Abstract
Xenotransplantation is one promising approach to bridge the gap between available human cells, tissues, and organs and the needs of patients with diabetes or end-stage organ failure. Based on recent progress using genetically-modified source pigs, improving results with conventional and experimental immunosuppression, and expanded understanding of residual physiologic hurdles, xenotransplantation appears likely to be evaluated in clinical trials in the near future for some select applications. This review offers a comprehensive overview of known mechanisms of xenograft injury, a contemporary assessment of preclinical progress and residual barriers, and our opinions regarding where breakthroughs are likely to occur.
Keywords: Antibody-mediated rejection; α1,3-galactosyltransferase gene-knockout; Coagulation; Genetic engineering, pig; Pancreatic islets, pig; Pig; Xenotransplantation
Introduction
Transplantation is one of medicine's greatest triumphs, saving thousands of lives each year, and improving the quality of life for many of those fortunate enough to obtain a scarce graft from another person. Unfortunately, the supply of human organs is insufficient to treat all the patients who present each year with organ failure, and who could benefit if a compatible graft were available. Similarly, the potential supply of islets from human donors will never be sufficient to treat the millions of people afflicted by diabetes, or even the minority with particularly brittle juvenile-onset disease who are currently considered candidates for pancreatic islet allotransplantation. Despite concerted, successful societal efforts to increase the availability of organs from cadaveric and living donors, it remains highly unlikely that human cellular or organ grafts will fulfill projected needs. And while substantial progress has been made toward harnessing the tremendous potential of stem cell biology and tissue engineering, most approaches based on these young technologies are at a proof-of-concept stage, and far from clinical application. These considerations provide a powerful impetus for preclinical “translational” research to develop clinical therapies using organ and islet xenografts.
The rationale for the choice of pigs as the preferred donor species is based on breeding characteristics as well as physiologic and organ size compatibility for use in humans, and because use of “concordant” primate species would pose very difficult ethical, infectious, and logistical issues [1-3]. Genetically-modified pigs expressing additional human complement pathway regulatory proteins (hCPRPs) [4,5] or with the α1,3-galactosyltransferase gene “knocked-out” (GalT-KO) [6,7] have been developed to address the initial barriers posed by anti-Gal antibody and complement. Work with hCPRP or GalTKO organs and tissues have significantly advanced survival results in preclinical organ and cell transplantation models. On the other hand, these studies reveal substantial residual obstacles that must be overcome before a xenograft is likely to improve human health, a benchmark that most experts agree should be a prerequisite for initiating a clinical xenotransplantation trial [8,9]. This review summarizes our current understanding of the immunobiology of xenotransplantation. Based on the available data and our working hypotheses regarding the major known obstacles, specific areas are identified as research priorities that, in our estimation, are likely to soon yield progress sufficient to justify initiation of safe clinical xenotransplantation trials.
Basic Mechanisms of Xenograft Injury
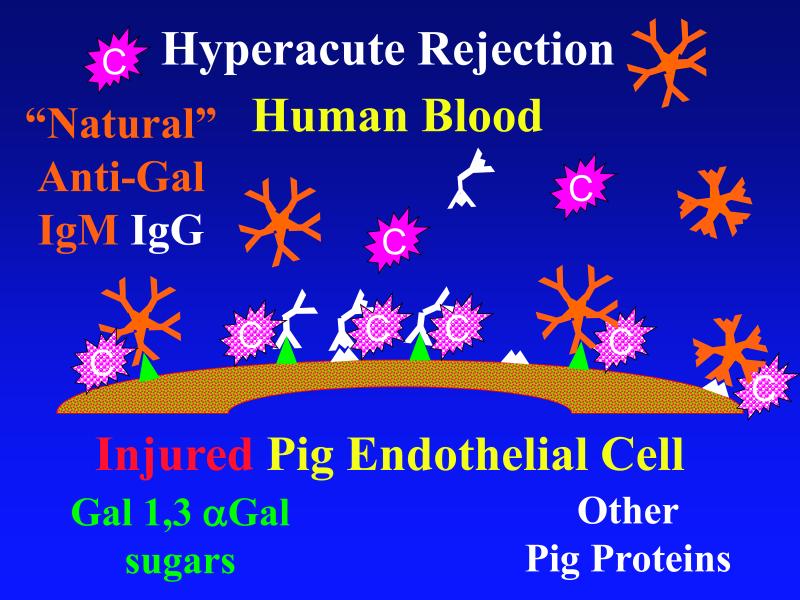
In most instances, when an organ from one species comes into contact with blood from another “discordant” species [10], preformed “natural” antibodies in the blood bind to foreign “xenogeneic” carbohydrates and proteins on the organ's endothelium. The endothelium responds by converting from an anti- to a pro-coagulant phenotype, while adherent antibodies in turn bind complement cascade proteins [11,12]. Activated complement causes further activation and lysis of endothelial cells, and attracts other proinflammatory immune system components. The result is “hyperacute rejection”, with immediate loss of organ function due to thrombosis and loss of endothelial barrier function. [Figure 1a]
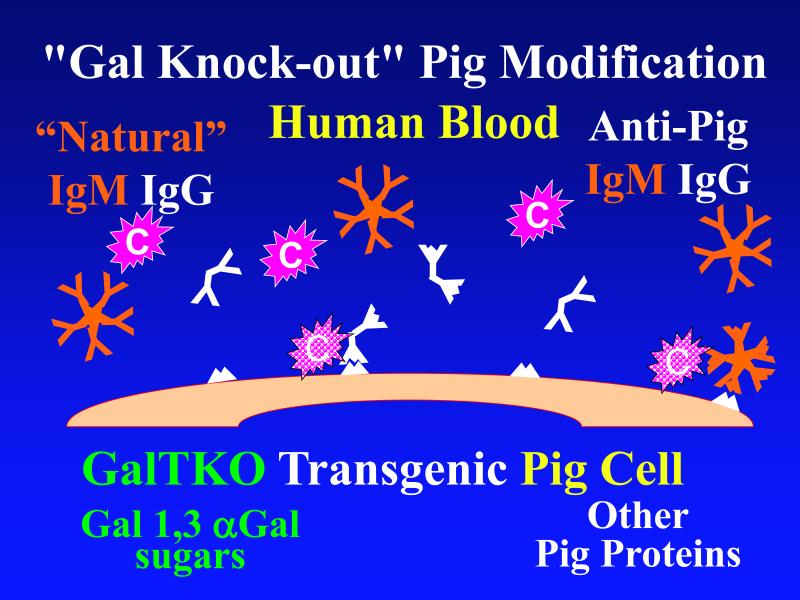
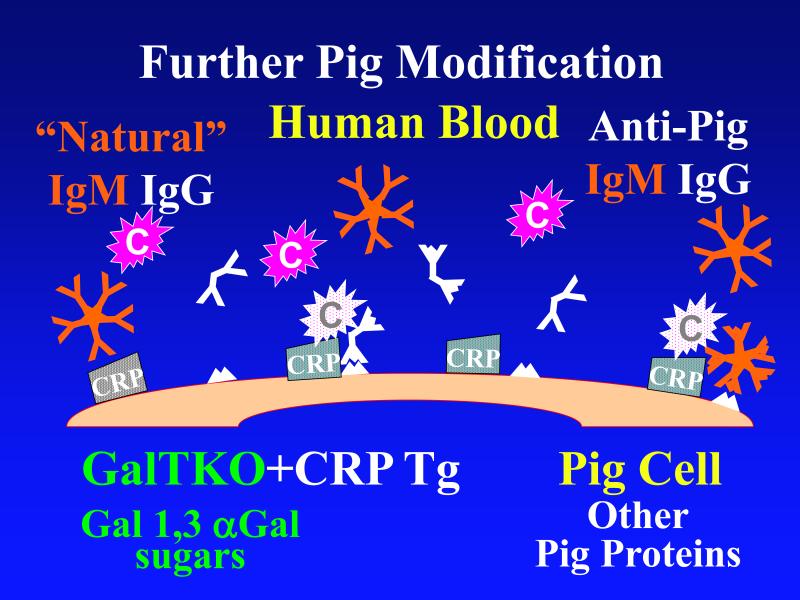
Figure 1. Genetic modifications to attenuate hyperacute rejection.
According to the conventional model, hyperacute rejection of pig organs in humans is triggered by binding of preformed human “natural” antibody, which is predominantly directed against Galα1-3Gal sugars that decorate many molecules on the surface of pig cells. Panel A: Complement is activated by bound antibody, triggering retraction and lysis of endothelial cells. Loss of endothelial barrier function contributes to leakage of blood into the interstitium. Blood clotting within vessels is activated by injured endothelium and exposed basement membrane, leading to downstream tissue ischemia and necrosis. Panel B: When the galactosyl transferase gene is disabled by gene knockout, pig cells lack the Galα1-3Gal target antigen. If present in sufficient quantity, antibody against other pig antigens that is either preformed or induced after transplant can trigger complement-mediated injury and associated coagulation pathway activation. Panel C: Human proteins that regulate (abort) complement activation (complement pathway regulatory proteins, CPRPs) disable human complement more efficiently than do their pig analogues. Organs from pigs genetically engineered to express human CPRPs are protected from hyperacute rejection, although this protection can be overcome by high-titer antibody, or if endothelial injury occurs due to other mechanisms (ischemia-perfusion injury or coagulation pathway activation, for example). GalT-KO, human CPRP, and perhaps additional gene modifications may be necessary to yield clinically useful, reliable protection of pig organs in man.
When hyperacute rejection does not occur, as in closely-related “concordant” primate species (e.g. chimpanzee-to-man), or if it is prevented by removing natural antibody or neutralizing complement, conventional immunosuppressive drugs prolong function of a xenogeneic organ from a few days to several weeks. In this setting xenografts usually fail due to a combination of “cellular” and “humoral” insults that together contribute to the phenomenon of “delayed xenograft rejection” (DXR, also interchangeably termed acute humoral xenograft rejection or acute vascular rejection) [13-18]. Cellular DXR histology resembles typical acute allograft rejection, with lymphocyte- and macrophage-rich perivascular infiltrates progressing to necrosis of graft parenchymal cells. Cellular DXR is typically not seen with intense but clinically-applicable immunosuppressive drug regimens [13-17,19-21].
Humoral DXR resembles humoral allograft rejection in the prominence of antibody and complement deposition with a paucity of immunocytes, and similarly often “breaks through” conventional immunosuppression. Humoral DXR is generally ascribed to antibodies produced rapidly and in high titer against donor xenoantigens recognized as foreign by the recipient's immune system. When such antibodies are detected in serum or in the graft, failure of the transplant is usually ascribed to incomplete control of antibody-producing B-cells or longer-lived “daughter” plasma cells. Evidence also supports a role for cytokines (interferon-γ, lipid mediators (thromboxane), proinflammatory cyclic nucleotides (adenosine mono- and diphosphate), and coagulation cascade molecules in humoral rejection, and these mediators should be evaluated in models where anti-donor antibody is not readily demonstrable.
Recent Progress with Pig Organ Xenografts
Role of Galα1,3Gal and α1,3-galactosyltransferase
The realization that preformed (“natural”) antibodies towards a particular carbohydrate, Galα1,3Gal (Gal), were largely responsible for triggering hyperacute rejection of pig organs transplanted into humans and Old World nonhuman primates [24,25] informed efforts to overcome this barrier. Many novel approaches that partially or completely blocked binding of anti-Gal antibodies and/or inhibited subsequent complement activation attenuated hyperacute rejection [reviewed in 26]. However none of these strategies prevented later DXR injury of Gal-expressing grafts.
Recently several groups genetically engineered “knock-out” pigs that lack the α1,3-galactosyltransferase gene (GalT-KO) and thus do not express the Gal oligosaccharide [6,7]. (Figure 1b) When the immune response to the Gal carbohydrate was thus eliminated from the equation, survival of GalT-KO kidneys [27,28] and hearts [29,30] was prolonged in nonhuman primates. (Figures 2, 3) Notably, conventional immunosuppression (FK506, rapamycin) at doses sufficient to protect an allograft did not prevent an elicited anti-non-Gal antibody response [28]. In contrast, an elicited antibody response against non-Gal pig epitopes could not usually be detected in the serum of baboons selected for low pretransplant anti-non-Gal antibody titers and treated with costimulation-based immunosuppression (ATG induction, anti-CD154, MMF and tapered steroids) that was associated with few infectious complications [27,29]. Similarly, the mixed lymphocyte reaction, reflecting cellular immunity to pig antigens, remained unresponsive. These observations suggest that the primary B- and T-cell immune responses to a pig organ xenograft were very efficiently controlled by the CD154 costimulation pathway-based regimens employed. However, focal deposits of immunoglobulins and complement were detected in the hearts and kidneys at the time of graft failure, and anti-non-Gal anti-pig antibodies were readily detected after heart graft removal [30]. If, as this observation suggests, residual “costimulatory” T-cell “help” for B cell immune function contributed to graft failure, improved control of costimulation-dependent adaptive immune responses will be required.
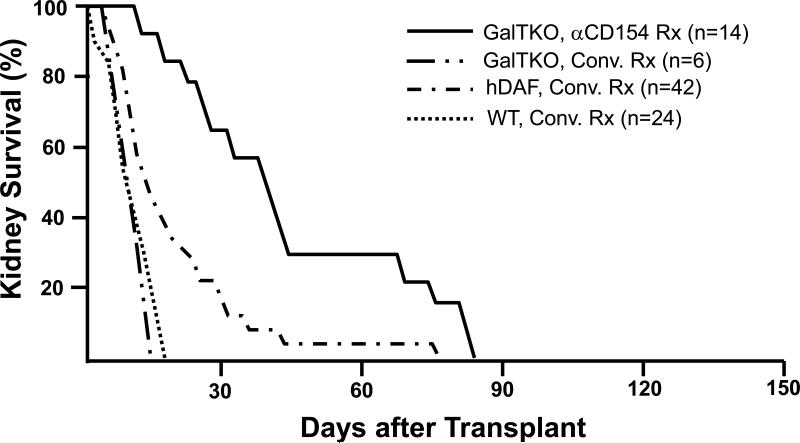
Figure 2. Effect of genetic modifications and treatment regimens on survival of pig kidneys in baboons.
Survival of life-supporting kidneys from wild-type (WT, n=24), hDAF transgenic (n=42), or GalT-KO pigs (n=20) in immunosuppressed baboons. With intensive conventional immunosuppression, with or without additional complement depletion, hDAF kidney recipients succumbed to infectious complications or developed DXR at a median of 15 to 25 days, significantly later than do similarly treated recipients of wild-type organs (~6-10 days). The incidence of early graft failure (<3 days) was reduced in recipients of GalT-KO kidneys. Six GalT-KO kidneys in baboons treated with conventional immunosuppression exhibited DXR associated with induction of anti-pig antibodies, demonstrating that the regimens tested in this experience were subtherapeutic. Using an anti-CD154 antibody, mycophenolate mofetil, T-cell depletion, and vascularized donor thymus tissue, 8 of 14 recipient baboons in the GalT-KO kidney group died with functioning grafts (serum creatinine <2.0mg/dL) from treatment complications. (Results in 3 recipients of hDAF kidneys treated with anti-CD154 antibody, which survived for 28, 29 and 29 days, are omitted from the figure due to the relatively small experience.)
Data for conventional immunosuppression is abstracted and aggregated from all peer-reviewed pig-to-baboon kidney transplant studies published since 1998. Pig phenotype (GalT-KO, hDAF, or WT) and treatment regimen (anti-CD154-based or conventional (Conv.) treatment (Rx)) are indicated. Similar results were achieved with conventional immunosuppression in over 250 cynomolgus recipients of hDAF kidneys that used comparable approaches.
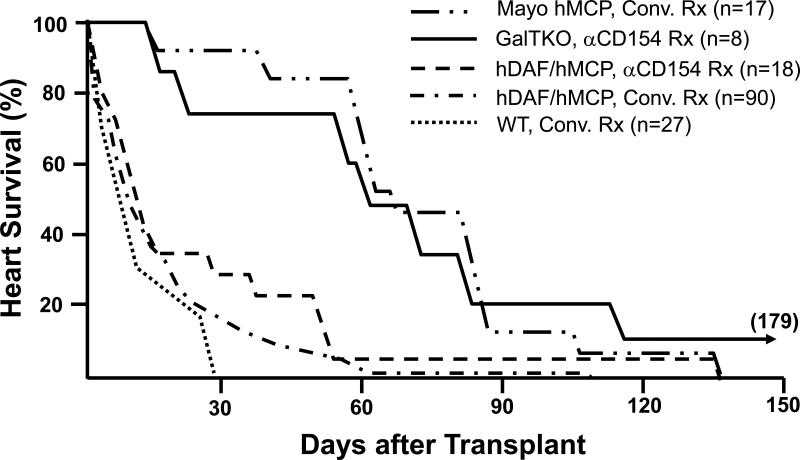
Figure 3. Effect of genetic modifications and treatment regimens on survival of pig hearts in baboons.
Survival in immunosuppressed baboons of heterotopic hearts from wild-type (WT) pigs (n=26), pigs transgenic for human decay-accelerating factor (hDAF), human membrane cofactor protein (hMCP), and/or human CD59 (n=125), and GalT-KO pigs (n=8). In conjunction with neutralization of anti-Gal antibodies, conventional or anti-CD154-based immunosuppression (using a monoclonal antibody that selectively blocks the CD154-CD40 “costimulation” pathway important to T- and B-cell activation) was associated with median survival of hDAF or hMCP transgenic hearts approaching one month in some series, and for over 2 months in the Mayo Clinic's recent experience. Infectious or treatment-associated complications caused the death of over 50% of recipients treated with conventional immunosuppression, but were unusual with anti-CD154-based treatment. Lymphocyte and antibody responses against pig antigens were generally undetectable during either anti-CD154 or intensive conventional therapy. Grafts failed with DXR histology in association with both conventional and CD154-based treatment regimens.
Eight GalT-KO hearts in baboons treated with anti-CD154 antibody, mycophenlate mofetil, and perioperative T-cell depletion, did not exhibit early graft failure (<3 days), and median survival exceeded two months. Typical DXR was rare in GalT-KO hearts, but thrombotic microangiopathy, which was also observed in long-surviving hDAF and hMCP hearts, was prominent in association with GalT-KO cardiac xenograft failure.
Data are abstracted from all peer-reviewed pig-to-baboon heart transplant studies published since 1988, aggregating data from studies that used similar therapeutic approaches. Pig phenotype (GalT-KO, hDAF, hMCP, or WT) and treatment regimen (anti-CD154-based or conventional (Conv.) treatment (Rx)) are indicated. Similar results were achieved with conventional immunosuppression in over 65 cynomolgus recipients of hDAF hearts.
Importantly, elicited anti-non-Gal immunity is often detected in association with consumptive coagulopathy or DXR of GalTKO pig organs when CD154 costimulation-based regimens are used in baboon recipients with higher pretransplant levels of anti-non-Gal antibody [31]. This finding underscores the importance of improved approaches to control secondary anti-non-Gal antibody responses to achieve clinically-applicable xenograft protection.
Controlling T cell-dependent anti-xeno responses
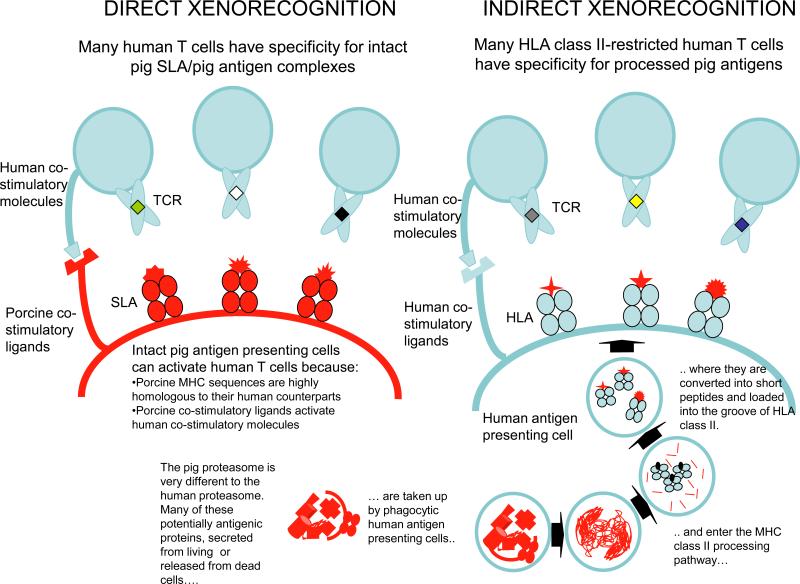
Human T-cells emerge from the thymus bearing a unique T-cell receptor selected on that individual's “self” major histocompatibility complex (MHC) class I or II molecules. They are activated in the periphery by a “foreign” – but not “self” – protein fragment antigenic peptide bound inside the peptide binding groove of MHC molecules on specialized antigen presenting cells. During evolution, this pathway of antigen recognition has allowed the “host” to mount a highly effective immune response to invading pathogens, including those with a high spontaneous mutation rate, because of the large number of antigens that can be recognized in this way. (Figure 4 Dorling antigen presentation)
Figure 4.
Available routes for presentation of pig antigens to primate T-cells (Editor's note: Legend is incorporate into the figure.)
In the context of transplantation between genetically-distinct humans, the transplanted organ releases proteins that can be processed into antigenic fragments by the same specialized cells, for display inside MHC molecules. If these are “foreign” to the recipient, they can be recognized by T cells in exactly the same way as a pathogenic antigen would be. Because of the way our understanding of transplantation immunology developed, this natural pathway of antigen recognition is known as the “indirect antigen presentation pathway” in the transplantation literature. This is to distinguish it from another pathway, the “direct pathway” in which mismatched donor MHC protein antigens on donor antigen presenting cells are engaged by the recipient's T-cell receptors directly, leading to T cell activation. This direct pathway is unique to the setting of transplantation. The combination of T cell activation by direct and indirect pathways makes T cell immunity to MHC-mismatched organs particularly strong. Similarly, for the clinically-relevant cross-species combination (pig-into-human), both the direct and indirect antigen presentation pathways operate relatively efficiently to activate a significant proportion of human T-cells.
Following the initial response, protective “memory” of prior infections is usually retained within a long-lived subset of the responding T-cell population. If the recipient has previously “seen” a pig xenograft, or has “memory” T-cells that happen to cross-react with pig antigens, the secondary immune response is likely to be vigorous and difficult to restrain using currently available immunosuppressive drugs or other methods.
For a T-cell to respond efficiently after its unique T-cell receptor encounters a foreign transplant or infectious protein antigen, one or more “costimulation” molecules must also adhere to a binding partner (ligand). Costimulation must occur in close physical (nearby on the same cell) and temporal (within minutes or hours) proximity to tight (“high-affinity”) T-cell receptor engagement by MHC bearing the antigenic peptide fragment. (Figure 4) CD40 and the B7 family proteins (CD80, CD86, and others), which are expressed on “antigen presenting cells”, bind to CD154 and CD28 or CTLA-4, respectively, ligands which are expressed mainly on responding T-cells; these are paradigmatic examples of costimulation pathway interactions that mediate effective protective immunity. For the indirect xenoantigen presentation, these interactions are always between human ligand pairs. For the direct xenoantigen presentation, efficient costimulation relies on porcine ligands interacting with their human counterparts and in general, all the important costimulatory molecular pairs have been shown to function efficiently between pigs and non-human primates or humans [30,32-34]. Thus for pig antigens, both the direct and indirect antigen presentation pathways are available to activate human or non-human primate T-cells, and in vivo adaptive immune responses to xenoantigens are robust due to efficient costimulatory function.
Several studies show that, relative to allo responses, the xeno immune response is particularly dependent on CD4 T-cells and on particular costimulation pathway interactions [35-38]. Thus adaptive xenoimmunity is potentially more vulnerable to suppression by selective blockade of these pathways. Blockade of the CD154 costimulation pathway, used with only low-dose mycophenolate mofetil and tapering corticosteroids, is associated with 2 to 6 month survival of GalT-KO pig hearts, notably better than in prior studies with intense immunosuppression using conventional agents [21], with few infectious or other drug-related complications [29]. Antibodies targeting the CD154/CD40 pathway were also a component of the four most successful regimens reported to date for protection of pig islet grafts in nonhuman primates [39-42]. We conclude that T-cell and B-cell responses to xenoantigens are particularly dependent upon efficient costimulatory function, especially pathways governed by CD154. By inference, safe, effective clinical strategies may depend on efficiently blocking this pathway. If so, the widely publicized thromboembolic complications associated with direct anti-CD154 therapy in humans [43,44] have directed current efforts toward CD40 blockade [45], and development of alternative approaches to target CD154.
Targeting other costimulation pathway molecules such as CD28 or the B7 family is one strategy that might prove effective for controlling T-cell-dependent immunity to xenoantigens [46]. Importantly, there is a negative-regulator pathway that functions inefficiently in the reverse direction - human B7 molecules fail to efficiently engage porcine CTLA-4. Soluble porcine CTLA-4 has been used as a tool to selectively block co-stimulatory interactions between porcine B7 and human CD28, one of the key interactions in the “direct” pathway, while its low affinity for human B7 theoretically preserves the human recipient's ability to respond to infectious antigens. The utility of this approach has been shown in a pig-to-mouse model of islet transplantation, and resulted in indefinite graft survival when the indirect pathway was simultaneously inhibited [47]. This observation raises the possibility that the organ-source pig could be genetically engineered to express a graft-specific CD28/B7-blocking agent (for example, porcine CTLA4-Ig), thus providing continuous protection of the graft from the direct T cell response [48]. Ideally, therapeutic gene expression would be controlled using a conditional or inducible gene promoter sequence, to allow control of protein expression in case potentially harmful systemic exposure was detected in the recipient's bloodstream.
Although “natural” anti-nonGal antibodies are typically associated with lower levels of cytotoxicity than anti-Gal antibodies [49,50], early graft failure (EGF, within three days of transplant) of GalT-KO organs in nonhuman primates is associated with anti-non-Gal antibody titers higher than are found in pooled human serum [31,51]. EGF incidence is markedly lower in recipients with low pretransplant anti-non-Gal antibody titers, or when GalT-KO organs express hCD46 or hCD55 [31]. Finally, when GalT-KO kidneys fail [27,28] or when TM develops in GalT-KO hearts [29,31], focal antibody and complement deposition are usually found, supporting the conclusion that both preformed and elicited anti-non-Gal antibodies are the principal mediators of Gal-TKO xenograft injury [17,28,52].
Importantly, and contrary to conventional immunological dogma, costimulation pathway inhibition modulates secondary antibody responses [21-23], albeit less efficiently than primary responses. Interestingly, depletion of B-cells with rituximab as induction therapy delays elicited immunity both with conventional immunosuppression [19] and when combined with costimulation pathway blockade (Mohiuddin and Pierson, personal communications). This observation suggests that CD20-bearing B-cells play a pivotal role in xenoantigen presentation, as recently suggested for alloantigens [53,54]. Targeting plasma cells with proteasome inhibitors such as bortezomib remains to be explored in xenotransplantation, but has shown efficacy in allosensitized renal allograft recipients [55,56].
Identification of non-Gal porcine antigens
It is likely that most humans’ immune systems will recognize a relatively restricted range of particularly antigenic pig proteins and carbohydrates [57-59]. Recent work has begun to elucidate the pig protein targets recognized by elicited anti-non-Gal antibody [60, 61]. In principle this restricted repertoire could be used to advantage to facilitate xenograft acceptance by administering the specific immunodominant antigen to the recipient before transplant under carefully controlled circumstances [62]. Alternatively each antigen can be genetically modified to the human form, peptide scavenging reagents fashioned to specifically block antibody binding, or masking accomplished, as by antigen-specific Fab fragments engineered for long serum half-life. In practice managing an array of multiple different antigens becomes exponentially more difficult, but would be addressed comprehensively by an effective tolerance induction approach.
Tolerance to promote xenograft acceptance
All available evidence suggests that induction of tolerance based on mixed hematopoetic chimerism should prevent adaptive immune injury to an allo- or xeno-graft [27,63,64]. With xenotransplantation, the genetically or pharmacologically manipulated donor can be identified well before the transplant is planned, and the recipient exposed to donor antigen under tolerogenic, controlled circumstances at consistent prescribed intervals, options not logistically feasible using organs from deceased human donors. Thus xenotransplantation offers a great opportunity to take advantage of major advances in immunology to reproducibly promote antigen-specific tolerance once the barriers to accomplishing this long-term goal are better defined [65]. Encouraging progress in rat- and human-to-mouse systems [66] has not yet been translated effectively into preclinical models, although significant progress is apparent in human renal allograft recipients [67] and with pig “thymokidney” grafts in baboons [27].
Blocking complement activation to protect GalT-KO organs
Most experts expect that initial organ xenograft trials will use GalT-KO source animals that additionally express one or more hCPRPs, based on the presumption that complement-activating antibodies against non-Gal antigens will contribute to the pathogenesis of DXR in GalT-KO organs, and that expression of these proteins will be protective. (Figure 1c) Indeed four groups have now presented data in support of this hypothesis. (R. Pierson, personal communications) Other strategies to efficiently prevent complement injury have emerged from the past decade of work in this field [26]. Recent studies show that complement activation within the “immunologic synapse” between the T-cell and antigen presenting cell provides non-classical costimulation, and that increased expression of CPRPs promotes antigen-specific immunoregulation [68,69]. This potentially pivotal observation suggests that high levels of hCPRP on the xenograft may mute xenoimmunity and promote xenograft acceptance.
Coagulation Dysregulation
Histology of the failed GalT-KO organs under anti-CD154 therapy exhibited a previously unusual form of DXR, “thrombotic microangiopathy” (TM) [15,23,29]. When GalT-KO pig organ xenografts fail, evidence of coagulation pathway activation is usually detected both in the graft and systemically [18,51,70], although some groups have not observed this phenomenon [19]. Thus considerable attention has been directed to understand the cause of coagulation pathway activation by a xenograft.
In other contexts TM is associated with abnormal intravascular coagulation due to impaired endothelial anticoagulant function. It is clear that antibody- and complement-mediated endothelial cell (EC) activation can be sufficient to initiate the coagulation abnormalities associated with xenograft rejection [11,17]. Assault by antibody and complement converts the EC from the normal anticoagulant phenotype to a procoagulant state, leading to loss of vascular integrity as well as infiltration by various immune cells.
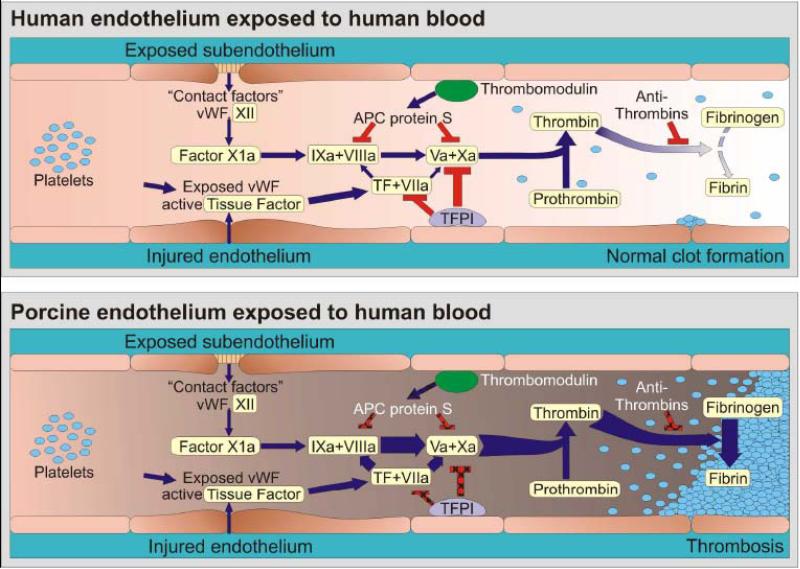
However, there is compelling in vitro data as well as increasing in vivo evidence that intrinsic cross-species coagulation cascade incompatibilities also contribute primarily to TM and that other coagulation cascade phenomena arise after organ xenografting without an obvious requirement for an antibody or complement immune trigger. As recently reviewed [71-74], human and pig coagulation pathway interactions are partially incompatible in several physiologically important ways. Interaction between GalT-KO pig endothelium, human platelets, and human monocytes promote clotting after interaction even in the absence of serum, although antibody and/or complement amplify some procoagulant mechanisms [75]. Several human clotting cascade proteins are activated “non-physiologically” (inappropriately) by quiescent, resting pig endothelium. In particular, quiescent porcine endothelium catalyzes formation of human thrombin from prothrombin [11,76]. In addition, quiescent human platelets interact constitutively with porcine von Willebrand factor, causing adhesion and activation, without first requiring GP1b receptor activation (which normally occurs after GPIIbIIIa receptor activation) as would be necessary for human GP1b to bind to human von Willebrand factor [77]. CD39, an endothelial nucleotidase that prevents local ADP-mediated vasoconstriction and platelet aggregation, is expressed at low levels on pig endothelium [78], and increased CD39 expression attenuates the associated procoagulant phenotype [79]. Porcine tissue factor pathway inhibitor (TFPI) may inefficiently disable activated primate tissue factor [74,80], while porcine thrombomodulin is inefficient to inhibit human thrombin due to inefficient binding to human Protein C [81]. The result is that porcine coagulation pathway regulatory molecules poorly protect pig endothelium in the context of primate coagulation pathway activation. In aggregate, these incompatibilities mean that clotting occurs more readily in a xenograft than in an allograft, and that, once the clotting cascade is initiated, mechanisms that would normally counteract intravascular clot propagation do not function well. (Figure 5 Rees Pierson cartoon coag) Similarly removing or temporarily disabling a pro-coagulant protein, such as fibrinogen-like protein 2, may protect pigs that do not express this inducible thrombin-generating enzyme from DXR [82].
Figure 5. Dysregulated coagulation in pig-to-primate xenotransplantation.
Coagulation is occurring continuously within the blood stream, but is normally restrained by a network of inhibitory pathways involving endothelial proteins such as thrombomodulin and tissue factor pathway inhibitor (TFPI) (Panel A). Increased coagulation is normally initiated when endothelium retracts or becomes “activated” by injury, in part because von Willebrand factor (vWF) is expressed and tissue factor (TF) is liberated into the circulation. The coagulation cascade then becomes amplified by the factors shown (VIIa/TF complex, IXa and Xa) which in turn activate thrombin. Thrombin amplifies the clotting cascade by a) activating XIa (not shown), b) activating platelets, c) cleaving fibrinogen into fibrin monomers that form the primary clot matrix, and d) activating factor XIIIa (not shown), which cross-links fibrin monomers into an insoluble clot.
TFPI and thrombomodulin normally inhibit coagulation on healthy endothelium, while soluble antithrombins inhibit thrombin by forming a complex with its active site.
Porcine EC activation – whether by xenoantibodies, complement, or other factors – results in loss of natural anticoagulant proteins (TFPI, thrombomodulin) and acquisition of a procoagulant phenotype (Panel B). In addition functional incompatibilities in the coagulation system between pigs and humans cause both inappropriate or accelerated thrombin formation and inefficient restraint of clot activation. Our current hypothesis is that xenografts succumb to an otherwise insignificant humoral or cellular immune response which amplifies endothelial injury and intravascular thrombosis, and becomes manifest as TM.
Blue arrows designate cascade amplification steps, while red lines identify loci of inhibition. The relative intensity of clot formation, the net product of coagulation pathway enzyme effects, is symbolized by arrow weight at the thrombin and fibrin steps. Pathways where pig endothelial proteins inefficiently dampen coagulation are indicated with hatchmarked red lines in Panel B. For simplicity, only the activated clotting factor intermediaries and key points at which regulation occurs are shown.
Several recent studies suggest that coagulation dysregulation may be playing a role not only in xenograft thrombosis but also by amplifying immune injury to the graft. In a mouse-to-rat model where control mouse hearts in immunosuppressed rats fail from DXR after 6 days, hearts from transgenic mice expressing membrane-tethered human TFPI (hTFPI) or leach-derived hirudin survived indefinitely (>100 days) [83]. No graft injury occurred despite antibody and complement deposition on the xenograft endothelium. This surprising result suggests that coagulation pathway activation on the graft endothelium is a critically important amplifier of the adaptive cellular and humoral immune responses to the xenograft. Increased expression of CD39 also dampens nonclassical costimulation and amplifies T-regulatory cell function [84], and could theoretically promote xenograft acceptance.
Overall, our current understanding suggests that strategies to prevent dysregulated intravascular coagulation are attractive targets to prevent both procoagulant and immune facets of DXR and TM. The constitutive and acquired molecular incompatibilities that together constitute the “coagulation barrier” might be overcome through systemic drug therapy, such as supplementation of antithrombin 3 [85] or selective inhibition of binding between porcine vWF and human GP1b [86]. In this context, the initial prostacyclin and prolonged high-dose heparin infusions used in most GalT-KO organ studies may have been an important therapeutic adjunct, perhaps protecting the grafts from earlier thrombotic injury and delaying consumptive coagulopathy in the organ recipients. On the other hand genetic manipulation of the organ-source pig, adding human coagulation regulatory proteins at the endothelial interface between graft and host, will likely be preferable to minimize recipient complications. These modifications should not only prevent EC activation by minimizing coagulation, but may also help control the immune response to the pig organ by modulating non-classical “costimulation” within the immunological synapse and thus muting T-cell proliferation. GalT-KO pigs are currently being engineered to express hTFPI, hirudin, thrombomodulin, and CD39; regulated or tissue-specific expression of one or more of these genes may be necessary to viability of some pig constructs. Currently, several groups are studying whether these modifications will yield clinically important graft protection from TM or other DXR phenomena in the context of the GalTKO background.
Cellular Non-T-cell Xenograft Responses
Macrophages: a bidirectional barrier to xeno-engraftment
Induction of central tolerance by infusion of donor bone marrow or mobilized hematopoietic progenitor cells into an irradiated host has already had some success in clinical organ allotransplantation [87] and is established therapy for hematopoetic malignancies. However, after infusion into baboons, porcine hematopoietic cells are avidly scavenged by primate macrophages and perhaps other reticuloendothelial system cells, a phenomenon not attenuated by absence of the Gal antigen (reviewed in [88]). Human Kupffer cells recognize and phagocytose porcine erythrocytes by a lectin-dependent mechanism [89]. A similar mechanism probably underlies the recognition and removal of porcine cells by host macrophages in vivo in baboons [89a]. Current work seeks to characterize the specific lectin-carbohydrate molecules involved in this recognition event, and to identify preventive strategies. In addition, absence of functional inhibitory interactions, such as those mediated by SIRP-1α and CD47, may promote xenogenic cell phagocytosis by macrophages [90].
This phenomenon also operates in the other direction: human erythrocytes are rapidly engulfed by pig macrophages by a carbohydrate-lectin interaction [91,92]. For example pig livers perfused ex vivo with human blood phagocytose approximately one unit of human erythrocytes from the circulation every 24h [93]. This process may be mediated by direct recognition of erythrocytes by pig Kupffer cells or endothelial cells, unrelated to antibody binding and complement activation [94]. A solution to this problem will be required if extracorporeal pig liver support of pig liver transplantation is to become an effective, practical clinical therapy. Similarly, severe thrombocytopenia has limited survival of pig GalTKO.hCD46 livers in baboons, probably mediated by “inappropriate” ligation of human GP1b by porcine vWF or “scavenging” associated with absence of “self” antigens [DKC Cooper, submitted]. Strategies to prevent these phenomena are currently being tested, and if successful, may justify the use of extracorporeal pig liver support of patients in fulminant hepatic failure as the first clinical trial involving xenogeneic solid organs.
Using a variety of techniques that essentially prevent the occurrence of hyperacute rejection, many investigators have demonstrated the crucial role of monocytes [95,96] and macrophages in mediating DXR [97-105]. Reciprocal “cross-talk” between macrophages and T cells links innate to adaptive immunity in xenograft rejection [106]. For example, activated macrophages elaborate chemokines that recruit T cells to initiate the adaptive immune response in the graft, and act as direct effectors of rejection in response to contact-dependent and cytokine-mediated T cell help. Xeno-specific strategies to regulate macrophage activation await identification of the exact molecular mechanisms involved so that these can be manipulated to protect the graft without crippling host defenses against environmental pathogens or neoplasia.
NK cell xenograft rejection mechanisms
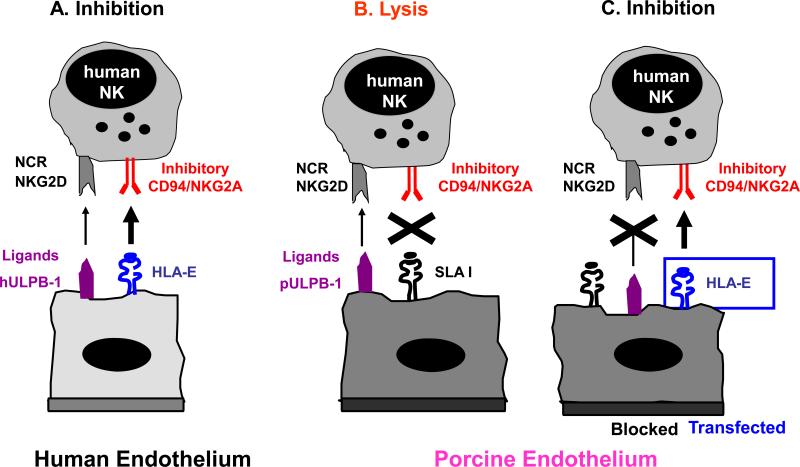
Several lines of evidence indicate that natural killer (NK) cells are involved in DXR [95,107-109]. In addition to possessing activating “non-self” receptors [110-113], NK cell activity is tightly regulated by inhibitory receptors that recognize “self” ligands on target cells, such as human MHC Class I antigens [114-117]. Adhesion and recruitment of human NK cells to porcine endothelium and subsequent tissue infiltration has been demonstrated in pig organs perfused ex vivo with human blood. Subsequently the molecular mechanisms involved in human NK cell-porcine endothelial cell interactions have been studied extensively in vitro by several groups in order to identify targets for therapeutic interventions. NK cell rolling is mediated by CD49d interacting with porcine CD106 (VCAM-1) and by CD62L and its porcine ligands, while the following firm adhesion mainly involves interactions between b2-integrins and ICAMs, as well as CD49d and CD106. Human NK cytotoxicity against porcine endothelial cells depends on the absence of interactions between human NK-MHC class I specific inhibitory receptors and porcine MHC class I molecules, and the cross-species binding of NK-activating receptors (NKp44 and NKG2D) to ligands on porcine EC such as porcine ULBP-1 [110-113]. Importantly, expression of Gal on pig endothelium does not seem to play a role in direct xenogeneic NK cell recognition [118, 119]. Strategies to overcome human anti-pig NK cell responses include (1) the blocking of the molecular events leading to recruitment (chemotaxis, adhesion, transmigration) [120], (2) the expression of human MHC class I molecules on pig endothelium [114-117,121,122] and (3) the elimination or blocking of pig ligands for activating human NK receptors (Figure 6 Seebach cartoon NK). Presently, the most advanced of these three options is the second with the advent of HLA-E transgenic pigs [123]. Whether pharmacological therapies are efficient to prevent xenogeneic NK responses remains to be elucidated; the effects of classical T-cell immunosuppressive drugs on NK cells are not known in sufficient detail.
Figure 6.
Molecular interactions between human NK cells and pig endothelial cells NK cells are tightly regulated through signals mediated by inhibiting and activating receptors expressed on their cell-surface. Many of the inhibitory NK receptors recognize MHC class I molecules and therefore allow NK cells to discriminate between self and non-self. Activating receptors on NK cells include natural cytotoxicity receptors (NCR) and the C-type lectin receptor NKG2D.
(A) Human endothelium is normally protected from NK cytotoxicity by dominant inhibitory signals provided by HLA, e.g. recognition of HLA-E by CD94/NKG2A. (B) In contrast, porcine endothelium is susceptible to human NK cell-mediated lysis likely due to the inability of swine leukocyte antigen (SLA) MHC class I molecules to signal through human NK inhibitory receptors. Human NK cytotoxicity against porcine EC is mediated primarily through NKG2D-pULBP-1 interactions and the NCR NKp44. (C) Transgenic expression of HLA class I molecules on porcine endothelium resulted in partial protection whereas additional blocking of activating NK receptors and their porcine ligands provides complete protection from human NK cytotoxicity.
Islet Xenografts
Life-supporting preclinical islet xenograft survival for over 6 months has recently been achieved in non-human primates by five different groups [123a]. Wild-type pig islets maintained insulin-independence for periods of up to 6 months in cynomolgus monkeys [39] and 9 months in rhesus monkeys [40-42] using CD154- or CD40-directed immunosuppression combined with induction IL2 receptor blockade and chronic administration of a “target of rapamycin” (TOR) inhibitor with either lefuonimide and FTY720 or a B7 inhibitor (belatacept, also known as LEA29Y). Embryonic pig pancreatic tissue transplantation restored long-term insulin independence in two monkeys using a similar immunosuppressive regimen without anti-CD154; one animal maintained excellent xenograft function for almost 400 days [124].
Insulin-independence for over one year was achieved using Gal-expressing islets expressing hCD46, using induction immunosuppression with polyclonal, depleting T cell antibodies and maintenance treatment with CD154 blockade and mycophenolate mofetile [125]. Immuno-isolation of whole islets by encapsulation has generally been less successful in primates than in rodents, but a recently reported islet disaggregation technique with subcutaneous implantation may represent an important advance, perhaps by improving beta cell nutrient access [126]. If success with either of these experimental regimens or with conventional immunosuppression can be replicated consistently, these results appear likely to approach IXA interim consensus recommendations for preclinical benchmarks necessary to justify initiation of a clinical islet xenograft trial [127].
The mechanisms of islet xenograft injury in primates and particularly the role of Gal antigen in islet cell injury are becoming better defined. Given that highly purified and cultured pancreatic islets from adult wild-type pigs express little or no Gal antigen [128,129] (although remaining intra-islet contaminating endothelial cells do) and absence of detectable anti-Gal antibody after failure of wild-type islet grafts [39, and B Hering, DKC Cooper, unpublished observations], use of islets from adult GalT-KO pigs appears to offer little or no advantage. In contrast, there is growing evidence for a role for anti-nonGal antibodies in islet xenograft injury [130-132], and for both cytokine [133] and T cell-mediated immune responses [38,134,135].
Innate immunity and in particular coagulation pathway dysregulation pose a substantial barrier to intravascular (usually portal vein) administration of xenogeneic islets. When islets from pigs, or from a concordant species, are infused into the recipient's portal vein, a phenomenon termed “immediate blood-mediated inflammatory response” (IBMIR) often occurs. IBMIR is characterized by rapid release of insulin, suggesting immediate islet destruction, and prolific complement activation. In an in vitro model, rapid upregulation of tissue factor expression on the islets has been observed [136], analogous to the proinflammatory coagulation pathway activation events observed with porcine endothelial cells exposed to human blood. IBMIR and the associated platelet consumption and fibrin generation are significantly inhibited by administration of low molecular weight dextran sulfate [137], supporting the hypothesis that platelet and coagulation cascade activation are integral to this phenomenon. IBMIR may also be amenable to pharmacologic anticoagulation, anti-inflammatory medication, and complement inhibition [138]. Alternatively, genetic manipulation of the source pigs to inhibit coagulation or complement pathway activation, or to selectively inhibit other local pathogenic mechanisms shown to participate in the response, may increase the resistance of the islets to this injury. As noted earlier, some of the best results to date with adult pig islet xenografts have been achieved using adult islets from Gal+ pigs that express hCPRPs, which may modulate both complement and T-cell-driven pathogenic pathways. Nevertheless, the relevance of IBMIR and its contributing factors remain poorly understood. IBMIR did not prevent the restoration of normoglycemia and insulin independence after intraportal transplantation of a high dose of cultured adult [39] or neonatal [40] islets in monkeys. It is conceivable that control of IBMIR would reduce the number of islets required to reverse diabetes after intraportal transplantation. Transplantation of islet grafts to nonvascular sites would also mitigate post-transplant activation of the coagulation cascade.
Infectious Disease Risks
An important concern for clinical xenotransplantation is the potential transmission of pathogens from the xenograft donor to the human recipient, and subsequently to the community at large [139]. The risk of disease transmission is a component of any form of transplantation and is enhanced by the immunosuppression required to maintain graft function. However, over the past decade significant progress has been made in identifying potential human pathogens from swine, defining their biology, and developing quantitative molecular assays for each. Such assays will be useful in the monitoring and safety of future clinical xenotransplant trials.
Of the potential pathogens specific to a pig donor, most are likely to be similar to those observed following human allotransplantation, such as herpesviruses including porcine cytomegalovirus (PCMV) and porcine lymphotropic herpesvirus (PLHV-1, -2, -3). Each of these pig herpesviruses has exhibited pathologic effects in preclinical studies: PCMV activation has been associated with consumptive coagulopathy in pig-to-primate models; PLHV causes a form of post-transplant lymphoproliferative disease in pigs, but has not been seen in primate xenograft recipients where the virus does not appear to replicate [140-143].
The safety of xenotransplantation will be enhanced by careful breeding of animals that are free of specific pathogens. For example, early weaning and hysterotomy derivation have yielded “defined pathogen free” herds that have no PCMV, parvovirus, trichinosis, or influenza virus. These swine may also be safer in some respects than human allotransplant donors in that many human pathogens are not present in swine (e.g., hepatitis viruses) and swine pathogens may not infect human cells.
A recent study addressed a different question: Does human cytomegalovirus (HCMV) infect and activate the porcine endothelium following pig-to-human solid organ transplantation? Indeed, HCMV cross-species infection was demonstrated to modulate MHC class-I and adhesion receptor expression on pig endothelial cells and induced the secretion of mediators promoting the recruitment and activation of human leukocytes, which are important contributors to xenograft rejection [144]. Current work to better understand the mechanisms of CMV infection may lead to strategies that render the porcine endothelium resistant to HCMV infection.
The recurring concern regarding the infectious safety of xenotransplantation revolves around the porcine endogenous retroviruses [139]. Porcine endogenous retrovirus (PERV) is the only identified, replication competent retrovirus of pigs with some potential to infect human cells. Three forms of PERV have been identified (PERV-A, -B, -C) and a recombinant form (PERV-AC) [145,146]. The infectious potential for human cells appears to be increased with serial passage in culture of PERV-AC and some transformed human cell lines are susceptible to PERV infection. In contrast, most normal human cells are relatively resistant to productive infection by PERV. In pig-to-primate preclinical studies, PERV transmission cannot be demonstrated. Further, infection with PERV has not been detected in a series of studies of humans exposed to living pig cells and tissues. The basis of the relative blocks to productive infection is under investigation. Importantly, exposure to PERV has not been associated with identifiable clinical sequelae, although insertional or other cellular effects cannot be conclusively excluded.
For clinical trials, monitoring strategies must be developed for potential pathogens as well as strategies to explore the possibility of transmission of unknown or unrecognized organisms. To this end, recipient surveillance must be coupled with archiving of tissues from xenograft donors and recipients to allow the future detection unknown organisms. The initial patients must be willing to participate in such a prolonged monitoring scheme and in the use of barrier protection against the potential transmission of pathogens with sexual contacts. The use of internationally acceptable standards [8,127,139] will enable epidemiologic investigation of any possible infectious syndromes in xenograft recipients or their close contacts. In this context, the risk-benefit ratio for society at large of expanded access to life-saving transplants will likely favor proceeding with clinical trials when preclinical data shows that the probable benefits of a particular clinical xenograft outweigh the likely risks for the individual.
Perspective on Current Prospects for Clinical Xenotransplantation
Heterotopic (non-life-supporting) hearts from transgenic pigs have survived for almost 6 months of in nonhuman primates [29]. In addition, 2 month survival of baboon recipients of orthotopic pig hearts has been achieved with a conventional, clinically acceptable immunosuppression strategy [147]. However these regimens have been associated with a relatively high rate of complications, particularly from infection, or with graft failure associated with DXR or TM [14,19,21,148]. An approach which achieves similar efficacy but with lower toxicity and reduced infectious complications could be considered for clinical trial, as patient monitoring and therapeutic interventions that are routine in the clinic are difficult or unavailable in preclinical trials. Recommended benchmarks for proceeding to clinical heart or lung transplant trials include prevalent life-supporting graft function of >3 months, associated with physiologic and histologic findings consistent with a high probability for intermediate-term (years) clinical success [149].
Based on the relatively limited risks associated with graft failure and expected progress in preventing early and late graft injury, we expect trials in islet [150] or perhaps other cell transplantation [151] to occur in the near future. Resumption of clinical trials will be predicated on identification of a clinically-tolerable immunosuppressive regimen. Preclinical success with a reduced immunosuppressive regimen may in turn be facilitated by cell implantation in an immune privileged site, encapsulation, or genetic modification of the islet donor, approaches designed to reduce immunogenicity and/or isolate cells from immune insults. If xenogeneic immunity can be controlled successfully and safely in clinical xeno islet recipients or in preclinical life-supporting organ transplant trials, the risk/benefit estimates for pig organ xenografts could become competitive with current conventional and allo islet-based therapies for some patients.
Extracorporeal wild-type pig liver support of patients in fulminant hepatic failure has demonstrated efficacy similar to that achieved with human liver perfusion (Table 1), and may be expected to be improved using GalTKO livers that express hCPRPs. Rapidly deteriorating liver transplant candidates may be an appropriate initial clinical trial population given that the patient will not necessarily receive immunosuppression during support, is likely to die without the intervention, and the organ can be rapidly disconnected if adverse effects attributable to the xenograft are appreciated. Further, new insights would result regarding immunologic and biochemical xenogeneic interactions.
Table 1.
Summary of Clinical Extracorporeal Hepatic Perfusion Experience
| Liver Donor Species | Number of treated Patients | Clinical Improvement* | Number of Survivors | Percent Patient Survival‡ | ||
|---|---|---|---|---|---|---|
| Neurologic #/total (%) | Ammonia #/total (%) | Bilirubin #/total (%) | ||||
| Baboon | 31 | 29/31 (94%) | 6/6 (100%) | 6/6 (100%) | 10 | 32% |
| Calf | 9 | 7/9 (78%) | 3/7 (43%) | 7/7 (100%) | 0 | 0% |
| Monkey | 2 | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0 | 0% |
| Human | 21 | 17/21 (81%) | 21/21 (100%) | 21/21 (100%) | 9 | 43% |
| Pig 1965-1979 | 114 | 59/88 (67%) | 63/74 (85%) | 90/91 (98%) | 21 | 18% |
| Pig 1980-1989 | 19 | 12/19 (63%) | 7/9 (78%) | 7/9 (78%) | 4 | 21% |
| Pig 1990-2001 | 18 | 7/7 (100%) | 16/17 (94%) | 17/17 (100%) | 9 | 50% |
Summarizes the subset of patients with improvement (#) divided by the total number of patients for whom this finding was reported (total).
Survival to hospital discharge or transplant. Derived from (51).
Heart or kidney xenografts may first be used in contexts where access to a subsequent human organ might be facilitated, such as in a highly-sensitized patient without secure dialysis access, as a “bridge” to a renal allograft. Alternatively, ”bridge” or “destination” heart xenografts could be considered if systemic anticoagulation were contraindicated in a patient likely to otherwise require long-term biventricular mechanical circulatory support. Demonstration in non-human primates of life-supporting heart function for almost two months, and of life-supporting renal function approaching 4 months suggests that physiologic, biochemical, or neurohumoral incompatibilities are unlikely to prevent use of these porcine organs to support life in humans.
Of importance to the planning of clinical trials, there is evidence that antibodies against HLA antigens do not generally cross-react with porcine antigens [152,153], and would therefore probably not constitute an additional barrier to efficacy for xenotransplantation, although this evidence is not definitive. Nor does prior exposure to a pig organ or cells appear to sensitize humans or primates to alloantigens [154]. Thus “bridge” therapy with a xenograft is unlikely to be detrimental to the immunologic outcome of a subsequent allotransplant as long as the immunomodulatory regimen used does not cause significant morbidity.
Ethical and scientific guidelines for undertaking clinical xenotransplant trials have been carefully developed [8,127,149,155-157]. Public acceptance will depend upon deliberate scientific progress supported by transparent peer review, ethical probity in trial design and conduct, and effective communication to the public of the societal risks, costs, and potential benefits associated with clinical xenotransplantation. Perhaps most worrisome with respect to the credibility of the xenotransplantation field, procedures that involve transfer of poorly characterized animal cell “products” to vulnerable or gullible patients are being advertised – and apparently performed – in unregulated markets without scientific justification, evidently for commercial gain. The IXA [127], TTS, and WHO [8] have published recommendations that articulate the strong case against unregulated, scientifically unjustified xenotransplantation ventures.
Summary
Substantial prolongation of Gal-expressing cell and GalT-KO organ xenograft survival in preclinical models has resulted from our improving understanding of the residual barriers to discordant xenograft survival. Thus, expression of hCPRPs or anticoagulant molecules on porcine passenger leukocytes or in association with pig antigens shed by the graft may not only attenuate inflammation and thrombodysregulatory phenomena, but also mute immune responses to the wild-type or GalT-KO graft. As new tools are rapidly becoming available to control well-defined pathogenic mechanisms, it is highly likely that sufficient additional progress will soon be achieved to justify resumption of clinical xenotransplantation trials. With effective, internationally-consistent regulation, robust infection surveillance, and systematic education and engagement of health care and lay communities, xenotransplantation is poised to emerge as an effective treatment option over the coming decade, and to deliver on its substantial promise to favorably impact public health.
Acknowledgements
The authors acknowledge their many colleagues, past and present, who contributed to the work from their own centers that is reviewed in this manuscript. RNP acknowledges the support of the American Heart Association, VA Merit Program, Office of Naval Research, American Society of Transplant Surgeons, University of Maryland General Clinical Research Center, and National Institutes of Health (NIH: UO1 AI 066335-01 and U01 AI 066719-01). AD was supported by the UK Medical Research Council (G0801965), the Garfield Weston Foundation, Kidney Research UK, the ROTRF, and the NIH (UO1 AI068642). JS was supported by research grants from the Swiss National Science Foundation (3200B0-109921 and 32003-114020), the Fondation Wilsdorf, and by an unconditional contribution of the late Christian Loepfe in support of transplantation research. MR received support from the NIH (R01 DK066160). JAF recognizes support by Public Health Services grant NIH-NIAID PO1-AI45897. BJH received support from the NIH (U19 AI067151), Juvenile Diabetes Research Foundation (PPG 7-2005-1167), Diabetes Research and Wellness Foundation, Eunice L. Dwan Diabetes Research Endowment and Immerge Biotherapeutics. DKCC acknowledges support from the NIH (UO1 AI068642 and R21 A1074844) and the American Diabetes Association. RNP, AD, and DKCC serve on the scientific advisory board of Revivicor, Inc. DA has a significant financial interest in Revivicor, Inc.
Abbreviations and definitions
- CPRP
complement pathway regulatory protein
- DXR
delayed xenograft rejection
- EC
endothelial cells
- Gal
Galα1,3Gal antigen
- GalT-KO
α1,3-galactosyltransferase gene-knockout
- hDAF
human decay-accelerating factor protein
- IBMIR
immediate blood-mediated inflammatory response
- mAb
monoclonal antibody
- MHC
major histocompatibility complex-associated antigen
- NK
natural killer
- PCMV
porcine cytomegalovirus
- PERV
porcine endogenous retrovirus
- PLHV
porcine lymphotropic herpesvirus
- TFPI
tissue factor pathway inhibitor
- TM
thrombotic microangiopathy
REFERENCES
- 1.Cooper DK, Dorling A, Pierson RN, III, et al. Alpha1,3-galactosyltransferase gene knockout pigs for xenotransplantation: where do we go from here? Transplantation. 2007;84(1):1–7. doi: 10.1097/01.tp.0000260427.75804.f2. [DOI] [PubMed] [Google Scholar]
- 2.Allan JS. The risk of using baboons as transplant donors: exogenous and endogenous viruses. Ann N Y Acad Sci. 1998;862:87–99. doi: 10.1111/j.1749-6632.1998.tb09120.x. [DOI] [PubMed] [Google Scholar]
- 3.Goodall J. Ethical concerns in the use of animals as donors. In: Hardy MA, editor. Xenograft 25. Excerpta Medica International Congress Series, Elsevier Science; Amsterdam, Netherlands: 1989. pp. 335–349. [Google Scholar]
- 4.Byrne GW, McCurry KR, Martin MJ, McClellan SM, Platt JL, Logan JS. Transgenic pigs expressing human CD59 and decay-accelerating factor produce an intrinsic barrier to complement-mediated damage. Transplantation. 1997;63(1):149–55. doi: 10.1097/00007890-199701150-00027. [DOI] [PubMed] [Google Scholar]
- 5.Langford GA, Yannoutsos N, Cozzi E, et al. Production of pigs transgenic for human decay accelerating factor. Transplant Proc. 1994;26(3):1400–1. [PubMed] [Google Scholar]
- 6.Phelps CJ, Koike C, Vaught TD, et al. Production of α1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolber-Simonds D, Lai L, Watt SR, et al. α1,3-galactosyltransferase null pigs via nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;19:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.First WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials: Changsha, China, 19-21 November 2008. The Changsha Communiqué. Xenotransplantation. 2009;16(2):61–3. doi: 10.1111/j.1399-3089.2009.00520.x. [DOI] [PubMed] [Google Scholar]
- 9.Pierson RN., III Current status of xenotransplantation. JAMA. 2009;301((9):967–9. doi: 10.1001/jama.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calne RY. Organ transplantation between widely disparate species. Transplant Proc. 1970;2(4):550–6. [PubMed] [Google Scholar]
- 11.Bach FH, Robson SC, Ferran C, et al. Endothelial cell activation and thromboregulation during xenograft rejection. Immunol Rev. 1994;141:5–30. doi: 10.1111/j.1600-065x.1994.tb00870.x. [DOI] [PubMed] [Google Scholar]
- 12.Saadi S, Platt JL. Role of complement in xenotransplantation. Clin Exp Pharmacol Physiol. 1999;26(12):1016–9. doi: 10.1046/j.1440-1681.1999.03184.x. [DOI] [PubMed] [Google Scholar]
- 13.McCurry KR, Parker W, Cotterell AH, et al. Humoral responses to pig-to-baboon cardiac transplantation: implications for the pathogenesis and treatment of acute vascular rejection and for accommodation. Hum Immunol. 1997;58(2):91–105. doi: 10.1016/s0198-8859(97)00229-2. [DOI] [PubMed] [Google Scholar]
- 14.Chen G, Sun H, Yang H, et al. The role of anti-non-Gal antibodies in the development of acute humoral xenograft rejection of hDAF transgenic porcine kidneys in baboons receiving anti-Gal antibody neutralization therapy. Transplantation. 2006;81(2):273–83. doi: 10.1097/01.tp.0000188138.53502.de. [DOI] [PubMed] [Google Scholar]
- 15.Houser SL, Kuwaki K, Knosalla C, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11(5):416–25. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 16.Byrne GW, Davies WR, Oi K, et al. Increased immunosuppression, not anticoagulation, extends cardiac xenograft survival. Transplantation. 2006;82(12):1787–91. doi: 10.1097/01.tp.0000251387.40499.0f. [DOI] [PubMed] [Google Scholar]
- 17.Gollackner B, Goh S-K, Qawi I, et al. Acute vascular rejection of xenografts: roles of natural and elicited xenoreactive antibodies in activation of vascular endothelial cells and induction of procoagulant activity. Transplantation. 2004;77:1735–1741. doi: 10.1097/01.tp.0000131167.21930.b8. [DOI] [PubMed] [Google Scholar]
- 18.Cowan PJ, Aminian A, Barlow H, et al. Protective effects of recombinant human antithrombin III in pig-to-primate renal xenotransplantation. Am J Transplant. 2002;2(6):520–5. doi: 10.1034/j.1600-6143.2002.20605.x. [DOI] [PubMed] [Google Scholar]
- 19.McGregor CG, Davies WR, Oi K, et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005;130(3):844–851. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Cozzi E, Vial C, Ostlie D, et al. Maintenance triple immunosuppression with cyclosporin A, mycophenolate sodium and steroids allows prolonged survival of primate recipients of hDAF porcine renal xenografts. Xenotransplantation. 2003;10(4):300–10. doi: 10.1034/j.1399-3089.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu G, Pfeiffer S, Schröder S, et al. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation. 2005;12(3):197–208. doi: 10.1111/j.1399-3089.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 22.Buhler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69:2296–2304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 23.Kuwaki K, Knosalla C, Dor FJ, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4(3):363–72. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 24.Good AH, Cooper DKC, Malcolm AJ, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in man. Transplant Proc. 1992;24:559–562. [PubMed] [Google Scholar]
- 25.Cooper DKC, Koren E, Oriol R. Genetically engineered pigs. Lancet. 1993;342:682–683. doi: 10.1016/0140-6736(93)91791-j. [DOI] [PubMed] [Google Scholar]
- 26.Pierson RN., 3rd Antibody-mediated xenograft injury: Mechanisms and protective strategies. Transpl Immunol. 2009;21(2):65–9. doi: 10.1016/j.trim.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 28.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11(12):1295–8. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 30.Tseng YL, Moran K, Dor FJ, et al. Elicited antibodies in baboons exposed to tissues from alpha1,3-galactosyltransferase gene-knockout pigs. Transplantation. 2006;81(7):1058–62. doi: 10.1097/01.tp.0000197555.16093.98. [DOI] [PubMed] [Google Scholar]
- 31.Azimzadeh AM, Kelishadi SS, Ezzelarab M, et al. Hyperacute rejection of Gal-TKO pig organs in baboons is associated with coagulation cascade activation. Xenotransplantation. in press. [Google Scholar]
- 32.Rogers NJ, Jackson IM, Jordan WJ, et al. Cross-species costimulation: relative contributions of CD80, CD86, and CD40. Transplantation. 2003;75(12):2068–76. doi: 10.1097/01.TP.0000069100.67646.08. [DOI] [PubMed] [Google Scholar]
- 33.Simon AR, Warrens AN, Yazzie NP, et al. Cross-species interaction of porcine and human integrins with their respective ligands: implications for xenogeneic tolerance induction. Transplantation. 1998;66(3):385–94. doi: 10.1097/00007890-199808150-00017. [DOI] [PubMed] [Google Scholar]
- 34.Pierson RN. Primate T-cell responses to porcine antigens: implications for clinical xenotransplantation. Xenotransplantation. 2006;13(1):14–8. doi: 10.1111/j.1399-3089.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 35.Pierson RN, III, Winn HJ, Russell PS, Auchincloss H., Jr Xenogeneic skin graft rejection is especially dependent on CD4+ T cells. J Exp Med. 1989;170:991–6. doi: 10.1084/jem.170.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moses RD, Pierson RN, III, Winn HJ, Auchincloss H., Jr Xenogeneic proliferation and lymphokine production are dependent on CD4+ helper T cells and self antigen-presenting cells in the mouse. J Exp Med. 1990;172(2):567–75. doi: 10.1084/jem.172.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popma SH, Krasinskas AM, Kreisel D, et al. Simultaneous blockade of B7-CD28 and CD40-CD40L costimulation eliminates the direct xenorestricted human anti-porcine T-cell response. Transplant Proc. 2001;33(1-2):767–9. doi: 10.1016/s0041-1345(00)02245-4. [DOI] [PubMed] [Google Scholar]
- 38.Gordon EJ, Woda BA, Shultz LD, Rossini AA, Greiner DL, Mordes JP. Rat xenograft survival in mice treated with donor-specific transfusion and anti-CD154 antibody is enhanced by elimination of host CD4+ cells. Transplantation. 2001;71(2):319–27. doi: 10.1097/00007890-200101270-00026. [DOI] [PubMed] [Google Scholar]
- 39.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–3. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 40.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–6. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 41.Russell M, Cardona K, Oliva V, Korbutt G, Cano J, Jiang W, Strobert E, Rajotte R, Pearson T, Larsen C. Engraftment of neonatal porcine islets in diabetic non-human primates by blockade of the CD28/40 costimulatory pathways.. 1st Joint Conference of CTS, IPITA, and IXA – Minneapolis, MN, 9/15-20; 2007; Abstract #305.8. [Google Scholar]
- 42.Cardona K, Milas Z, Strobert E, et al. Engraftment of adult porcine islet xenografts in diabetic nonhuman primates through targeting of costimulation pathways. Am J Transplant. 2007;7(10):2260–8. doi: 10.1111/j.1600-6143.2007.01933.x. [DOI] [PubMed] [Google Scholar]
- 43.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6(2):114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 44.Kirk AD, Harlan DM. Reply: Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2001;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 45.Aoyagi T, Yamashita K, Suzuki T, et al. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. Am J Transplant. 2009 Jun 10; doi: 10.1111/j.1600-6143.2009.02693.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Dorling A, Lechler RI. T cell-mediated xenograft rejection: specific tolerance is probably required for long term xenograft survival. Xenotransplantation. 1998;5:234–245. doi: 10.1111/j.1399-3089.1998.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 47.Mirenda V, Golshayan D, Read J, et al. Achieving permanent survival of islet xenografts by independent manipulation of direct and indirect T-cell responses. Diabetes. 2005;54:1048–1055. doi: 10.2337/diabetes.54.4.1048. [DOI] [PubMed] [Google Scholar]
- 48.Phelps C, Ball S, Vaught T, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. doi: 10.1111/j.1399-3089.2009.00533.x. In press. [DOI] [PubMed] [Google Scholar]
- 49.Rood PPM, Hara H, Ezzelarab M, et al. Preformed antibodies to α1,3-galactosyltransferase gene-knockout (GT-KO) pig cells in humans, baboons, and monkeys: Implications for xenotransplantation. Transplant Proc. 2005;37:3514–3515. doi: 10.1016/j.transproceed.2005.09.082. [DOI] [PubMed] [Google Scholar]
- 50.Baumann BC, Stussi G, Huggel K, et al. Human natural antibodies directed against endothelial cells derived from Gal-alpha(1,3)Gal knock-out pigs. Xenotransplantation. 2005;12:375. (Abstract O1:6). [Google Scholar]
- 51.Wu G, Pfeiffer S, Schröder C, et al. Coagulation cascade activation triggers early failure of pig hearts expressing human complement regulatory genes. Xenotransplantation. 2007;14(1):34–47. doi: 10.1111/j.1399-3089.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 52.Hisashi Y, Yamada K, Kuwaki K, et al. Rejection of cardiac xenografts transplanted from alpha1,3-galactosyltransferase gene-knockout (GalT-KO) pigs to baboons. Am J Transplant. 2008;8(12):2516–26. doi: 10.1111/j.1600-6143.2008.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelishadi SS, Azimzadeh AM, Zhang T, et al. Coagualation Cascade Activation and Formation of Non-Gal Antibodies Contribute to Early Xenograft Rejection using Gal-TKO Pig Organs in Baboons. Transplantation. 2007 abstract. [Google Scholar]
- 54.Liu C, Noorchashm H, Sutter JA, et al. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nat Med. 2007;13(11):1295–8. doi: 10.1038/nm1673. [DOI] [PubMed] [Google Scholar]
- 55.Perry DK, Burns JM, Pollinger HS, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009;9(1):201–9. doi: 10.1111/j.1600-6143.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 56.Everly MJ, Everly JJ, Susskind B, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86(12):1754–61. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 57.Cramer DV, Wu GD, Kearns-Jonker M, et al. The humoral response to xenografts is controlled by a restricted repertoire of immunoglobulin VH genes. Transplantation. 1998;66(10):1375–83. doi: 10.1097/00007890-199811270-00019. [DOI] [PubMed] [Google Scholar]
- 58.Bouhours D, Pourcel C, Bouhours JE. Simultaneous expression by porcine aorta endothelial cells of glycosphinogolipids bearing the major epitope for human xenoreactive antibodies (Galα1-3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J. 1996;13:947–953. doi: 10.1007/BF01053190. [DOI] [PubMed] [Google Scholar]
- 59.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 60.Byrne GW, Stalboerger PG, Davila E, et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation. 2008;15(4):268–76. doi: 10.1111/j.1399-3089.2008.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Puga Yung G, Schneider M, Seebach JD. Immune responses to α1,3 galactosyltransferase knockout pigs. Current Opinion in Organ Transplantation. 2009;14:154–160. doi: 10.1097/MOT.0b013e328329250d. [DOI] [PubMed] [Google Scholar]
- 62.Karim M, Feng G, Wood KJ, Bushell AR. CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood. 2005;105(12):4871–7. doi: 10.1182/blood-2004-10-3888. [DOI] [PubMed] [Google Scholar]
- 63.Cosimi AB, Sachs DH. Mixed chimerism and transplantation tolerance. Transplantation. 2004;77(6):943–6. doi: 10.1097/01.tp.0000117779.23431.3f. [DOI] [PubMed] [Google Scholar]
- 64.Sykes M, Shimizu I, Kawahara T. Mixed hematopoietic chimerism for the simultaneous induction of T and B cell tolerance. Transplantation. 2005;79(3 Suppl):S28–9. doi: 10.1097/01.tp.0000153296.80385.e7. [DOI] [PubMed] [Google Scholar]
- 65.Muller YD, Golshayan D, Ehirchiou D, Wekerle T, Seebach JD, Bühler LH. T regulatory cells in xenotransplantation. Xenotransplantation. 2009;16:121–128. doi: 10.1111/j.1399-3089.2009.00531.x. [DOI] [PubMed] [Google Scholar]
- 66.Murakami M, Ito H, Harada E, Enoki T, Sykes M, Hamano K. Long-term survival of xenogeneic heart grafts achieved by costimulatory blockade and transient mixed chimerism. Transplantation. 2006;82(2):275–81. doi: 10.1097/01.tp.0000226221.53161.10. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto S, Lavelle JM, Vagefi PA, et al. Vascularized thymic lobe transplantation in a pig-to-baboon model: a novel strategy for xenogeneic tolerance induction and T-cell reconstitution. Transplantation. 2005;80(12):1783–90. doi: 10.1097/01.tp.0000184445.70285.4b. [DOI] [PubMed] [Google Scholar]
- 68.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, Xu Y, Medof ME. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201(10):1523–30. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lalli PN, Zhou W, Sacks S, Medof ME, Heeger PS. Locally produced and activated complement as a mediator of alloreactive T cells. Front Biosci (Schol Ed) 2009;1:117–24. doi: 10.2741/S11. [DOI] [PubMed] [Google Scholar]
- 70.Buhler LH, Basker M, Alwayn IPJ, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70:1323–1331. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 71.Schulte am Esch J, Rogiers X, Robson SC. Molecular incompatibilities in hemostasis between swine and men—impact on xenografting. Ann Transplant. 2001;6:12–16. [PubMed] [Google Scholar]
- 72.Robson SC, Cooper DKC, d'Apice AJF. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation. 2000;7:166–176. doi: 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 73.Chen D, Dorling A. Microcoagulation processes after xenotransplantation. Curr Opin Organ Transplant. 2005;10:240–245. [Google Scholar]
- 74.Cowan PJ, Roussel JC, d'Apice AJ. The vascular and coagulation issues in xenotransplantation. Curr Opin Organ Transplant. 2009;14(2):161–7. doi: 10.1097/mot.0b013e3283279591. [DOI] [PubMed] [Google Scholar]
- 75.Lin CC, Chen D, McVey JH, Cooper DK, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86(5):702–9. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lawson JH, Daniels LJ, Platt JL. The evaluation of thrombomodulin activity in porcine to human xenotransplantation. Transplant Proc. 1997;29:884–885. doi: 10.1016/s0041-1345(96)00192-3. [DOI] [PubMed] [Google Scholar]
- 77.Schulte am Esch J, Cruz MA, Siegel JB, Anrather J, Robson SC. Activation of human platelets by the membrane-expressed A1 domain of von Willebrand factor. Blood. 1997;90:4425–4437. [PubMed] [Google Scholar]
- 78.Khalpey Z, Yuen AH, Lavitrano M, et al. Mammalian mismatches in nucleotide metabolism: implications for xenotransplantation. Mol Cell Biochem. 2007;304(1-):109–17. doi: 10.1007/s11010-007-9491-9. [DOI] [PubMed] [Google Scholar]
- 79.Dwyer KM, Mysore TB, Crikis S, et al. The transgenic expression of human CD39 on murine islets inhibits clotting of human blood. Transplantation. 2006;82(3):428–32. doi: 10.1097/01.tp.0000229023.38873.c0. [DOI] [PubMed] [Google Scholar]
- 80.Kopp CW, Siegel JB, Hancock WW, et al. Effect of porcine endothelial tissue factor pathway inhibitor on human coagulation factors. Transplantation. 1997;63:749–758. doi: 10.1097/00007890-199703150-00023. [DOI] [PubMed] [Google Scholar]
- 81.Roussel JC, Moran CJ, Salvaris EJ, Nandurkar HH, d'Apice AJ, Cowan PJ. Pig thrombomodulin binds human thrombin but is a poor cofactor for activation of human protein C and TAFI. Am J Transplant. 2008;8(6):1101–12. doi: 10.1111/j.1600-6143.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- 82.Mendicino M, Liu M, Ghanekar A, et al. Targeted deletion of Fgl-2/fibroleukin in the donor modulates immunologic response and acute vascular rejection in cardiac xenografts. Circulation. 2005;112(2):248–56. doi: 10.1161/CIRCULATIONAHA.105.534271. [DOI] [PubMed] [Google Scholar]
- 83.Chen D, Weber M, McVey JH, Kemball-Cook G, Tuddenham EG, Lechler RI, Dorling A. Complete inhibition of acute humoral rejection using regulated expression of membrane-tethered anticoagulants on xenograft endothelium. Am J Transplant. 2004;4(12):1958–63. doi: 10.1111/j.1600-6143.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 84.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cowan PJ, Aminian A, Barlow H, et al. Protective effects of recombinant human antithrombin III in pig-to-primate renal xenotransplantation. Am J Transplant. 2002;2(6):520–5. doi: 10.1034/j.1600-6143.2002.20605.x. [DOI] [PubMed] [Google Scholar]
- 86.Fontayne A, Meiring M, Lamprecht S, et al. The humanized anti-glycoprotein Ib monoclonal antibody h6B4-Fab is a potent and safe antithrombotic in a high shear arterial thrombosis model in baboons. Thromb Haemost. 2008 Oct;100(4):670–7. [PubMed] [Google Scholar]
- 87.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tseng Y-L, Sachs DH, Cooper DKC. Porcine hematopoietic progenitor cell transplantation in nonhuman primates: a review of progress. Transplantation. 2005;79:1–9. doi: 10.1097/01.tp.0000146504.73727.13. [DOI] [PubMed] [Google Scholar]
- 89.Ide K, Ohdan H, Kobayashi T, Hara H, Ishiyama K, Asahara T. Antibody- and complement-independent phagocytotic and cytolytic activities of human macrophages toward porcine cells. Xenotransplantation. 2005;12:181–188. doi: 10.1111/j.1399-3089.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 89a.Rees MA. A novel role for lectins in xenotransplantation. Invited commentary. Xenotransplantation. 2005;12(1):7–12. doi: 10.1111/j.1399-3089.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 90.Ide K, Wang H, Tahara H, et al. Role for CD47-SIRPα signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104(12):5062–6. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burlak C, Twining LM, Rees MA. Terminal sialic acid residues on human glycophorin A are recognized by porcine Kupffer cells. Transplantation. 2005;80:344–352. doi: 10.1097/01.tp.0000162974.94890.9f. [DOI] [PubMed] [Google Scholar]
- 92.Rees MA, Butler AJ, Brons IG, et al. Evidence of macrophage receptors capable of direct recognition of xenogeneic epitopes without opsonization. Xenotransplantation. 2005;12:13–19. doi: 10.1111/j.1399-3089.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 93.Rees MA, Butler AJ, Chavez-Cartaya G, et al. Prolonged function of extracorporeal hDAF transgenic pig livers perfused with human blood. Transplantation. 2002;73((8):1194. doi: 10.1097/00007890-200204270-00003. [DOI] [PubMed] [Google Scholar]
- 94.Rees MA, Butler AJ, Negus MC, Davies HF, Friend PJ. Classical pathways complement destruction is not responsible for the loss of human erythrocytes during porcine liver perfusion. Transplantation. 2004;77:1416–1423. doi: 10.1097/01.tp.0000121135.24688.a3. [DOI] [PubMed] [Google Scholar]
- 95.Schneider M, Seebach JD. Current cellular innate immune hurdles in pig-to-primate xenotransplantation. Current Opinion in Organ Transplantation. 2008;13:171–177. doi: 10.1097/MOT.0b013e3282f88a30. [DOI] [PubMed] [Google Scholar]
- 96.Schneider MK, Ghielmetti M, Rhyner DM, Antsiferova MA, Seebach JD. Human leukocyte transmigration across Gal α(1,3)Gal-negative porcine endothelium is regulated by human CD18 and CD99. Transplantation. 2009;87:491–499. doi: 10.1097/TP.0b013e318195fb8d. [DOI] [PubMed] [Google Scholar]
- 97.Fryer JP, Leventhal JR, Dalmasso AP, et al. Cellular rejection in discordant xenografts when hyperacute rejection is prevented: analysis using adoptive and passive transfer. Transpl Immunol. 1994;2(2):87–93. doi: 10.1016/0966-3274(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 98.Blakely ML, Van der Werf WJ, Berndt MC, Dalmasso AP, Bach FH, Hancock WW. Activation of intragraft endothelial and mononuclear cells during discordant xenograft rejection. Transplantation. 1994;58(10):1059–66. [PubMed] [Google Scholar]
- 99.Satake M, Korsgren O, Ridderstad A, Karlsson-Parra A, Wallgren AC, Moller E. Immunological characteristics of islet cell xenotransplantation in humans and rodents. Immunol Rev. 1994;141:191–211. doi: 10.1111/j.1600-065x.1994.tb00878.x. [DOI] [PubMed] [Google Scholar]
- 100.Wallgren AC, Karlsson-Parra A, Korsgren O. The main infiltrating cell in xenograft rejection is a CD4+ macrophage and not a T lymphocyte. Transplantation. 1995;60(6):594–601. doi: 10.1097/00007890-199509270-00013. [DOI] [PubMed] [Google Scholar]
- 101.Lin Y, Vandeputte M, Waer M. Contribution of activated macrophages to the process of delayed xenograft rejection. Transplantation. 1997;64(12):1677–83. doi: 10.1097/00007890-199712270-00008. [DOI] [PubMed] [Google Scholar]
- 102.Itescu S, Kwiatkowski P, Artrip JH, et al. Role of natural killer cells, macrophages, and accessory molecule interactions in the rejection of pig-to-primate xenografts beyond the hyperacute period. Hum Immunol. 1998;59(5):275–86. doi: 10.1016/s0198-8859(98)00026-3. [DOI] [PubMed] [Google Scholar]
- 103.Fox A, Koulmanda M, Mandel TE, van Rooijen N, Harrison LC. Evidence that macrophages are required for T-cell infiltration and rejection of fetal pig pancreas xenografts in nonobese diabetic mice. Transplantation. 1998;66(11):1407–16. doi: 10.1097/00007890-199812150-00002. [DOI] [PubMed] [Google Scholar]
- 104.Xu H, Gundry SR, Hancock WW, et al. Prolonged discordant xenograft survival and delayed xenograft rejection in a pig-to-baboon orthotopic cardiac xenograft model. J Thorac Cardiovasc Surg. 1998;115(6):1342–9. doi: 10.1016/S0022-5223(98)70218-1. [DOI] [PubMed] [Google Scholar]
- 105.Xia G, Ji P, Rutgeerts O, Waer M. Natural killer cell- and macrophage mediated discordant guinea pig-- >rat xenograft rejection in the absence of complement, xenoantibody and T cell immunity. Transplantation. 2000;70(1):86–93. [PubMed] [Google Scholar]
- 106.Fox A, Mountford J, Braakhuis A, et al. Innate and adaptive immune responses to nonvascular xenografts: evidence that macrophages are direct effectors of xenograft rejection. J Immunol. 2001;166:2133–2140. doi: 10.4049/jimmunol.166.3.2133. [DOI] [PubMed] [Google Scholar]
- 107.Inverardi L, Clissi B, Stolzer AL, et al. Human natural killer lymphocytes directly recognize evolutionarily conserved oligosaccharide ligands expressed by xenogeneic tissues. Transplantation. 1997;63:1318–1330. doi: 10.1097/00007890-199705150-00021. [DOI] [PubMed] [Google Scholar]
- 108.Rieben R, Seebach JD. Xenograft rejection: IgG1, complement and NK cells team up to activate and destroy the endothelium. Trends Immunol. 2005;26:2–5. doi: 10.1016/j.it.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 109.Li S, Waer M, Billiau AD. Xenotransplantation: role of natural immunity. Transpl Immunol. 2009;21(2):70–4. doi: 10.1016/j.trim.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 110.Forte P, Lilienfeld BG, Baumann BC, Seebach JD. Human NK cytotoxicity against porcine cells is triggered by NKp44 and NKG2D. J. Immunol. 2005;178:5463–5470. doi: 10.4049/jimmunol.175.8.5463. [DOI] [PubMed] [Google Scholar]
- 111.Lilienfeld BG, Garcia-Borges C, Crew MD, Seebach JD. Porcine UL16-binding protein 1 expressed on the surface of endothelial cells triggers human NK cytotoxicity through NKG2D. J Immunol. 2006;177(4):2146–52. doi: 10.4049/jimmunol.177.4.2146. [DOI] [PubMed] [Google Scholar]
- 112.Lilienfeld BG, Schildknecht A, Imbach LL, Mueller NJ, Schneider MK, Seebach JD. Characterization of porcine UL16-binding protein 1 endothelial cell surface expression. Xenotransplantation. 2008;15(2):136–44. doi: 10.1111/j.1399-3089.2008.00453.x. [DOI] [PubMed] [Google Scholar]
- 113.Tran PD, Christiansen D, Winterhalter A, et al. Porcine cells express more than one functional ligand for the human lymphocyte activating receptor NKG2D. Xenotransplantation. 2008;15(5):321–32. doi: 10.1111/j.1399-3089.2008.00489.x. [DOI] [PubMed] [Google Scholar]
- 114.Seebach JD, Comrack C, Germana S, LeGuern C, Sachs DH, DerSimonian H. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J Immunol. 1997;159:3655–3661. [PubMed] [Google Scholar]
- 115.Sharland A, Patel A, Lee JH, et al. Genetically modified HLA class I molecules able to inhibit human NK cells without provoking alloreactive CD8+ CTLs. J Immunol. 2002;168:3266–3274. doi: 10.4049/jimmunol.168.7.3266. [DOI] [PubMed] [Google Scholar]
- 116.Dorling A, Monk NJ, Lechler RI. HLA-G inhibits the transendothelial migration of human NK cells. Eur J Immunol. 2000;30:586–593. doi: 10.1002/1521-4141(200002)30:2<586::AID-IMMU586>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 117.Forte P, Baumann BC, Weiss EH, Seebach JD. HLA-E expression on porcine cells: protection from human NK cytotoxicity depends on peptide loading. Am J Transplant. 2005;5:2085–2093. doi: 10.1111/j.1600-6143.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 118.Baumann BC, Forte P, Hawley RJ, Rieben R, Schneider MK, Seebach JD. Lack of galactose-α1,3-galactose expression on porcine endothelial cells prevents complement-induced lysis but not direct xenogeneic NK cytotoxicity. J Immunol. 2004;172:6460–6467. doi: 10.4049/jimmunol.172.10.6460. [DOI] [PubMed] [Google Scholar]
- 119.Christiansen D, Mouhtouris E, Milland J, Zingoni A, Santoni A, Sandrin MS. Recognition of a carbohydrate xenoepitope by human NKRP1A (CD161). Xenotransplantation. 2006;13(5):440–6. doi: 10.1111/j.1399-3089.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- 120.Crew MD, Cannon MJ, Phanavanh B, Garcia-Borges CN. An HLA-E single chain trimer inhibits human NK cell reactivity towards porcine cells. Mol Immunol. 2005;42:1205–1214. doi: 10.1016/j.molimm.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 121.Crew MD. Play it in E or G: utilization of HLA-E and -G in xenotransplantation. Xenotransplantation. 2007;14(3):198–207. doi: 10.1111/j.1399-3089.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- 122.Forte P, Baumann BC, Schneider MK, Seebach JD. HLA-Cw4 expression on porcine endothelial cells reduces cytotoxicity and adhesion mediated by CD158a+ human NK cells. Xenotransplantation. 2009;16(1):19–26. doi: 10.1111/j.1399-3089.2009.00510.x. [DOI] [PubMed] [Google Scholar]
- 123.Weiss EH, Lilienfeld BG, Müller S, et al. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation. 2009;87(1):35–43. doi: 10.1097/TP.0b013e318191c784. [DOI] [PubMed] [Google Scholar]
- 123a.Hering BJ, Walawalkar N. Pig-to-nonhuman primate islet xenotransplantation. Transplantation Immunology. 2009;21:81–86. doi: 10.1016/j.trim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 124.Hecht G, Eventov-Friedman S, Rosen C, et al. Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proc Natl Acad Sci U S A. 2009;106(21):8659–64. doi: 10.1073/pnas.0812253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ayares DL, Trucco M, Cooper DKC, et al. Genetic engineering of pigs for improved transplant outcomes. Transplantation Abstract #404.2. 1st Joint Conference of CTS, IPITA, and IXA – Minneapolis, MN, 9/15-20; 2007. [Google Scholar]; van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, Murase N, Hara H, Ball S, Loveland' BE, Ayares D, Lakkis FG, Cooper DKC, Trucco M. Diabetic nonhuman primates transplanted with hCD46 transgenic porcine islets maintain long-term normoglycemia under limited immunosuppression. Am J Transplantation. doi: 10.1111/j.1600-6143.2009.02850.x. May replace with. submitted. [DOI] [PubMed] [Google Scholar]
- 126.Gianello G, Dufrane D, et al. Encapsulation of adult pig islets in monolayer cellular device to correct STZ diabetes in primates without immunosuppression. Bull Mem Acad R Med Belg. 2007;162:439–449. [PubMed]
- 125.Interim Recommendations for Preclinical Data to support Clinical Islet Xenotransplantation. http://www.transplantation soc.org/sections.php?s=01#recommendations. [Google Scholar]
- 128.McKenzie IF, Xing PX, Vaughan HA, Prenzoska J, Dabkowski PL, Sandrin MS. Distribution of the major xenoantigen (Gal[α1-3]Gal) for pig to human xenografts. Transpl Immunol. 1994;2:81–86. doi: 10.1016/0966-3274(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 129.Dor FJ, Cheng J, Alt A, et al. Galα1,3Gal expression on porcine pancreatic islets, testis, spleen, and thymus. Xenotransplantation. 2004;11:101–106. doi: 10.1111/j.1399-3089.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 130.McKenzie IF, Koulmanda M, Mandel TE, Sandrin MS. Pig islet xenografts are susceptible to “anti-pig” but not Galα(1,3)Gal antibody plus complement in Galo/o mice. J Immunol. 1998;161:5116–5119. [PubMed] [Google Scholar]
- 131.Komoda H, Miyagawa S, Kubo T, et al. A study of the xenoantigenicity of adult pig islets cells. Xenotransplantation. 2004;11:237–246. doi: 10.1111/j.1399-3089.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- 132.Komoda H, Miyagawa S, Omori T, et al. Survival of adult islet grafts from transgenic pigs with N-acetylglucosaminyltransferase-III (GnT-III) in cynomolgus monkeys. Xenotransplantation. 2005;12:209–216. doi: 10.1111/j.1399-3089.2005.00206.x. [DOI] [PubMed] [Google Scholar]
- 133.Safley SA, Kapp JA, Weber CJ. Proliferative and cytokine responses in CTLA4-Ig-treated diabetic NOD mice transplanted with microencapsulated neonatal porcine ICCs. Cell Transplant. 2002;11(7):695–705. doi: 10.3727/000000002783985413. [DOI] [PubMed] [Google Scholar]
- 134.Soderlund J, Wennberg L, Castanos-Velez E, et al. Fetal porcine islet-like cell clusters transplanted to cynomolgus monkeys: an immunohistochemical study. Transplantation. 1999;67:784–791. doi: 10.1097/00007890-199903270-00002. [DOI] [PubMed] [Google Scholar]
- 135.Safley SA, Kapp LM, Tucker-Burden C, Hering B, Kapp JA, Weber CJ. Inhibition of cellular immune responses to encapsulated porcine islet xenografts by simultaneous blockade of two different costimulatory pathways. Transplantation. 2005;79(4):409–18. doi: 10.1097/01.tp.0000150021.06027.dc. [DOI] [PubMed] [Google Scholar]
- 136.Johansson H, Lukinius A, Moberg L, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54(6):1755–62. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 137.Johansson H, Goto M, Siegbahn A, Elgue G, Korsgren O, Nilsson B. Low molecular weight dextran sulfate: a strong candidate drug to block IBMIR in clinical islet transplantation. Am J Transplant. 2006;6(2):305–12. doi: 10.1111/j.1600-6143.2005.01186.x. [DOI] [PubMed] [Google Scholar]
- 138.Bennet W, Sundberg B, Lundgren T, et al. Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: protective effects of sCR1 and heparin. Transplantation. 2000;69(5):711–9. doi: 10.1097/00007890-200003150-00007. [DOI] [PubMed] [Google Scholar]
- 139.Fishman JA, Patience C. Xenotransplantation: infectious risk revisited. Am J Transplant. 2004;4(9):1383–90. doi: 10.1111/j.1600-6143.2004.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mueller N, Barth RN, Yamamoto S, et al. Activation of cytomegalovirus in pig-to-primate organ xenotransplantation. J Virol. 2002;76:4734–4740. doi: 10.1128/JVI.76.10.4734-4740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gollackner B, Mueller NJ, Houser S, et al. Porcine Cytomegalovirus And Coagulopathy In Pig-To-Primate Xenotransplantation. Transplantation. 2003;75(11):1841–1847. doi: 10.1097/01.TP.0000065806.90840.C1. [DOI] [PubMed] [Google Scholar]
- 142.Huang CA, Fuchimoto Y, Gleit ZL, et al. Post-transplant lymphoproliferative disease (PTLD) in miniature swine after allogeneic hematopoietic cell transplantation: Similarity to human PTLD and association with porcine gamma herpesvirus. Blood. 2000;97:1467–1473. doi: 10.1182/blood.v97.5.1467. [DOI] [PubMed] [Google Scholar]
- 143.Cho PS, Mueller NJ, Cameron AM, et al. Risk Factors for the Development of Post-Transplant Lymphoproliferative Disorder in a Large Animal Model. Am J Transplant. 2004;4(8):1274–1282. doi: 10.1111/j.1600-6143.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- 144.Ghielmetti M, Millard AL, Haeberli L, et al. Human CMV infection of porcine endothelial cells increases adhesion receptor expression and human leukocyte recruitment. Transplantation. 2009;87(12):1792–800. doi: 10.1097/TP.0b013e3181a75a41. [DOI] [PubMed] [Google Scholar]
- 145.Wood JC, Quinn G, Suling KM, et al. Identification of exogenous recombinant human-tropic porcine endogenous retrovirus. J Virol. 2004;78:2494–2501. doi: 10.1128/JVI.78.5.2494-2501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martin SI, Wilkinson RA, Fishman JA. Genomic presence of recombinant porcine endogenous retrovirus in transmitting miniature swine. Virology J. 2006;3:1743–422. doi: 10.1186/1743-422X-3-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McGregor CGA, Byrne GW, Vlasin M, et al. Preclinical orthotopic cardiac xenotransplantation. JHLT. 2009;28(2S):S224. [Google Scholar]
- 148.Teotia SS, Walker RC, Schirmer JM, et al. Prevention, detection, and management of early bacterial and fungal infections in a preclinical cardiac xenotransplantation model that achieves prolonged survival. Xenotransplantation. 2005;12:127–133. doi: 10.1111/j.1399-3089.2005.00205.x. [DOI] [PubMed] [Google Scholar]
- 149.Cooper DK, Keogh AM, Brink J, et al. Report of the Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation: the present status of xenotransplantation and its potential role in the treatment of end-stage cardiac and pulmonary diseases. J Heart Lung Transplant. 2000;19(12):1125–65. doi: 10.1016/s1053-2498(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 150.Groth CG, Korsgren O, Tibell A, et al. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344(8934):1402–4. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 151.Nagata H, Nishitai R, Shirota C, et al. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology. 2007;132(1):321–9. doi: 10.1053/j.gastro.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 152.Hara H, Ezzelarab M, Rood PPM, et al. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation. 2006;13:357–365. doi: 10.1111/j.1399-3089.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 153.Wong BS, Yamada K, Koumi M, et al. Allosensitization does not increase the risk of xenoreactivity to ά1,3-Galactosyltransferase gene-knockout (GalT-KO) miniature swine in patients on transplantation waiting lists. Transplantation. 2006;82:314–319. doi: 10.1097/01.tp.0000228907.12073.0b. [DOI] [PubMed] [Google Scholar]
- 154.Cooper DKC, Tseng Y-L, Saidman SL. Allo- and xeno-antibody cross-reactivity in transplantation. Transplantation. 2004;77:1–5. doi: 10.1097/01.TP.0000105116.74032.63. [DOI] [PubMed] [Google Scholar]
- 155.Guidance for Industry Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans. April 3, 2003 (68 FR 16542) http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation /Guidances/Xenotransplantation/ucm074354.htm.
- 156.Sykes M, d'Apice A, Sandrin M. IXA Ethics Committee. Position paper of the Ethics Committee of the International Xenotransplantation Association. Transplantation. 2004;78(8):1101–7. doi: 10.1097/01.tp.0000142886.27906.3e. [DOI] [PubMed] [Google Scholar]
- 157.U.S. Department Of Health And Human Services Secretary's Advisory Committee On Xenotransplantation Informed Consent In Clinical Research Involving Xenotransplantation. http://www4.od.nih.gov/oba/SACX/reports/IC_draft_030905.pdf.