Abstract
Pentamidine is a small molecule inhibitor of the Ca2+ binding protein S100B and disrupts the S100B-p53 protein-protein interaction; this is thought to restore wild type p53 tumour suppressor function in melanoma. Additional anti-cancer effects may be the result of inhibition of PRL family phosphatases.
In this study we have used a standardised ATP Tumour Chemosensitivity Assay (ATP-TCA) to investigate the effect of pentamidine on cells derived from 18 skin melanoma samples, and 1 uveal melanoma sample. The cells were tested at six concentrations from which the IC50 and IC90 were calculated. To allow comparison between samples, an IndexSUM was calculated based on percentage tumour growth inhibition at each concentration.
Of the skin melanoma samples tested, 78% exhibited an IndexSUM<300 indicating strong inhibition. The median IndexSUM of 237 also indicates strong inhibition. The median IC90 was 79.5% of the test drug concentration (30.2 μM) consistent with a strong response at a clinically achievable drug concentration. The uveal melanoma sample exhibited and IndexSUM=333, indicating moderate inhibition, and 86% inhibition at test drug concentration (30.2 μM).
These results support the prospect of a therapeutic use for pentamidine in melanoma, and a phase II clinical trial is in progress.
Keywords: Chemosensitivity, melanoma, pentamidine, ATP
Introduction
1,5-Bis(4-amidinophenoxy)pentane (Pentamidine) is an aromatic diamidine pharmacologically active as an antiprotazoal agent. It is used in the treatment and prevention of Pneumocystis carinii pneumonia (PCP), particularly in patients with HIV infection, and in the treatment of trypanosomiasis and visceral leishmaniasis. Pentamidine has recently been highlighted as a potential anti-cancer drug, particularly in the context of melanoma where it is thought to inhibit the S100B-p53 protein-protein interaction [1, 2].
S100B is a highly conserved 21.5kDa homodimer belonging to the Ca2+-binding EF-hand motif superfamily, structurally related to other Ca2+ binding proteins such as calmodulin and troponin C [3, 4]. S100B interacts with p53 at its C terminus in a Ca2+ dependent manner and binds through hydrophobic interactions with exposed residues, and salt bridges [2]. In addition to this interaction, the C terminus of p53 has been demonstrated to be a substrate of protein kinase C [5, 6]. These findings serve to link p53 activity to calcium signaling. It is thought that S100B inhibits the transcriptional activity of p53 by inhibiting tetramerisation and phosphorylation of the C terminus by PKC [7, 8]. p53 has long been recognized as a vital transcriptional activator of many genes involved in apoptosis and cell cycle control; stabilization and activation of this protein (by tetramerisation and modifications such as C terminus phosphorylation) halts inappropriate growth and cell cycling; a tumour suppressor function [9].
Pentamidine has been identified as a molecule that binds to the p53 binding site on S100B, and models of pentamidine bound to S100B have been produced [2, 12–14]. Pentamidine may therefore act to prevent S100B-p53 binding and prevent loss of tetramerisation (stabilization) and C terminus phosphorylation caused by this protein-protein interaction.
High levels of S100B are associated with melanoma and are commonly used in diagnosis by immunohistochemistry [7, 10, 11]. Lin et al. [1] have demonstrated a direct relationship between levels of p53 and S100B protein in 6 melanoma cell lines (LOX-IM, UACC-62, SK-MEL-5, UACC-2571, C8146A, Malme-3M) with a wild type p53 genotype, where a high S100B level is directly related to a low level of p53, and a low level of S100B is directly related to a high level of p53 as measured by western blot [1]. Furthermore these authors develop a physiological theory of S100B, suggesting that p53 binds the S100B promoter at levels above which are required for most p53 transcriptional targets, and the generation of S100B acts as a negative feedback on p53, in a functionally similar manner to hdm2 [1, 7].
There is some evidence that pentamidine may also exert its anticancer effects by acting as an inhibitor of phosphatase of regenerating liver (PRL) family phosphatases whose biological functions are poorly understood, but which are overexpressed in many cancers [15]. Pathak et al. report Pentamidine inhibits all three PRLs in vitro and exhibits an inhibitory effect against WM9 human melanoma cell line xenografts in nude mice [15, 16]. Wang et al. found high levels of PRL-1 expression in five of six melanoma cell lines studied by quantitative RT-PCR [17].
However, all of the in vitro and xenograft data are based on cell lines, which are highly passaged and adapted to the cell culture environment, resulting in high growth rates and greater sensitivity to chemotherapeutic agents [18, 19]. The use of tumour-derived cells or low passage number cell lines can offset this disadvantage, as we have previously shown in ovarian cancer [18] and in melanoma [Fernando et al. unpublished]. We therefore took the opportunity to study the activity of pentamidine against human tumour-derived melanoma cells in vitro using the ATP-TCA [20].
Materials and Methods
Tumours
A total of eighteen metastatic skin melanoma samples (10 males, 8 females), and one uveal melanoma sample (female) were tested in the study. These samples were obtained at debulking surgery for regional lymph node metastasis. The individual tumours are described in Table 1. Only one patient had received previous chemotherapy (Temozolomide). In each case, only tumour material not required for diagnosis was used in the ATP-TCA. Individual patient consent was obtained for all samples and permission for tissue use granted by the local ethics committee.
Table 1.
Data for individual patient samples including the site of primary tumour and lymph node metastasis, IC50, IC90, and IndexSUM
| Sample Type | Age | Sex | Primary Tumour site | Lymph Node Metastasis site | IC50 μM | IC90 μM | IndexSUM |
|---|---|---|---|---|---|---|---|
| Uveal Melanoma | 37 | F | Left Ciliary body | N/A | 22.776 | 50.11 | 333 |
| 49 | M | Right thigh | Right thigh | 27.0 | 63.4 | 366 | |
| 36 | F | Left groin | Left groin | 14.0 | 22.8 | 393 | |
| 49 | F | Back | Back | 8.73 | 32.3 | 235 | |
| 64 | M | Left leg | Left leg | 6.83 | 11.8 | 192 | |
| 33 | F | Unknown | Unknown | 17.1 | 35.7 | 274 | |
| 27 | M | Unknown | Groin | 21.3 | 35.3 | 266 | |
| 63 | M | Left leg | Left Leg | 4.56 | 16.3 | 138 | |
| 63 | F | Unknown | Left Inguinal | 16.7 | 40.6 | 310 | |
| Skin Melanoma | 72 | F | Unknown | Unknown | 3.80 | 22.0 | 140 |
| 81 | M | Unknown | Groin | 3.80 | 11.0 | 126 | |
| 45 | M | Unknown | Left axila | 4.56 | 17.5 | 156 | |
| 61 | F | Unknown | Right groin | 9.11 | 34.5 | 239 | |
| 60 | M | Unknown | Left axila | 15.9 | 55.4 | 306 | |
| 75 | M | Scalp | Right neck | 9.11 | 28.1 | 233 | |
| 73 | M | Right leg | Right groin | 14.8 | 34.5 | 275 | |
| 85 | F | Fingertip | Left axila | 4.18 | 14.0 | 141 | |
| 66 | F | Lateral malleolus | Left groin | 3.80 | 14.0 | 123 | |
| 65 | M | Chest | Left axila | 14.0 | 35.7 | 277 | |
| Median n=18 | 9.11 | 30.2 | 237 | ||||
ATP-TCA (Tumour Chemosensitivity Assay)
The ATP-TCA assay was performed as previously described [20, 21]. Cells were obtained by enzymatic dissociation of solid tumour tissue by collagenase (Sigma, C8051). These cells were diluted in serum-free complete assay medium (CAM; available from DCS Innovative Diagnostik Systeme, Hamberg, Germany) and plated in 96 well polypropylene plates (Corning Life Sciences, High Wycombe, UK) at 20000 cells per well.
Pentamidine was added in triplicate to wells at serial dilutions of 200%, 100%, 50%, 25%, 12.5%, 6.25% Test Drug Concentration (TDC). For Pentamidine the TDC was set at 37.96 μM based on previous in vitro experiments defining the inhibition of S100B-p53 interaction [2]. Each plate included two controls: a medium only row (MO) which contained no drug, and a row to which a maximum inhibitor (MI: available from DCS Innovative Diagnostik Systeme) was added, killing all cells present.
Cells were incubated for six days at 37 °C in 5% CO2. After the incubation period, cells were lysed by the addition of a cell extraction reagent (available from DCS Innovative Diagnostik Systeme). An aliquot of lysate (0.05 ml) from each well was added to the corresponding well of a white 96 well microplate (Thermo Life Sciences, Basingstoke, UK), to which 0.05 ml Luciferin-luciferase counting reagent (D-luciferin and recombinant luciferase (R&D systems Abingdon, UK)) was then added. The light output corresponding to the level of ATP present was measured using a luminometer (MPLX, Berthold Diagnostic Systems, Hamberg, Germany). The light output data was transferred to a spreadsheet and the % inhibition at each concentration was calculated using the equation: 1- (test-MI)/(MO-MI).
To compare results between tumours, a simple logarithmic sum index (Indexsum) was calculated by summing the % inhibition at each of the six % TDC used and subtracting this from 600: Index = 600-SUM[%Inhibition6.25, 12.5…200]. Total inhibition across all concentrations produces an IndexSUM=0, while no inhibition produces an IndexSUM=600.
Pentamidine
The Pentamidine (Pentamidine Isethionate salt) used in the ATP-TCA assay was sourced from Sigma Aldrich (PO547). The drug was dissolved in DMSO at 0.18 g/μl, and stock aliquots stored at −20 °C. The stock was diluted in CAM for testing in the ATP-TCA at six concentrations ranging from 6.25% TDC (2.37 μM) to 200% TDC (75.92 μM)
Data Analysis
The data for the 19 samples was input to an Access database (Microsoft) and transferred to Excel (Microsoft) for further analysis. The median % inhibition and interquartile range was calculated at each % TDC. The % TDC was converted to μM concentrations and a Concentration-Response curve plotted using the natural logarithmic scale produced by serial drug dilution. The IC50 and IC90 for Pentamidine in each tumour sample were calculated by the trapezoidal rule.
Results
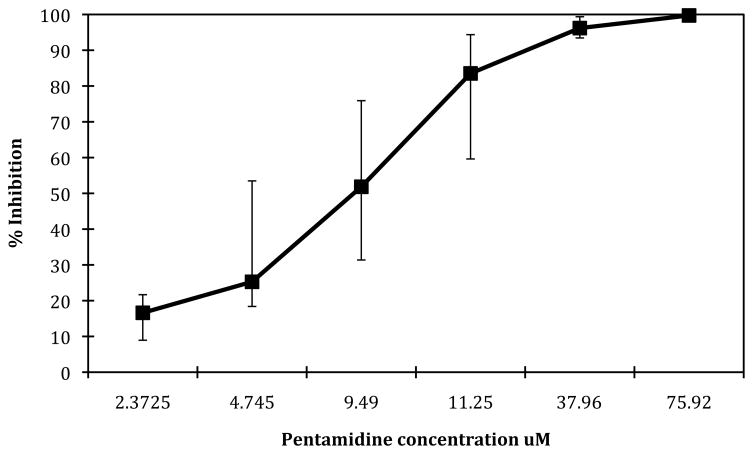
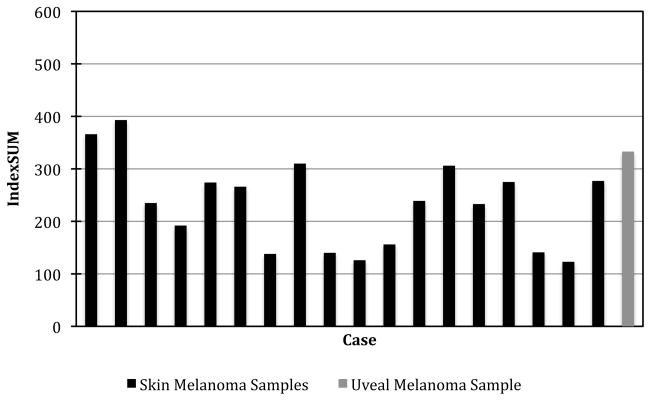
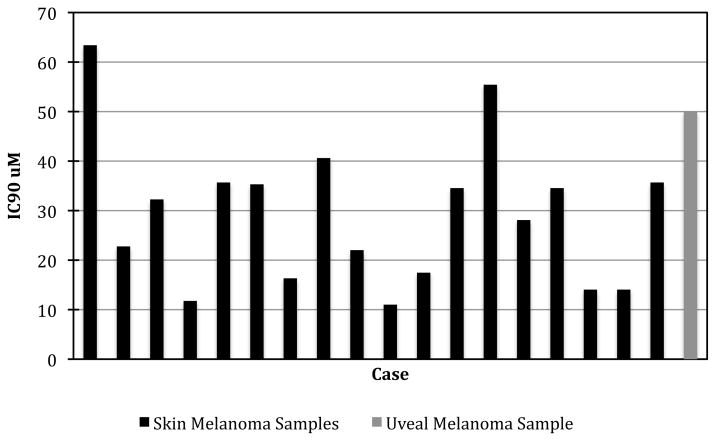
Pentamidine exhibited strong inhibition in all of the melanoma tumour samples tested, though with some heterogeneity between samples and less activity than reported in cell lines. There was increasing inhibition with increasing drug concentration (Fig 1). In all, 50% of the skin melanoma samples tested exhibited greater than 95% inhibition at 100% TDC (37.96 μM) while 22% (4/18) exhibited greater than 95% inhibition at 50% TDC (11.25 μM). Pentamidine exhibited increasing inhibition of the uveal melanoma sample at increasing concentrations, and 86% inhibition at 100% TDC (37.96 μM). There was heterogeneity of response (shown in fig 2). Heterogeneity of response to single agents has been observed previously in melanoma [22], and was expected in this study. Indexsum<300 corresponding to 50% inhibition across the range of concentrations tested is a useful way to compare samples, and 78% (14/18) of the skin melanoma samples exhibited an Indexsum<300 indicating strong inhibition (Fig 2). The median Indexsum=237, and the median IC90 of 79.5% TDC (30.2 μM) also demonstrate a strong response in skin melanoma samples. However a considerable range of IC90 values (11.0–55.4 μM) was observed (Fig 3). The uveal melanoma sample exhibited an IndexSUM=333 indicating moderate inhibition (Fig 2) and an IC90 of 132% TDC (30.2 μM) (Fig 3).
Figure 1.
Median percentage inhibition at increasing pentamidine concentrations, with interquartile range, in skin melanoma samples n=18.
Figure 2.
IndexSUM of individual samples.
Figure 3.
IC90 for individual samples
Discussion
Several studies have linked pentamidine to anti-cancer effects in melanoma [1, 2, 7, 14, 16], however these effects have been observed in cell lines whose characteristics are not directly comparable to the original tissue [18]. This study investigated the effects of pentamidine on tumour-derived melanoma cells in the ATP-TCA, and as a result gives a more accurate indication of the potential effect of pentamidine on melanoma in vivo than can be achieved with cell lines. Despite heterogeneity of response, the median Indexsum remained low (<300), and at high concentrations (100% TDC) the interquartile range of inhibition was low (fig 2), showing a decreased heterogeneity of response at higher concentrations and considerable pentamidine activity at the concentrations measured.
The results of this study show pentamidine to be active against melanoma over a range of concentrations which are probably just clinically attainable. It has been shown that in patients with leishmaniasis, a peak plasma concentration (Cmax) on day 7 of 751 nmol/L is achievable (AUC: 6,738 nmol/h/L), based on a dose of 3–9 mg/kg/day given by IV infusion over 4 hours once daily [23]. Others have shown higher values within 8 hours of administation. In trypanosomiasis patients, given a 2 hour IV infusion of 3.0 to 4.8 mg/kg, Cmax was noted to be 713 – 2,461 nmol/L (median 923 nmol/L) [24]. Other studies show similar figures for both Cmax and AUC [25, 26]. Metabolism is by cytochrome P450 and excretion is mainly via the kidney [24]. This compares with our data showing IC90 values of 11.0 – 55.4 μM, suggesting that the concentrations observed to be active could be achieved in patients. However, little protein is present in CAM, and pentamidine is 70% protein bound in plasma. Much depends on the degree to which pentamidine is taken up by tumour tissue, and this is unknown.
There may be several mechanisms by which pentamidine exerts its anticancer effects: The binding of pentamidine to S100B has been rigorously established [1, 2, 14] and it is likely that pentamidine restores wild type p53 tumour suppressor function. A second potential mechanism, inhibition of PRL family phosphatases, may halt cell cycle progression; PRL-1 has been shown to be required for normal cell cycle progression [17], and Lee et al. [27] report that when used in conjunction with chlorpromazine, pentamidine has a synergistic effect in halting mitosis in tumours. Both mechanisms likely play a role in tumour suppression, and further mechanistic studies must be conducted to conclude which is the most important, and which can be best targeted. Both of these targets are involved in cell cycle progression, however given the extremely important role of p53 as an upstream regulator of the cell cycle, this target is likely to be the most significant.
This study shows pentamidine to be active in vitro against tumour derived melanoma cells and supports the prospect of its future therapeutic use in patients with metastatic melanoma, though the concentration required is only just clinically achievable. Its frequent and serious side effects, particularly renal and pancreatic damage are a concern [28, 39, 30], but are no worse and probably better than many anticancer agents or combinations in use for melanoma. They may be ameliorated by careful scheduling [31]. A phase II trial is being conducted investigating the effect of pentamidine against melanoma with wild type p53 and detectable S100B in human participants with relapsed or refractory melanoma (www.clinicaltrials.gov Identifier: NCT00729807).
Acknowledgments
Sponsorship: This work was supported by the Royal Navy (JS), Cantech Ltd, and the Skin Cancer Research Fund.
References
- 1.Lin J, Yang Q, Yan Z, Markowitz J, Wilder PT, Carrier F, et al. Inhibiting S100B restores p53 levels in primary malignant melanoma cancer cells. The Journal of biological chemistry. 2004;279(32):34071–7. doi: 10.1074/jbc.M405419200. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz J, Chen I, Gitti R, Baldisseri DM, Pan Y, Udan R, et al. Identification and characterization of small molecule inhibitors of the calcium-dependent S100B-p53 tumor suppressor interaction. Journal of medicinal chemistry. 2004;47(21):5085–93. doi: 10.1021/jm0497038. [DOI] [PubMed] [Google Scholar]
- 3.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. The international journal of biochemistry & cell biology. 2001;33(7):637–68. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 4.Zimmer DB, Wright Sadosky P, Weber DJ. Molecular mechanisms of S100-target protein interactions. Microscopy research and technique. 2003;60(6):552–9. doi: 10.1002/jemt.10297. [DOI] [PubMed] [Google Scholar]
- 5.Baudier J, Delphin C, Grunwald D, Khochbin S, Lawrence JJ. Characterization of the tumor suppressor protein p53 as a protein kinase C substrate and a S100b-binding protein. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(23):11627–31. doi: 10.1073/pnas.89.23.11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilder PT, Rustandi RR, Drohat aC, Weber DJ. S100B(betabeta) inhibits the protein kinase C-dependent phosphorylation of a peptide derived from p53 in a Ca2+-dependent manner. Protein science : a publication of the Protein Society. 1998;7(3):794–8. doi: 10.1002/pro.5560070330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin J, Blake M, Tang C, Zimmer D, Rustandi RR, Weber DJ, et al. Inhibition of p53 transcriptional activity by the S100B calcium-binding protein. The Journal of biological chemistry. 2001;276(37):35037–41. doi: 10.1074/jbc.M104379200. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Fernandez MR, Veprintsev DB, Fersht AR. Proteins of the S100 family regulate the oligomerization of p53 tumor suppressor. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(13):4735–40. doi: 10.1073/pnas.0501459102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vousden KH, Lane DP. P53 in Health and Disease. Nature reviews. Molecular cell biology. 2007;8(4):275–83. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 10.Cochran AJ, Lu H, Li P, Saxton R, Wen D. S-100 protein remains a practical marker for melanocytic and other tumours. Melanoma Research. 1993;3:325–330. doi: 10.1097/00008390-199310000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hauschild A, Engel G, Brenner W, Gläser R, Mönig H, Henze E, et al. S100B protein detection in serum is a significant prognostic factor in metastatic melanoma. Oncology. 1999;56(4):338–44. doi: 10.1159/000011989. [DOI] [PubMed] [Google Scholar]
- 12.Charpentier TH, Wilder PT, Liriano MA, Varney KM, Pozharski E, MacKerell AD, et al. Divalent metal ion complexes of S100B in the absence and presence of pentamidine. Journal of molecular biology. 2008;382(1):56–73. doi: 10.1016/j.jmb.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gieldon A, Mori M, Del Conte R. Theoretical study on binding of S100B protein. Journal of molecular modeling. 2007;13(11):1123–31. doi: 10.1007/s00894-007-0231-6. [DOI] [PubMed] [Google Scholar]
- 14.Charpentier Thomas H, Wilder Paul T, Varney Kristen M, Toth Eric A, Weber David J. Crystal structure of pentamidine bound to Ca2+-S100B. AACR Meeting Abstracts; 2006; 2006. p. 456-b. [Google Scholar]
- 15.Stephens BJ, Han H, Gokhale V, Von Hoff DD. PRL phosphatases as potential molecular targets in cancer. Molecular cancer therapeutics. 2005;4(11):1653–61. doi: 10.1158/1535-7163.MCT-05-0248. [DOI] [PubMed] [Google Scholar]
- 16.Pathak MK, Dhawan D, Lindner DJ, Borden EC, Farver C, Yi T, et al. Pentamidine is an inhibitor of PRL phosphatases with anticancer activity. Molecular cancer therapeutics. 2002;1(14):1255–64. [PubMed] [Google Scholar]
- 17.Wang J, Kirby CE, Herbst R. The Tyrosine Phosphatase PRL-1 Localizes to the Endoplasmic Reticulum and the Mitotic Spindle and Is Required for Normal Mitosis*. Biochemistry. 2002;277(48):46659–46668. doi: 10.1074/jbc.M206407200. [DOI] [PubMed] [Google Scholar]
- 18.Fernando A, Glaysher S, Conroy M, Pekalski M, Smith J, Knight La, et al. Effect of culture conditions on the chemosensitivity of ovarian cancer cell lines. Anti-cancer drugs. 2006;17(8):913–9. doi: 10.1097/01.cad.0000224445.23953.d9. [DOI] [PubMed] [Google Scholar]
- 19.Andreotti PE, Linder D, Hartmann DM, Cree Ia, Pazzagli M, Bruckner HW, et al. TCA-100 tumour chemosensitivity assay: differences in sensitivity between cultured tumour cell lines and clinical studies. Journal of bioluminescence and chemiluminescence. 1994;9(6):373–8. doi: 10.1002/bio.1170090604. [DOI] [PubMed] [Google Scholar]
- 20.Andreotti PE, Cree Ia, Kurbacher CM, Hartmann DM, Linder D, Harel G, et al. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer research. 1995;55(22):5276–82. [PubMed] [Google Scholar]
- 21.Cree IA. Luminescence-Based cell viability testing. In: LaRossa RA, editor. Bioluminescence Methods and Protocols. Totowa, NJ: Humana Press Inc; 1998. pp. 169–177. [DOI] [PubMed] [Google Scholar]
- 22.Cree Ian A, Neale Michael H, Andreotti PE. Heterogeneity of chemosensitivity of metastatic cutaneous melanoma. Anti-cancer drugs. 1999;10:437–444. doi: 10.1097/00001813-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Lidman C, Bronner U, Gustafsson LL, Rombo L. Plasma pentamidine concentrations vary between individuals with Pneumocystis carinii pneumonia and the drug is actively secreted by the kidney. The Journal of antimicrobial chemotherapy. 1994;33(4):803–10. doi: 10.1093/jac/33.4.803. [DOI] [PubMed] [Google Scholar]
- 24.Bronner U, Gustafsson LL, Doua F, Ericsson O, Miezan TW, Rais M, et al. Pharmacokinetics and adverse reactions after a single dose of pentamidine in patients with Trypanosoma gambiense sleeping sickness. Br J clin Pharmac. 1995;39:289–295. doi: 10.1111/j.1365-2125.1995.tb04451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard EM, Donnelly HJ, Maher MP, Armstrong D. Use of a New Bioassay to Study Pentamidine Pharmacokinetics. October. 2009;152(4):750–754. doi: 10.1093/infdis/152.4.750. [DOI] [PubMed] [Google Scholar]
- 26.Bronner U, Doua F, Ericsson O, Gustafsson LL, Miezan TW, Rais M, et al. Pentamidine concentrations in plasma, whole blood and cerebrospinal fluid during treatment of Trypanosoma gambiense infection in Cote d’Ivoire. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1991;85:608–611. doi: 10.1016/0035-9203(91)90364-5. [DOI] [PubMed] [Google Scholar]
- 27.Lee MS, Johansen L, Zhang Y, Wilson A, Keegan M, Avery W, et al. The novel combination of chlorpromazine and pentamidine exerts synergistic antiproliferative effects through dual mitotic action. Cancer research. 2007;67(23):11359–67. doi: 10.1158/0008-5472.CAN-07-2235. [DOI] [PubMed] [Google Scholar]
- 28.Lidman C, Bronner U, Gustafsson LL, Rombo L. Plasma pentamidine concentrations vary between individuals with Pneumocystis carinii pneumonia and the drug is actively secreted by the kidney. The Journal of antimicrobial chemotherapy. 1994;33(4):803–10. doi: 10.1093/jac/33.4.803. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz MS, Cappell MS. Pentamidine-associated pancreatitis. Digestive Diseases and Sciences. 1989;34(10):1617–1620. doi: 10.1007/BF01537122. [DOI] [PubMed] [Google Scholar]
- 30.Sands M, Kron MA, Brown RB. Pentamidine: A Review. Reviews of Infectious Diseases. 1985;7(5):625–634. doi: 10.1093/clinids/7.5.625. [DOI] [PubMed] [Google Scholar]
- 31.Lachaal M, Venuto RC. Nephrotoxicity and hyperkalemia in patients with acquired immunodeficiency syndrome treated with pentamidine. The American journal of medicine. 1989;87(3):260–3. doi: 10.1016/s0002-9343(89)80147-0. [DOI] [PubMed] [Google Scholar]