Abstract
To assess the utility and precision of GFR measurements in multicenter trials, the test performance and variability of GFR were analyzed in 2,250 patients enrolled in 44 clinical centers participating in either the Modification of Diet in Renal Disease (MDRD) Study or the Diabetes Control and Complications Trial (DCCT). GFR was measured as the renal clearance of (125I)iothalamate after an sc injection without epinephrine. The studies used similar protocols for obtaining blood and urine, training clinical center staff, and processing specimens in central laboratories. The performance of GFR measurements, assessed from adherence to protocol and quality control analyses, was excellent. The variability among the four clearance periods (intratest coefficient of variation (CV)) was acceptable; the median intratest CV for GFR was 9.4% in the MDRD Study and 11.7% in the DCCT. The pattern of decline in serum counts was better approximated by an exponential rather than a linear relationship. The cause of the intratest variability in GFR measurements was explored by univariate and multivariate analysis. The intratest CV was highest at the extremes of GFR. Among patients with a high GFR (>90 mL/min per 1.73 m2), most of whom were participants in the DCCT, the higher intratest GFR was due, in part, to a systematic decline in GFR during the test. Among patients with a very low GFR (<13 mL/min per 1.73 m2), technical difficulties in urine collections contributed substantially to the higher intratest CV. Other patient characteristics, including age, gender, weight, serum glucose, renal diagnosis, and use of diuretics, were not strongly correlated with the intratest CV. The precision of GFR measurements was assessed from the variability from measurement to measurement (intertest CV). Among MDRD Study subjects, in whom two measurements of GFR were performed over a 3-month interval, the median intertest CV was relatively low (6.3%) and was only weakly related to the intratest CV. Thus, GFR measurements are reasonably precise, even if the intratest CV is high. Given the relatively high intratest CV that is characteristic of GFR measurements, the estimate of GFR in an individual is more precise if multiple clearance periods, rather than a single period, are included. Similarly, the estimate of mean GFR for a population is also more precise if multiple clearance periods are included. In conclusion, by the use of standardized methods, an acceptable precision of GFR results can be obtained in multicenter trials. The same methods can be applied in clinical practice. The usefulness of GFR measurements in practice depends, in part, on the results of these and other ongoing clinical trials investigating therapeutic interventions to prevent the onset or retard the progression of renal disease.
Keywords: Renal function, GFR, diabetes, chronic renal disease, clinical trial
The growing interest in assessing therapies to slow the progression of renal disease and the recognition of the potential limitations of serum creatinine as an index of renal function have renewed attention to measuring GFR. Although numerous studies have documented the utility of radioisotope-labeled filtration markers of renal clearance (1), only recently have these markers been used in multicenter clinical trials. The objective of our report is to describe the experience of two large trials sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) in which GFR is measured as the renal clearance of [125I]iothalamate. The analysis of these measurements, performed in 2,250 patients in 44 clinical centers, provides an opportunity to assess the feasibility of GFR measurements in clinical trials, the sources of intratest variability of GFR measurements, and the relationship of intratest variability to the precision of GFR results. The experience also permits us to make recommendations for the performance of GFR measurements in clinical trials and in practice.
METHODS
Objectives and Analysis Plan
We had three objectives. (1) To assess the feasibility of GFR measurements in clinical trials, we analyzed the performance of the measurements, including adherence to protocol, quality control, changes in components of the measurement during clearance periods, and intratest variability across clinical centers. (2) To assess sources of the intratest variability of GFR results, we analyzed the relationship of the intratest coefficient of variation (CV) of GFR to components of the clearance measurement, autocorrelation among clearance periods, and the contribution of patient characteristics by univariate and multivariate techniques. (3) To assess the relationship of intratest variability to the precision of GFR measurements, we examined changes in the intratest CV over time, the relationship of the intratest CV to the intertest CV. and the relationship of the number of clearance periods to the intertest CV and to the interindividual (population) mean and CV.
Patients
This report includes GFR measurements in 2,250 patients who were participating in two NIDDK-sponsored multicenter trials and who were undergoing their first measurement of GFR in the trial. Of these patients, 1,760 were enrolled in the Modification of Diet in Renal Disease (MDRD) Study, a controlled clinical trial of the effects of low-protein and low-phosphorus diets and lower than usual blood pressure on the progression of renal disease (2–4). Entry requirements were as follows: age, 18 to 70 yr; chronic renal disease; mean arterial pressure less than 125 mm Hg; and reduced renal function, as judged by either an elevated serum creatinine level (1.2 to 7.0 mg/dL in women or 1.4 to 7.0 mg/dL in men) or reduced creatinine clearance (<70 mL/min per 1.73 m2). Patients with Type 1 diabetes mellitus were excluded. Patients were classified by provisional renal diagnosis obtained solely from a limited chart review. This report includes the first (N = 1.760) and second (N= 1.065) measurements of GFR during the baseline period. (Some patients [N = 152] entered the baseline period a second time. This report includes results from only their first baseline period measurements.)
One thousand four hundred forty-one patients were randomized in the Diabetes Control and Complications Trial (DCCT), a controlled clinical trial of the effects of intensive insulin therapy (three or more daily injections of insulin or a continuous sc infusion of insulin, designed to achieve glucose bevels as close to normal as possible) on the development and progression of microvascular complications of Type 1 diabetes (5,6). Entry requirements for all patients included age of 13 to 39 yr, Type 1 diabetes with a duration of diabetes of 1 to 15 yr. normal renal function as judged by a serum creatinine level of 1.2 mg/dL or less or a creatinine clearance of 100 mL/min per 1.73 m2 or more, and blood pressure less than 140/90 mm Hg. Additionally, the primary prevention cohort had diabetes of only 1 to 5 yr in duration, no retinopathy, and a urine albumin excretion rate of less than 2.8 μg/min at baseline. The secondary intervention cohort had diabetes of 5 to 15 yr in duration, minimal retinopathy (<P2 according to the modified Airlie House criteria [7]), and a urine albumin excretion rate of less than 13.8 μg/min at baseline. The GFR protocol was implemented after the DCCT was already in progress. This report includes both baseline GFR measurements (N = 490) and GFR measurements approximately 3 yr later (N = 265).
The demographic and clinical characteristics of the patients included in this report are shown in Table 1. The range of GFR (as determined from [125I]iothalamate clearance) was wide, extending from 5 to 287 mL/min per 1.73 m2 at the initial (baseline) measurement. Because the patients in the MDRD Study and DCCT have different characteristics, patients in the two studies were considered separately for some analyses. However, for other analyses, particularly for variables affected by the level of GFR, patients in the two studies were combined. For these analyses, the following subgroups were defined by level of GFR: <13 mL/min per 1.73 m2 (N = 77; MDRD Study only); 13 to 24 mL/min per 1.73 m2 (N = 389, MDRD Study only); 25 to 55 mL/min per 1.73 m2 (N = 922; MDRD Study, N = 919; DCCT, N = 3); 56 to 90 mL/min per 1.73 m2 (N = 348; MDRD Study, N = 340; DCCT, N = 8); 91 to 125 mL/min per 1.73 m2 (N = 245; MDRD Study, N = 30; DCCT, N = 215); >125 mL/min per 1.73 m2 (N = 269; MDRD Study, N = 5; DCCT, N = 264).
TABLE 1.
Demographic and clinical characteristics of patientsa
| Characteristic | MDRD (N = 1,760) | DCCT (N = 490) |
|---|---|---|
| Age (yr) | 50 ± 13 | 28 ± 7 |
| % Male | 60 | 55 |
| % of Standard Weightb | 110 ± 17 | 105 ± 13 |
| Selected Diagnoses (%) | ||
| Polycystic kidney disease | 22 | 0c |
| Hypertensive nephrosclerosis | 17 | 0c |
| Glomerular disease | 27 | 0c |
| Tubulointerstitial disease | 7 | 0c |
| Hereditary nephritis | 2 | 0c |
| Diabetic nephropathy (non-insulin-dependent) | 3 | 0c |
| Urinary tract disease | 3 | 0c |
| Absence of one kidney (without other known cause) | 4 | 0c |
| Other renal disease or not specified | 15 | 0c |
| % Type 1 Diabetes With Retinopathy in Addition to Microaneurysms, Duration of Diabetes 1–15 yr (Secondary Intervention, N = 65) | 0d | 13 |
| % Type 1 Diabetes With Microaneurysms Only, Duration of Diabetes 1–15 yr (Secondary Intervention, N = 90) | 0d | 18 |
| % Type 1 Diabetes With No Retinopathy, Duration of Diabetes 1–5 yr (Primary Prevention, N = 335) | 0d | 68 |
| Systolic BP (mm Hg) | 134 ± 20 | 114 ± 11 |
| Diastolic BP (mm Hg) | 82 ± 11 | 73 ± 8 |
| Mean Arterial Pressure (mm Hg) | 100 ± 12 | 87 ± 8 |
| Initial Baseline GFR (mL/min per 1.73 m2) | 40 ± 21 | 130 ± 24 |
| Serum Creatinine (mg/dL) | 2.2 ± 1.2 | 0.8 ± 0.2 |
| Protein Excretione (mg/24 h) | 1,208 ± 1,890 | 13.3 ± 12.5 |
| Creatinine Clearance (mL/min per 1.73 m2) | 49 ± 24 | 127 ± 29 |
| Hemoglobin A1c (%) | 5.7 ± 0.9 | 8.7 ± 1.6 |
| Serum Glucose (mg/dL) | 92 ± 23 | 216 ± 82 |
| Duration of Diabetes (months) | NA | 54 ± 44 |
| Percent on Diuretics | 39 | 0c |
± values are means ± SD. NA. not available; BP. blood pressure.
Percent of ideal body weight for DCCT.
Exclusion criteria for DCCT.
Exclusion criteria for MDRD Study.
Albumin excretion for DCCT.
Procedure for GFR Measurement
GFR was measured as the renal clearance of [125I]iothalamate after an sc injection of 35 μCi of 125I, without epinephrine (8–11). [125I]iothalamate was selected because it is comparable to inulin as a filtration marker and can be assayed accurately and precisely in a central laboratory (1). The [125I]iothalamate was administered as an sc bolus, rather than as a continuous or bolus iv injection, because of the convenience of administration (8–11). A simultaneous sc injection of epinephrine was omitted to avoid any possible effects on systemic or renal hemodynamics that might affect GFR. Nonsteroidal anti-inflammatory agents were not ingested within 48 h of the measurement. GFR was measured in the morning (in most cases), after fasting (MDRD Study) or a light breakfast containing less than 15 g of protein but no caffeine (DCCT), and after a water load (10 mL/kg) to increase urine flow rate (UFR). After an equilibration period of at least 1 h, four consecutive urine collections were obtained by voluntary voiding and five serum samples bracketing the urine collections were obtained from an indwelling iv catheter in the arm contralateral to the [125I]iothalamate injection.
GFR measurements were performed by study personnel (nurses and technicians) who were trained in a common protocol at a central training site (Department of Hypertension and Nephrology, Cleveland Clinic Foundation, Cleveland, OH). The training manual specified the duration of the radioisotope equilibration period (≥1 h) and the subsequent clearance periods (≥30 mm in the MDRD Study and ≥20 min in the DCCT) and the desired UFR during the equilibration period (≥3 mL/min) and during the subsequent clearance periods (≥1 mL/min). Serum and urine radioactivities were analyzed at central laboratories (MDRD Study, Department of Hypertension and Nephrology, Cleveland Clinic Foundation, Cleveland, OH; DCCT, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN).
GFR was calculated as the renal clearance for each individual period (see Appendix, Equation 1); the overall GFR was calculated as if the clearance periods were one long period (see Appendix, Equations 2 and 3). Individual and overall GFR values were adjusted for body surface area (see Appendix, Equation 4) (12). Only GFR measurements with at least three clearance periods were included in this analysis. In the MDRD Study, 1,666 GFR measurements had four periods (94.7%). In the DCCT, 474 measurements had four periods (96.7%).
STATISTICAL ANALYSES
Performance of GFR Measurements
Summary of Data Across Clearance Periods
Results were described as the mean for each subgroup for each of the four clearance periods. Comparisons among periods were performed by the use of paired t tests.
Quality control
Split samples were analyzed in the central laboratories of both studies.
Pattern of decline in serum counts
Because of a marked decline in serum counts during the clearance periods in subgroups with a high GFR, different methods for the estimation of the average plasma concentration during the clearance periods were compared. On the basis of a two-compartment model for the distribution and excretion of a filtration marker after an iv bolus injection, the pattern of decline in serum counts is expected to follow a biexponential decline (13). After an equilibration between plasma and extracellular fluid, the pattern can be approximated by a monoexponential decline. However, after an sc bolus, with the concomitant administration of epinephrine, only a slight decline in serum level was observed in patients with a GFR of more than 30 mL/min per 1.73 m2 and a stable serum level was observed in patients with a GFR of less than 30 mL/min per 1.73 m2 (9). These findings justify the common practice of assuming a linear decline in serum level. The pattern of decline in patients not receiving epinephrine, however, is unknown. We compared linear and monoexponential models over successive clearance periods by computing, for each patient, the value of r2 both for a linear regression of serum count on time and for the exponential model (see Appendix, Equation 5). The median r2 values for these two models were compared for each subgroup by use of the sign test.
Sources of Intratest Variability of GFR Results
Intratest CV for GFR and UFR
The intratest (i.e., between period) variability of GFR was quantified by the CV of the clearances for the individual measurement periods (14). The intratest CV for UFR was calculated similarly.
Relationship of Intratest CV for GFR to GFR Level
The intratest CV for GFR for subgroups was compared by the Kruskall-Wallis test (15).
Relationship of Intratest CV for GFR and UFR
During water diuresis, the UFR should be relatively constant. Variability in UFR may indicate technical difficulties in urine collections. To assess the effect of technical difficulties in urine collection on intratest variability in GFR, we analyzed the relationship between intratest variabilities in UFR and GFR by correlating the log intratest CV. Intratest CV were log transformed to reduce positive skewness. Because the relationship was not linear, a nonlinear relationship (cubic spline) was used (16).
Autocorrelation (Correlation Among Clearance Periods)
In principle, if intratest variability in GFR is the result of incomplete bladder emptying in one period followed by more complete bladder emptying in the next period, then the GFR in successive periods should exhibit significant negative autocorrelation (a clearance period with a lower GFR should be followed by a period with a higher GFR). If, on the other hand, variability among collection periods is random, the expected value for the correlation between residuals for successive periods should be −0.333. Correlations that are smaller (more negative) than −0.333 indicate negative autocorrelation, whereas larger correlations (less negative) indicate positive autocorrelation.
For each of the six GFR ranges, the extent of autocorrelation was explored by first standardizing the log GFR measurements and then obtaining the residuals (see Appendix, Equation 6). The Pearson correlations of the residuals among the four periods were obtained for each of the six GFR ranges and pooled by use of the Fisher Z transformation (17). Confidence intervals for the correlations were obtained by the bootstrap method (18) with 1,000 replications.
Relationship of Patient Characteristics to Intratest CV for GFR and UFR
Nonparametric methods (i.e., Spearman correlations, rank-sums tests, etc. [15]) were used to relate the intratest CV for GFR and UFR to each other and to patient characteristics in univariate analyses. Because of the expected relationship between the intratest CV for GFR and UFR, two separate multiple regressions were used to relate them to patient characteristics. In these multiple regressions, the intratest CV for GFR and UFR were log transformed, as discussed above. The variables examined in univariate and multiple regression analyses included GFR, age, gender, weight, serum glucose, use of diuretics (MDRD Study only), diagnosis (as classified in Table 1), and study (MDRD Study or DCCT).
Relationship of Intratest Variability to Precision of GFR Measurements
The precision of GFR tests in individuals is most properly assessed from a comparison of GFR results over time. The precision of GFR tests in a population can be assessed from the interindividual (population) variability.
Changes in Intratest Variability Over Time
If the intratest variability in GFR in an individual is an indication of the precision of GFR measurements in that individual, then the intratest CV should be relatively constant over time. Spearman rank correlations were used to compare intratest CV for both GFR and UFR in measurements at two times.
Relation of Intertest Variability to Intratest Variability
If the intratest variability in GFR in an individual is an indication of the precision of GFR measurements, then the intertest variability (i.e., variability between two different measurement days) and the intratest variability should be related. The intertest variability was quantified by the intertest CV. The relationship of the intertest variability to the intratest variability was explored by computing the Spearman rank correlation of the intertest and intratest CV and by comparing the intertest CV of patients with different ranges of intratest CV by use of the Kruskall-Wallis test.
Relationship of the Number of Clearance Periods to Intertest and Interindividual Variability in GFR
The relationship between the number of periods used to compute an overall GFR and the precision of this estimate was evaluated by computing overall GFR on the basis of measurements from one, two, three, and four periods. For each number of periods, we summarized (1) the intertest variability (CV) between the first and second overall GFR measured 3 months apart in the MDRD Study, and (2) the interindividual (population) variability (standard deviation and CV) of the MDRD Study and the DCCT for the overall observed GFR at the initial baseline measurement. Because of the stabilizing effects of averaging results of periods, a reduction in both types of variability with increasing number of periods would be expected.
RESULTS
Performance of GFR Measurements
Adherence to Protocol
We assessed adherence to protocol regarding the duration of the equilibration period and subsequent clearance periods and the desired UFR during each period. Results showed acceptable adherence to the protocol. In 96.7% of patients, the equilibration period exceeded 1 h. UFR was 3 mL/min or more in 96.8% of DCCT patients, in whom GFR values were generally higher, and in 76.3% of MDRD Study patients, in whom GFR values were generally lower. In the subgroup of MDRD Study patients with the lowest GFR (<13 mL/min per 1.73 m2), a UFR of 3 mL/min or more was achieved in only 52.9%, but was more than 1 mL/min in 97.1 % of patients. The duration of clearance periods was 20 min or more in 100% of DCCT patients and 30 min or more in 99.6% of MDRD Study patients. UFR during clearance periods was 1 mL/min or more in 99.9% of all patients.
Quality Control
As a laboratory quality control measure in the DCCT, serum and urine samples from 43 baseline GFR measurements were split in the central laboratory and analyzed separately and the results were compared. The median difference (in absolute value, expressed as a percentage of the mean of the two split sample measurements) was 2.8 and 2.4% for the serum and urine counts, respectively. In the MDRD Study, serum and urine samples from GFR procedures were split at the clinical centers and analyzed and calculated by central laboratory personnel without knowledge that the split samples were from the same patient. The median difference (in absolute value) between the split samples for 42 GFR measurements was 1.0 mL/min per 1.73 m2, with a maximal difference of 7.9 mL/min per 1.73 m2. The median difference, expressed as a percentage of the mean of the two split sample GFR values, was 2.0%, with a maximum of 16.2%.
Summary of Data Across Clearance Periods
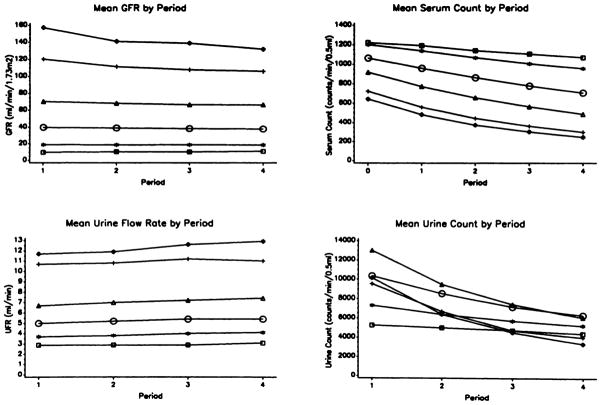
We examined trends in the mean values of GFR and the individual components of the clearance equation (Figure 1). The results show that the mean GFR declined during the four clearance periods in groups with higher overall GFR, with the maximal change being from the first to second periods. The mean drop in GFR between the first and fourth periods in the highest GFR group was 26 mL/min per 1.73 m2 (P < 0.0001). The decline in GFR during clearance periods was not apparent in patients with GFR of less than 25 mL/min per 1.73 m3.
Figure 1.
Summary of data across clearance periods. Subgroups are defined by level of GFR (in milliliters per minute per 1.73 m2): >125 (diamonds), 91 to 125 (cross), 56 to 90 (triangles), 25 to 55 (circles), 13 to 24 (asterisks), and <13 (squares).
The changes during clearance periods in components of the GFR measurement (UFR, serum counts, and urine counts) were as expected for a substance excreted by glomerular filtration during a water diuresis. The mean UFR was highest among patients with the highest GFR. The mean UFR rose slightly over time in all subgroups (P < 0.05). Mean serum counts were highest initially in the subgroup with the lowest GFR and subsequently declined in all groups because of the excretion of the isotope. The extent of the decline was greatest in subgroups with the highest GFR. In the subgroup with GFR of more than 125 mL/min per 1.73 m2, the mean (±SD) final serum count was only 245 (±102) cpm/0.5 ml, but 84% of patients had final serum counts exceeding 150 cpm/0.5 mL, which is approximately five times the background value and is adequate for counting. There was not a simple relationship between initial urine counts and GFR, probably because of the effects of the level of GFR on serum counts and urinary dilution. Because of stable or declining GFR and declining serum counts, mean urine counts declined in subsequent clearance periods in all subgroups.
Pattern of Decline in Serum Counts
We next compared the pattern of decline in serum counts in individual patients by the use of two different mathematical models, a linear and a monoexponential decline (Table 2). For subgroups with GFR of 25 mL/min per 1.73 m2 or more, the pattern of serum counts was significantly more closely approximated by the exponential rather than by the linear decline.
TABLE 2.
Pattern of serum radioactivity count over time
| Group | N | Median r2 of individual Regressions |
Proportion of Exponential Model r2 > Linear Model r2 | P Value Sign Test | |
|---|---|---|---|---|---|
| Linear Model Serum Count Vs. Time | Exponential Model Serum Count Vs. Time | ||||
| GFR<13 | 70 | 0.943 | 0.939 | 0.46 | 0.550 |
| GFR 13–24 | 363 | 0.951 | 0.956 | 0.47 | 0.248 |
| GFR 25–55 | 878 | 0.971 | 0.980 | 0.60 | <0.0001 |
| GFR 55–90 | 333 | 0.976 | 0.989 | 0.72 | <0.0001 |
| GFR 91–125 | 237 | 0.964 | 0.992 | 0.80 | <0.0001 |
| GFR > 125 | 259 | 0.960 | 0.992 | 0.78 | <0.0001 |
Intratest Variability in GFR Among Clearance Periods
We assessed the intratest CV for GFR among clearance periods in each center. In the MDRD Study, the median intertest CV was 9.4% and ranged from 6.5 to 12.0% among the 15 clinical centers. In the DCCT, the median intratest CV was 11.7% and ranged from 6.0 to 16.5% among the 29 clinical centers. As an indirect assessment of the contribution of the systematic decline in GFR during the clearance periods to the intratest CV for GFR in both studies, we computed the mean GFR for each period and then evaluated the variability of the mean GFR across the four clearance periods. The CV for the mean GFR by period was 2.6 and 6.5%, respectively, for the MDRD Study and DCCT, indicating that the decline in GFR during collection periods contributes substantially to the intratest GFR CV.
Sources of Intratest Variability of GFR Results
Relationship of Intratest CV for GFR to level of GFR
The intratest CV for GFR was slightly, but significantly higher in subgroups with GFR of more than 90 mL/min per 1.73 m2 and with GFR of less than 13 mL/min per 1.73 m2 (P < 0.0001) (Figure 2).
Figure 2.
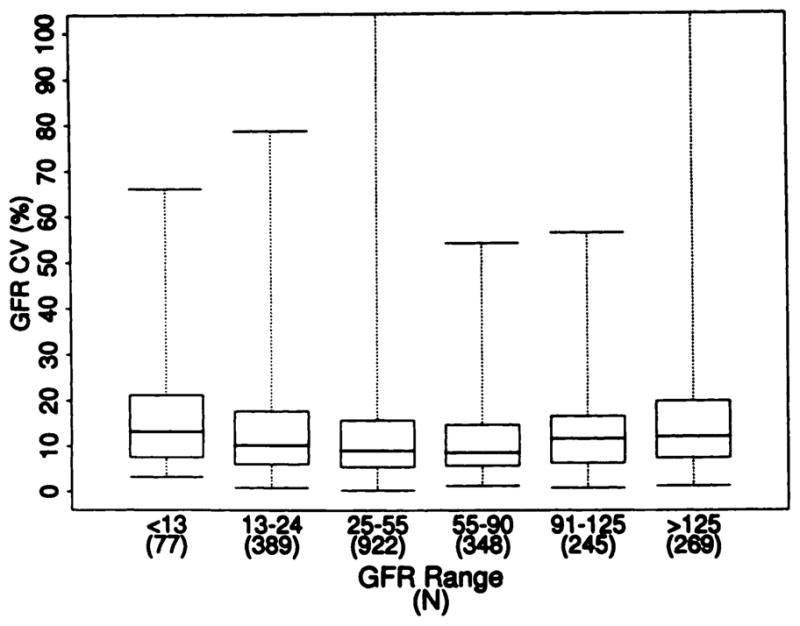
Relationship of intratest CV for GFR to level of GFR. Box plots show the minimum value (bottom line). maximum value (top line), middle 50% of values (box), and median value (middle line). Kruskall-Wallis test, P < 0.0001. (Four values for intratest CV >100% are not shown.)
Relationship of Intratest CV for GFR and UFR
The variability in UFR may indicate technical difficulties in urine collection. The correlation between intratest variabilities in UFR and GFR was examined in all 2,250 patients. Because GFR is calculated from an equation including UFR, the intratest CV for UFR and GFR CV are positively related (Spearman r = 0.46). Interestingly, the relationship is strongest at lower GFR and becomes weaker as GFR increases (r was 0.62 for patients with GFR <13 mL/min per 1.73 m2 and 0.30 for patients with GFR >125 mL/min per 1.73 m2). These results suggest that technical difficulties related to urine collection may affect the variability of GFR results, especially at low GFR values.
Autocorrelation Among GFR Results Across Clearance Periods
Technical difficulties in urine collection, such as incomplete bladder emptying in one period followed by more complete bladder emptying in the subsequent period, may be reflected in negative autocorrelation across clearance periods. We computed the 95% confidence intervals for the correlations between residuals for adjacent periods and for periods separated by one or two periods. For all adjacent periods and for periods separated by one period, the correlations did not differ significantly from their expected values (r = −0.333) under the assumption of random variability. Thus, intratest variability in GFR results did not appear to be the result of predictable errors in bladder emptying. On the other hand, the correlation (and 95% confidence interval) for periods separated by two periods (i.e., Periods 1 and 4) was −0.44 (−0.50, −0.37), indicating a significant negative autocorrelation. However, this may have been the result of the systematic decline in GFR during the clearance periods rather than incomplete bladder emptying.
Relationship of Patient Characteristics to Intratest CV for GFR and UFR
As discussed earlier, without adjustment for the level of GFR and other patient characteristics, the median intratest CV for GFR was higher in the DCCT (11.7%) than in the MDRD Study (9.4%) (P < 0.001; median test). Similarly, the median intratest CV for UFR was also higher in the DCCT (19.0%) than in the MDRD Study (16.5%) (P = 0.016; median test). We assessed the contribution to the intratest CV for GFR and UFR of patient characteristics, including age, gender, weight, serum glucose, diagnosis (as classified in Table 1), and use of diuretics (MDRD Study only), in univariate and multiple regression analyses. The multiple regression analyses confirmed the higher intratest CV for GFR (P = 0.012), but not for UFR, among patients in the DCCT. Because of the large number of patients studied, weak relationships involving several patient characteristics were found to be significant. In addition, there was an almost significant relationship between the intratest CV for GFR and the severity of retinopathy (P = 0.060): the intratest CV was 11.1, 12.4, and 14.4%, respectively, among patients with no retinopathy, microaneurysms only, and retinopathy in addition to microaneurysms. Nonetheless, the value for r2, the proportion of variability in log intratest CV for GFR and UFR, accounted for by variability in the patient characteristics that we included in the multiple regression models, was only 0.048 and 0.033, respectively. Hence, these patient characteristics explained little of the intratest variability in GFR and UFR results.
Relation of Intratest Variability to Precision of GFR Estimates
Changes in Intratest Variability Over Time
We compared the intratest CV for both GFR and UFR in two measurements in 1,056 MDRD Study patients and 256 DCCT patients (Table 3). In MDRD Study patients, in whom the interval between tests was approximately 3 months, the correlations were significant, but relatively weak (Spearman r was 0.31 and 0.30, respectively, for the intratest CV for GFR and UFR). In DCCT patients, in whom the interval between tests was approximately 3 yr, the correlations were significant, but even weaker (r was 0.15 and 0.16, respectively, for the intratest CV for GFR and UFR). The wide variability in intratest CV for GFR, even over a relatively short interval, is consistent with our observation that patient characteristics have little effect on the intratest CV for GFR and UFR.
TABLE 3.
Comparison of intratest variability in subjects who had two GFR measurements
| Study | N | Interval | Median UFR CV (%) |
ra | Median GFR CV (%) |
ra | ||
|---|---|---|---|---|---|---|---|---|
| First | Second | First | Second | |||||
| MDRD Studyb | 1,056 | 3 months | 16.1 | 15.3 | 0.30c | 9.3 | 8.9 | 0.31c |
| DCCTd | 265 | 3 yr | 20.5 | 18.9 | 0.16e | 12.2 | 10.5 | 0.15e |
Spearman correlation coefficients.
Repeat GFP measurements were performed after 3 months.
P < 0.0001.
Repeat GFP measurements were performed after 3 yr.
P =0.01.
Relation of Intertest Variability to Intratest Variability
We examined variability in GFR results in 957 MDRD Study subjects in whom complete four-period GFR measurements were obtained twice over an interval of approximately 3 months and related the intertest CV to the intratest CV (Figure 3). The median difference (absolute value) between the two tests was only 8.9%. This corresponds to a median intertest CV of 6.3%, with a 95% confidence interval of 5.8 to 6.7%. If a high intratest CV is a measure of imprecision of the GFR measurement, then the intertest CV should be, on average, substantially larger for patients with higher intratest CV than for patients with lower intratest CV. The intertest CV was related to the intratest CV (Spearman r = 0.15; P < 0.0001); however, as shown in Figure 3, the difference in intertest CV was not large. For example, the median intertest CV were 5.47 and 8.56%, respectively, for patients with intratest CV of 0 to 10% and 20 to 30%. These data indicate that intratest variability in GFR was only weakly rebated to the precision of the GFR measurements. This implies that GFR results with a high intratest CV were not necessarily imprecise.
Figure 3.
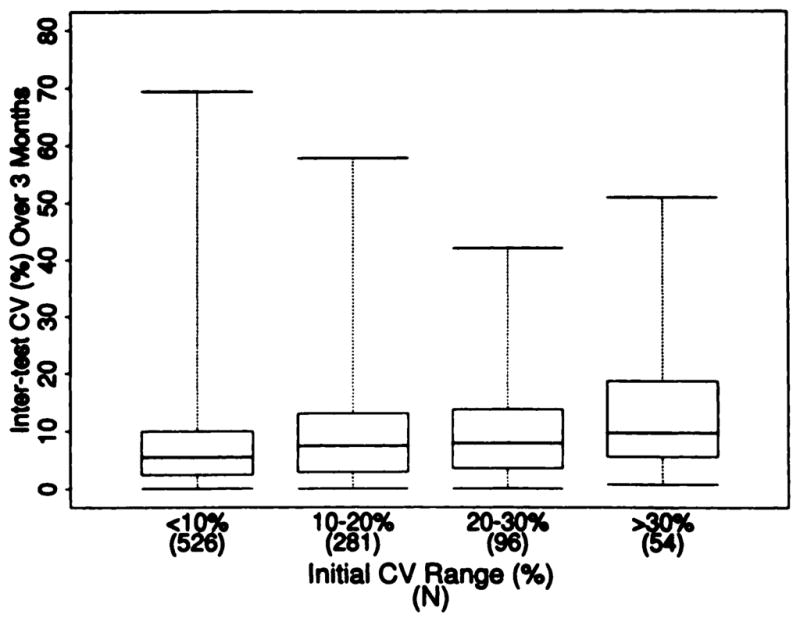
Relationship of intertest and intratest CV for GFR in the MDRD Study. Box plots show the minimum value, maximum value, middle 50% of values, and median value (as described in legend to Figure 2). Kruskall-Wallis test, P < 0.0001.
Relationship of Number of Clearance Periods to Intertest CV and Population Mean and CV
Although we have demonstrated that a high intratest CV may not necessarily imply an imprecise overall GFR, this conclusion was derived from estimates of GFR based on four collection periods. The inclusion of four collection periods tends to average out differences in GFR results among the periods. Table 4 summarizes the intertest CV between the two GFR measurements for the 957 MDRD Study patients with four period GFR as a function of the number of periods used to compute the GFR. Assessing GFR with more than one period leads to a substantial decrease in the intertest CV. Although most of the decrease in median intertest CV is associated with using two periods rather than one period to estimate GFR, the 75th and 90th percentiles continue to decrease when three or four periods are used, indicating further improvement in precision with the inclusion of more periods.
TABLE 4.
Relationship of number of periods to intertest GFR variability (patients with four-period GFR for both measurements)
| Clearance Periods | Intertest GFR CV (%) |
||
|---|---|---|---|
| Median | 75th Percentile | 95th Percentile | |
| GFR Range <13 mL/min per 1.73 m2 (N = 9) | |||
| 1 | 14.7 | 18.6 | 67.5 |
| 1–2 | 15.5 | 34.2 | 64.8 |
| 1–3 | 4.4 | 15.8 | 55.7 |
| 1–4 | 6.8 | 15.2 | 49.9 |
| GFR Range 13–24 mL/min per 1.73 m2 (N = 253) | |||
| 1a | 11.1 | 19.4 | 40.6 |
| 1–2a | 8.4 | 15.8 | 33.5 |
| 1–3 | 8.2 | 14.1 | 32.7 |
| 1–4 | 8.4 | 13.6 | 29.6 |
| GFR Range 25–55 mL/min per 1.73 m2 (N = 649) | |||
| 1a | 9.4 | 17.8 | 34.4 |
| 1–2a | 6.9 | 13.1 | 25.1 |
| 1–3a | 6.0 | 11.4 | 22.8 |
| 1–4 | 5.8 | 10.6 | 21.8 |
| GFR Range 56–90 mL/min per 1.73 m2 (N = 46) | |||
| 1 | 5.6 | 13.5 | 28.9 |
| 1–2 | 4.5 | 7.5 | 19.0 |
| 1–3 | 3.5 | 5.9 | 15.8 |
| 1–4 | 4.0 | 8.4 | 13.7 |
| All Subjects (N = 957) | |||
| 1a | 9.6 | 17.8 | 36.8 |
| 1–2a | 7.2 | 13.6 | 27.6 |
| 1–3a | 6.3 | 12.3 | 25.1 |
| 1–4 | 6.3 | 11.4 | 23.8 |
Differs from distribution of intertest GFR CV based on four-period GFP by sign test (P < 0.05).
In addition, as shown in Table 5, the interindividual (population) variability in GFR for each study also declined as the number of clearance periods increased. Because the GFR during the first period was generally higher than that in subsequent periods (as discussed earlier), the population mean also declined as the number of collection periods increased. Because the standard error of the mean of a sample of GFR measurements is proportional to the interindividual (population) standard deviation, the decline in the interindividual standard deviations with the number of periods indicates a corresponding increase in the precision with which the population mean GFR can be estimated. Thus, given the relatively high intratest variability that is characteristic of GFR measurements, the calculation of the result from four clearance periods improves the precision of the estimate of GFR both for individuals and for the population mean.
TABLE 5.
Relationship of number of periods to GFR mean and variability
| Clearance Periods | Mean | SD | CV (%) | 5th Percentile | 95th Percentile |
|---|---|---|---|---|---|
| MDRD Patients With Four-Period GFR (N = 1,666) | |||||
| 1 | 41.5a | 23.2b | 55.8 | 12.4 | 84.9 |
| 2 | 40.9a | 22.1b | 54.1 | 12.6 | 82.4 |
| 3 | 40.5a | 21.5b | 53.1 | 12.7 | 86.0 |
| 4 | 40.2 | 21.2 | 52.6 | 12.9 | 78.9 |
| DCCT Patients with Four-Period GFR (N = 474) | |||||
| 1 | 139.7a | 36.7b | 26.2 | 95.0 | 197.9 |
| 2 | 134.1a | 27.5b | 20.5 | 100.5 | 177.2 |
| 3 | 131.6a | 26.1b | 19.8 | 99.6 | 171.9 |
| 4 | 129.6 | 24.0 | 18.5 | 98.3 | 166.0 |
Differs by comparison to four-period GFR by paired t test (P < 0.05).
Differs by comparison to four-period GFR by test comparing standard deviations of paired observations (13) (P < 0.05).
DISCUSSION
GFR is generally considered the best overall index of renal function in health and disease (19,20). The renal clearance of inulin during a continuous iv infusion is the traditional method of measuring GFR, but technical demands in performing clearance measurements and high intratest variability have discouraged its routine use in practice [20–22]. Instead, most physicians rely on the measurement of the serum creatinine level as an index of renal function. However, recent emphasis on the potential limitations of serum creatinine level to estimate GFR led investigators in clinical trials to use radioisotope-labeled filtration markers and modifications of the traditional clearance method to avoid a continuous iv infusion and bladder catheterization. One such method, the renal clearance of [125I]iothalamate after sc injection, without the concomitant infusion of epinephrine, was selected for use in two large, ongoing clinical trials sponsored by the NIDDK. Although this method of measuring GFR had been validated in single-center studies (8–10) and in a pilot study for the MDRD Study (11), it had not been used previously in multicenter clinical trials, and the extent to which it could be standardized across clinical centers was unknown.
The results obtained from 2,250 patients studied at 44 clinical centers participating in the MDRD Study and DCCT demonstrate acceptable adherence to protocol, as judged by the evaluation of the duration of clearance periods and UFR and by the analysis of split samples. The median value for intratest CV, long regarded as a marker of technical performance, was 9.4% in the MDRD Study, similar to results in single-center studies also measuring renal clearance with voluntary bladder emptying and only slightly higher than in studies using the traditional method of inulin clearance including bladder catheterization (23). The median intratest CV in the DCCT was 11.7%, which is slightly, but significantly, higher than that in the MDRD Study. These data demonstrate that the GFR procedure can be implemented in multicenter clinical trials and performed with a similar degree of consistency, as reported in single-center studies.
The causes for the high intratest CV in renal clearance measurements have not been carefully investigated, although it is widely regarded that a high intratest CV indicates an imprecise result. Our results demonstrate that variation in GFR over clearance periods is due, in part, to a systematic decline in GFR during the interval of the test. This decline is most pronounced in subjects with high GFR (Figure 1). This may account, in part, for the higher intratest CV observed in patients with higher GFR (>90 mL/min per 1.73 m2) (Figure 2).
In other studies, simultaneous measurements of renal and plasma clearance during a continuous iv infusion of the filtration marker demonstrate lower intratest and interindividual (population) CV by the plasma clearance method, suggesting that technical difficulties in urine collections are the cause of higher variability of renal clearance measurements (24,25). The correlation that we observed between the intratest CV for GFR and UFR (r = 0.46) is consistent with this explanation, although, as discussed above, a correlation between these parameters is to be expected because GFR is calculated from an equation including the UFR. However, an analysis of autocorrelation among clearance periods does not support the simple explanation that the high intratest CV for GFR is the result of alternating high and low clearances due to incomplete bladder empyting. Our data suggest that technical difficulties related to urine collection contributed substantially to the higher intratest CV observed in patients with a very low level of GFR (Figure 2). In the patients with GFR of less than 13 mL/min per 1.73 m2, the intratest CV for GFR and UFR were highly correlated.
Results of univariate and multivariate analyses reveal little overall dependence of the intratest CV for GFR and UFR on patient characteristics, including age, gender, weight, serum glucose, use of diuretics, and renal diagnosis. However, after taking into account the level of GFR, the intratest CV for UFR, and other patient characteristics, patients in the DCCT have significantly higher intratest CV for GFR. The higher intratest CV among diabetics has been observed in other studies and has been attributed to difficulties in bladder emptying as the result of autonomic neuropathy (26). Possibly, the presence of autonomic neuropathy affecting bladder emptying in DCCT patients, especially among patients with more severe retinopathy, may have contributed to their higher intratest CV for GFR. However, multivariate analysis did not show that the intratest CV for UFR was significantly higher among DCCT patients, and the relationship between intratest CV for GFR and UFR was less strong in patients with GFR in the range found in the DCCT, suggesting that factors other than bladder emptying affected the variability in GFR measurements in the diabetic patients that we observed. We speculate that the higher intratest CV for GFR among DCCT patients compared with MDRD Study patients is, in part, the result of the more rapid decline in GFR during the procedure observed in patients with higher GFR.
Despite a high intratest CV, the estimate of GFR would be precise if the average of the four clearance periods is consistent over time. Indeed, the median intertest CV for two measurements in the MDRD Study performed 3 months apart was only 6.3% (Table 4), indicating that the GFR determinations performed in these studies are reasonably precise. Even for patients with an initial baseline intratest GFR CV as high as 20 to 30%, the median intertest GFR CV is only 8.56%. Furthermore, there is substantial variability in the intratest CV for GFR and UFR from test to test (Table 3). Therefore, these data do not support the practice of excluding patients or GFR results with high intratest CV from clinical studies. Instead, we recommend interpreting the overall time-weighted clearance as a relatively precise estimate of the GFR.
As shown in Table 4, the relatively low intertest CV is, in part, the result of averaging the result of four clearance periods for each determination of GFR. Table 5 also shows the effect of increasing the number of clearance periods on the interindividual (population) variation for the MDRD Study and DCCT. As expected, the greater the number of clearance periods, the lower the intertest CV and the interindividual (population) variability. In clinical trials, in which sample size and cost vary inversely with the precision of measurements used in the trial, it is advantageous to use multiple clearance periods to measure GFR. One practical recommendation is to use the four-period GFR protocol that is being used in the MDRD Study and the DCCT.
Other practical recommendations for performing GFR measurements in clinical trials relate to the patterns of decline in serum counts and in GFR that we observed during the clearance periods. First, the pattern of decline in serum counts after an sc infusion without epinephrine is more closely fit by an exponential than by a linear function. The difference is most marked in patients with the highest levels of GFR, in whom the decline in serum counts is most rapid. Thus, in calculating the renal clearance, it is more accurate to estimate the plasma concentration from the natural logarithmic mean, rather than the arithmetic mean, of the values for serum counts at the beginning and end of the clearance period.
Second, to increase the precision of the serum radioactivity assay in patients with anticipated normal GFR or “hyperfiltration,” we recommend a higher dose of [125I]iothalamate, for example, 50 μCi, to achieve higher final plasma serum counts.
Third, at GFR levels of more than 25 mL/min per 1.73 m2, GFR tends to decline during the procedure, with the highest value occurring during the first clearance period (Figure 1). The effect is most marked in patients with the highest levels of GFR (>125 mL/min per 1.73 m2), in whom the mean difference between the first and fourth period was 26 mL/min per 1.73 m2, representing 17.9% of the mean overall GFR. The decline in GFR level during the procedure was noted in the early investigations of Smith and colleagues using a continuous infusion of inulin and was ascribed to decreasing hydration (27). This effect seems unlikely because we did not observe a decline in the UFR during the procedure. Possibly, the effect of the overnight fast (in MDRD Study patients) or the avoidance of high-protein foods (in DCCT patients) that was part of the protocol in these trials contributed to the decline in GFR during the measurement. In fed subjects, there is a diurnal variation in GFR, with the lowest values observed during the night and a rise in GFR that begins in the early to midmorning (28). If the normally higher GFR during the day reflects “protein-induced hyperfiltration” associated with meals, then the fall in GFR during clearance measurements performed in the morning may be the result of the interruption in the diurnal pattern of GFR by fasting. However, this explanation also seems unlikely because the duration of abstinence from high-protein feeding was approximately 8 to 12 h, and the most pronounced decline in GFR occurred consistently after the first clearance period. A more likely contributing factor is a systematic overestimation of clearance as the result of an underestimation of the plasma level because of the the rapid decline early in the procedure. The effects of a rapidly declining plasma level on the estimation of the average value have been discussed extensively by Smith (21) and others (29,30). These effects include an inaccurate estimation of average plasma level by mathematical equations, disequilibrium between arterial and venous concentrations because of rapid renal excretion of the filtration marker, and failure to take into account the transit time from the renal tubules to the urinary bladder. Nelson and colleagues have presented preliminary data suggesting a systematic overestimation of the renal clearance of iothalamate after a bolus injection compared with that obtained from a continuous iv infusion (31). Additional studies are required to determine whether these limitations apply to GFR studies using an sc bolus.
Irrespective of its cause, the decline in GFR throughout the procedure has important implications for the design of studies including measurements of GFR. First, measurements should be performed under standardized conditions with respect to food intake and time of day. Second, it may be advisable to standardize the duration of the equilibration period so that the rate of decline in serum counts is more uniform among patients with similar GFR. Third, and most important, it is necessary to include a “time control” in studies of the effect of acute interventions on GFR. For example, the GFR after an intervention should be compared with the GFR in the same subject at the same time on another day without receiving the intervention or in other subjects at the same time of day who did not receive the intervention.
In summary, this analysis of the performance of GFR measurements from the MDRD Study and DCCT demonstrates the feasibility of including these measurements in multicenter clinical trials, clarifies the sources of intratest variability of GFR results, demonstrates the precision of the estimates of GFR, and provides practical recommendations for the performance of GFR measurements using the renal clearance of [125I]iothalamate after an sc bolus, without concomitant epinephrine. Although the studies that we analyzed were performed as part of clinical research studies, with appropriate attention to training and quality control, the same protocols could be established in renal function laboratories to provide GFR results in clinical practice. The direct cost of disposable supplies and a technician for a GFR measurement in a hospital clinical research laboratory, excluding the cost of the filtration marker, is approximately $100. [125I]iothalamate (Glofil; Isotex Diagnostics, Friendswood, TX) costs approximately $700 for a 1,000-μCi vial, has a shelf-life of 45 days, and is prepared by the manufacturer once monthly. If 20 GFR measurements per month (240/yr) are performed, the cost of the filtration marker would be $35 per measurement, and therefore, the total direct cost of the measurement would be $135, including the cost of a technician paid on a half-time basis. Apparently, the reimbursement currently provided by third-party payors in several states would be adequate to cover costs. Thus, GFR measurements could be implemented in hospital clinical laboratories for use in practice. The ultimate importance of measuring GFR in practice will depend, in part, on the results of the MDRD Study, the DCCT, and other ongoing clinical trials investigating therapeutic interventions to prevent the onset or retard the progression of renal disease.
Acknowledgments
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases through cooperative agreements. The authors are grateful to Henry A. Rolin, M.T. (ASCAP), M.S., and Diane S. Pexa, M.T. (ASCAP), of the Central GFR Laboratory for the MDRD Study; Jean Bucksa, M.T. (ASCAP), and Maren L. Nowicki, M.T. (ASCAP), of the Central Biochemistry Laboratory of the DCCT; Judith R. Leatherman, B.S., of the Data Coordinating Center for the MDRD Study; Susan Sorlie, M.S., and Walter Owen, B.A., of the Biostatistics Coordinating Center for the DCCT; Alice A. Martin, B.S.N., R.N., David Furlong, B.S., and Christopher Moleske, B.A., of New England Medical Center, Boston, MA; and the GFR technicians and study coordinators at the MDRD Study and DCCT Clinical Centers for their assistance in performing GFR measurements.
APPENDIX
Calculations
Clearance
Clearance (Ci) for the ith individual urine collection period was calculated according to the formula
| Equation 1 |
where U and P are defined as urine and plasma concentrations of [125I]iothalamate (counts per minute per 0.5 mL), V is the urine flow rate (0.5 mL/min), and the subscripts i0 and i1 indicate samples obtained at the beginning and end, respectively, of the ith collection period. (The justification for calculating the average plasma concentration as the natural logarithmic mean, rather than the arithmetic mean, is given in the Results.)
Overall clearance during the four clearance periods was calculated as if the four periods were one period, that is, as the ratio of the time-weighted mean of the urine excretion rates
| Equation 2 |
and the time-weighted mean of the serum counts
| Equation 3 |
where t1 is the duration of the ith clearance period.
The clearance for each individual period and that for the entire period were adjusted for body surface area (BSA) by multiplying each clearance by the factor 1.73/BSA, calculated according to the formula
| Equation 4 |
where W = weight (in kilograms) and H = height (in centimeters) (12).
Pattern of decline in serum counts
The monoexponential decline in serum counts was calculated as follows:
| Equation 5 |
where B0 is the serum count at the beginning of the first clearance period and B1 is the proportional rate of decline in serum counts over time.
Autocorrelation among clearance periods
The extent of the autocorrelation of GFR results among clearance periods was analyzed by standardizing the log GFR measurements to have unit variance and mean 0 for each period and obtaining the residuals
| Equation 6 |
where lgfrij represents the standardized log GFR for the jth period for patient i and lgfri represents the mean of the standardized log GFR for patient i over the four periods.
Footnotes
Presented in part at the 24th Annual Meeting of the American Society of Nephrology, Battimore, MD, November 19, 1991.
References
- 1.Levey AS. Use of glomerular filtration rate measurements to assess the progression of renal disease. Semin Nephrol. 1989;9:370–379. [PubMed] [Google Scholar]
- 2.Klahr S. The Modification of Diet in Renal Disease Study. N Engl J Med. 1989;320:864–866. doi: 10.1056/NEJM198903303201310. [DOI] [PubMed] [Google Scholar]
- 3.Modification of Diet in Renal Disease Study Group. The Modification of Diet in Renal Disease (MDRD) Study: Design, methods and results from the feasibility study. Am J Kidney Dis. 1992;20:18–33. doi: 10.1016/s0272-6386(12)80313-1. [DOI] [PubMed] [Google Scholar]
- 4.Modification of Diet in Renal Disease Study Group (Prepared by. Beck GJ, Berg RL, Coggins CH, Gassman JJ, Hunsicker LG, Schluchter MD, Williams GW. Design and statistical issues of the Modification of Diet in Renal Disease Trial. Con Clin Trials. 1991;12:566–586. doi: 10.1016/0197-2456(91)90069-x. [DOI] [PubMed] [Google Scholar]
- 5.DCCT Research Group. The Diabetes Control and Complications Trial (DCCT): Design and methodologic consideration for the feasibility phase. Diabetes. 1986;35:530–545. [PubMed] [Google Scholar]
- 6.DCCT Research Group. The Diabetes Control and Complications Trial (DCCT): Update. Diabetes Care. 1990;13:427–433. doi: 10.2337/diacare.13.4.427. [DOI] [PubMed] [Google Scholar]
- 7.The Diabetic Retinopathy Study Research Group. A modification of the Airlie House Classification of diabetic retinopathy. Diabetic Retinopathy Study (DRS) report no. 7. Invest Ophthalmol. 1981;21:210–226. [PubMed] [Google Scholar]
- 8.Israelit AH, Long DL, White MG, Hull AR. Measurement of glomerular filtration rate utilizing a single subcutaneous injection of 125I-iothalamate. Kidney Int. 1973;4:346–349. doi: 10.1038/ki.1973.127. [DOI] [PubMed] [Google Scholar]
- 9.Ott NT, Wilson DM. A simple technique for estimating glomerular filtration rate with subcutaneous injection of [125I]iothalamate. Mayo Clin Proc. 1975;50:664–668. [PubMed] [Google Scholar]
- 10.Adefuin PY, Gur A, Siegel NJ, Spencer RP, Hayslett JP. Single subcutaneous injection of iothalamate sodium 125I to measure glomerular filtration rate. JAMA. 1976;235:1467–1469. [PubMed] [Google Scholar]
- 11.Perrone RD, Steinman TI, Beck GJ, et al. Utility of radioisotopic filtration markers in chronic renal insufficiency: Simultaneous comparison of 125I-iothalamate, 169Yb-DTPA, 99mTc-DTPA and inulin. Am J Kidney Dis. 1990;16:224–235. doi: 10.1016/s0272-6386(12)81022-5. [DOI] [PubMed] [Google Scholar]
- 12.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 13.Bianchi C, Donadio C, Tramonti G. Noninvasive methods for the measurement of total renal function. Nephron. 1981;28:53–57. doi: 10.1159/000182104. [DOI] [PubMed] [Google Scholar]
- 14.Snedecor GW, Cochran WG. Statistical Methods. 7. Ames: Iowa State University Press; 1980. pp. 190–191. [Google Scholar]
- 15.Hollander M, Wolfe DA. Nonparametric Statistical Methods. New York: John Wiley and Sons; 1973. [Google Scholar]
- 16.Eubank RL. Spline Smoothing and Nonparametric Regression. New York: Marcel Dekker, Inc; 1988. [Google Scholar]
- 17.Hotelling H. New light on the correlation coefficient and its transforms (with discussion) J R Stat Soc B. 15:193–232. [Google Scholar]
- 18.Efron B. Bootstrap methods: Another look at the jackknife. Ann Stat. 1979;7:1–26. [Google Scholar]
- 19.Smith HW. The Kidney. Structure and Function in Health and Disease. New York: Oxford University Press; 1951. Diseases of the kidney and urinary tract; pp. 836–887. [Google Scholar]
- 20.Levey AS. Nephrology forum: Measurement of renal function in chronic renal disease. Kidney Int. 1990;38:167–184. doi: 10.1038/ki.1990.182. [DOI] [PubMed] [Google Scholar]
- 21.Smith HW. The Kidney: Structure and Function in Health and Disease. New York: Oxford University Press; 1951. Measurement of the filtration rate; pp. 39–62. [Google Scholar]
- 22.Smith HW. The Kidney: Structure and Function in Health and Disease. New York: Oxford University Press; 1951. The reliability of inulin as a measure of glomerular filtration; pp. 231–238. [Google Scholar]
- 23.Davies DF, Shock NW. The variability of measurement of inulin and diodrast tests of kidney function. J Clin Invest. 1950;29:491–495. doi: 10.1172/JCI102285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnurr E, Lahme W, Kuppers H. Mesaurement of renal clearance of inulin and PAH in the steady state without urine collection. Clin Nephrol. 1980;13:26–29. [PubMed] [Google Scholar]
- 25.van Acker BAC, Koomen GCM, Koopman MG, Krediet RT, Arisz L. Discrepancy between circadian rhythms of inulin and creatinine clearance. J Lab Clin Med. 1992;120:400–410. [PubMed] [Google Scholar]
- 26.Loon NR, Morelli E, Myers BD. Determinants and monitoring of GFR in diabetic glomerular injury [Abstract] Kidney Int. 1989;35:210. [Google Scholar]
- 27.Smith HW. The Kidney: Structure and Function in Health and Disease. New York: Oxford University Press; 1951. Comparative physiology of the kidney; pp. 520–574. [Google Scholar]
- 28.Sirota JH, Baldwin DS, Villarreal H. Diurnal variations of renal function in man. J Clin Invest. 1950;29:187–192. doi: 10.1172/JCI102245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levinsky NG, Levy M. Clearance techniques. In: Orloff J, Berliner RW, editors. Handbook of Physiology. Section 8: Renal Physiology. Washington, DC: American Physiological Society; 1973. pp. 103–117. [Google Scholar]
- 30.Levey AS, Madaio MP, Perrone RD. Laboratory assessment of renal disease: Clearance, urinalysis and renal biopsy. In: Brenner BM, Rector FC Jr, editors. The Kidney. 4. Philadelphia: WB Saunders; 1991. pp. 919–968. [Google Scholar]
- 31.Nelson RG, Beck GJ, Myers BD DRDS Study. Limitations of plasma clearance of iothalamate as a measure of GFR [Abstract] J Am Soc Nephrol. 1991;2:241. [Google Scholar]