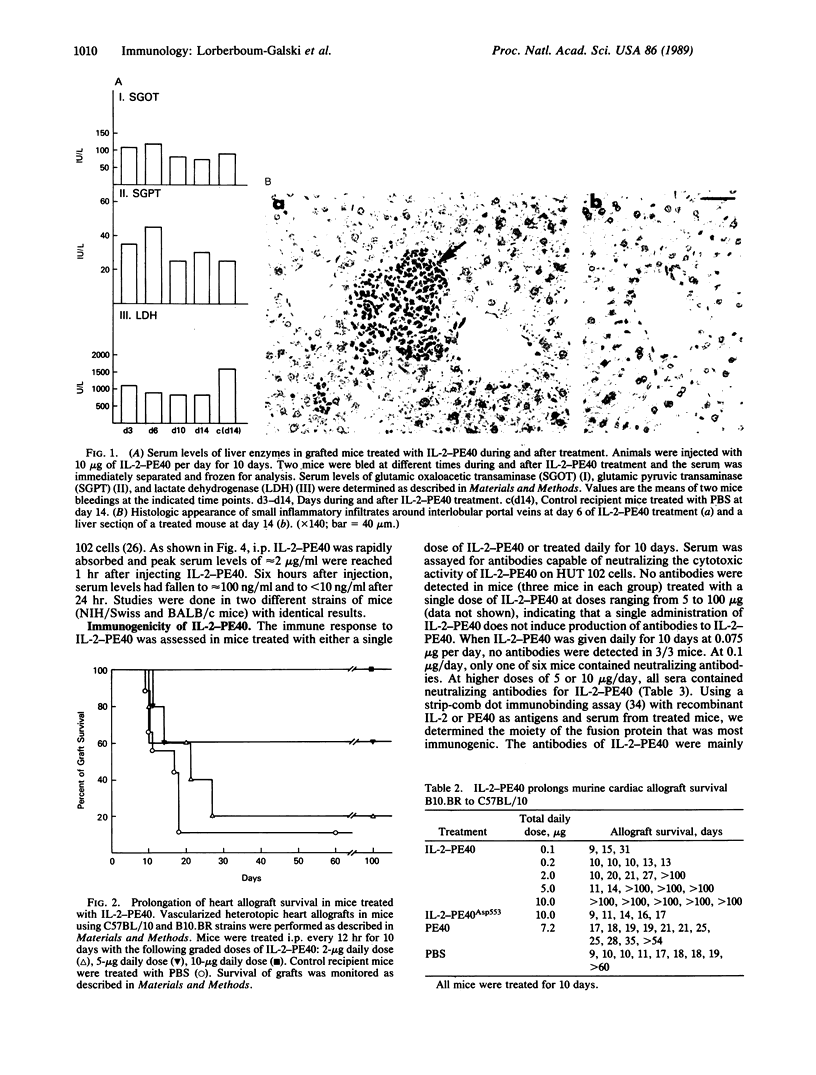
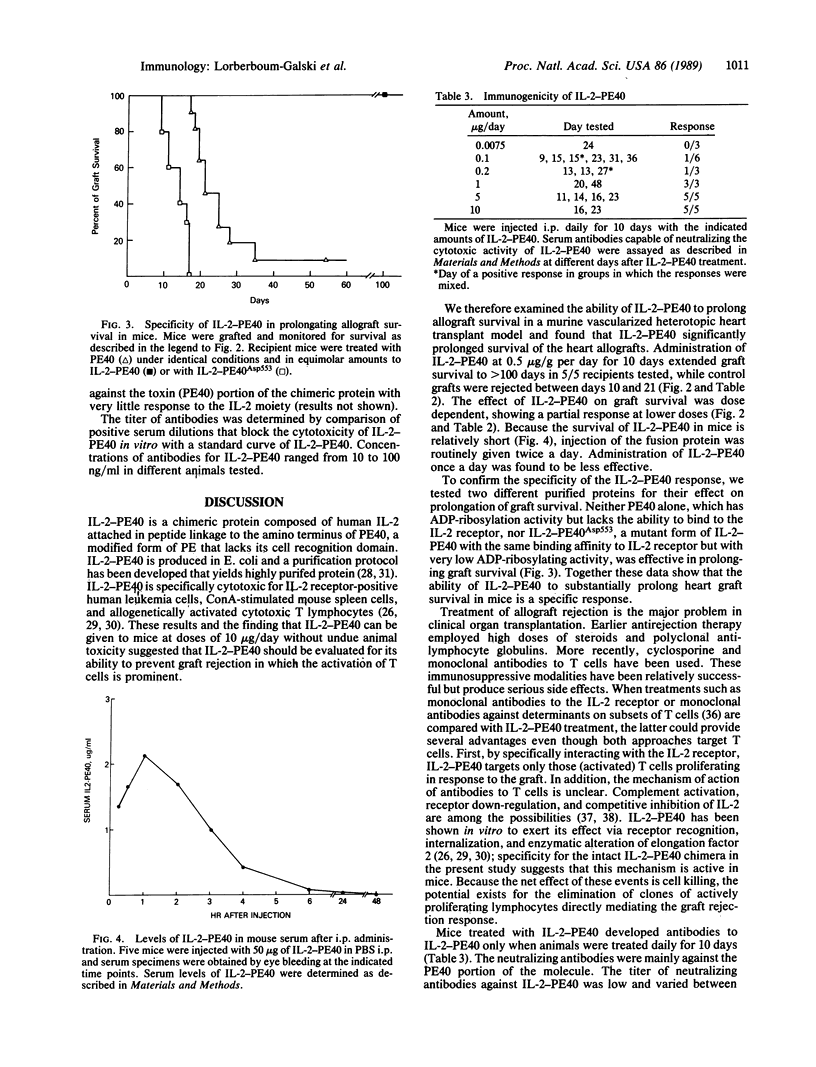
Abstract
IL-2-PE40 is a chimeric protein composed of human interleukin 2 (IL-2) genetically fused to the amino terminus of a modified form of Pseudomonas exotoxin lacking its cell recognition domain. IL-2-PE40, which is extremely cytotoxic to IL-2 receptor-positive cells, was examined for its ability to prevent graft rejection in mice in which activation of T cells is prominent. We demonstrate that intraperitoneally administered IL-2-PE40 specifically and significantly prolongs the survival of vascularized heart allografts in mice. The chimeric toxin, IL-2-PE40, offers an alternative approach to the treatment of autoimmune diseases and transplant rejection in humans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacha P., Williams D. P., Waters C., Williams J. M., Murphy J. R., Strom T. B. Interleukin 2 receptor-targeted cytotoxicity. Interleukin 2 receptor-mediated action of a diphtheria toxin-related interleukin 2 fusion protein. J Exp Med. 1988 Feb 1;167(2):612–622. doi: 10.1084/jem.167.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnard G. D., Yasaka K., Jacobson D. Ligand-activated T cell growth factor-induced proliferation: absorption of T cell growth factor by activated T cells. J Immunol. 1979 Dec;123(6):2704–2708. [PubMed] [Google Scholar]
- Bottazzo G. F., Dean B. M., McNally J. M., MacKay E. H., Swift P. G., Gamble D. R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Case J. P., Lorberboum-Galski H., Lafyatis R., FitzGerald D., Wilder R. L., Pastan I. Chimeric cytotoxin IL2-PE40 delays and mitigates adjuvant-induced arthritis in rats. Proc Natl Acad Sci U S A. 1989 Jan;86(1):287–291. doi: 10.1073/pnas.86.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., FitzGerald D. J., Adhya S., Pastan I. Activity of a recombinant fusion protein between transforming growth factor type alpha and Pseudomonas toxin. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4538–4542. doi: 10.1073/pnas.84.13.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Mizukami T., Fuerst T. R., FitzGerald D. J., Moss B., Pastan I., Berger E. A. Selective killing of HIV-infected cells by recombinant human CD4-Pseudomonas exotoxin hybrid protein. Nature. 1988 Sep 22;335(6188):369–372. doi: 10.1038/335369a0. [DOI] [PubMed] [Google Scholar]
- Corry R. J., Winn H. J., Russell P. S. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973 Oct;16(4):343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- Cotner T., Williams J. M., Christenson L., Shapiro H. M., Strom T. B., Strominger J. Simultaneous flow cytometric analysis of human T cell activation antigen expression and DNA content. J Exp Med. 1983 Feb 1;157(2):461–472. doi: 10.1084/jem.157.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. M., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: substitution of glutamic acid 553 with aspartic acid drastically reduces toxicity and enzymatic activity. J Bacteriol. 1987 Nov;169(11):4967–4971. doi: 10.1128/jb.169.11.4967-4971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar J. J., Benjamin W. R., Hilfiker M. L., Howard M., Farrar W. L., Fuller-Farrar J. The biochemistry, biology, and role of interleukin 2 in the induction of cytotoxic T cell and antibody-forming B cell responses. Immunol Rev. 1982;63:129–166. doi: 10.1111/j.1600-065x.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Hahn H. J., Kuttler B., Dunger A., Klöting I., Lucke S., Volk H. D., von Baehr R., Diamantstein T. Prolongation of rat pancreatic islet allograft survival by treatment of recipient rats with monoclonal anti-interleukin-2 receptor antibody and cyclosporin. Diabetologia. 1987 Jan;30(1):44–46. doi: 10.1007/BF01788907. [DOI] [PubMed] [Google Scholar]
- Heidecke C. D., Kupiec-Weglinski J. W., Lear P. A., Abbud-Filho M., Araujo J. L., Araneda D., Strom T. B., Tilney N. L. Interactions between T lymphocyte subsets supported by interleukin 2-rich lymphokines produce acute rejection of vascularized cardiac allografts in T cell deprived rats. J Immunol. 1984 Aug;133(2):582–588. [PubMed] [Google Scholar]
- Jinno Y., Chaudhary V. K., Kondo T., Adhya S., FitzGerald D. J., Pastan I. Mutational analysis of domain I of Pseudomonas exotoxin. Mutations in domain I of Pseudomonas exotoxin which reduce cell binding and animal toxicity. J Biol Chem. 1988 Sep 15;263(26):13203–13207. [PubMed] [Google Scholar]
- Kelley V. E., Bacha P., Pankewycz O., Nichols J. C., Murphy J. R., Strom T. B. Interleukin 2-diphtheria toxin fusion protein can abolish cell-mediated immunity in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3980–3984. doi: 10.1073/pnas.85.11.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman R. L., Barrett L. V., Gaulton G. N., Kelley V. E., Ythier A., Strom T. B. Administration of an anti-interleukin 2 receptor monoclonal antibody prolongs cardiac allograft survival in mice. J Exp Med. 1985 Jul 1;162(1):358–362. doi: 10.1084/jem.162.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman R. L., Barrett L. V., Koltun W. A., Diamantstein T. Prolongation of murine cardiac allograft survival by the anti-interleukin-2 receptor monoclonal antibody AMT-13. Transplant Proc. 1987 Feb;19(1 Pt 1):618–619. [PubMed] [Google Scholar]
- Kupiec-Weglinski J. W., Diamantstein T., Tilney N. L., Strom T. B. Therapy with monoclonal antibody to interleukin 2 receptor spares suppressor T cells and prevents or reverses acute allograft rejection in rats. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2624–2627. doi: 10.1073/pnas.83.8.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupiec-Weglinski J. W., Hahn H. J., Kirkman R. L., Volk H. D., Mouzaki A., DiStefano R., Tellides G., Dallman M., Morris P. J., Strom T. B. Cyclosporine potentiates the immunosuppressive effects of anti-interleukin 2 receptor monoclonal antibody therapy. Transplant Proc. 1988 Apr;20(2 Suppl 2):207–216. [PubMed] [Google Scholar]
- Larsson E. L. Mechanism of T cell activation. II. Antigen- and lectin-dependent acquisition of responsiveness to TCGF is a nonmitogenic, active response of resting T cells. J Immunol. 1981 Apr;126(4):1323–1326. [PubMed] [Google Scholar]
- Lemm G., Warnatz H. Evidence for enhanced interleukin 2 (IL-2) secretion and IL-2 receptor presentation by synovial fluid lymphocytes in rheumatoid arthritis. Clin Exp Immunol. 1986 Apr;64(1):71–79. [PMC free article] [PubMed] [Google Scholar]
- Lorberboum-Galski H., FitzGerald D., Chaudhary V., Adhya S., Pastan I. Cytotoxic activity of an interleukin 2-Pseudomonas exotoxin chimeric protein produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1922–1926. doi: 10.1073/pnas.85.6.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouzaki A., Volk H. D., Osawa H., Diamantstein T. Blocking of interleukin 2 (IL 2) binding to the IL 2 receptor is not required for the in vivo action of anti-IL 2 receptor monoclonal antibody (mAb). I. The production, characterization and in vivo properties of a new mouse anti-rat IL 2 receptor mAb that reacts with an epitope different to the one that binds to IL 2 and the mAb ART-18. Eur J Immunol. 1987 Mar;17(3):335–341. doi: 10.1002/eji.1830170306. [DOI] [PubMed] [Google Scholar]
- Murphy J. R., Bishai W., Borowski M., Miyanohara A., Boyd J., Nagle S. Genetic construction, expression, and melanoma-selective cytotoxicity of a diphtheria toxin-related alpha-melanocyte-stimulating hormone fusion protein. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8258–8262. doi: 10.1073/pnas.83.21.8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T., Cheng S. Y. Strip-comb dot immunobinding: a rapid, easy and sensitive method to screen monoclonal antibodies. Biotechniques. 1988 Apr;6(4):299–303. [PubMed] [Google Scholar]
- Ranges G. E., Sriram S., Cooper S. M. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985 Sep 1;162(3):1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984 Jan;25(1):32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. E., Kirkman R. L., Reed M. H., Puskas J. D., Mazoujian G., Letvin N. L., Carpenter C. B., Milford E. L., Waldmann T. A., Strom T. B. Monoclonal anti-IL-2 receptor antibody in primate renal transplantation. Transplant Proc. 1987 Feb;19(1 Pt 1):594–598. [PubMed] [Google Scholar]
- Shizuru J. A., Gregory A. K., Chao C. T., Fathman C. G. Islet allograft survival after a single course of treatment of recipient with antibody to L3T4. Science. 1987 Jul 17;237(4812):278–280. doi: 10.1126/science.2955518. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Gillis S., Baker P. E., McKenzie D., Ruscetti F. W. T-cell growth factor-mediated T-cell proliferation. Ann N Y Acad Sci. 1979;332:423–432. doi: 10.1111/j.1749-6632.1979.tb47136.x. [DOI] [PubMed] [Google Scholar]
- Tellides G., Dallman M. J., Kupiec-Weglinski J. W., Diamantstein T., Morris P. J. Functional blocking of the interleukin-2 receptor (IL-2R) may be important in the efficacy of IL-2R antibody therapy. Transplant Proc. 1987 Oct;19(5):4231–4233. [PubMed] [Google Scholar]
- Waldor M. K., Sriram S., Hardy R., Herzenberg L. A., Herzenberg L. A., Lanier L., Lim M., Steinman L. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science. 1985 Jan 25;227(4685):415–417. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- Wall J. R., Baur R., Schleusener H., Bandy-Dafoe P. Peripheral blood and intrathyroidal mononuclear cell populations in patients with autoimmune thyroid disorders enumerated using monoclonal antibodies. J Clin Endocrinol Metab. 1983 Jan;56(1):164–169. doi: 10.1210/jcem-56-1-164. [DOI] [PubMed] [Google Scholar]
- Williams D. P., Parker K., Bacha P., Bishai W., Borowski M., Genbauffe F., Strom T. B., Murphy J. R. Diphtheria toxin receptor binding domain substitution with interleukin-2: genetic construction and properties of a diphtheria toxin-related interleukin-2 fusion protein. Protein Eng. 1987 Dec;1(6):493–498. doi: 10.1093/protein/1.6.493. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Loertscher R., Cotner T., Reddish M., Shapiro H. M., Carpenter C. B., Strominger J. L., Strom T. B. Dual parameter flow cytometric analysis of DNA content, activation antigen expression, and T cell subset proliferation in the human mixed lymphocyte reaction. J Immunol. 1984 May;132(5):2330–2337. [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985 Feb 1;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]



