Abstract
Sensation seeking is a personality trait that is linked to use and abuse of drugs. Laboratory studies have established that high sensation seekers, as measured by different instruments, are more likely to report abuse liability-related subjective effects from drugs such as nicotine, alcohol, and d-amphetamine than low sensation seekers. One class of drugs that has not been studied to date in this fashion is opioids. Accordingly, a retrospective analysis encompassing five studies that examined oxycodone effects, including its abuse liability-related effects, was conducted in subjects categorized as high or low sensation seekers. In addition, because there appear to be sex differences in how males and females respond to opioids, this factor was taken into account in the analysis. Seventy one subjects who scored on the lower end (15 and 19 low sensation seeking males and females, respectively) or the higher end (23 and 14 high sensation seeking males and females) of the Disinhibition subscale of the Sensation Seeking Scale-Form V were studied for their responses to 0, 10, and 20 mg of oral oxycodone. Ratings of “pleasant bodily sensations” were significantly higher after oxycodone administration than placebo only in male and female high sensation seekers. Ratings of “take again,” “drug liking,” “carefree,” and “elated (very happy)” also tended to differentiate high from low sensation seekers although Group × Dose interactions were only marginally significant with the latter three ratings. Male and female low sensation seekers and female high sensation seekers reported dysphoric effects (e.g., ratings of nauseated) particularly after administration of the 20-mg oxycodone dose. The results of this analysis provide suggestive evidence that high sensation seekers are more likely to experience greater positive subjective effects from oxycodone than low sensation seekers, but likelihood of experiencing negative effects is more complex (involving both sensation seeking status and sex).
Keywords: opioid, oxycodone, prescription opioid, sensation seeking, personality, subjective, abuse liability, human
1. Introduction
There is a large body of literature that supports sensation seeking as a risk factor for drug use and abuse. Sensation seeking (SS) has been defined as a biologically based personality trait that involves the tendency to seek varied, novel, complex, and intense sensations and experiences (Zuckerman, 1994). Several questionnaires have been used to assess SS, most notably the Sensation Seeking Scale (SSS-Form V) (Zuckerman, 1979) with four subscales (Thrill and Adventure Seeking, Experience Seeking, Disinhibition, and Boredom Susceptibility), the Tridimensional Personality Questionnaire (one of its dimensions is Novelty Seeking and is considered to be a measure of SS) (Cloninger, 1987), and more recently the Zuckerman-Kuhlman Personality Questionnaire (ZKPQ) (one of its scales used as a metric of SS is the Impulsiveness Sensation-Seeking scale) (Zuckerman et al., 1993). A number of studies examining whether a link exists between drug abuse and SS have been correlational in nature; subjects complete a SS instrument and also report their drug use, and consistently those subjects who are high sensation seekers (HS) have a greater likelihood of using drugs or using drugs more heavily than subjects who are low sensations seekers (LS). This finding applies to children (e.g., Stephenson et al., 2003), adolescents (e.g., Andrucci et al., 1989; Teichman et al., 1989; Martin et al., 2002), college students (e.g., Jaffe and Archer, 1987; Alterman et al., 1990; Erblich and Earleywine, 2003) and adults (e.g., West, 2002; Franques et al., 2003). Males typically have higher SS scores than females (Zuckerman et al., 1978), but the link between SS and drug use is found in both sexes, in general. The link between high SS and drug abuse has been found with many drugs including nicotine, alcohol, opioids, hallucinogens, and stimulants (Pedersen et al., 1989; Bobes et al., 2002). Several studies have established that HSs are more sensitive to the subjective, reinforcing and/or physiological effects of drugs: the drugs studied included alcohol (de Wit et al., 1987; Fillmore et al., 2009), amphetamines (Hutchison et al., 1999; Kelly et al., 2006, 2009; Stoops et al., 2007), methylphenidate (Chait, 1994), benzodiazepines (Kelly et al., 2009), and nicotine (Perkins et al., 2000).
Preclinical studies also support the link between SS and drug effects. Rats who displayed a greater preference for a novel environment were more sensitive to the discriminative stimulus effects of amphetamine as well as to its suppressant effects on response rate (Bevins et al., 1997). Piazza and his colleagues have shown that animals who are designated as high responders (who show a great deal of activity in a novel environment) are more sensitive to the reinforcing and/or psychomotor effects of amphetamines and opioids (Piazza et al., 1989, 1990; Deroche et al., 1993). Both high responding and novelty preference are considered models for human SS. The biological link underlying the trait of SS has not been definitively determined, but a number of preclinical studies have implicated the role of dopamine in the mesolimbic region of the brain, an area that includes the nucleus accumbens and is thought to be involved in mediating the reinforcing effects of a number of abused drugs. In these studies, animals that differ in their novelty-seeking behavior also show differences in dopamine activity in this brain region (e.g., Hooks and Kalivas, 1994; Bardo et al., 1996; Saigusa et al., 1999).
A number of studies have been conducted in my laboratory with the prescription opioid, oxycodone, and at the end of those studies, subjects completed several questionnaires including the SSS-Form V. The studies have had different purposes but the methodologies as well as oxycodone doses studied have been similar, so data from the separate studies were pooled together. The present retrospective analysis examined whether subjects who scored higher on a subscale of the SSS-Form V, Disinhibition, were more sensitive to the abuse liability-related subjective effects of oxycodone (e.g., euphoria, drug liking) than those who scored lower. Other subjective effects, and psychomotor/cognitive and physiological effects were also included in the analysis.
The Disinhibition subscale of the SSS-V is comprised of 10 items describing a social-hedonistic orientation, with the pursuit of sensation through parties, drinking, and sex (e.g., “A person should have considerable sexual experience before marriage,” “Keeping the drinks full is the key to a good party”). This subscale was chosen to classify subjects as low or high SSs in the retrospective analysis because 1) several studies in the drug abuse literature have determined that this subscale is predictive of substance use and abuse (Clapper et al., 1994; Beck et al., 1995; Liraud and Verdoux, 2000; Franques et al., 2003; Hittner and Swickert, 2006), and 2) laboratory studies in healthy volunteers have also demonstrated a linkage of scores on this subscale to positive effects of drugs (Hutchison et al., 1999; Perkins et al., 2000). Hittner and Swickert (2006) conducted a meta-analysis of 61 studies to investigate the relationship between SS and alcohol consumption and found that of the four subscales that comprise the SSS, the Disinhibition subscale was most strongly correlated to alcohol use. In one of the laboratory studies, subjects who scored high on the Disinhibition subscale experienced more abuse liability-related subjective effects of amphetamine than subjects who scored low (Hutchison et al., 1999), and in the other study, scores on the this subscale were significantly correlated with several subjective effects of nasal spray nicotine in non-smokers that have been linked to greater nicotine self-administration in smokers (e.g., increase in VAS ratings of “pleasant”) (Perkins et al., 2000).
The retrospective analysis was done for two reasons. First, although the link between SS and psychopharmacological effects of different classes of drugs have been established, the extent to which SS is associated with effects of opioids, in this case the prescription opioid oxycodone, has not been determined. Second, in the last decade nonmedical use and abuse of prescription opioids have been on the rise, and is of concern to law enforcement officials, medical, regulatory, pain relief advocacy, and drug abuse organizations, as well as the general public (Zacny et al., 2003; Birnbaum et al., 2006; Wright et al., 2006). Oral oxycodone is particularly relevant to study at this time because prevalence of non-medical use of this prescription opioid in several formulations is high (Cicero et al., 2005; Dasgupta et al., 2006; Gilson et al., 2004; Substance Abuse and Mental Health Services Administration, 2008), and is part of a major public health problem in the United States. Given the evidence for a link between novelty seeking and drug consumption in nonhumans, the link between SS and propensity to use drugs of abuse in humans, and the effects of SS on abuse liability-related effects of drugs from several CNS drug classes, it was hypothesized that HSs would show greater abuse liability-related effects from a prescription opioid (oxycodone) than would LSs. Sex of the subject was also included into the analysis. This was because in one of the studies that comprise this retrospective analysis, there were significant sex differences with females reporting more dysphoric effects from oxycodone (e.g., nausea) than males (Zacny and Drum, 2009).
2. Methods
2.1. Studies from which the subject pool was gathered
The 71 subjects came from five studies; Table 1 lists the studies and sample sizes, the drugs and doses tested in a study, and the number of subjects from each study that were included in this retrospective analysis. The Zacny and Gutierrez (2009) study was included in the retrospective analysis even though oxycodone was combined with acetaminophen. Acetaminophen by itself did not have any effects, and this lack of psychoactive effects has been documented in several other studies (Bradley and Nicholson, 1987; Eade and Lasagna, 1967; Pickworth et al., 1991; Zacny et al., 2005).
Table 1.
Oral opioid studies that comprised the retrospective analysis.
| Study (n: males [m]/females [f]) | n* (m/f)a | Drugs and doses |
|---|---|---|
| Zacny and Gutierrez, 2003 (9 m/9 f) | 8 m/8 f | 0, 10, 20, 30 mg OXY; 40 mg MOR; 2 mg LZP |
| Zacny and Lichtor, 2008 (10 m/10 f) | 5 m/6 f | 0, 10, 20 mg OXY; 30, 60 mg MOR |
| Zacny and Gutierrez, 2009 (10 m/10 f) | 9 m/7 f | 0, 10 mg OXY/487 mg ACET, 20 mg OXY/975 mg ACET; 15 mg HYD/487 mg ACET; 30 mg HYD/975 mg ACET |
| Zacny and de Wit, 2009 (6 m/6 f) | 6 m/4 f | 0, 5, 10, 20 mg OXY |
| Zacny and Drum, 2009b (16 m/13 f) | 10 m/8 f | 0, 10, 20 mg OXY |
Abbreviations: OXY=oxycodone; MOR=morphine; LZP=lorazepam; ACET=acetaminophen
‘n*’ refers to number of subjects from a given study that were included in the retrospective analysis
study sample consisted of light and moderate alcohol drinkers
2.2. Subjects, experimental design, and procedures
Subject inclusion/exclusion criteria, procedural aspects of the five studies and dependent measures have been previously reported in detail (Zacny and Gutierrez, 2003) and will be briefly described below. In order to be eligible for the studies, volunteers needed to 1) be within the age range of 21-39 years; 2) have a high school education or an equivalent; 3) be verbally fluent in English; 4) have a body mass index between 18 and 27; and 5) have at least some current level of recreational drug usage. With the exception of the alcohol-drinking status study (Zacny and Drum, 2009), usage was defined as consumption of 3 or more alcoholic drinks within a month or some [1 joint/week] but not daily use of marijuana. In the drinking status study, light drinkers had to consume at least one but no more than four drinks/month, and drink two or fewer drinks/occasion. Moderate drinkers had to meet two or more of the following criteria (Evans et al., 1996; Stoops et al., 2003): 1) ingest at least seven drinks/week, 2) drink at least three drinks/occasion at least once/week, and/or 3) consume alcohol at least four days/week. All subjects had to be in good health, which was ascertained by passing a physical examination and having a normal resting EKG. Subjects were excluded for having a history of psychiatric or substance use disorders as determined from a structured interview using DSM-IV diagnostic criteria (American Psychiatric Association, 2000) and smoking more than five cigarettes daily. The local Institutional Review Board approved the five studies. Before beginning the study, written informed consent was obtained from each subject. In the consent form, volunteers were told they would receive an FDA-approved, non-experimental drug or drugs that could come from one or more classes of drugs, and examples given included sedative/tranquilizers (for example, Valium®), stimulants (for example, amphetamine or speed), opiates (for example, morphine), or placebo (no active drug at all).
A randomized, placebo-controlled, double-blind, crossover trial consisting of 4-6 sessions was conducted across the five studies. Sessions were approximately 5.5 h in duration (0900-1430 h) and spaced at least a week apart. All sessions took place in a departmental laboratory with subjects in a semi-recumbent position in a hospital bed. Urine toxicology screening, breath-alcohol testing, and pregnancy testing (for females) were conducted prior to the start of each session. Then subjects had their physiological status checked and completed several subjective effects forms and psychomotor tests. After this baseline period, subjects were given 150 ml of water with three #00 identical (size and color) capsules and were told, “The capsules you are about to ingest may or may not contain a drug.” An anesthetist administered the capsules. At fixed time intervals for 300 min after capsule ingestion, mood, psychomotor performance, and physiological status were assessed.
2.3. Classification of volunteers into low and high SS groups
The Disinhibition subscale consists of ten items, with scores ranging from 0 to 10. Those volunteers who scored on the lower end of the subscale, i.e., 0-4, were defined as LSs, and those who scored on the higher end of the subscale, i.e., 7-10, were defined as HSs. The United States mean for both males (6.5) and females (5.1) falls between the strata that were created for this analysis (Zuckerman, 1994).
2.4. Dependent measures
2.4.1. Subjective effects
Subjective effects were measured by five forms: a computerized, short form of the Addiction Research Center Inventory (ARCI) (Haertzen, 1966; Martin et al., 1971), a locally developed 12-item opiate adjective rating scale (OARS) derived from two questionnaires sensitive to the somatic and subjective effects of opioids (Fraser et al., 1961; Preston et al., 1989), a locally developed visual analog scale (VAS) consisting of 26 or 28 items (the 26 items that the studies had in common were analyzed), a Drug Effect/Drug Liking/Take Again (DEL/TA) questionnaire, and a locally developed 20-item post-session sequelae questionnaire (PSQ) that assessed residual effects of the drug that subjects were asked to complete 24 h after the session. The ARCI and OARS were completed before capsule ingestion (baseline) and 60, 120, 180, 240, and 300 min afterwards. The VAS and DEL/TA questionnaire in addition to those time points were administered at 15, 30, 90, 150, 210, and 270 min after capsule ingestion. The DEL/TA assessed the extent to which subjects currently felt a drug effect on a scale of 1 (I feel no effect from it at all) to 5 (I feel a very strong effect); assessed drug liking and disliking on a 100-mm line (0 mm=dislike a lot; 50 mm=neutral; 100 mm=like a lot); and assessed how much subjects “would want to take the drug you received today again on another session, if given the opportunity” on a 100-mm line [0 mm=definitely would not; 50 mm=neutral (don't care); 100 mm=definitely would]. Overall drug liking and overall “want to take drug again” were also assessed at the end of each session and 24 hours later on a modified version of the DEL/TA.
2.4.2. Cognitive/psychomotor performance
In four of the five studies included in this retrospective analysis, performance was measured with five tests: an eye-hand coordination test (Nuotto and Korttila, 1991), the Digit Symbol Substitution Test (DSST) (Wechsler, 1958), an auditory reaction test (Nuotto and Korttila, 1991), a logical reasoning test (Baddeley, 1968), and a locally developed recall memory test. In one or more of the four studies, performance on the eye hand coordination test, DSST, and logical reasoning test were affected by oxycodone, and were therefore analyzed in this study. These tests were administered before capsule ingestion (baseline) and 60, 120, 180, 240, and 300 min afterwards. The DSST was also administered at 15, 30, 90, 150, 210, and 270 min after capsule ingestion. The Zacny and de Wit (2009) study did not assess these cognitive/psychomotor measures.
2.4.3. Physiological measures
Six physiological measures were assessed: heart rate, blood pressure, arterial oxygen saturation, respiration rate, exophoria, and pupil size. Physiological assessments with the exception of pupil size were made at baseline and at hourly time points up to 5 h after capsule administration. Pupil size was measured in with the room darkened at baseline and 60, 120, 180, and 300 min post-capsule ingestion.
2.4.4. Personality measures
At least 24 h after the final experimental session, subjects were asked to complete a post-study packet of questionnaires, including the Sensation Seeking Scale (SSS-Form V, Zuckerman 1994), the Tridimensional Personality Questionnaire (Cloninger, 1987), the Eysenck Personality Inventory (Eysenck and Eysenck, 1968), and the Drug Attitudes Scale (Goodstadt et al., 1978). The Tridimensional Personality Questionnaire has three main dimensions, Novelty Seeking, Harm Avoidance, and Reward Dependence, each divided into subscales. Novelty Seeking which has been used as a measure of SS consists of four subscales, each scored separately: exploratory excitability (NS1), impulsiveness (NS2), extravagance (NS3), and disorderliness (NS4). A total score is also calculated for each dimension by summing scores on the subscales. The Eysenck Personality Inventory (Form A) consists of three subscales termed Extraversion/Introversion, Neuroticism/Stability, and Lie. The Drug Attitudes Scale consists of scales concerning Ethanol, Cannabis, Hallucinogens, Speed, Tranquilizers, Barbiturates, Heroin, Opiates, Tobacco, and General. A general drug use of all these scales is also calculated as an overall measure of drug attitudes. Higher scores on these scales indicate more positive attitudes.
2.5. Data analysis
The subjects were divided into four groups based on their SS status and sex: LS males, LS females, HS males, and HS females. Demographic variables were analyzed where appropriate with chi square tests for categorical variables and Student's two-sample t-tests for continuous variables. A mixed model analysis of variance (ANOVA) was used to examine the subjective, psychomotor/cognitive, and physiological effects of oxycodone (3 dosing levels: 0, 10, 20 mg) as a function of the grouping variable (4 levels) (SigmaStat, Point Richmond, CA). The analysis compared peak (highest value obtained), trough (lowest value obtained), or mean effects of the three drug conditions. In the peak and trough analyses, only post-capsule administration values were included, and values were determined for each subject independent of time point. Mean effect analyses were done on those measures that were assessed only once either during or after experimental sessions. When significant Group × Dose interactions were obtained, Holm-Sidak post hoc testing was used to determine the nature of the interactions. F values were considered significant for p≤0.05.
3. Results
A total of 99 subjects completed the five studies in Table 1, 71 who met criteria for a LS (n=34, 15 males) or a HS (n=37, 23 males). Table 2 shows the demographics of those subjects, including their current self-reported drug use history, selected subscales of the personality questionnaires, and lifetime drug use history of certain drugs. First, male LSs were compared to female LSs, and male HSs compared to female HSs. Both male LSs and HSs weighed more than female LSs and HSs. Female LSs had significantly lower Disinhibition scores than male LSs, and female HSs had lower BMIs than male HSs. No other differences were detected between males and females within the LS or HS categorization schema in Table 2, so data were then collapsed across sex, and chi square and t tests were repeated to compare LSs to HSs on the demographic measures. For the sake of brevity, only lifetime use of drugs and subscales of the personality questionnaires that showed significant differences between the LSs and HSs are listed. HSs reported consuming more alcoholic drinks per week than LSs, and a greater proportion of HSs than LSs reported lifetime time use of stimulants, hallucinogens, and “ecstasy”. A greater proportion of LSs reported not using marijuana in their lifetime than HSs, and conversely, a greater proportion of HSs than LSs reported lifetime use of 10 to 50 times. Also, a greater proportion of HSs than LSs reported lifetime use of marijuana greater than 50 times, as well as current use, but the differences were marginally significant (lifetime use: p=0.06; current use: p=0.06). It should be noted that self-reported prevalence of lifetime nonmedical use of prescription opioids was similar in the LS and HS groups, (8.9% and 13.5%, respectively).
Table 2.
Participant demographics
| LS | HS | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Group size (n) | 15 | 19 | 23 | 14 |
| Age (years; M±SD) | 24.8 (3.0) | 24.3 (3.1) | 24.4 (3.9) | 23.9 (3.2) |
| Weight (kg; M±SD) | 71.2 (12.0) | 59.6 (6.4)* | 76.9 (9.3) | 59.0 (9.7)* |
| BMI (M±SD) | 22.3 (2.8) | 21.9 (2.1) | 23.5 (1.9) | 21.3 (2.8)* |
| Race | ||||
| Caucasian | 10 | 13 | 17 | 10 |
| African American | 2 | 5 | 1 | 2 |
| Asian | 1 | 0 | 2 | 2 |
| American Indian/Alaskan Native | 1 | 1 | 0 | 0 |
| Multiracial | 1 | 0 | 3 | 0 |
| Current drug use (past 30 days) | ||||
| Alcohol (drinks/week; M±SD)a | 2.9 (2.4) | 2.4 (2.2) | 6.0 (4.1) | 5.2 (4.9) |
| Caffeine (drinks/week; M±SD) | 5.4 (5.0) | 6.0 (4.3) | 5.0 (4.2) | 5.0 (5.2) |
| Smokers (n; all<5 cigarettes/day) | 6 | 6 | 6 | 3 |
| Marijuana (n) | 3 | 3 | 8 | 6 |
| Lifetime recreational drug use (n) | ||||
| Marijuana | ||||
| Never usedb | 5 | 9 | 5 | 1 |
| Used <10 times | 3 | 6 | 2 | 2 |
| Used 10-50 timesb | 4 | 1 | 6 | 7 |
| Used >50 times | 3 | 3 | 10 | 4 |
| Stimulantsb | 2 | 0 | 7 | 7 |
| Hallucinogensb | 2 | 4 | 11 | 5 |
| “Ecstasy”b | 0 | 1 | 7 | 4 |
| Sensation Seeking Scale-V | ||||
| Experience Seekinga | 6.3 | 6.5 | 7.2 | 7.7 |
| Disinhibitiona | 3.5 | 2.6* | 7.9 | 7.6 |
| Boredom Susceptibilitya | 2.9 | 2.6 | 4.7 | 3.3 |
| Totala | 18.5 | 18.4 | 27.4 | 25.5 |
| Eysenck Personality Inventory | ||||
| Extraversiona | 10.9 | 10.7 | 14.3 | 13.9 |
| Liea | 3.6 | 3.5 | 1.9 | 2.7 |
| Tridimensional Personality Questionnaire | ||||
| Novelty Seeking Scale | ||||
| Impulsiveness (NS2)a | 1.9 | 2.2 | 3.3 | 2.6 |
| Disorderliness (NS4)a | 4.1 | 3.7 | 6.1 | 5.8 |
| Totala | 14.1 | 15.2 | 19.5 | 18.6 |
| Drug Attitudes Scale | ||||
| Alcohola | 21.1 | 18.4 | 23.4 | 22.0 |
| Cannabisa | 19.9 | 17.5 | 23.3 | 23.9 |
| Hallucinogensa | 17.9 | 15.0 | 19.8 | 21.6 |
| Speeda | 14.1 | 12.4 | 15.1 | 16.9 |
| General drug usea | 16.9 | 14.2 | 19.0 | 18.6 |
Abbreviations: LS = low sensation seeker; HS = high sensation seeker; BMI = body mass index
Significant difference from males
Significant difference between SS groups collapsed across Sex as determined by t tests
Significant difference between SS groups collapsed across Sex as determined by chi square tests
On the SSS-Form V, HSs as defined by the Disinhibition subscale, also had higher scores on the subscales ES and BS and the total SS score than did the LSs. HSs had significantly higher scores than did LSs on two subscales of the Novelty Seeking dimension of the Tridimensional Personality Questionnaire, as well as total (summed) scores on that dimension. On the Eysenck Personality Inventory, HSs had higher extraversion scores but lower Lie scores than LSs. The mean Lie scores in both groups did not reach a score of 4, which Eysenck and Eysenck (1964) viewed as a cutoff indicative of dissimulation (“faking good”). HSs had more positive attitudes towards alcohol, cannabis, hallucinogens, speed, and drugs in general than LSs, as measured by the Drug Attitudes Scale, and this was consistent with their usage pattern of these drugs.
Table 3 shows those variables in which there were significant Group × Dose interactions, in addition to three abuse liability-related variables that had marginally significant interactions. The interactions were limited to subjective effects. There was one positive drug effect that was reported only by male and female HSs after one or both doses of oxycodone relative to placebo – peak VAS ratings of “pleasant bodily sensations” (Figure 1, top left frame) (Group × Dose: p<0.01). There were an additional two abuse liability-related effects that showed this pattern, VAS ratings of “elated (very happy)” (Group × Dose: p=0.07) (Figure 1, bottom left frame) and the OARS rating of “carefree” (Group × Dose: p=0.08) (Figure 1, bottom right frame). Ratings of “take again” (referring to wanting to take the drug again on another session, if given the opportunity) were significantly increased by one or both doses of active oxycodone relative to placebo in all four groups, but female HSs had significantly higher ratings than did either male or female LSs (Figure 1, top right frame) (Group × Dose: p<0.05). Female and male HSs did not differ on this rating. Drug liking ratings were also significantly increased by one or both doses of active oxycodone, but female and male HSs tended to have higher scores than did female and male LSs (Group × Dose: p=0.11). There were a number of Group × Dose interactions on measures that could be considered as dysphoric in nature. In most cases, female LSs and HSs and male LSs especially at the high oxycodone dose reported higher ratings of dysphoria (e.g., nauseated, dislike [trough liking], and a desire not to take the drug again) in contrast to the male HSs who did not report such effects.
Table 3.
Variables in which there was a Group × Dose interaction except where otherwise noted.a
| LS | HS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||||
| Oxycodone dose (mg) | 0 | 10 | 20 | 0 | 10 | 20 | 0 | 10 | 20 | 0 | 10 | 20 |
| VAS | ||||||||||||
| Dizzy | 5.1 | 21.3 | 26.1b | 1.5 | 13.2 | 41.7b,c | 5.0 | 14.2 | 16.2 | 1.9 | 15.1 | 46.4b,c |
| Elated (very happy)d | 39.3 | 36.9 | 41.4 | 21.5 | 26.6 | 20.9 | 11.2 | 19.1 | 26.3 | 16.6 | 27.9 | 30.8 |
| Feel bad | 9.0 | 13.0 | 30.5b,c | 4.6 | 20.3b | 43.9b,c | 9.5 | 11.6 | 10.2 | 5.0 | 10.6 | 27.9b |
| Nauseated | 1.7 | 16.4 | 28.7b | 1.4 | 14.5 | 46.3b,c | 2.4 | 8.2 | 14.2 | 6.1 | 15.7 | 41.1b,c |
| Pleasant bodily sensations | 23.5 | 24.9 | 28.1 | 20.6 | 29.6 | 25.7 | 16.2 | 27.4 | 37.3b | 14.1 | 38.4b | 47.7b |
| Sleepy (drowsy, tired) | 31.4 | 58.2b | 56.7b | 33.4 | 59.5b | 82.4b | 37.3 | 47.7 | 61.0b | 52.4 | 55.4 | 70.1 |
| Unpleasant bodily sensations | 4.1 | 14.6 | 29.3b | 7.4 | 19.2 | 46.4b,c | 9.4 | 10.5 | 13.8 | 3.6 | 16.9 | 39.6b,c |
| OARS | ||||||||||||
| Carefreee | 1.9 | 1.9 | 1.9 | 1.2 | 1.4 | 1.2 | 1.3 | 1.8 | 2.0 | 0.9 | 1.3 | 1.8 |
| Turning of stomach | 0.2 | 0.9 | 0.9 | 0.2 | 0.6 | 1.9b,c,f | 0.4 | 0.3 | 0.6 | 0.4 | 0.6 | 1.6b,c |
| DEL/TA | ||||||||||||
| Likingg | 53.5 | 61.3 | 65.4 | 56.3 | 66.9 | 64.1 | 54.5 | 65.5 | 72.3 | 54.5 | 70.7 | 77.0 |
| Likingh | 46.3 | 39.3 | 25.4b | 47.9 | 32.2b | 21.7b,i | 42.5 | 39.4 | 36.7 | 44.9 | 40.4 | 25.3b |
| Take again | 55.6 | 67.3b | 67.5b | 58.0 | 70.2b | 66.0 | 57.0 | 67.7b | 74.4b | 57.1 | 74.4b | 83.4b,f,k |
| End-of-session likingj | 48.6 | 41.8 | 36.1i | 56.2 | 44.7 | 32.5b,i | 48.8 | 51.5 | 56.5 | 48.6 | 52.4 | 40.6 |
| End-of-session take againj | 48.5 | 51.0 | 31.4i | 60.9 | 44.5b | 33.2b,i | 52.2 | 52.2 | 57.3 | 52.9 | 56.0 | 41.8i |
| 24-h likingj | 47.8 | 42.0 | 26.9b,i | 50.1 | 44.3 | 28.3b,i | 49.9 | 43.0 | 53.4 | 44.5 | 46.7 | 34.5i |
| 24-h take againj | 48.1 | 43.5 | 24.2b,i | 53.1 | 44.4 | 25.5b,i | 50.0 | 42.7 | 55.4 | 46.9 | 42.9 | 39.5 |
| PSQj | ||||||||||||
| Down | 0.0 | 0.1 | 0.5b,c,l | 0.0 | 0.0 | 0.2 | 0.0 | 0.1 | 0.0 | 0.0 | 0.3 | 0.0 |
| Feel bad | 0.0 | 0.7b | 1.7b,c,k | 0.1 | 0.3 | 0.7b | 0.2 | 0.3 | 0.2 | 0.1 | 0.6 | 0.9b,c |
| Headache | 0.1 | 0.3 | 1.1b,c,k | 0.3 | 0.4 | 0.1 | 0.3 | 0.6 | 0.3 | 0.1 | 0.6 | 0.7 |
| Lightheaded | 0.1 | 0.3 | 0.6 | 0.2 | 0.2 | 0.1 | 0.3 | 0.3 | 0.2 | 0.1 | 0.1 | 0.9b,c,k |
| Nausea | 0.1 | 0.6b | 1.5b,c | 0.1 | 0.6 | 1.0b,c | 0.1 | 0.0 | 0.3 | 0.0 | 0.3 | 1.1b,c |
| Vomiting | 0.0 | 0.2 | 1.1b,c,k,l | 0.0 | 0.1 | 0.6b,c | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5b,c |
Abbreviations: LS = low sensation seeker; HS = high sensation seeker; VAS = Visual Analog Scale; OARS = Opiate Adjective Rating Scale; DEL/TA = Drug effect/Drug liking/Take again scale; PSQ = Post Session Sequelae questionnaire.
Measures are peak values, except where otherwise noted.
Holm-Sidek post hoc analysis determined significant difference from placebo
higher than HS male
Group × Dose interaction: p=0.07
Group × Dose interaction: p=0.08
higher than LS male
Group × Dose interaction: p=0.11
trough measure
lower than HS male
mean (single) measure
higher than LS female
higher than HS female
Figure 1.
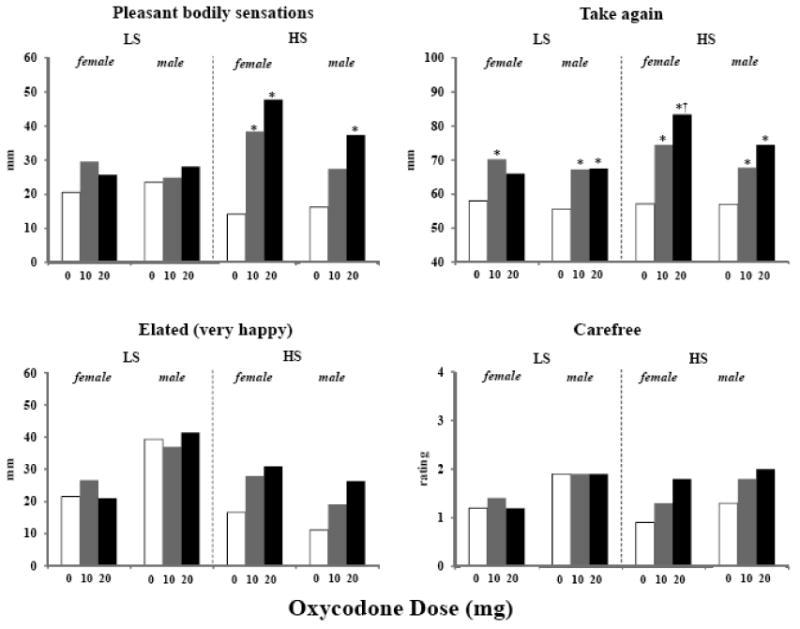
Peak ratings of “pleasant bodily sensations” (top left frame), “take again” (top right frame), “elated (very happy)” (bottom left frame), and “carefree” (bottom right frame) in female and male low sensation seekers (LS) and high sensation seekers (HS) when administered 0, 10, and 20 mg oral oxycodone. The visual analog scale for “pleasant bodily sensations” and “elated (very happy)” ratings ranged from 0 (“not at all”) to 100 (“extremely”). The visual analog scale for “take again” ratings was bipolar and labels at the far ends (0, 100) and the middle (50) were “definitely would not,” “definitely would,” and “neutral (don't care).” Subjects rated “carefree” from the OARS on a 5-point scale from 0 (“not at all”) to 4 (“extremely”). Asterisks indicate a significant difference from 0 mg oxycodone. The dagger represents a significantly higher rating of “take again” in female HSs than in male and female LSs in the 20-mg oxycodone condition.
Besides the significant and marginally significant Group × Dose interactions described above, there were a number of significant Dose effects. In the majority of cases, both doses of oxycodone differed significantly from that of placebo, and magnitude of effect was related to dose. On the ARCI, peak PCAG, A, MBG, and LSD scores were increased, and trough BG scores were decreased by oxycodone relative to placebo. On the VAS, peak ratings of “coasting (‘spaced out’),” “confused,” “difficulty concentrating,” “dreamy,” “drunk,” “floating,” “having unpleasant thoughts,” “high,” “heavy or sluggish feeling,” “lightheaded,” “sedated (calm, tranquil),” and “tingling” were increased, and trough ratings of “in control of body,” and “in control of thoughts” were decreased by oxycodone relative to placebo. On the OARS, peak ratings of “dry mouth,” “flushing,” “nodding,” “numb,” “skin itchy,” “sweating,” and “vomiting” were increased, and trough ratings of “drive” were decreased by oxycodone relative to placebo. On the DEL/TA, peak ratings of “feel drug effect” were increased, and trough ratings of “take again” were decreased by oxycodone, relative to placebo. On the PSQ, mean ratings of “coasting,” “confused,” “difficulty concentrating,” “dreaminess,” “drowsiness,” “dry mouth,” “heavy,” “skin itchy” were increased, and ratings of “feel good” were decreased, by oxycodone relative to placebo. Both doses of oxycodone impaired cognitive and psychomotor performance relative to placebo, as measured by the DSST, eye-hand coordination test, and the logical reasoning test but grouping status did not modulate degree of impairment. Oxycodone increased exophoria ratings and decreased pupil size, systolic blood pressure, respiration rate, heart rate, and arterial oxygen saturation. Oxycodone's effects on the latter four measures were clinically insignificant.
4. Discussion
It was hypothesized for the reasons cited in the introduction that HSs would report greater abuse liability-related subjective effects than would LSs. In general HSs reported more of these effects or reported effects of a greater magnitude than did LSs. For example, male and female HSs reported increased ratings of “pleasant bodily sensations” in one or both of the active oxycodone conditions, relative to placebo whereas male and female LSs did not. Similar patterns were noted with “carefree” and “elated (very happy),” although the Group × Dose interactions were only marginally significant. Female and male HSs had higher “take again” and drug liking ratings than did female and male LSs, and in the case of “take again,” female HSs had significantly higher ratings than did male and female LSs. These data provide suggestive evidence that high SS status is associated with a propensity to report more positive effects from oxycodone. This is consistent with other laboratory studies that have established that high sensation seekers are more sensitive to abuse-liability related effects of drugs, including alcohol (de Wit et al., 1987; Fillmore et al., 2009), nicotine (Perkins et al., 2000), d-amphetamine (Hutchison et al., 1999; Kelly et al. 2006, 2009; Stoops et al., 2007), and benzodiazepines (Kelly et al., 2009).
There was a rather more complex relationship between SS status, sex, and self-reported negative effects of oxycodone. Specifically, male HSs did not report the negative effects that male LSs and female LSs and HSs reported, as shown in Table 3. As stated in the Introduction, sex of the subject was taken into account in this retrospective analysis because in one of the studies that comprise it (Zacny and Drum, 2009), sex differences to the dysphoric effects of oxycodone were documented, with females reporting greater effects than males. Sex differences in nausea (and/or vomiting) after opioid administration have also been noted in both laboratory and clinical studies (Zun et al., 2002; Cepeda et al., 2003; Fillingim et al., 2005). Therefore the fact that both low and high SS females reported dysphoric effects in the larger analysis was not totally unexpected. It is admittedly unclear, however, why male LSs reported nausea as well as other dysphoric effects. Although the magnitude of dysphoric effects was lesser than that reported by female LSs, the differences were not significant, and residual effects as measured by the PSQ in some cases were significantly larger in the male LS group than in the female LS group. One might argue that perhaps females reported dysphoric effects because they were receiving higher doses than were males, since dosing was not adjusted for body weight. However this would not explain why male LSs reported more dysphoric effects than male HSs, because their weights did not differ. Second, in the Zacny and Drum (2009) study which comprised part of this study, weight was included as a covariate, because there were sex differences of a similar nature reported in this analysis – the sex differences remained with weight controlled for in the analysis of covariance.
Sensation-seeking status was based on the Disinhibition scale of the SSS-Form V. There is precedent for doing this as was elaborated upon in the Introduction. However, it should be acknowledged that more recent studies have used the Impulsive Sensation-Seeking scale from the ZKPQ as the metric to define low and high SS (Kelly et al., 2006, 2009; Stoops et al., 2007; Fillmore et al., 2009). Our grouping strategy (LS: 0-4; HS: 7-10) has face validity in that the LSs and HSs as defined in this retrospective analysis differed in their self-reported drug use history in the expected direction. One would expect HSs to have more extensive drug use than LSs based on the body of literature reviewed in the Introduction. Indeed, in this retrospective analysis, significantly greater proportion of HSs than LSs reported lifetime use of stimulants, hallucinogens, and “ecstasy.” A greater proportion of HSs than LSs reported using marijuana more than ten times in their lifetime (10-50 times, p<0.05; greater than 50 times, p=0.06), and a greater proportion of HSs than LSs also reported current use (p=0.06). HSs also reported drinking more alcohol in the 30 days preceding the study than did LSs. Perhaps not surprisingly, the HSs had more favorable attitudes towards these drugs than did LSs. Another piece of evidence supporting the validity of the categorization schema used in this analysis is that the LSs and HSs differed on total scores and scores on two subscales of the dimension of Novelty Seeking, also considered to be a measure of SS, from the Tridimensional Personality Questionnaire (Cloninger, 1987). Finally, Pearson product-moment correlations were determined between scores on the Disinhibition subscale and scores on the other subscales of the SSS-V, the total score on the SSS-V, and total scores on the Novelty Seeking scale and its four subscales. Correlations were significant between the Disinhibition subscale and the total score on the SSS-V (r=0.71, p<0.001); two of the other three subscales of the SSS-V (Experience Seeking: r=0.32, p<0.01; Boredom Susceptibility: p=0.34, p<0.005); and scores on the Novelty Seeking scale (r=0.48, p<0.001) and its four subscales (Exploratory excitability: r=0.26, p<0.05; Impulsiveness: r=0.28, p<0.05; Extravagance: r=0.25, p<0.05; Disorderliness: r=0.53, p<0.001). These data showing significant correlations between the Disinhibition subscale and other measures of SS lend further support for the classification of high and low sensation seekers using the Disinhibition subscale.
In conclusion, the results from this analysis provide suggestive evidence that HSs (as measured by the Disinhibition subscale of the SSS-Form V) are more likely to experience positive effects from the prescription opioid, oxycodone, than are LSs. Dysphoric effects appear to be linked to both SS status and sex. The notion has been put forth by others that the abuse liability of a drug is related to both its positive and negative effects (Evans et al., 1990; McColl and Sellers, 2006; Comer et al., 2008). Thus a person who experiences both positive and negative subjective effects from a drug may be less likely to use that drug for recreational purposes than a person who experiences positive effects and little negative effect. Although speculative, it is possible that male HSs may be more at risk of nonmedical use of oxycodone, and perhaps other prescription opioids, than male LSs and females, regardless of their SS status. It would be worthwhile to systematically replicate this analysis to determine if similar results are found when the Impulsive Sensation-Seeking Scale of the ZKPQ is used to categorize people as low and high sensation seekers.
Acknowledgments
This research was supported by NIDA grant DA-08573. The author would like to thank Jenny Jun, Sandra Gutierrez, and Melinda Drum for their assistance in analyzing the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alterman AI, Hall JG, Purtill JJ, Searles JS, Holahan JM, McLellan AT. Heavy drinking and its correlates in young men. Addict Behav. 1990;15:95–103. doi: 10.1016/0306-4603(90)90012-m. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR (text revision) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Andrucci GL, Archer RP, Pancoast DL, Gordon RA. The relationship of MMPI and Sensation Seeking Scales to adolescent drug use. J Pers Assess. 1989;53:253–66. doi: 10.1207/s15327752jpa5302_4. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. A three-minute reasoning test based on grammatical transformation. Psychonom Sci. 1968;10:341–2. [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–4. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Beck KH, Thombs DL, Mahoney CA, Fingar KM. Social context and sensation seeking: gender differences in college student drinking motivations. Int J Addict. 1995;30:1101–15. doi: 10.3109/10826089509055830. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Klebaur JE, Bardo MT. Individual differences in response to novelty, amphetamine-induced activity and drug discrimination in rats. Behav Pharmacol. 1997;8:113–23. [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Reynolds JL, Greenberg PE, Zhang M, Vallow S, Schein J, Katz NP. Estimated costs of prescription opioid analgesic abuse in the United States in 2001. Clin J Pain. 2006;22:667–76. doi: 10.1097/01.ajp.0000210915.80417.cf. [DOI] [PubMed] [Google Scholar]
- Bobes J, Saiz PA, Gonzalez MP, Bascaran MT, Bousono M, Ricaurte GA, McCann UD. Use of MDMA and other illicit drugs by young adult males in northern Spain. A five-year study. Eur Addict Res. 2002;8:147–54. doi: 10.1159/000059385. [DOI] [PubMed] [Google Scholar]
- Bradley CM, Nicholson AN. Studies on performance with aspirin and paracetamol and with the centrally acting analgesics meptazinol and pentazocine. Eur J Clin Pharmacol. 1987;32:135–9. doi: 10.1007/BF00542185. [DOI] [PubMed] [Google Scholar]
- Cepeda MS, Farra JY, Baumgarten M, Boston R, Carr DB, Strom BL. Side effects of opioids during short-term administration: effects of age, gender and race. Clin Pharmacol Ther. 2003;74:102–12. doi: 10.1016/S0009-9236(03)00152-8. [DOI] [PubMed] [Google Scholar]
- Chait LD. Reinforcing and subjective effects of methylphenidate in humans. Behav Pharmacol. 1994;5:281–8. doi: 10.1097/00008877-199406000-00005. [DOI] [PubMed] [Google Scholar]
- Cicero TK, Inciardi JA, Munoz A. Trends in abuse of OxyContin and other opioid analgesics in the United States: 2002-2004. J Pain. 2005;6:662–72. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Clapper RL, Martin CS, Clifford PR. Personality, social environment, and past behavior as predictors of late adolescent drug use. J Subst Abuse. 1994;6:305–13. doi: 10.1016/s0899-3289(94)90491-x. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. Arch Gen Psychiatry. 1987;4:573–85. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology. 2008;33:1179–91. doi: 10.1038/sj.npp.1301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta N, Kramer ED, Zalman MA, Carino S, Jr, Smith MY, Haddox JD, Wright C., 4th Association between non-medical and prescriptive usage of opioids. Drug Alcohol Depend. 2006;82:135–42. doi: 10.1016/j.drugalcdep.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Le Moal M, Simon H. Individual differences in the psychomotor effects of morphine are predicted by reactivity to novelty and influenced by corticosterone secretion. Brain Res. 1993;623:341–4. doi: 10.1016/0006-8993(93)91451-w. [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Pierri J, Johanson CE. Individual differences in behavioral and subjective responses to alcohol. Alcohol Clin Exp Res. 1987;11:52–9. doi: 10.1111/j.1530-0277.1987.tb01263.x. [DOI] [PubMed] [Google Scholar]
- Eade NR, Lasagna L. A comparison of acetophenetidin and acetaminophen. II. Subjective effects in healthy volunteers. J Pharmacol Exp Ther. 1967;155:301–8. [PubMed] [Google Scholar]
- Erblich J, Earleywine M. Behavioral undercontrol and subjective stimulant and sedative effects of alcohol intoxication: independent predictors of drinking habits? Alcohol Clin Exp Res. 2003;27:44–50. doi: 10.1097/01.ALC.0000047300.46347.CE. [DOI] [PubMed] [Google Scholar]
- Evans SM, Funderburk FR, Griffiths RR. Zolpidem and triazolam in humans: behavioral and subjective effects and abuse liability. J Pharmacol Exp Ther. 1990;255:1246–55. [PubMed] [Google Scholar]
- Evans SM, Griffiths RR, de Wit H. Preference for diazepam, but not buspirone, in moderate drinkers. Psychopharmacology. 1996;123:154–63. doi: 10.1007/BF02246172. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Inventory. London: University of London; 1964. [Google Scholar]
- Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, Staud R. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–24. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Ostling EW, Martin CA, Kelly TH. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug Alcohol Depend. 2009;100:91–9. doi: 10.1016/j.drugalcdep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franques P, Auriacombe M, Piquemal E, Verger M, Brisseau-Gimenez S, Grabot D, Tignol J. Sensation seeking as a common factor in opioid dependent subjects and high risk sport practicing subjects. A cross sectional study. Drug Alcohol Depend. 2003;69:121–6. doi: 10.1016/s0376-8716(02)00309-5. [DOI] [PubMed] [Google Scholar]
- Fraser HF, van Horn GD, Martin WR, Wolbach AB, Isbell H. Methods for evaluating addiction liability. (a) “attitude” of opiate addicts toward opiate-like drugs, (b) a short-term “direct” addiction test. J Pharmacol Exp Ther. 1961;133:371–87. [PubMed] [Google Scholar]
- Gilson AM, Ryan KM, Joranson DE, Dahl JL. A reassessment of trends in the medical use and abuse of opioid analgesics and implications for diversion control: 1997-2002. J Pain Symptom Manage. 2004;28:176–88. doi: 10.1016/j.jpainsymman.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Goodstadt MS, Cook G, Magid S, Gruson V. The Drug Attitude Scale (DAS): Its development and evaluation. Int J Addict. 1978;13:1307–17. doi: 10.3109/10826087809039344. [DOI] [PubMed] [Google Scholar]
- Haertzen CA. Development of scales based on patterns of drug effects, using the Addiction Research Center Inventory (ARCI) Psychol Rep. 1966;18:163–94. doi: 10.2466/pr0.1966.18.1.163. [DOI] [PubMed] [Google Scholar]
- Hittner JB, Swickert R. Sensation seeking and alcohol use: A meta-analytic review. Addict Behav. 2006;31:1383–1401. doi: 10.1016/j.addbeh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Kalivas PW. Involvement of dopamine and excitatory amino acid transmission in novelty-induced motor activity. J Pharmacol Exp Ther. 1994;269:976–88. [PubMed] [Google Scholar]
- Hutchison KE, Wood MD, Swift R. Personality factors moderate subjective and psychophysiological responses to d-amphetamine in humans. Exp Clin Psychopharmacol. 1999;7:493–501. doi: 10.1037//1064-1297.7.4.493. [DOI] [PubMed] [Google Scholar]
- Jaffe LT, Archer RP. The prediction of drug use among college students from MMPI, MCMI, and sensation seeking scales. J Pers Assess. 1987;51:243–53. doi: 10.1207/s15327752jpa5102_8. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Delzer T, Martin CA, Harrington NG, Hays LR, Bardo MJ. Performance and subjective effects of diazepam and d-amphetamine in high and low sensation seekers. Behav Pharmacol. 2009;20:505–17. doi: 10.1097/FBP.0b013e3283305e8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington HG, Rush CR. Individual differences in drug abuse vulnerability: d-amphetamine and sensation-seeking status. Psychopharmacology. 2006;189:17–25. doi: 10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liraud F, Verdoux H. Which temperamental characteristics are associated with substance use in subjects with psychotic and mood disorders? Psychiatry Res. 2000;93:63–72. doi: 10.1016/s0165-1781(99)00120-1. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J Am Acad Child Adolesc Psychiatry. 2002;41:1495–502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–58. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McColl S, Sellers EM. Research design strategies to evaluate the impact of formulations on abuse liability. Drug Alcohol Depend. 2006;83 1:S52–62. doi: 10.1016/j.drugalcdep.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Nuotto EJ, Korttila K. Evaluation of a new computerized psychomotor test battery: effects of alcohol. Pharmacol Toxicol. 1991;68:360–5. doi: 10.1111/j.1600-0773.1991.tb01253.x. [DOI] [PubMed] [Google Scholar]
- Pedersen W, Clausen SE, Lavik NJ. Patterns of drug use and sensation-seeking among adolescents in Norway. Acta Psychiatr Scand. 1989;79:386–90. doi: 10.1111/j.1600-0447.1989.tb10274.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Grobe JE, Wilson A. Greater sensitivity to subjective effects of nicotine in nonsmokers high in sensation seeking. Exp Clin Psychopharmacology. 2000;8:462–71. doi: 10.1037//1064-1297.8.4.462. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol. 1990;1:339–45. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Klein SA, George FR, Henningfield JE. Acetaminophen fails to inhibit ethanol-induced subjective effects in human volunteers. Pharmacol Biochem Behav. 1991;41:189–94. doi: 10.1016/0091-3057(92)90081-p. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Bickel WK, Liebson IA. Drug discrimination in human postaddicts: agonist-antagonist opioids. J Pharmacol Exp Ther. 1989;250:184–96. [PubMed] [Google Scholar]
- Saigusa T, Tuinstra T, Koshikawa N, Cools AR. High and low responders to novelty: effects of a catecholamine synthesis inhibitor on novelty-induced changes in behaviour and release of accumbal dopamine. Neuroscience. 1999;88:1153–63. doi: 10.1016/s0306-4522(98)00275-9. [DOI] [PubMed] [Google Scholar]
- Stephenson MT, Hoyle RH, Palmgreen P, Slater MD. Brief measures of sensation seeking for screening and large-scale surveys. Drug Alcohol Depend. 2003;72:279–86. doi: 10.1016/j.drugalcdep.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Fillmore MT, Poonacha MS, Kingery JE, Rush CR. Alcohol choice and amphetamine effects in light and moderate drinkers. Alcohol Clin Exp Res. 2003;27:804–11. doi: 10.1097/01.ALC.0000067977.23096.6A. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: influence of sensation-seeking status. Addict Behav. 2007;32:1177–88. doi: 10.1016/j.addbeh.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Office of Applied Studies. [November 6, 2009];2008 Results from the 2007 National Survey on Drug Use and Health: Detailed Tables, http://www.oas.samhsa.gov/NSDUH/2k7NSDUH/tabs/Sect1peTabs88to92.pdf.
- Teichman M, Barnea Z, Ravav G. Personality and substance use among adolescents: a longitudinal study. Br J Addict. 1989;84:181–90. doi: 10.1111/j.1360-0443.1989.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The measurement and appraisal of adult intelligence. Baltimore MD: Williams and Wilkins; 1958. [Google Scholar]
- West MM. Early risk indicators of substance abuse among nurses. J Nurs Scholarship. 2002;34:187–93. doi: 10.1111/j.1547-5069.2002.00187.x. [DOI] [PubMed] [Google Scholar]
- Wright CW, IV, Kramer ED, Zalman MA, Smith MY, Haddox JD. Risk identification, risk assessment, and risk management of abusable drug formulations. Drug Alcohol Depend. 2006;83S:S68–S76. doi: 10.1016/j.drugalcdep.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215–32. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Zacny JP, de Wit H. The prescription opioid, oxycodone, does not alter behavioral measures of impulsivity in healthy volunteers. Pharmacol Biochem Behav. 2009;94:108–13. doi: 10.1016/j.pbb.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Drum M. Psychopharmacological effects of oxycodone in healthy volunteers: roles of alcohol-drinking status and sex. Drug Alcohol Depend. 2009 Nov 28; doi: 10.1016/j.drugalcdep.2009.10.012. Epub, ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S. Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non-drug-abusing volunteers. Psychopharmacology. 2003;170:242–54. doi: 10.1007/s00213-003-1540-9. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S. Within-subject comparison of the psychopharmacological profiles of oral hydrocodone and oxycodone combination products in non-drug-abusing volunteers. Drug Alcohol Depend. 2009;101:107–14. doi: 10.1016/j.drugalcdep.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S, Bolbolan SA. Profiling the subjective, psychomotor, and physiological effects of a hydrocodone/acetaminophen product in recreational drug users. Drug Alcohol Depend. 2005;78:243–52. doi: 10.1016/j.drugalcdep.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Lichtor S. Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacology. 2008;196:105–16. doi: 10.1007/s00213-007-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America: cross-cultural, age, and sex comparisons. J Consult Clin Psychol. 1978;46:139–49. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation-Seeking: Beyond the Optimal Level of Arousal. Hillsdale NJ: Erlbaum; 1979. [Google Scholar]
- Zuckerman M, Kuhlman DM, Joireman J, Teta P, Kraft M. A comparison of three structural models for personality: the big three, the big five, and the alternative five. J Pers Soc Psychol. 1993;65:757–68. [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. New York: Cambridge University Press; 1994. [Google Scholar]
- Zun LS, Downey LV, Gossman W, Rosenbaumdagger J, Sussman G. Gender differences in narcotic-induced emesis in the ED. Am J Emerg Med. 2002;20:151–4. doi: 10.1053/ajem.2002.32631. [DOI] [PubMed] [Google Scholar]


