Eye. New York: Academic Press; 1974. pp. 71–121. [Google Scholar]
- Fautsch MP, Bahler CK, Jewison DJ, Johnson DH. Recombinant TIGR/MYOC increases outflow resistance in the human anterior segment. Invest. Ophthalmol. Vis. Sci. 2000;41:4163–4168. [PubMed] [Google Scholar]
- Fautsch MP, Bahler CK, Vrabel AM, Howell KG, Loewen N, Teo WL, Poeschla EM, DH J. Perfusion of His-tagged eukaryotic myocilin increases outflow resistance in human anterior segments in the presence of aqueous humor. Invest. Ophthalmol. Vis. Sci. 2006a;47:213–221. doi: 10.1167/iovs.05-0334. [DOI] [PubMed] [Google Scholar]
- Fautsch MP, Johnson DH. Characterization of myocilin-myocilin interactions. Invest. Ophthalmol. Vis. Sci. 2001;42:2324–2331. [PubMed] [Google Scholar]
- Fautsch MP, Vrabel AM, Johnson DH. The identification of myocilin-associated proteins in the human trabecular meshwork. Exp Eye Res. 2006b;82:1046–1052. doi: 10.1016/j.exer.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Fautsch MP, Vrabel AM, Peterson SL, Johnson DH. In vitro and in vivo characterization of disulfide bond use in myocilin complex formation. Mol Vis. 2004;10:417–425. [PubMed] [Google Scholar]
- Filla MS, Liu X, Nguyen TD, Polansky JR, Brandt CR, Kaufman PL, Peters DM. In vitro localization of TIGR/MYOC in trabecular meshwork extracellular matrix and binding to fibronectin. Invest. Ophthalmol. Vis. Sci. 2002;43:151–161. [PubMed] [Google Scholar]
- Fingert JH, Heon E, L JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum. Mol. Genet. 1999;8:899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- Fingert JH, L Y, Swiderski RE, Nystuen AM, Arbour NC, Alward WL, Sheffield VC, Stone EM. Characterization and comparison of the human and mouse GLC1A glaucoma genes. Genome Res. 1998;8:377–384. doi: 10.1101/gr.8.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil S, Rodrique M-A, Moisan S, Nguyen TD, Polansky JR, Morissette J, Raymond V. Intracellular sequestration of hetero-oligomers formed by wild-type and glaucoma-causing myocilin mutants. Invest. Ophthalmol. Vis. Sci. 2004;45:3560–3567. doi: 10.1167/iovs.04-0300. [DOI] [PubMed] [Google Scholar]
- Goldwich A, Baulmann DC, Ohlmann A, Flugel-Koch C, Schocklmann H, Tamm ER. Myocilin is expressed in the glomerulus of the kidney and induced in mesangioproliferative glomerulonephritis. Kidney Int. 2005;67:140–151. doi: 10.1111/j.1523-1755.2005.00064.x. [DOI] [PubMed] [Google Scholar]
- Goldwich A, Ethier CR, Chan DW-H, Tamm ER. Perfusion with the olfactomedin domain of myocilin does not affect outflow facility. Invest. Ophthalmol. Vis. Sci. 2003;44:1953–1961. doi: 10.1167/iovs.02-0863. [DOI] [PubMed] [Google Scholar]
- Huang W, Jaroszewski J, Ortego J, Escribano J, Coca-Prados M. Expression of the TIGR gene in the iris, ciliary body, and trabecular meshwork of the human eye. Ophthalmic Genet. 2000;21:155–169. [PubMed] [Google Scholar]
- Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Sheffield VC. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum. Mol. Genet. 2001;10:117–125. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- Joe MK, Sohn S, Choi YR, Park H, Kee C. Identification of flotillin-1 as a protein interacting with myocilin: Implications for the pathogenesis of primary open-angle glaucoma. Biochemical & Biophsyical Research Communications. 2005;336:1201–1206. doi: 10.1016/j.bbrc.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Karali A, Russell P, Stefani FH, Tamm ER. Localization of myocilin/trabecular meshwork inducible glucocorticoid response protein in the human eye. Invest. Ophthalmol. Vis. Sci. 2000;41:729–740. [PubMed] [Google Scholar]
- Kubota R, Noda S, Wang Y, Minoshima S, Asakawa S, Kudoh J, Mashima Y, Oguchi Y, N S. A novel myosin-like protein (myocilin) expressed in the connecting cilium of the photoreceptor: Molecular cloning, tissue expression, and chromosomal mapping. Genomics. 1997;41:360–369. doi: 10.1006/geno.1997.4682. [DOI] [PubMed] [Google Scholar]
- Li Y, Aroca-Aguilar JD, Ghosh S, Sanchez-Sanchez F, Escribano J, Coca-Prados M. Interaction of myocilin with the C-terminal region of hevin. Biochemical & Biophsyical Research Communications. 2006;339:797–804. doi: 10.1016/j.bbrc.2005.11.082. [DOI] [PubMed] [Google Scholar]
- Loewen N, Barraza R, Whitwam T, Saenz DT, Kemler I, Poeschla EM. FIV vectors. Methods Mol. Biol. 2003;229:251–271. doi: 10.1385/1-59259-393-3:251. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR. Gene structure and properties of an olfactomedin-related glycoprotein, TIGR, cloned from glucocorticoid-induced trabecular meshwork cells. J. Biol. Chem. 1998;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Huang W, Bloom E, Polansky JR. Glucocorticoid effects on HTM cells: Molecular biology approaches. In: Lutjen-Drecoll E, Rohen JW, editors. Basic aspects of glaucoma research III. Stuttgart: Schattaeur Press; 1993. pp. 331–343. [Google Scholar]
- Noda S, Mashima Y, Obazawa M, Kubota R, Oguchi Y, Kudoh J, Minoshima S, Shimizu N. Myocilin expression in the astrocytes of the optic nerve head. Biochemical & Biophsyical Research Communications. 2000;276:1129–1135. doi: 10.1006/bbrc.2000.3591. [DOI] [PubMed] [Google Scholar]
- O’Brien ET, Ren X-O, Wang Y. Localization of myocilin to the Golgi apparatus in Schlemm’s canal cells. Invest. Ophthalmol. Vis. Sci. 2000;41:3842–3849. [PubMed] [Google Scholar]
- Ohlmann A, Goldwich A, Flugel-Koch C, Fuchs AV, Schwager K, Tamm ER. Secreted glycoprotein myocilin is a component of the myelin sheath in peripheral nerves. Glia. 2003;43:128–140. doi: 10.1002/glia.10233. [DOI] [PubMed] [Google Scholar]
- Ortego J, Escribano J, Coca-Prados M. Cloning and characterization of subtracted cDNAs from a human ciliary body library encoding TIGR, a protein involved in juvenile open angle glaucoma with homology to myosin and olfactomedin. FEBS Lett. 1997;413:349–353. doi: 10.1016/s0014-5793(97)00934-4. [DOI] [PubMed] [Google Scholar]
- Rao PV, Allingham RR, Epstein DL. TIGR/myocilin in human aqueous humor. Exp Eye Res. 2000;71:637–641. doi: 10.1006/exer.2000.0920. [DOI] [PubMed] [Google Scholar]
- Russell P, Tamm ER, Grehn FJ, Picht G, Johnson M. The presence and properties of myocilin in the aqueous humor. Invest. Ophthalmol. Vis. Sci. 2001;42:983–986. [PubMed] [Google Scholar]
- Saenz DT, Barraza R, Loewen N, Teo W, Poeschla ECSH. NY., Production and Use of Feline Immunodeficiency Virus (FIV)-based lentiviral vectors. In: Rossi J, Friedman T, editors. Gene Transfer: A Laboratory Manual. Cold Spring, Harbor, NY: Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- Sakai H, Shen X, Koga T, Park BC, Noskina Y, Tibudan M, Yue BY. Mitchondrial association of myocilin, product of a glaucoma gene, in human trabecular meshwork cells. J. Cell. Physiol. 2007;213:775–784. doi: 10.1002/jcp.21147. [DOI] [PubMed] [Google Scholar]
- Sanchez-Sanchez F, Martinez-Redondo F, Aroca-Aguilar JD, Coca-Prados M, Escribano J. Characterization of the intracellular proteolytic cleavage of myocilin and identification of calpain II as a myocilin-processing protease. J. Biol. Chem. 2007;282:27810–27824. doi: 10.1074/jbc.M609608200. [DOI] [PubMed] [Google Scholar]
- Sohn S, Hur W, Joe MK, J.-H., K., Lee Z-W, Ha K-S, Kee C. Expression of wild-type and truncated myocilins in trabecular meshwork cells: Their subcellular localizations and cytotoxicities. Invest. Ophthalmol. Vis. Sci. 2002;43:3680–3685. [PubMed] [Google Scholar]
- Stamer WD, Perkumas KM, Hoffman EA, Roberts BC, Epstein DL, McKay BS. Coiled-coil targeting of myocilin to intracellular membranes. Exp Eye Res. 2006;83:1386–1395. doi: 10.1016/j.exer.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Surgucheva I, Park BC, Yue BY, Tomarev S, Surguchov A. Interaction of myocilin with gamma-synuclein affects its secretion and aggregation. Cell. Mol. Neurobiol. 2005;25:1009–1033. doi: 10.1007/s10571-005-8471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiderski RE, Ying I, Cassell MD, Alward WL, Stone EM, Sheffield VC. Expression pattern and in situ localization of the mouse homologue of the human MYOC (GLCIA) gene in adult brain. Molecular Brain Research. 1999;68:64–72. doi: 10.1016/s0169-328x(99)00085-6. [DOI] [PubMed] [Google Scholar]
- Torrado M, Trivedi R, Zinovieva R, Karavanova I, Tomarev SI. Optimedin: A novel olfactomedin-related protein that interacts with myocilin. Hum. Mol. Genet. 2002;11:1291–1301. doi: 10.1093/hmg/11.11.1291. [DOI] [PubMed] [Google Scholar]
- Tripathi RC, Millard CB, Tripathi BJ. Protein composition of human aqueous humor: SDS-PAGE analysis of surgical and post-mortem samples. Exp Eye Res. 1989;48:117–130. doi: 10.1016/0014-4835(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Ueda J, Wentz-Hunter K, Cheng E, Fukuchi T, Abe H, Yue Y. Ultrastructural localization of myocilin in human trabecular meshwork cells and tissues. J. Histochem. Cytochem. 2000;48:1321–1329. doi: 10.1177/002215540004801003. [DOI] [PubMed] [Google Scholar]
- Ueda J, Wentz-Hunter K, Yue BY. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Invest. Ophthalmol. Vis. Sci. 2002;43:1068–1076. [PubMed] [Google Scholar]
- Wentz-Hunter K, Ueda J, Shimizu N, Yue BY. Myocilin is associated with mitochondria in human trabecular meshwork cells. J. Cell. Physiol. 2002a;190:46–53. doi: 10.1002/jcp.10032. [DOI] [PubMed] [Google Scholar]
- Wentz-Hunter K, Ueda J, Yue BY. Protein interactions with myocilin. Invest. Ophthalmol. Vis. Sci. 2002b;43:176–182. [PubMed] [Google Scholar]
- Zeng L-C, Han Z-G, Ma W-J. Elucidation of subfamily segregation and intramolecular coevolution of the olfactomedin-like proteins by comprehensive phylogenetic analysis and gene expression pattern assessment. FEBS Lett. 2005;579:5443–5453. doi: 10.1016/j.febslet.2005.08.064. [DOI] [PubMed] [Google Scholar]