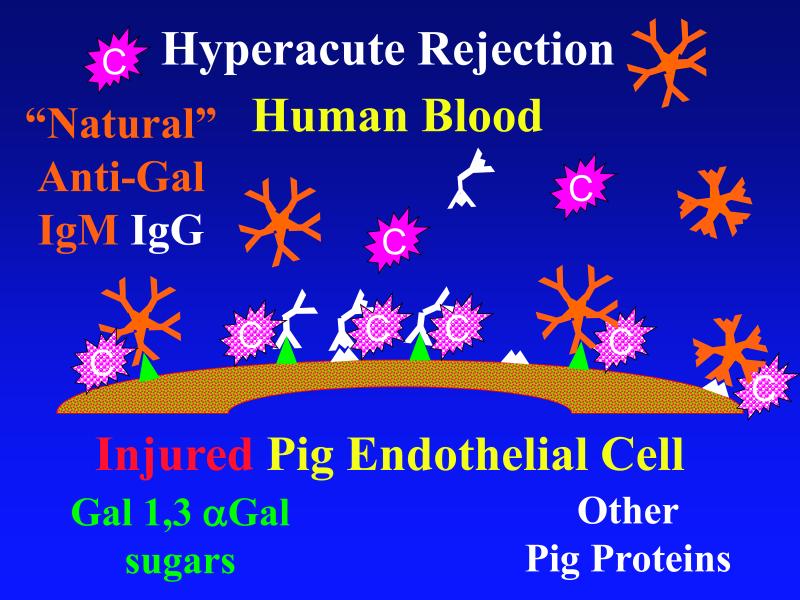
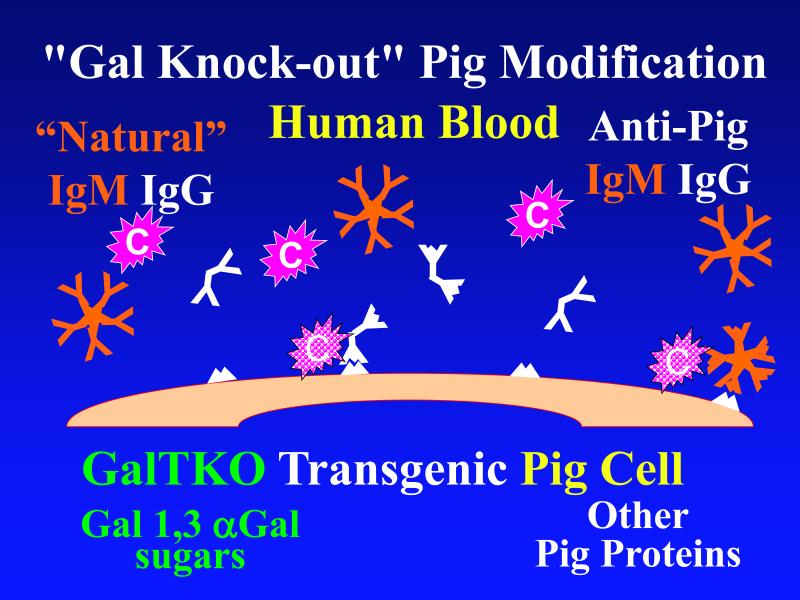
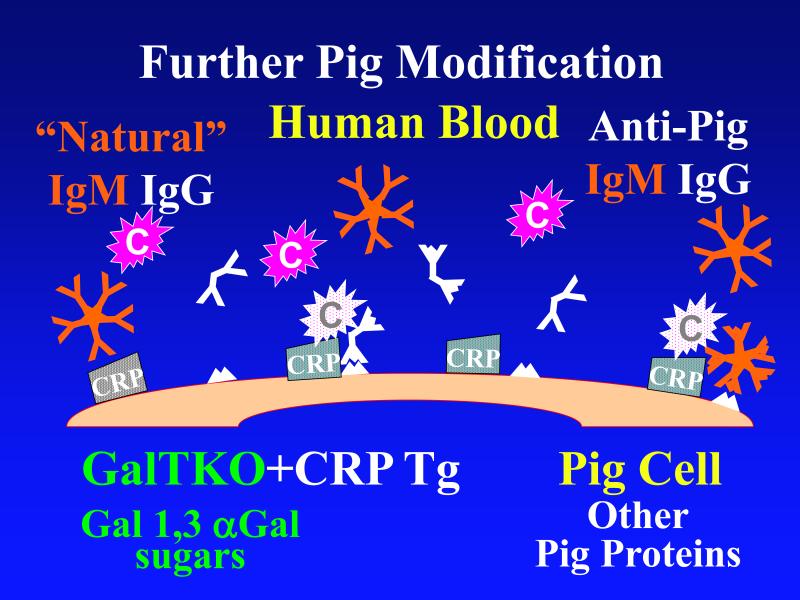
Figure 1. Genetic modifications to attenuate hyperacute rejection.
According to the conventional model, hyperacute rejection of pig organs in humans is triggered by binding of preformed human “natural” antibody, which is predominantly directed against Galα1-3Gal sugars that decorate many molecules on the surface of pig cells. Panel A: Complement is activated by bound antibody, triggering retraction and lysis of endothelial cells. Loss of endothelial barrier function contributes to leakage of blood into the interstitium. Blood clotting within vessels is activated by injured endothelium and exposed basement membrane, leading to downstream tissue ischemia and necrosis. Panel B: When the galactosyl transferase gene is disabled by gene knockout, pig cells lack the Galα1-3Gal target antigen. If present in sufficient quantity, antibody against other pig antigens that is either preformed or induced after transplant can trigger complement-mediated injury and associated coagulation pathway activation. Panel C: Human proteins that regulate (abort) complement activation (complement pathway regulatory proteins, CPRPs) disable human complement more efficiently than do their pig analogues. Organs from pigs genetically engineered to express human CPRPs are protected from hyperacute rejection, although this protection can be overcome by high-titer antibody, or if endothelial injury occurs due to other mechanisms (ischemia-perfusion injury or coagulation pathway activation, for example). GalT-KO, human CPRP, and perhaps additional gene modifications may be necessary to yield clinically useful, reliable protection of pig organs in man.