Abstract
A sensitive immunohistochemical method for phosphorylated α-synuclein was used to stain sets of sections of spinal cord and tissue from 41 different sites in the bodies of 92 subjects, including 23 normal elderly, 7 with incidental Lewy body disease (ILBD), 17 with Parkinson’s disease (PD), 9 with dementia with Lewy bodies (DLB), 19 with Alzheimer’s disease with Lewy bodies (ADLB) and 17 with Alzheimer’s disease with no Lewy bodies (AD-NLB). The relative densities and frequencies of occurrence of phosphorylated α-synuclein histopathology (PASH) were tabulated and correlated with diagnostic category. The greatest densities and frequencies of PASH occurred in the spinal cord, followed by the paraspinal sympathetic ganglia, the vagus nerve, the gastrointestinal tract and endocrine organs. The frequency of PASH within other organs and tissue types was much lower. Spinal cord and peripheral PASH was most common in subjects with PD and DLB, where it appears likely that it is universally widespread. Subjects with ILBD had lesser densities of PASH within all regions, but had frequent involvement of the spinal cord and paraspinal sympathetic ganglia, with less-frequent involvement of end-organs. Subjects with ADLB had infrequent involvement of the spinal cord and paraspinal sympathetic ganglia with rare involvement of end-organs. Within the gastrointestinal tract, there was a rostrocaudal gradient of decreasing PASH frequency and density, with the lower esophagus and submandibular gland having the greatest involvement and the colon and rectum the lowest.
Keywords: Parkinson’s disease, Parkinsonism, Dementia with Lewy bodies, Alzheimer’s disease, Incidental Lewy bodies, α-Synuclein, Spinal cord, Sympathetic nervous system, Peripheral nervous system, Autonomic nervous system, Enteric nervous system, Submandibular gland, Esophagus, Adrenal gland, Heart, Stomach, Gastrointestinal system
Introduction
The topographical distribution and density of Lewy bodies and their associated abnormal neurites are much greater than formerly appreciated [8, 28, 31, 33, 37, 44, 46, 61, 64, 70, 71, 77, 82]. Furthermore, it is also now more clearly apparent that Lewy body pathology frequently extends to the spinal cord and peripheral nervous system [12, 14, 17–19, 29, 40, 48, 81]. Despite these recent achievements, there has not yet been published a wide survey of the distribution of Lewy-type histopathological changes in the peripheral nervous system. A sensitive immunohistochemical method for phosphorylated α-synuclein [8, 10] was used to stain sets of spinal cord and peripheral nervous system sections from 92 subjects that had previously received neuropathological diagnoses and Lewy body central nervous system (CNS) staging. The densities and frequencies of occurrence of phosphorylated α-synuclein histopathology (PASH) in multiple regions of spinal cord, sympathetic ganglia and tissue from the major organ systems were tabulated and correlated with diagnostic category. The results are presented below.
Materials and methods
Human subjects
Deceased human subjects were autopsied at Sun Health Research Institute (SHRI), a division of the not-for-profit health care provider Banner Health, located in the Sun Cities retirement communities of northwest metropolitan Phoenix, Arizona. All subjects had volunteered for the SHRI Brain and Body Donation Program (BBDP) [5, 9]. The majority of BBDP subjects are clinically characterized at SHRI with annual standardized test batteries that include functional, neuropsychological and neuromotor components, including the Mini Mental State Examination (MMSE) and Unified Parkinson’s Disease Rating Scale (UPDRS). In addition, private medical records are requisitioned and reviewed for each subject and the postmortem Dementia Questionnaire [32] or an adaptation of the Clinical Dementia Rating Scale (CDR) are administered to subject contacts to help determine the presence or absence of dementia for those subjects lacking standardized antemortem evaluations. Subjects sign consent that has been approved by the Banner Health Institutional Review Board.
Subjects were chosen by searching the BBDP database for all cases that had received a whole-body autopsy, a completed neuropathologist’s examination and a neuropathologic diagnosis of a Lewy body disorder, including Parkinson’s disease (PD), dementia with Lewy bodies (DLB), incidental Lewy body disease (ILBD) and Alzheimer’s disease with Lewy bodies (ADLB). Comparison groups were composed of subjects without evidence of dementia or parkinsonism (normal elderly subjects) and subjects with AD but no Lewy body pathology (ADNLB).
Subjects received standardized neuropathological examinations. Specific clinicopathologic diagnostic criteria were used for AD [1], PD [36], and DLB [58]. For both AD and DLB, cases received the diagnosis if they were classified as “intermediate” or “high” probabilities in their respective classification schemes. Cases with PASH, but not meeting these diagnostic criteria were designated as either ILBD, if they had no clinical history of parkinsonism or dementia, or ADLB if they had dementia, Alzheimer’s disease and Lewy bodies in any brain region, but failed to meet clinicopathologic criteria for DLB or PD.
Gross and microscopic neuropathologic assessments were made by a single observer (TGB) without knowledge of the clinical history or clinical diagnosis; subsequently the clinical history was reviewed with the neurologists (CHA, JNC, HAS, MNS) in order to make an appropriate clinicopathologic diagnosis.
Histologic methods
Diagnostic histologic methods were performed on standard blocks of tissue that were fixed in 3.75% neutral-buffered formaldehyde and then either dehydrated and embedded in paraffin or cryoprotected and cut on a freezing, sliding microtome. Each case was first staged according to the Unified Staging System for Lewy body disorders with a standard set of brain sections stained with an immunohistochemical method for phosphorylated α-synuclein as previously described [8]. The Unified Staging System is a modification of the scheme first devised by the Dementia with Lewy Bodies Consortium [8, 58, 59].
Paraffin-embedded sections from multiple body sites (Table 1) were stained in an identical fashion as the brain sections, using a polyclonal antiserum raised against an α-synuclein peptide fragment phosphorylated at serine 129, after epitope exposure with proteinase K [34]. The process leading to the choice of immunohistochemical method, as well as details of the method, has been described in a previous publication [10]. In each body region, the density of α-synuclein-immunoreactive perikaryal neuronal cytoplasmic inclusions as well as puncta and neurites was scored, at the site of highest density, by a single observer (TGB) without knowledge of diagnosis, as none, sparse, moderate, frequent and very frequent, using the templates provided by the Dementia with Lewy Bodies Consortium [58]. The total number of body sites examined varied between subjects as an initially broad sampling scheme was progressively reduced to those regions showing a greater likelihood to have positive staining. To evaluate the relative frequency of immunoreactivity in the different body regions, paraffin sections on a single stained slide from each body site listed in Table 1 were used. Following this analysis, selected regions of interest were further evaluated with up to five additional paraffin sections and/or 80 µm thick formalin-fixed, frozen sections.
Table 1.
Body sites, organs and tissue types investigated with an immunohistochemical method for phosphorylated α-synuclein histopathology
| Body region | Sites investigated within region |
|---|---|
| Spinal cord | Cervical (C4–5), thoracic (T6–7), lumbar (L3–4) and sacral (S1–5) spinal cord |
| Sympathetic ganglia | Middle cervical ganglia, middle ganglia of thoracic chain |
| Vagus nerve | Alongside the common carotid artery at the level of the larynx |
| Sciatic nerve | Overlying the external iliac artery in the pelvic cavity |
| Gastrointestinal system | Upper third and lower third of esophagus, stomach (body), duodenum, jejunum, ileum, transverse colon, sigmoid colon, rectum, submandibular gland, liver, pancreas (head), gallbladder |
| Respiratory system | Larynx, primary bronchus, lung |
| Endocrine system | Adrenal gland, thyroid gland, parathyroid gland, ovary, testis |
| Cardiovascular system | Thoracic and abdominal aorta, left and right ventricle and epicardium of heart at apex |
| Genitourinary tract | Kidney, urinary bladder, uterus, vagina |
| Musculoskeletal system | Rib (bone and associated muscle and soft tissue), psoas muscle, diaphragm |
| Skin | Abdominal skin, scalp |
| Miscellaneous | Spleen, lymph nodes (parabronchial), small bowel mesentery, breast |
Alzheimer’s disease histopathology was staged and graded on 40 µm thick sections stained with the Gallyas method for neurofibrillary tangles and the Campbell–Switzer and thioflavine-S methods for senile plaques [15]. Braak’s neurofibrillary tangle stages and CERAD neuritic plaque densities were assigned as described [16, 62].
Statistical analysis
Statistical analyses consisted of, for comparing group means, analysis of variance (ANOVA), or, for non-parametric data, Kruskall–Wallis ANOVA. Proportional measures were compared using χ2 tests.
Results
Ninety-two subjects had a full autopsy as well as neuropathological diagnoses within the targeted groups. All data from the study are given within the supplementary online table, for brevity some of the data are grouped for summary here. Descriptive measures of these groups are given in Tables 2, 3 and 4. The subjects were all of advanced age (Table 2) and the group means differed significantly (P < 0.0001), with the youngest group (PD) having a mean age of 79.3 while the oldest group (ILBD) mean was 86.7. As with all subjects of this advanced age, all had at least some neurofibrillary tangles in the brain [13] and even the elderly control group had a mean Braak stage of 2.6. All diagnostic groups had more male subjects and this was pronounced in the PD and DLB groups. The median postmortem intervals were uniformly short, ranging from 2.7 to 5.9 h and the group means were not significantly different.
Table 2.
General characteristics of the study subjects, by neuropathologic diagnosis, age, gender, postmortem interval (PMI), Braak neurofibrillary stage and CERAD neuritic plaque (NP) density
| Diagnosis (N) | Agea | Gender (% male) | PMI (h) | Braak stagea | CERAD NP densitya |
|---|---|---|---|---|---|
| Normal (23) | 81.0 (14.1) | 56.5 | 3.9 (2.5) | 2.6 (1.3) | 1.0 (1.3) |
| ILBD (7) | 86.7 (8.5) | 57.1 | 3.8 (2.9) | 3.0 (1.0) | 1.3 (1.4) |
| PD (17) | 79.3 (7.5) | 76.5 | 4.9 (4.1) | 2.5 (1.1) | 0.8 (1.2) |
| DLB (9) | 83.2 (9.5) | 67 | 2.7 (0.4) | 4.4 (0.7) | 3.0 (0.0) |
| ADLB (19) | 84.0 (5.0) | 53.0 | 4.3 (3.8) | 5.2 (1.0) | 2.7 (0.7) |
| ADNLB (17) | 84 (5.9) | 53.0 | 5.9 (10.2) | 4.4 (1.2) | 2.8 (0.4) |
Means and standard deviations (SD) are given
Subjects did not differ significantly in terms of gender distribution or PMI but differed significantly in terms of age, Braak stage and CERAD neuritic plaque density
Group means were significantly different (P<0.0001)
Table 3.
Classification of subjects with phosphorylated α-synuclein histopathology by the Unified Staging System for Lewy body disorders
| Diagnosis | Olfactory bulb only (I) | Brainstem predominant (IIa) | Limbic predominant (IIb) | Brainstem and limbic (III) | Neocortical (IV) |
|---|---|---|---|---|---|
| ILBDa | 0 | 1 (17%) | 2 (33%) | 3 (50%) | 0 |
| PDa | 0 | 2 (12.5%) | 0 | 10 (62.5%) | 4 (25%) |
| DLB | 0 | 0 | 0 | 1 (11%) | 8 (89%) |
| ADLB | 2 (10%) | 3 (16%) | 11 (58%) | 3 (16%) | 0 |
Number and percentage of subjects in each stage are given
One subject was not classifiable due to missing brain regions needed for staging
Table 4.
Mean (SD) of phosphorylated α-synuclein histopathology regional brain density scores for all subjects by diagnostic classification, with an aggregate total brain load (last row) given by the sum of the mean regional density scores
| Brain region | ILBD | PD | DLB | ADLB |
|---|---|---|---|---|
| Olfactory bulb | 2.5 (1.0) | 2.6 (0.9) | 3.6 (1.1) | 3.0 (0.9) |
| Medulla | 1.6 (1.6) | 3.2 (0.7) | 3.4 (0.7) | 1.0 (1.2) |
| Pons | 1.1 (1.5) | 3.0 (0.9) | 3.1 (1.0) | 0.3 (0.6) |
| Midbrain | 0.4 (0.8) | 3.0 (0.9) | 2.9 (1.5) | 0.2 (0.7) |
| Amygdala | 2.5 (1.6) | 3.1 (0.9) | 3.9 (0.3) | 2.4 (1.7) |
| Transentorhinal | 0.8 (1.8) | 2.3 (1.1) | 3.6 (0.7) | 2.4 (1.8) |
| Cingulate cortex | 0.3 (0.8) | 1.8 (1.0) | 2.9 (1.2) | 0.3 (0.4) |
| Mid. temp. gyrus | 0.3 (0.5) | 1.2 (1.1) | 2.6 (0.9) | 0.2 (0.4) |
| Mid. front. gyrus | 0.3 (0.5) | 1.1 (0.9) | 1.7 (1.1) | 0.05 (0.2) |
| Inf. par. lobule | 0.3 (0.5) | 1.0 (1.0) | 1.8 (1.0) | 0.05 (0.2) |
| Sum of mean scores | 10.1 | 22.3 | 29.5 | 9.9 |
The distribution of PASH within the brain is summarized in Table 3 using the Unified Staging System for Lewy Body Disorders and the mean densities of PASH within the ten scored brain regions are given in Table 4. An estimate of the aggregate CNS load of microscopic disease was also determined, using the sum of the mean PASH density scores in all ten evaluated brain regions (Table 4). More than 80% of subjects with PD and DLB were in the two highest stages, involving either brainstem through limbic regions (Stage III) or brainstem through neocortical regions (Stage IV). Most subjects with ILBD were in the brainstem-predominant (IIa) or limbic-predominant (IIb) stages while the most frequent stage for ADLB subjects was limbic predominant (IIb). Subjects with ILBD and ADLB had similar overall low aggregate PASH load scores while subjects with PD and DLB had overall high aggregate PASH load scores.
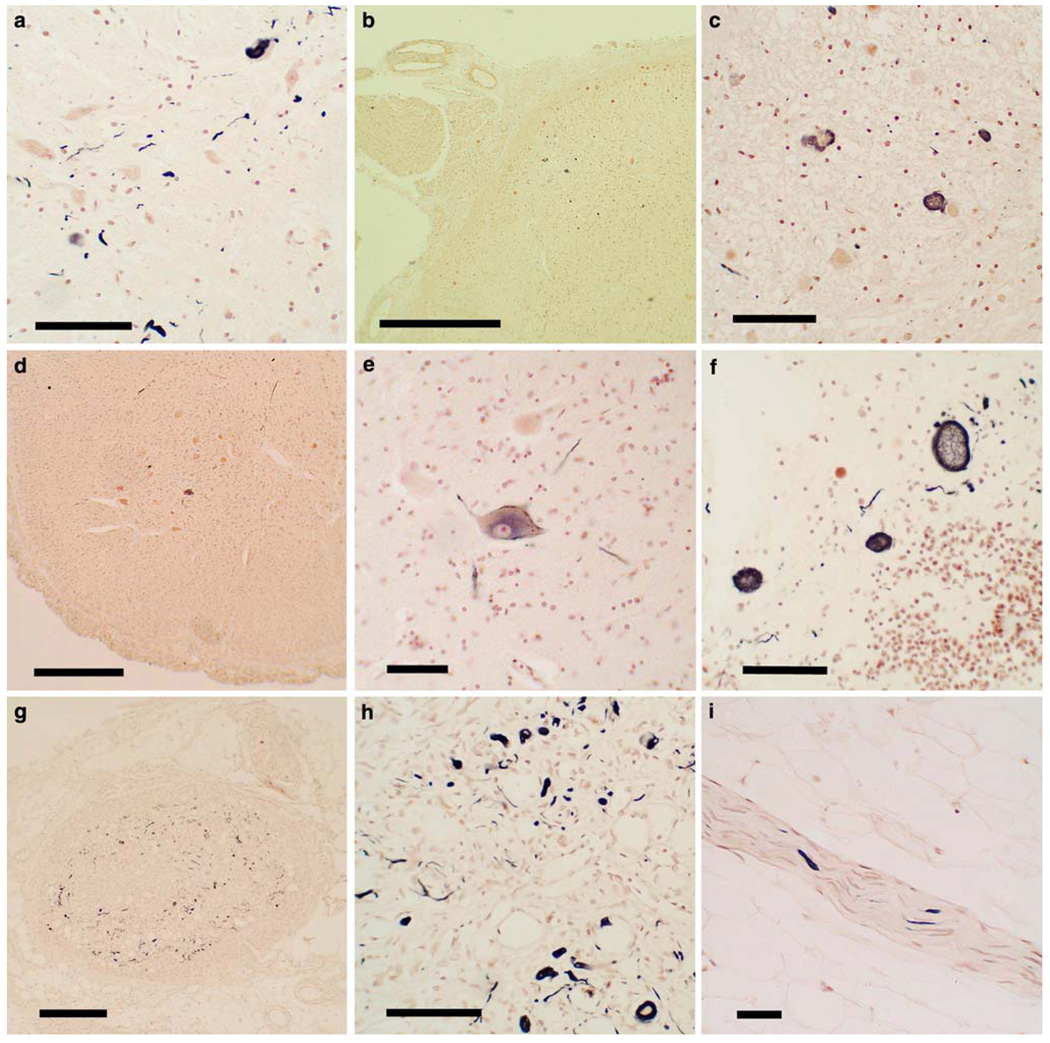
Figure 1 shows photomicrographs of the immunohistochemical staining for α-synuclein in the spinal cord, sympathetic ganglia and vagus nerve. Generally, sections with positive staining contained fibers, puncta and neuronal perikaryal staining (a, c, e, f, h) but occasionally sections or regions contained just fibers (i) or just perikaryal staining. The perikaryal staining was either diffusely distributed in the cytoplasm (e) or condensed into defined inclusions (h), only a subset of which resembled classical Lewy bodies. Within the vagus and peripheral nerves, and generally within nerves seen elsewhere in other body regions, positive staining was only in the form of nerve fibers (i). Within the spinal cord, positively-stained structures were found in all gray matter regions, including posterior horn (b, c), anterior horn (d, e), intermediolateral region (a) and adjacent to the central canal (f). Although not formally analyzed here, the most frequent regions affected appeared to be the thoracolumbar intermediolateral horn and the base of the posterior horn of the sacral cord.
Fig. 1.
Photomicrographs of slides of immunohistochemical staining for phosphorylated α-synuclein histopathology (PASH) in paraffin sections of the spinal cord, sympathetic ganglia and vagus nerve. Positive staining is black, the counterstain is neutral red. a The intermediolateral horn of the thoracic spinal cord of a subject with PD, showing immunoreactive fibers, puncta and perikaryal cytoplasmic inclusions. Calibration bar 80 µm. b, c Low and higher magnification images of the posterior root entry into the sacral spinal cord of a subject with DLB, showing immunoreactive neuronal perikaryal staining. Calibration bar in b 0.3 mm; in c 80 µm. d, e Low and higher magnification images of the anterior horn of the sacral spinal cord in a subject with PD, showing diffuse cytoplasmic perikaryal immunoreactivity of a large motorneuron. Calibration bar in d 0.5 mm; in e 60 µm. f Sacral spinal cord adjacent to the central canal of a subject with PD, showing immunoreactive swollen degenerating neurons or neurites. Calibration bar 80 µm. g, h Low and higher magnification images of a middle cervical sympathetic ganglion of a subject with DLB, showing frequent immunoreactive dystrophic neurites, puncta and neuronal perikaryal cytoplasmic inclusions. Calibration bar in g 0.1 mm; calibration bar in h 100 µm. i Small branch of the vagus nerve in a subject with PD, showing several immunoreactive nerve fibers. Calibration bar 60 µm
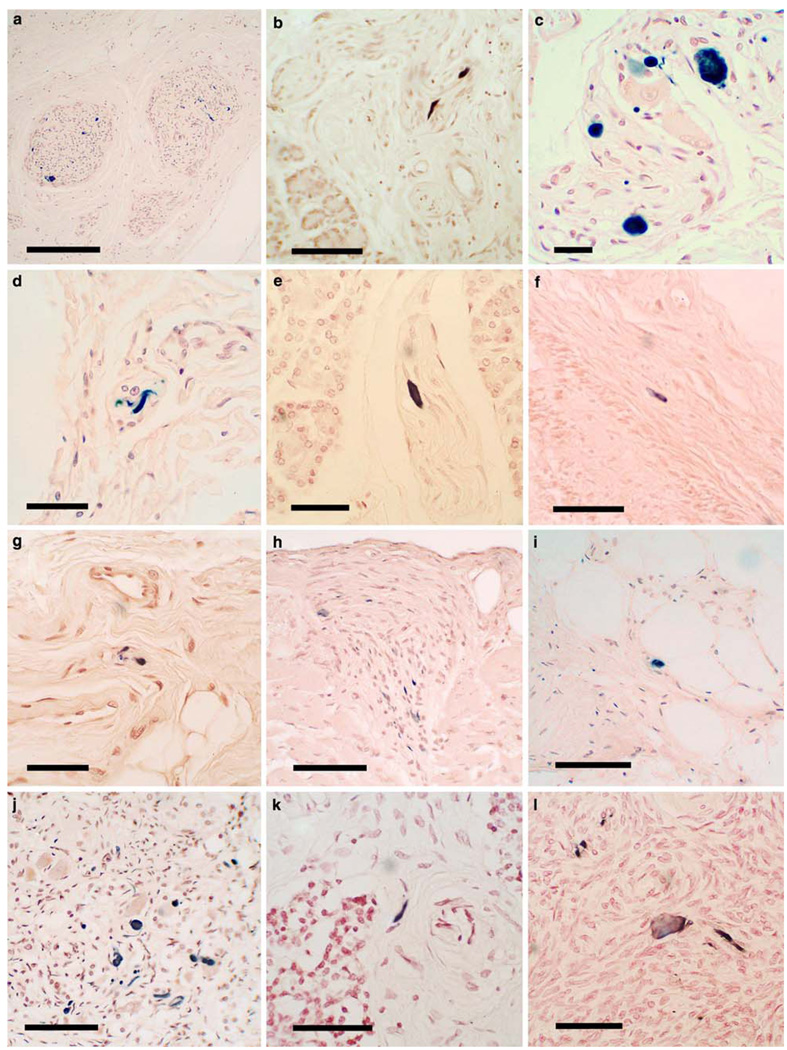
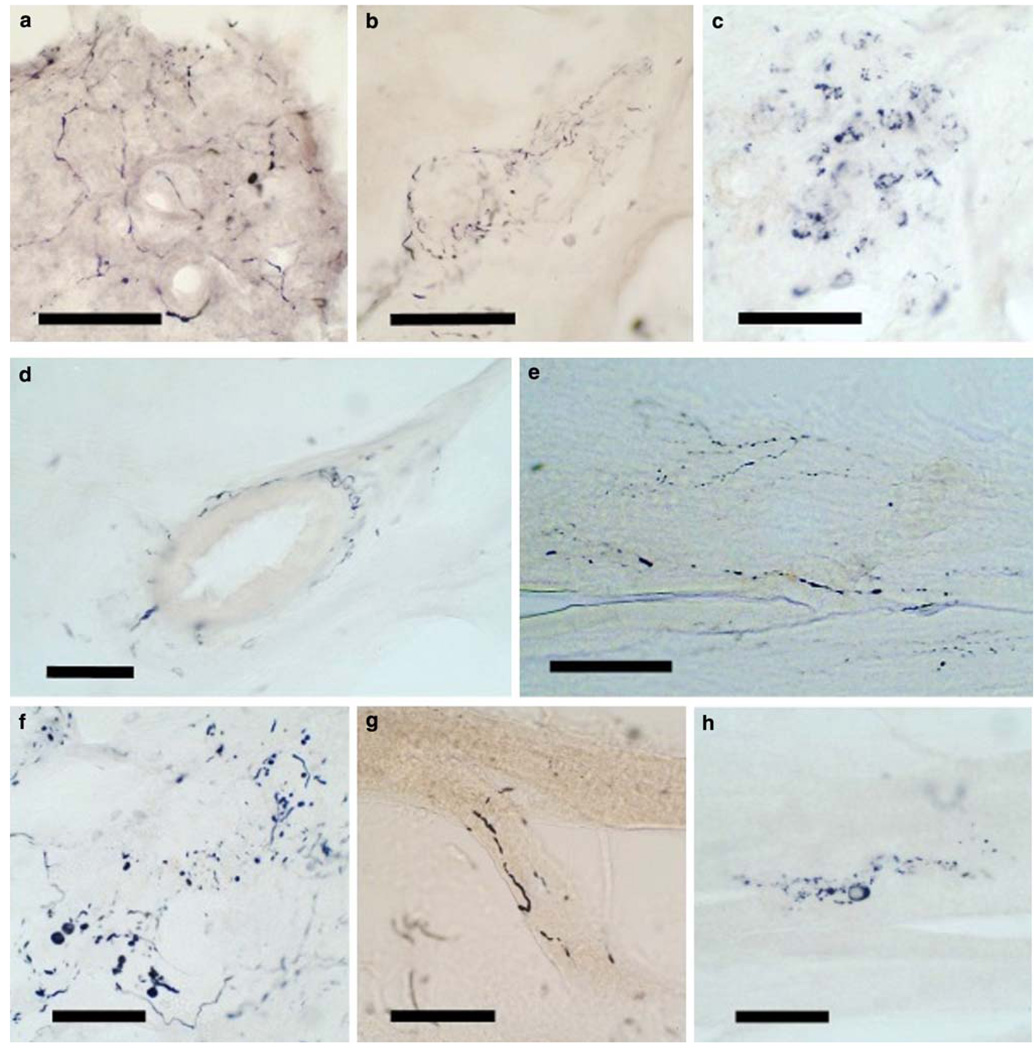
Figure 2 shows photomicrographs of the immunohistochemical staining for α-synuclein in multiple bodily organs and tissue types. As described for the spinal cord and sympathetic ganglia above, sections with positive staining contained either fibers, puncta or neuronal (ganglion cell) perikaryal staining, but ganglion cell staining was much less frequently seen, with the great majority of immunoreactive elements being fibers and puncta. A much more detailed morphologic delineation was seen in 80-µm thick sections of submandibular gland and lower esophagus (Fig. 3), where extensive fiber networks and moderately frequent ganglion cells were often visualized, especially in subjects with PD or DLB. Generally, immunoreactive structures in the gastrointestinal tract were concentrated in the myenteric plexus of Auerbach and the submucosal plexus of Meissner. In the submandibular gland and pancreas, positively stained elements tended to be concentrated in nerve bundles within the connective tissue stroma. In the submandibular gland, 80-µm section staining occasionally showed nerve fibers apparently investing arterioles (Fig. 3d). Adrenal gland staining was exclusively seen in the medulla and in nerve bundles in the surrounding fatty tissue, with no staining seen in the adrenal cortex.
Fig. 2.
Photomicrographs of immunohistochemical staining for phosphorylated α-synuclein in paraffin sections of bodily organs and tissue. Positive staining is black, the counterstain is neutral red. a, b Low and higher magnification images of the submandibular gland in two subjects with PD, showing immunoreactive nerve fibers within the stroma of the gland. Calibration bar in a 0.2 mm; in b 100 µm. c The submucosa of the lower esophagus of a subject with PD, showing immunoreactive puncta, fibers and perikaryal cytoplasmic inclusions in ganglion cells. d Immunostaining of fibers in the duodenal submucosa of a subjects with DLB. Calibration bar 20 µm. e A single immunoreactive fiber in the stroma of the pancreas from a subject with PD. Calibration bar 40 µm. f A single immunoreactive fiber in the submucosa of a primary bronchus of a subject with PD. Calibration bar 40 µm. g A few immunoreactive fibers in the submucosa of the larynx of a subject with PD. Calibration bar 20 µm. h Several immunoreactive fibers in an epicardial nerve twig entering the myocardium in a subject with DLB. Calibration bar 100 µm. i A single immunoreactive fiber in the intermyenteric plexus of the urinary bladder of a subject with DLB. Calibration bar 20 µm. j Frequent immunoreactive fibers, puncta as well as cells with diffusely stained perikaryal cytoplasm in the adrenal medulla of a subject with PD. Calibration bar 100 µm. k A single immunoreactive fiber in the stroma of the parathyroid gland of a subject with PD. Calibration bar 10 µm. l The ovary of a woman with PD showing diffuse perikaryal immunostaining of a neuron-like cell with adjacent immunoreactive fibers and puncta. Calibration bar 10 µm
Fig. 3.
Photomicrographs of immunohistochemical staining for phosphorylated α-synuclein in 80-µm thick frozen sections of formalin-fixed, cryoprotected tissue blocks of submandibular gland and lower esophagus. Positive staining is black. There is no counterstain. Submandibular gland from subjects with DLB (a–c) and PD (d) showing frequent immunoreactive fibers in the gland parenchyma (a), stroma (b) and around a small artery (d), while frequent immunoreactive puncta are seen in c. Lower esophagus from subjects with PD (e, g, h) and DLB (f) showing immunoreactive nerve fibers in e, nerve fibers and puncta in f, a thickened nerve fiber adherent to a bundle of smooth muscle fibers in g and a ganglion cell with diffuse perikaryal cytoplasmic staining in h
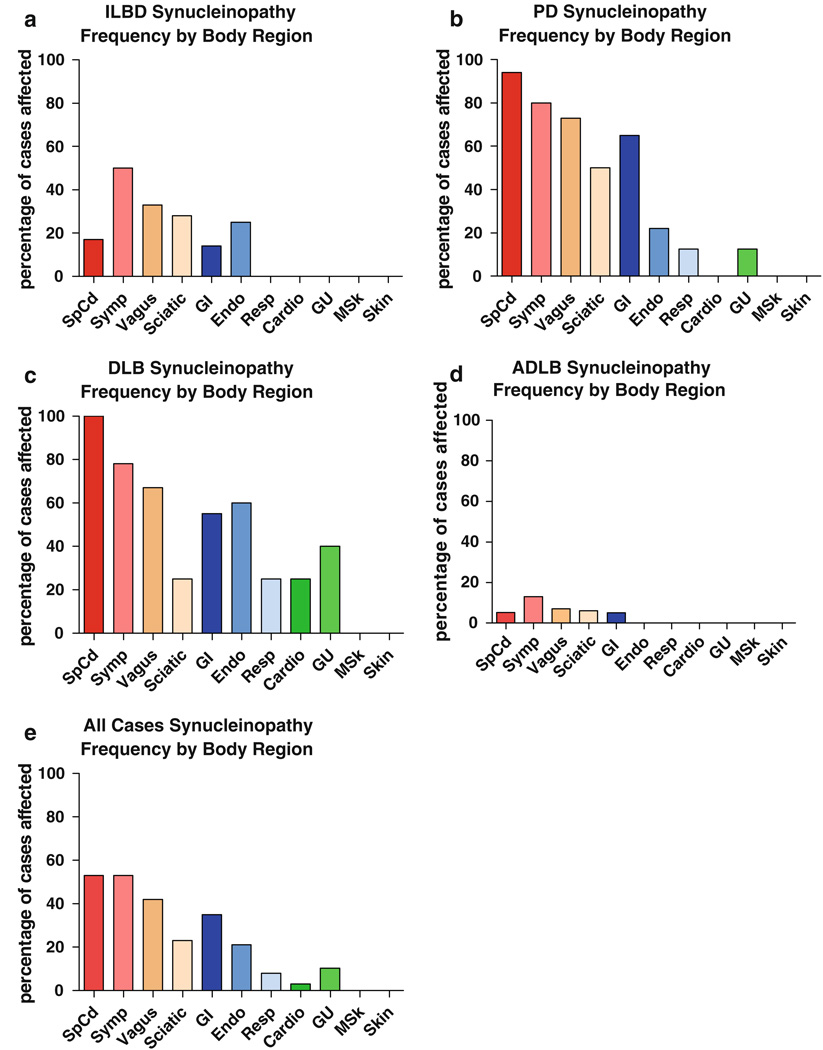
The regional distribution of PASH, as evaluated in single microscopic slides from each body site, is shown in Table 5 and Fig. 4. No immunoreactive elements were present in any subject that had previously been classified, on the basis of the brain examination, as being free of PASH. Subjects with DLB and PD had similar profiles (Fig. 4b, c) and will be discussed together. In both the groups, the most frequently affected body region was the spinal cord, with 25 of 26 subjects involved, followed by the sympathetic ganglia (19/24), the vagus nerve (15/21), the gastrointestinal system (16/26), the sciatic nerve (10/22) and endocrine system (5/14), with other organ systems and tissues following at generally much lower frequencies. No positive staining was observed in the abdominal skin in any of the 14 subjects with DLB or PD. Examination of additional sets of immunostained serial paraffin and 80-µm sections of submandibular gland and esophagus from DLB and PD subjects showed positive staining in 22 of 23 subjects for which the extra sections were stained.
Table 5.
Regional frequency of phosphorylated α-synuclein histopathology in single slides of different body regions, with subjects grouped by neuropathological diagnosis
| Dx | SpCd | Sym | Vagus | Sciat | GI | Resp | Endo | Cardio | GU | MSK | Skin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ILBD | 1/6a | 3/6 | 2/7 | 1/6 | 1/7 | 0/4 | 1/4 | 0/6 | 0/4 | N/A | 0/2 |
| PD | 16/17 | 12/15 | 11/15 | 8/16 | 11/17b | 1/8 | 2/9 | 0/9 | 1/8 | 0/6 | 0/8 |
| DLB | 9/9 | 7/9 | 4/6 | 2/8 | 5/9c | 1/4 | 3/5 | 1/4 | 2/5 | 0/3 | 0/3 |
| ADLB | 1/19 | 2/15 | 1/15 | 0/17 | 1/19d | 0/10 | 0/11 | 0/12 | 0/10 | 0/7 | 0/9 |
| All | 27/51 | 24/45 | 18/43 | 11/47 | 18/52 | 2/26 | 6/29 | 1/31 | 3/29 | 0/16 | 0/22 |
See Table 1 for listing of individual sites sampled and Fig. 4 for graphic representation; see supplementary online table for frequency of PASH within individual sites
Dx diagnosis, SpCd spinal cord, Sym sympathetic ganglia, Vagus vagus nerve, Sciat sciatic nerve, GI gastrointestinal system, Resp respiratory tract, Endo endocrine system, Cardio cardiovascular, GU genitourinary tract, MSK musculoskeletal
6/7 when multiple slides of paraffin-embedded spinal cord were examined
14/15 when multiple slides of paraffin-embedded and 80-µm frozen sections of esophagus and submandibular gland were examined
8/8 when multiple slides of paraffin-embedded and 80-µm frozen sections of esophagus and submandibular gland were examined
3/15 when multiple slides of paraffin-embedded and 80-µm frozen sections of esophagus and submandibular gland were examined
Fig. 4.
Relative frequency of PASH by diagnostic group for phosphorylated α-synuclein in different body regions including spinal cord, sympathetic ganglia, vagus nerve, sciatic nerve and multiple organs and tissues. Relative frequency is the percentage of subjects that showed immunoreactive tissue elements of any kind (fibers, puncta, perikaryal diffuse staining, perikaryal cytoplasmic inclusions) in single slides from each of the sites evaluated. For list of individual sites within each body region or organ system, see Table 1 and supplementary online table. Frequency was investigated further for some sites (see text)
Subjects with ILBD and ADLB had much lower frequencies of positive staining in body regions (Fig. 4a, d). In subjects with ADLB, positive staining was limited entirely to the spinal cord and sympathetic ganglia, with only 1/18 and 2/18 subjects affected, respectively. Subjects with ILBD showed higher frequencies of positive staining than ADLB subjects in the spinal cord, sympathetic ganglia, vagus nerve and gastrointestinal tract while all other areas, as with the ADLB cases, showed no positive staining. When several microscopic slides from each spinal cord subdivision were stained, 5/6 of the ILBD cases had PASH present while on single-slide analysis, only 1 of 6 subjects had been positive. For the ADLB subjects for which serial paraffin and 80-µm sections of submandibular gland and esophagus were examined, 3/15 were found to be positive, whereas examination of single paraffin sections had found no positively stained structures. Figure 4e depicts the regional staining distribution, derived from analysis of a single slide per region, when data from all subjects were combined. This shows generally the same pattern as for PD and DLB subjects.
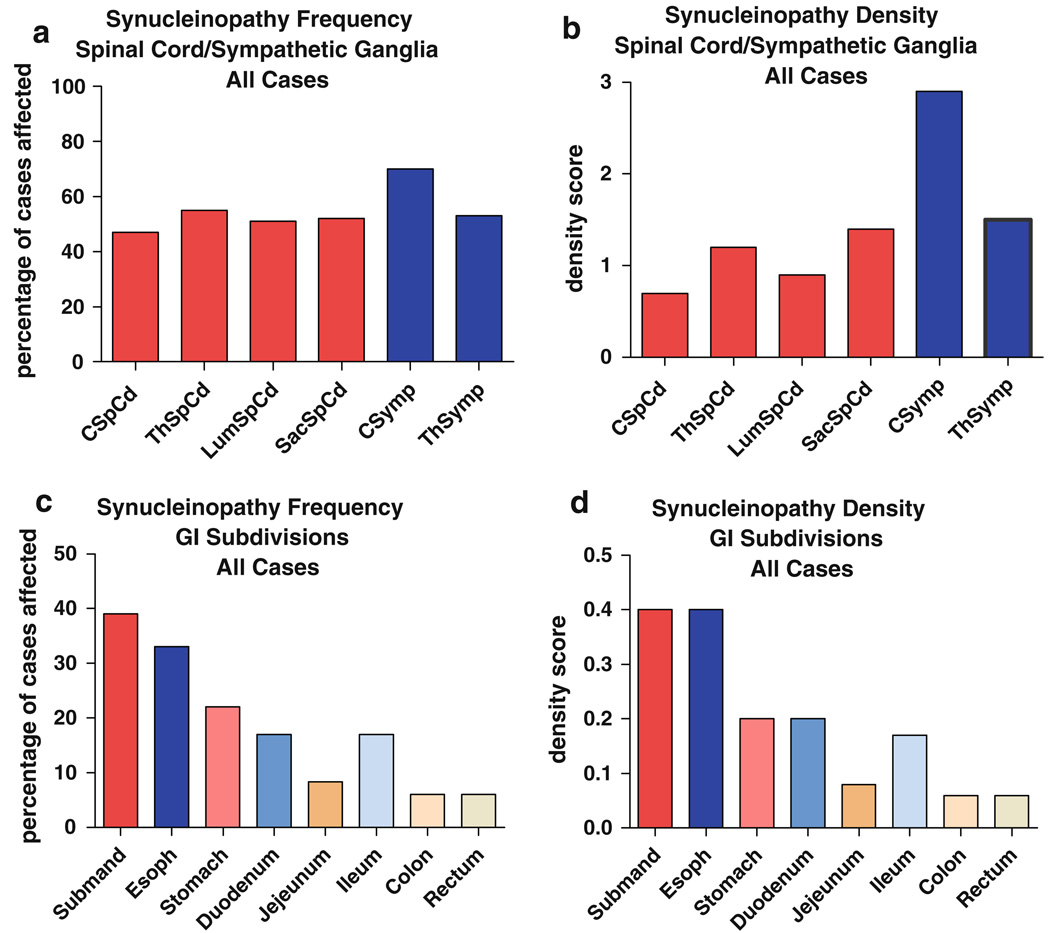
Table 6 and Fig. 5a and b show the regional distribution and density of PASH within paraffin sections on single slides of the major subdivisions of the spinal cord as well as cervical and thoracic sympathetic ganglia, for all subjects combined. The frequency of positive staining between different cord regions is similar, with the cervical cord being the least-often affected (Fig. 5a). In terms of the density scores, the thoracic and sacral cord regions have higher mean scores than the cervical and lumbar regions. Cervical sympathetic ganglia had the highest frequency and mean density scores of any region.
Table 6.
Frequency and mean (SD) density of with phosphorylated α-synuclein histopathology in single slides from major spinal cord and sympathetic nervous system subdivisions (all cases considered together)
| Cervical | Thoracic | Lumbar | Sacral | CSymp | ThSymp | |
|---|---|---|---|---|---|---|
| Frequency | 23/49 (47%) | 26/47 (55%) | 25/49 (51%) | 25/48 (52%) | 14/20 (70%) | 19/36 (53%) |
| Density score | 0.7 (0.9) | 1.2 (1.3) | 0.9 (1.1) | 1.4 (1.5) | 2.1 (1.6) | 1.5 (1.6) |
See Fig. 5 for a graphic representation
CSymp cervical sympathetic, ThSymp thoracic sympathetic
Fig. 5.
Relative frequency and density of PASH by diagnostic group in different spinal cord regions and sympathetic nervous system subdivisions. Relative frequency is the percentage of subjects that showed immunoreactive tissue elements of any kind (fibers, puncta, perikaryal diffuse staining, perikaryal cytoplasmic inclusions) in single slides taken from each of the sites evaluated. Density was calculated only for sites with immunoreactivity, negative or “zero” scores were not included
Table 7 and Fig. 5c and d show the regional distribution and density of PASH within major sites within the gastrointestinal (GI) system. There is a marked trend for a diminishing rostrocaudal gradient. The submandibular gland and lower esophagus have the highest frequency of PASH, followed by the stomach, small bowel regions, large bowel regions and rectum. The data shown for submandibular gland and esophagus do not include, to provide an unbiased comparison between GI regions, results from the serial paraffin or 80-µm sections. As mentioned previously, when staining from these extra sections was considered, a higher percentage of subjects were graded as positive. A striking finding was the complete absence of positive staining in upper esophagus and therefore all data for esophagus shown are derived from analysis of the lower esophagus. Although not quantitatively assessed here, it was apparent that PASH was more frequently present in the myenteric plexus than in the submucosal plexus.
Table 7.
Frequency of and mean density of with phosphorylated α-synuclein histopathology in single slides of subdivisions of the gastroin-testinal system (all cases considered together)
| Submand | Esoph | Stomach | Duodenum | Jejunum | Ileum | Colon | Rectum | |
|---|---|---|---|---|---|---|---|---|
| Frequency | 11/37 (39%) | 17/51 (33%) | 7/31 (22%) | 5/30 (17%) | 2/24 (8.3%) | 4/24 (17%) | 3/50 (6%) | 3/50 (6%) |
| Density score | 0.4 (0.7) | 0.4 (0.7) | 0.2 (0.4) | 0.2 (0.6) | 0.08 (0.28) | 0.17 (0.4) | 0.06 (0.2) | 0.06 (0.2) |
See Fig. 5 for a graphic representation
Submandib submandibular gland, esoph lower esophagus
Discussion
The results of this investigation support prior studies [12, 14, 17–19, 29, 40, 48, 81] that have indicated that PASH is widespread throughout the spinal cord and peripheral nervous system of subjects with PD. The present study adds to this body of knowledge by providing data for other Lewy body disorders including DLB, ILBD and ADLB, by surveying many more sites than had previously been investigated, and using large enough sample sizes to provide preliminary estimates of the relative frequency and density of α-synuclein histopathology at these locations. In addition, the use of a method that is specific and sensitive for phosphorylated α-synuclein, which is detected only in pathological structures [34] effectively eliminates the ambiguity of staining results derived using antibodies that recognize normal α-synuclein.
For spinal cord and sympathetic ganglia, subjects with PD as well as DLB invariably have PASH in the regions of the cord containing preganglionic autonomic neurons as well as within sympathetic ganglia. Because these neurons project widely throughout the body, it is highly probable that, for subjects with PD and DLB, that α-synuclein histopathology is also very widely present within the end-organ targets of the autonomic nervous system, although a full test of this hypothesis will require multiple-section examination of each of the many sites [49]. This study has found that the relative frequency of PASH in end-organs was generally much lower than in the spinal cord or sympathetic ganglia, but when sampling was expanded considerably through the use of multiple paraffin or 80-µm sections, such as was done for the submandibular gland and esophagus, the frequency of positive staining was increased.
For subjects with ILBD, PASH has a more limited distribution, being more likely to be confined to the spinal cord, sympathetic ganglia, vagus nerve and a subset of end-organs. Given the wide projections of the involved spinal preganglionic and sympathetic ganglion neurons, a wide but sparse involvement of the peripheral nervous system is likely to exist in ILBD, although again a more definitive investigation with multiple-section analysis will be required to confirm this. Subjects with ADLB have a very restricted frequency and distribution of PASH, with very sparse involvement outside the spinal cord and sympathetic ganglia, detectable only when multiple sections are examined.
Other groups have reported higher frequencies for PASH in selected body regions including skin, adrenal medulla, urinary bladder and cardiac epicardium [35, 43, 45, 61]. Each of these earlier studies stained multiple slides from each site, however, probably accounting for most of the differences from the present work, in which for most of these regions only a single slide was stained. Differing sites of sampling may also have been responsible for differing results, for example Iwanaga et al. [45] sampled the heart from around the coronary arteries while in the present study the cardiac apex was sampled. Differences in staining methods may also have contributed to the different findings. Minguez-Castellano et al. [61] used an antibody against normal (unphosphorylated) α-synuclein while in the present study the antibody was specific for α-synuclein phosphorylated at serine 129, which is found only in pathological α-synuclein deposits [34]. Most other groups have used formic acid pretreatment for epitope exposure while the present study used proteinase K pretreatment [10, 41]. Proteinase-K would theoretically destroy normal α-synuclein and thus further eliminate non-specific (non-pathological) staining.
A critical question has been whether or not α-synuclein histopathology begins in the brain or within elements of the peripheral nervous system [20, 38, 55]. The stimulus for this intriguing hypothesis has come largely from clinical studies of PD that have found a wide range of non-motor signs and symptoms that accompany the disease [4, 78]. Many of these non-motor accompaniments are related to dysfunction of the peripheral autonomic system. These may occur early in the motor progression and there is suggestive evidence that some may even occur in the premotor prologue [2, 3, 47, 66, 74]. The description of Lewy bodies within the sympathetic and parasympathetic ganglia, adrenal medulla and GI tract within autopsied subjects with PD [17, 21, 29, 35, 39, 50, 75] has shown that peripheral nervous system α-synuclein histopathology is certainly present but there has been insufficient data regarding the findings in prodromal phases of disease. Autopsy studies of relatively small numbers of subjects with ILBD have demonstrated a high prevalence of α-synuclein histopathology within the spinal cord, sympathetic ganglia, adrenal medulla and upper GI tract [12, 17, 21, 48, 61], consistent with accumulating reports of premotor autonomic dysfunction in PD [78], but only two subjects, out of more than a thousand examined in recent studies, have had α-synuclein histopathology in the spinal cord or peripheral nervous system in the absence of brain involvement. Fumimura et al. [35] reported one case out of 783 with adrenal medulla as the only site with α-synuclein histopathology, but the olfactory bulb was not examined. Miki et al. [60] reported a single subject with α-synuclein histopathology restricted to the heart and stellate ganglion; in this case, the olfactory bulb was examined. The present work is in general agreement with these prior studies as, of the 40 subjects without brain and olfactory bulb PASH, none had PASH within the spinal cord or peripheral nervous system sites sampled. However, owing to the relatively small sample size and single-section analysis at many sites, it cannot be excluded that α-synuclein pathology may rarely begin in the peripheral nervous system prior to CNS involvement.
A derivative of the “body-first” hypothesis has been the conjecture as to whether an exogenous pathogen might be the cause of disease and gain entry through peripheral nerve endings [20, 38], either through the olfactory epithelium or GI mucosa. The findings of the present study are not incompatible with a GI entry for PD, ILBD and DLB, but as subjects with ADLB have relatively rare or sparse involvement of the caudal neuraxis this seems unlikely for that group. The universal and primal involvement of the olfactory bulb in all of the Lewy body disorders [8, 11] is highly compatible with the exogenous pathogen hypothesis. If an exogenous pathogen is involved, whether it be a virus, micro-organism or toxin, if it was able to induce aggregation of α-synuclein in exposed neurons, this change could then be propagated throughout the remainder of the PNS and CNS, generating the observed brain and spinal cord regional pattern of α-synuclein histopathology through trans-synaptic transmission. Recently, there have been reports of PD subjects that have developed Lewy bodies within non-host neurons transplanted into the stri-atum more than a decade earlier, supporting the possibility that α-synuclein histopathology might be acquired and passed along from neuron to neuron [51–53, 57]. In relation to this, experimental studies have recently shown that aggregated α-synuclein may be transferred between neurons by endocytosis [26, 27, 30].
The rostrocaudal gradient of PASH within the gastrointestinal system is an interesting finding of the present work and confirms a previous report by Wakabayashi et al. [81] who mapped Lewy bodies in the alimentary tract of seven PD subjects using classical stains. The reason for this rostrocaudal gradient may be of interest. It could be due to the known distribution of vagal innervation, which extends only as far as the proximal colon and which has been documented to be more heavily distributed to the lower esophagus and stomach than to the small bowel or proximal colon [25, 42]. If so, this would suggest that, for the gastrointestinal tract, α-synuclein histopathology within vagal efferents predominates over α-synuclein histopathology originating from sympathetic ganglia or from enteric neurons. However, as Lewy bodies and α-synuclein histopathology have been demonstrated to occur within sympathetic ganglia as well as intrinsic neurons of the enteric nervous system [17, 54, 69, 80, 81] an alternative explanation is that either or both of these have a selective rostrocaudal vulnerability to α-synuclein histopathology. A notable exception to the rostrocaudal α-synuclein histopathology gradient is the upper esophagus, which on single-slide analysis was always negative for PASH. This may be due to the fact that while most of the vagal innervation of the GI tract is derived from neuronal cell bodies located in the dorsal motor nucleus of the vagus, the cell bodies giving rise to the vagal innervation of the upper esophagus arise in the nucleus ambiguus. The former develop α-synuclein histopathology but the latter do not [20].
Spinal cord involvement with α-synuclein histopathology has usually been found to be concentrated in the preganglionic parasympathetic cell columns of the thoracolumbar intermediolateral horn and its clinical expression has, therefore, been assumed to be mainly autonomic dysfunction, but there have also been reports localizing substantial α-synuclein histopathology to the dorsal horn, where this might conceivably be a cause of neuropathic pain, and to the ventral horn, where it may affect motor neuron function [12, 21, 48, 63, 79]. The findings of the present study support these earlier works, as PASH, although most densely and frequently seen within the intermediolateral horn, was also not uncommonly present within posterior and anterior horns. The large size of some affected anterior horn neurons is consistent with involvement of small numbers of α-motor neurons, a surprising finding because there are, to our knowledge, no prior reports of Lewy bodies or α-synuclein histopathology in either spinal cord or brainstem motor neurons. Several electrophysiological studies, however, have been consistent with mild spinal motor neuron disease in PD, with evidence of motor unit reinnervation and dropout [22–24, 72, 73].
To our knowledge, this is the first report of α-synuclein histopathology within many of the sites studied. Of these, the most interesting location was the submandibular gland, which, together with the lower esophagus, was the most frequent non-neural peripheral structure affected. Sialorrhea, or drooling, has long been recognized as a characteristic sign of PD. Rather than being due to overproduction of saliva, however, sialorrhea appears to be primarily due to decreased swallowing and hence oral accumulation, and that secretion of saliva is actually decreased in subjects with PD [6, 7, 32, 56, 65, 67, 68, 76]. As both sympathetic and parasympathetic innervation of salivary glands have stimulatory effects on saliva production, it is possible that α-synuclein histopathology within either or both of these could be responsible for the decreased production observed in PD. Of further and perhaps more practical interest is the accessibility of the submandibular gland to biopsy, which could theoretically improve the low clinical diagnostic accuracy for early PD and DLB.
Supplementary Material
Acknowledgments
This research is supported by Grants to the Sun Health Research Institute Brain and Body Donation Program and the Arizona Parkinson’s Disease Consortium by the Michael J. Fox Foundation for Parkinson’s Research (The Prescott Family Initiative), the Arizona Biomedical Research Commission (contracts 4001, 0011 and 05-901) and the National Institute on Aging (Arizona Alzheimer’s Disease Consortium, P30 AG19610). We would like to thank other members of the Arizona Parkinson’s Disease Consortium, including Erika Driver-Dunckley, MD, Virgilio Evidente, MD, and Donald Connor, PhD.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00401-010-0664-3) contains supplementary material, which is available to authorized users.
Contributor Information
Thomas G. Beach, Sun Health Research Institute, 10515 West Santa Fe Drive, Sun City, AZ 85351, USA thomas.beach@bannerhealth.com
Charles H. Adler, Mayo Clinic, Scottsdale, AZ, USA
Lucia I. Sue, Sun Health Research Institute, 10515 West Santa Fe Drive, Sun City, AZ 85351, USA
Linda Vedders, Sun Health Research Institute, 10515 West Santa Fe Drive, Sun City, AZ 85351, USA.
LihFen Lue, Sun Health Research Institute, 10515 West Santa Fe Drive, Sun City, AZ 85351, USA.
Charles L. White, III, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Haru Akiyama, Tokyo Institute of Psychiatry, Tokyo, Japan.
John N. Caviness, Mayo Clinic, Scottsdale, AZ, USA
Holly A. Shill, Sun Health Research Institute, 10515 West Santa Fe Drive, Sun City, AZ 85351, USA
Marwan N. Sabbagh, Sun Health Research Institute, 10515 West Santa Fe Drive, Sun City, AZ 85351, USA
Douglas G. Walker, Sun Health Research Institute, 10515 West Santa Fe Drive, Sun City, AZ 85351, USA
References
- 1.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 2.Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, Popper JS, Ross GW. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57:456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- 3.Abbott RD, Ross GW, Petrovitch H, Tanner CM, Davis DG, Masaki KH, Launer LJ, Curb JD, White LR. Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord. 2007 doi: 10.1002/mds.21560. [DOI] [PubMed] [Google Scholar]
- 4.Adler CH. Nonmotor complications in Parkinson’s disease. Mov Disord. 2005;20 Suppl 11:S23–S29. doi: 10.1002/mds.20460. [DOI] [PubMed] [Google Scholar]
- 5.Adler CH, Hentz JG, Joyce JN, Beach T, Caviness JN. Motor impairment in normal aging, clinically possible Parkinson’s disease, and clinically probable Parkinson’s disease: longitudinal evaluation of a cohort of prospective brain donors. Parkinsonism Relat Disord. 2002;9:103–110. doi: 10.1016/s1353-8020(02)00012-3. [DOI] [PubMed] [Google Scholar]
- 6.Bagheri H, Damase-Michel C, Lapeyre-Mestre M, Cismondo S, O’Connell D, Senard JM, Rascol O, Montastruc JL. A study of salivary secretion in Parkinson’s disease. Clin Neuropharmacol. 1999;22:213–215. [PubMed] [Google Scholar]
- 7.Bateson MC, Gibberd FB, Wilson RS. Salivary symptoms in Parkinson disease. Arch Neurol. 1973;29:274–275. doi: 10.1001/archneur.1973.00490280086013. [DOI] [PubMed] [Google Scholar]
- 8.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CLIII, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, Dickson DW, Roncaroli F, Buttini M, Hladik CL, Sue LI, Noorigian JV, Adler CH. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol. 2008;116:277–288. doi: 10.1007/s00401-008-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beach TG, White CLIII, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, Sue LI, Sasse J, Bachalakuri J, Henry-Watson J, Akiyama H, Adler CH. Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol. 2009;117:169–174. doi: 10.1007/s00401-008-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 13.Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex. 1994;4:138–150. doi: 10.1093/cercor/4.2.138. [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K. Stanley Fahn Lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord. 2006;21:2042–2051. doi: 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;1:213–216. doi: 10.1111/j.1750-3639.1991.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 16.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 17.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auer-bach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Braak H, Del Tredici K. Invited Article: nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- 19.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 20.Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 21.Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 2007;113:421–429. doi: 10.1007/s00401-007-0193-x. [DOI] [PubMed] [Google Scholar]
- 22.Brait K, Fahn S, Schwarz GA. Sporadic and familial parkinsonism and motor neuron disease. Neurology. 1973;23:990–1002. doi: 10.1212/wnl.23.9.990. [DOI] [PubMed] [Google Scholar]
- 23.Caviness JN, Smith BE, Clarke SJ, Adler CH, Caselli RJ, Hentz JG, Manfred MS, Muenter D. Motor unit number estimates in idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2002;8:161–164. doi: 10.1016/s1353-8020(01)00007-4. [DOI] [PubMed] [Google Scholar]
- 24.Caviness JN, Smith BE, Stevens JC, Adler CH, Caselli RJ, Reiners CA, Hentz JG, Muenter MD. Motor unit changes in sporadic idiopathic Parkinson’s disease. Mov Disord. 2000;15:238–243. doi: 10.1002/1531-8257(200003)15:2<238::aid-mds1006>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 25.Christensen J, Stiles MJ, Rick GA, Sutherland J. Comparative anatomy of the myenteric plexus of the distal colon in eight mammals. Gastroenterology. 1984;86:706–713. [PubMed] [Google Scholar]
- 26.Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danzer KM, Krebs SK, Wolff M, Birk G, Hengerer B. Seeding induced by alpha-synuclein oligomers provides evidence for spreading of alpha-synuclein pathology. J Neurochem. 2009;111:192–203. doi: 10.1111/j.1471-4159.2009.06324.x. [DOI] [PubMed] [Google Scholar]
- 28.Del Tredici K, Rub U, de Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 29.den Hartog Jager WA, Bethlem J. The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiatry. 1960;23:283–290. doi: 10.1136/jnnp.23.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002;52:205–210. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 32.Edwards LL, Quigley EM, Harned RK, Hofman R, Pfeiffer RF. Characterization of swallowing and defecation in Parkinson’s disease. Am J Gastroenterol. 1994;89:15–25. [PubMed] [Google Scholar]
- 33.Fujishiro H, Tsuboi Y, Lin WL, Uchikado H, Dickson DW. Co-localization of tau and alpha-synuclein in the olfactory bulb in Alzheimer’s disease with amygdala Lewy bodies. Acta Neuropathol. 2008;116:17–24. doi: 10.1007/s00401-008-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. Alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 35.Fumimura Y, Ikemura M, Saito Y, Sengoku R, Kanemaru K, Sawabe M, Arai T, Ito G, Iwatsubo T, Fukayama M, Mizusawa H, Murayama S. Analysis of the adrenal gland is useful for evaluating pathology of the peripheral autonomic nervous system in Lewy body disease. J Neuropathol Exp Neurol. 2007;66:354–362. doi: 10.1097/nen.0b013e3180517454. [DOI] [PubMed] [Google Scholar]
- 36.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 37.Giasson BI, Duda JE, Forman MS, Lee VM, Trojanowski JQ. Prominent perikaryal expression of alpha- and beta-synuclein in neurons of dorsal root ganglion and in medullary neurons. Exp Neurol. 2001;172:354–362. doi: 10.1006/exnr.2001.7805. [DOI] [PubMed] [Google Scholar]
- 38.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hishikawa N, Hashizume Y, Hirayama M, Imamura K, Washimi Y, Koike Y, Mabuchi C, Yoshida M, Sobue G. Brainstemtype Lewy body disease presenting with progressive autonomic failure and lethargy. Clin Auton Res. 2000;10:139–143. doi: 10.1007/BF02278018. [DOI] [PubMed] [Google Scholar]
- 40.Hishikawa N, Hashizume Y, Yoshida M, Sobue G. Clinical and neuropathological correlates of Lewy body disease. Acta Neuropathol. 2003;105:341–350. doi: 10.1007/s00401-002-0651-4. [DOI] [PubMed] [Google Scholar]
- 41.Hladik CL, White CL. Comparison of digestive enzyme and formic acid pretreatment for optimal immunohistochemical demonstration of alpha-synuclein-immunoreactive cerebral cortical Lewy neurites. J Neuropathol Exp Neurol. 2003;62:554. [Google Scholar]
- 42.Hopkins DA, Bieger D, deVente J, Steinbusch WM. Vagal efferent projections: viscerotopy, neurochemistry and effects of vagotomy. Prog Brain Res. 1996;107:79–96. doi: 10.1016/s0079-6123(08)61859-2. [DOI] [PubMed] [Google Scholar]
- 43.Ikemura M, Saito Y, Sengoku R, Sakiyama Y, Hatsuta H, Kanemaru K, Sawabe M, Arai T, Ito G, Iwatsubo T, Fukayama M, Murayama S. Lewy body pathology involves cutaneous nerves. J Neuropathol Exp Neurol. 2008;67:945–953. doi: 10.1097/NEN.0b013e318186de48. [DOI] [PubMed] [Google Scholar]
- 44.Iseki E, Marui W, Kosaka K, Akiyama H, Ueda K, Iwatsubo T. Degenerative terminals of the perforant pathway are human alpha-synuclein-immunoreactive in the hippocampus of patients with diffuse Lewy body disease. Neurosci Lett. 1998;258:81–84. doi: 10.1016/s0304-3940(98)00856-8. [DOI] [PubMed] [Google Scholar]
- 45.Iwanaga K, Wakabayashi K, Yoshimoto M, Tomita I, Satoh H, Takashima H, Satoh A, Seto M, Tsujihata M, Takahashi H. Lewy body-type degeneration in cardiac plexus in Parkinson’s and incidental Lewy body diseases. Neurology. 1999;52:1269–1271. doi: 10.1212/wnl.52.6.1269. [DOI] [PubMed] [Google Scholar]
- 46.Jellinger KA. Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm. 2004;111:1219–1235. doi: 10.1007/s00702-004-0138-7. [DOI] [PubMed] [Google Scholar]
- 47.Kaufmann H, Nahm K, Purohit D, Wolfe D. Autonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodies. Neurology. 2004;63:1093–1095. doi: 10.1212/01.wnl.0000138500.73671.dc. [DOI] [PubMed] [Google Scholar]
- 48.Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, DeLucia MW, Dickson DW. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology. 2006;66:1100–1102. doi: 10.1212/01.wnl.0000204179.88955.fa. [DOI] [PubMed] [Google Scholar]
- 49.Knowles CH, De GR, Kapur RP, Bruder E, Farrugia G, Geboes K, Gershon MD, Hutson J, Lindberg G, Martin JE, Meier-Ruge WA, Milla PJ, Smith VV, Vandervinden JM, Veress B, Wedel T. Gastrointestinal neuromuscular pathology: guidelines for histological techniques and reporting on behalf of the Gastro 2009 International Working Group. Acta Neuropathol. 2009;118:271–301. doi: 10.1007/s00401-009-0527-y. [DOI] [PubMed] [Google Scholar]
- 50.Koike Y, Takahashi A. Autonomic dysfunction in Parkinson’s disease. Eur Neurol. 1997;38 Suppl 2:8–12. doi: 10.1159/000113470. [DOI] [PubMed] [Google Scholar]
- 51.Kordower JH, Brundin P. Lewy body pathology in long-term fetal nigral transplants: is Parkinson’s disease transmitted from one neural system to another? Neuropsychopharmacology. 2009;34:254. doi: 10.1038/npp.2008.161. [DOI] [PubMed] [Google Scholar]
- 52.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 53.Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov Disord. 2008;23:2303–2306. doi: 10.1002/mds.22369. [DOI] [PubMed] [Google Scholar]
- 54.Kupsky WJ, Grimes MM, Sweeting J, Bertsch R, Cote LJ. Parkinson’s disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology. 1987;37:1253–1255. doi: 10.1212/wnl.37.7.1253. [DOI] [PubMed] [Google Scholar]
- 55.Langston JW. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- 56.Leopold NA, Kagel MC. Pharyngo-esophageal dysphagia in Parkinson’s disease. Dysphagia. 1997;12:11–18. doi: 10.1007/pl00009512. [DOI] [PubMed] [Google Scholar]
- 57.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 58.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del ST, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 59.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 60.Miki Y, Mori F, Wakabayashi K, Kuroda N, Orimo S. Incidental Lewy body disease restricted to the heart and stellate ganglia. Mov Disord. 2009;24:2299–2301. doi: 10.1002/mds.22775. [DOI] [PubMed] [Google Scholar]
- 61.Minguez-Castellanos A, Chamorro CE, Escamilla-Sevilla F, Ortega-Moreno A, Rebollo AC, Gomez-Rio M, Concha A, Munoz DG. Do alpha-synuclein aggregates in autonomic plexuses predate Lewy body disorders? A cohort study. Neurology. 2007;68:2012–2018. doi: 10.1212/01.wnl.0000264429.59379.d9. [DOI] [PubMed] [Google Scholar]
- 62.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 63.O’Sullivan SS, Holton JL, Massey LA, Williams DR, Revesz T, Lees AJ. Parkinson’s disease with Onuf’s nucleus involvement mimicking multiple system atrophy. J Neurol Neurosurg Psychiatry. 2008;79:232–234. doi: 10.1136/jnnp.2007.133314. [DOI] [PubMed] [Google Scholar]
- 64.Parkkinen L, Pirttila T, Alafuzoff I. Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol. 2008;115:399–407. doi: 10.1007/s00401-008-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Persson M, Osterberg T, Granerus AK, Karlsson S. Influence of Parkinson’s disease on oral health. Acta Odontol Scand. 1992;50:37–42. doi: 10.3109/00016359209012744. [DOI] [PubMed] [Google Scholar]
- 66.Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003;2:107–116. doi: 10.1016/s1474-4422(03)00307-7. [DOI] [PubMed] [Google Scholar]
- 67.Pinnington LL, Muhiddin KA, Ellis RE, Playford ED. Non-invasive assessment of swallowing and respiration in Parkinson’s disease. J Neurol. 2000;247:773–777. doi: 10.1007/s004150070091. [DOI] [PubMed] [Google Scholar]
- 68.Proulx M, de Courval FP, Wiseman MA, Panisset M. Salivary production in Parkinson’s disease. Mov Disord. 2005;20:204–207. doi: 10.1002/mds.20189. [DOI] [PubMed] [Google Scholar]
- 69.Qualman SJ, Haupt HM, Yang P, Hamilton SR. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology. 1984;87:848–856. [PubMed] [Google Scholar]
- 70.Saito Y, Kawashima A, Ruberu NN, Fujiwara H, Koyama S, Sawabe M, Arai T, Nagura H, Yamanouchi H, Hasegawa M, Iwatsubo T, Murayama S. Accumulation of phosphorylated alpha-synuclein in aging human brain. J Neuropathol Exp Neurol. 2003;62:644–654. doi: 10.1093/jnen/62.6.644. [DOI] [PubMed] [Google Scholar]
- 71.Saito Y, Ruberu NN, Sawabe M, Arai T, Kazama H, Hosoi T, Yamanouchi H, Murayama S. Lewy body-related alpha-synucleinopathy in aging. J Neuropathol Exp Neurol. 2004;63:742–749. doi: 10.1093/jnen/63.7.742. [DOI] [PubMed] [Google Scholar]
- 72.Sica RE, Herskovits E, Aguilera N, Poch G. An electro-physiological investigation of skeletal muscle in Parkinson’s disease. J Neurol Sci. 1973;18:411–420. doi: 10.1016/0022-510x(73)90135-4. [DOI] [PubMed] [Google Scholar]
- 73.Sica RE, Sanz OP. An electrophysiological study of the functional changes in the spinal motoneurones in Parkinson’s disease. Electromyogr Clin Neurophysiol. 1976;16:409–417. [PubMed] [Google Scholar]
- 74.Siddiqui MF, Rast S, Lynn MJ, Auchus AP, Pfeiffer RF. Autonomic dysfunction in Parkinson’s disease: a comprehensive symptom survey. Parkinsonism Relat Disord. 2002;8:277–284. doi: 10.1016/s1353-8020(01)00052-9. [DOI] [PubMed] [Google Scholar]
- 75.Takeda S, Yamazaki K, Miyakawa T, Arai H. Parkinson’s disease with involvement of the parasympathetic ganglia. Acta Neuropathol. 1993;86:397–398. doi: 10.1007/BF00369454. [DOI] [PubMed] [Google Scholar]
- 76.Tumilasci OR, Cersosimo MG, Belforte JE, Micheli FE, Benarroch EE, Pazo JH. Quantitative study of salivary secretion in Parkinson’s disease. Mov Disord. 2006;21:660–667. doi: 10.1002/mds.20784. [DOI] [PubMed] [Google Scholar]
- 77.Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–697. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient-reported autonomic symptoms in Parkinson disease. Neurology. 2007;69:333–341. doi: 10.1212/01.wnl.0000266593.50534.e8. [DOI] [PubMed] [Google Scholar]
- 79.Wakabayashi K, Takahashi H. The intermediolateral nucleus and Clarke’s column in Parkinson’s disease. Acta Neu-ropathol. 1997;94:287–289. doi: 10.1007/s004010050705. [DOI] [PubMed] [Google Scholar]
- 80.Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990;79:581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- 81.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988;76:217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- 82.Zaccai J, Brayne C, McKeith I, Matthews F, Ince PG. Patterns and stages of alpha-synucleinopathy: relevance in a population-based cohort. Neurology. 2008;70:1042–1048. doi: 10.1212/01.wnl.0000306697.48738.b6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.