Abstract
Purpose
To characterize optic nerve head (ONH) connective tissue deformation following acute (15 or 30 minutes) intraocular pressure (IOP) elevation within six adult normal monkeys using 3-D histomorphometry.
Methods
Trephinated ONH and peripapillary sclera from both eyes of six monkeys, each perfusion fixed with one eye at IOP 10 mmHg and the other at IOP 30 or 45 mmHg by anterior chamber manometer were serial sectioned, 3-D reconstructed, 3-D delineated and quantified using standard parameters. For each monkey, inter-eye differences (high IOP eye minus IOP 10 eye) for each parameter were calculated and compared by ANOVA and EPIDmax both overall and regionally. EPIDmax deformations for each parameter were defined to be those statistically significant differences that exceeded the maximum physiologic inter-eye difference within six bilaterally normal monkeys of a previous report.
Results
Regional EPIDmax laminar thinning, posterior bowing of the peripapillary sclera, thinning and expansion of the scleral canal were present in most high IOP eyes and were colocalized in those demonstrating the most deformation. Laminar deformation was minimal and not only posterior but in some cases anterior in the high IOP eyes. No increase in deformation was seen in the IOP-45 versus the IOP-30 eyes.
Conclusion
ONH connective tissue alterations following acute IOP elevation involve regional thinning, stretching and deformation of the lamina cribrosa and peripapillary sclera which are minimal to modest in magnitude. The time-dependent character of these alterations, as well as their compressive, expansile, and shear effects on the contained axons, astrocytes, laminar and posterior ciliary circulations remain to be determined.
Keywords: Glaucoma, Acute IOP elevation, Optic Nerve Head, Neural Canal Offset and Depth, Lamina Cribrosa Position and Thickness, Peripapillary Scleral Position and Thickness, Post-NCO
Introduction
The neural, vascular, and connective tissues of the optic nerve head (ONH) make up a dynamic environment wherein 1.2 to 2.0 million retinal ganglion cell (RGC) axons converge, turn, and exit the eye through the neural canal opening (NCO; Figure 2 and Figure 7). Within the scleral portion of the neural canal, the bundled axons pass through the lamina cribrosa, a three-dimensional (3-D) meshwork of astrocyte-covered, capillary-containing, connective tissue beams. While glaucomatous damage to the visual system likely includes important pathophysiologies within the RGC body 1-6, photoreceptors7-11, lateral geniculate body12-14 and visual cortex14, strong evidence suggests that damage to the retinal ganglion cell axons within the lamina cribrosa of the ONH15-20 is the central pathophysiology underlying glaucomatous vision loss. Recent studies in human21, 22, monkey19, 20, 23-28, rat29-31 and mouse32, support the importance of the ONH by describing significant alterations within the prelaminar, laminar and peripapillary scleral tissue of the ONH at the earliest detectable stage of experimental glaucoma. While the interaction between ONH connective tissue deformation19, 20, 24, 26, 33, altered astrocyte function34-38 and ischemia 39-42 are beginning to be elucidated, the multifactorial insults to the RGC axons remain to be characterized. These insults may include intraocular pressure (IOP)-related27 and non IOP-related components43-46 that do not require IOP-induced connective tissue deformation, damage, and/or remodeling to occur.
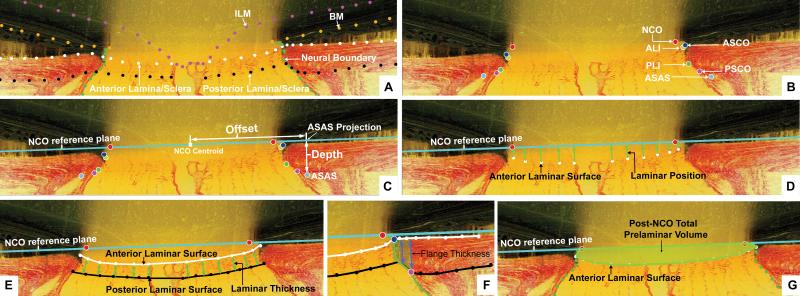
Figure 2. Parameter Definitions.
(A) A representative digital sagittal slice showing the internal limiting membrane (ILM, pink dots), Bruch's membrane (BM, orange dots), anterior laminar/scleral surface (white dots), posterior laminar/scleral surface (black dots) and neural boundary (green dots). (B) A representative digital sagittal slice showing neural canal architectures. The neural canal includes neural canal opening (NCO, the opening in the Bruch's Membrane/Retinal Pigment Epithelial complex, red), the anterior scleral canal opening (ASCO, dark blue), the anterior laminar insertion (ALI, dark yellow, partly hidden behind the ASCO in dark blue), the posterior laminar insertion (PLI, green), the posterior scleral canal opening (PSCO, pink). The anterior-most aspect of the subarachnoid space (ASAS, light blue) was also delineated. (C) Definitions of the offset and depth using ASAS as an example. Right ASAS point was projected to NCO zero reference plane (cyan line), the distance between NCO centroid to the projection of ASAS is defined as offset. The distance between the ASAS to the projection is defined as depth of ASAS. The offset and depth of all other neural canal architectures were defined in the same way. (D) Laminar position (green arrow) is defined as the shortest distance from the delineated anterior laminar surface point (white dot) to the NCO zero reference plane. (E) Lamina cribrosa thickness at each delineated anterior surface point is determined by fitting a continuous surface (white line) to all anterior surface points and then measuring the distance along a normal vector of the anterior surface (green arrow) from each anterior delineated point to the posterior surface. (F) The thickness of the scleral flange at each delineated anterior surface point (white dots) is defined as the distance between the neural canal boundary points (green line), along a vector parallel to the PSCO normal vector (blue arrow). (I) Post-NCO total prelaminar volume (light green: a measure of the laminar or connective tissue component of cupping) is the volume beneath the NCO zero reference plane in cyan, above the lamina cribrosa and within the neural canal wall.
Figure 7. A peripapillary scleral-lamina cribrosa dynamic underlies ONH biomechanics48.
We propose that there are peripapillary scleral and lamina cribrosa contributions to ONH connective tissue behavior and that the manner in which the sclera canal and lamina cribrosa deform following a given level of IOP elevation in a given ONH will be determined by its level and duration and the structural stiffness of each tissue. Top Diagram. Normal lamina cribrosa (light blue), peripapillary sclera (slanted lines), Bruch's membrane (solid pink line), NCO (red points - in this schematic diagram, Bruch's membrane extends into the canal and is thus considered the neural canal opening (NCO)); Anterior scleral opening (blue points),NCO reference plane (small dotted red line), Border tissue of Elschnig (light green), choroid (black circles) and scleral portion of neural canal (orange). We define the structural stiffness of a tissue to be the combined function of both its connective tissue architecture (the quantity and distribution of load-bearing tissue) and material properties (the stiffness or compliance of the tissue) Bottom Diagram. Changes following acute IOP elevation in the monkey eye as depicted in this diagram can include: 1) posterior bowing of the ONH and circum-papillary sclera relative to the more peripheral peripapillary sclera (which manifest as an anterior deformation of the peripapillary sclera relative to the NCO reference plane); 2) axial thinning (orange lines are smaller in the high IOP eye) and radial expansion (angle theta has increased at high IOP) of the posterior scleral portion of the neural canal; 3) laminar thinning (solid blue at high IOP is less than low IOP); and 4) small anterior or posterior deformations of the anterior lamina cribrosa surface (black arrows and dotted blue lines). The effects of these deformations on astrocyte physiology, posterior ciliary artery blood flow and retinal ganglion cell axon axoplasmic transport and flow remain to be determined.
To characterize the ONH as a biomechanical structure, deformation of the lamina cribrosa following acute IOP elevations has been studied in human cadaver eyes21, 22, 47 and in monkeys19, 48. Levy and Crapps22 reported a 12 μm average posterior (outward) movement of the central lamina with acute IOP elevations from 10 to 25 mmHg for short periods in human cadaver eyes. Yan et al.21 found that increasing IOP from 5 to 50 mmHg for 24 hours produced an average posterior deformation of the central lamina of 79 μm, and small contractions of the scleral canal and thinning of the lamina which did not achieve significance.
We have previously studied acute IOP-induced deformation of the monkey ONH connective tissues using 2-D histomorphometry within two groups of monkey eyes 19, 48. In the first study 48, Bellezza demonstrated that anterior laminar position was significant more anterior (towards Bruch's membrane), the lamina cribrosa was thinner, and the scleral canal diameter was larger in a group of monkey eyes that had been perfusion fixed at IOP-10 mmHg compared with a group of immersion fixed (IOP-0 mmHg) eyes. These results suggested that the lamina cribrosa and scleral canal wall act like an expandable trampoline at low levels of IOP, with the canal expanding and the lamina thinning and becoming more tautly stretched as IOP is elevated from 0 to 10 mmHg.
In a subsequent study 19, young adult normal monkeys were perfusion fixed 15 to 80 minutes after one eye was set to 10 mmHg and the other to 30 or 45 mmHg by anterior chamber manometer. This study demonstrated an overall posterior laminar deformation of 10 to 23 μm in the high IOP eyes relative to their contralateral IOP-10 control eyes. However, this deformation did not exceed the magnitude of inter-eye differences in laminar surface position in a separate group of bilaterally normal monkeys immersion fixed following enucleation.
These human and monkey studies, considered together, suggest that the lamina cribrosa and scleral canal wall deform following acute IOP elevation in the normal monkey and human eye, that the deformation of the lamina may be anterior rather than posterior within the canal, and that this deformation may increase as the period of acute IOP elevation is extended. However, the measurements in most of these studies were taken from two dimensional (2-D) histologic sections and were therefore subject to artifacts introduced by the section angle through the tissue, section distortion during mounting, and the lack of a consistent reference plane for measurements, all of which may have limited their accuracy. Levy's study using 2-D x-ray images also suffered from artifacts induced by the angle of imaging and the fact that the position of the platinum wire was not certain relative to the anterior lamina cribrosa surface.
While we have previously emphasized that IOP-related axonal trauma within the lamina cribrosa may be unrelated to actual connective tissue deformation25, 33, it remains important to identify the biomechanical determinants of laminar and peripapillary scleral displacement at a given IOP. The purpose of this study is to characterize connective tissue deformation of the lamina, scleral canal wall, and peripapillary sclera within 3-D ONH reconstructions following acute (15 minutes) IOP elevation (from 10 to 30 or 45 mmHg) within six young adult normal monkeys using 3-D histomorphometry24-26, 28
Materials and Methods
Animals
All animals were treated in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. One female and five male normal monkeys (5 - 14 years old), were used for this study (Table 1). Prior to sacrifice, IOP and axial length were measured in both eyes of each animal on 3 to 5 separate occasions. IOP was defined to be the mean of three measurements by Tono-pen (Tono-Pen XL; Bio-Rad, Glendale, CA). Axial length was defined to be the mean of the longest 3 measurements out of 10 ultrasonic axial length measurements (Model A1500; Sonomed, Lake Success, NY).
Table 1.
Animal and Eye Data
| Monkey | Histomorphometric Optic Disc Size $ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | ID | Weight (kg) | Age (y) | Species | Sex | Eye | IOP# (mm Hg) | Fixation Pressure (mm Hg) | Axial Length (mm) | Duration of IOP elevation (min) | Number of serial Section Images | Vertical (μm) & | Horizontal (μm) * | Area (mm2) ! |
| 1 | 20852 | 4.32 | 14 | Rhesus | Female | Right | 11 | 45 | NA | 15 | 686 | 1214 | 869 | 0.828 |
| Left | 11 | 10 | NA | - | 554 | 1216 | 864 | 0.825 | ||||||
| 2 | 5889 | 5.9 | 6 | Rhesus | Male | Right | 9 | 45 | 20.51 | 15 | 316 | 1415 | 1042 | 1.158 |
| Left | 8 | 10 | 20.2 | - | 349 | 1384 | 1009 | 1.097 | ||||||
| 3 | 7484 | 5.9 | 5 | Rhesus | Male | Right | 9 | 45 | 21.39 | 15 | 283 | 1641 | 1143 | 1.473 |
| Left | 8 | 10 | 21.02 | - | 306 | 1577 | 1131 | 1.401 | ||||||
| 4 | AA3P | 5.5 | 9 | Cynomolgus | Male | Right | 12 | 30 | 18.49 | 15 | 325 | 1238 | 828 | 0.805 |
| Left | 12 | 10 | 18.29 | - | 290 | 1263 | 808 | 0.801 | ||||||
| 5 | 278 | 6 | 5 | Rhesus | Male | Right | 4 | 30 | 19.85 | 15 | 217 | 1534 | 1112 | 1.340 |
| Left | 4 | 10 | 19.6 | - | 170 | 1487 | 1073 | 1.253 | ||||||
| 6 | 528 | 7.2 | 6 | Rhesus | Male | Right | 11 | 30 | 19.89 | 30 | 280 | 1368 | 999 | 1.074 |
| Left | 11 | 10 | 18.54 | - | 265 | 1318 | 914 | 0.946 | ||||||
Mean IOP of all N = 3 to 9 baseline measurements under ketamine/xylazine anesthesia
Optic disc dimension as determined by the clinical visible optic disc margin which is Neural Canal Opening in these monkey eyes at the perfusion pressure
Vertical length as determined by the major length of the NCO ellipse
Horizontal length as determined by the minor length of the NCO ellipse
Disc Area is determined by the area of the NCO ellipse
Monkey Euthanasia and Perfusion Fixation at Prescribed IOP
Both eyes of each monkey were cannulated with a 27-gauge needle under deep pentobarbital anesthesia and the IOP was set to 10 mmHg using an adjustable saline reservoir. After a minimum of 30 minutes, IOP was raised to either 30 or 45 mmHg in one eye for 15 minutes (30 minutes for monkey 6) and the monkey was perfusion fixed via the descending aorta with 1 L of 4% buffered hypertonic paraformaldehyde solution followed by 6 L of 5% buffered hypertonic glutaraldehyde solution pressurized during perfusion to approximately 4 psi for 5 monkeys, and 15 psi for one monkey (monkey 1 – the only monkey in which perfusion pressure was monitored via the brachial artery during perfusion). Following perfusion, IOP was maintained for one hour, and then each eye was enucleated, all extraocular tissues were removed, and the intact anterior chamber was excised 2 to 3 mm posterior to the limbus. By gross inspection, perfusion was excellent in all six IOP-10 mmHg eyes. However, blood was variably present in the retinal vessels, posterior ciliary arteries and vortex veins of the six high IOP eyes. The posterior scleral shell with intact ONH, choroid and retina were placed in 5% glutaraldehyde solution for storage.
Generation of the Aligned Serial Section Images for Each ONH and 3-D ONH Reconstruction
These steps have been described in detail within our previous reports20, 24. Briefly, the ONH and peripapillary sclera were trephinated (6-mm-diameter), pierced with alignment sutures, embedded in paraffin, mounted to a microtome and sectioned. After each section was taken, the block surface was stained with a 1:1 (v/v) mixture of Ponceau S and acid fuchsin stains, and then imaged at a resolution of 2.5 × 2.5 μm per pixel. Sections were serially cut at 3.0 μm thickness, and the staining and imaging process was repeated after each cut. Imaging began at the vitreoretinal interface and continued approximately 200 μm into the retrolaminar orbital optic nerve.
Serial section images of ONH were aligned in the anterior-to-posterior direction and stacked at 3.0-μm intervals into a 3-D reconstruction of the ONH and peripapillary scleral connective tissue (approximately 1080 × 1520 × 400 voxels, each 2.5 × 2.5 × 3.0 μm in size).
3-D Delineation of ONH and Peripapillary Scleral Landmark Points
Our 3-D delineation technique has been described in detail in previous reports24-26 (see Figure 1). Briefly, using custom software (based on the Visualization Toolkit, Clifton Park, NY), the 3-D ONH reconstruction was loaded and the delineator designated the approximate center of the neural canal as the center of rotation, around which 40, 7-voxel-thick, digital, radial, sagittal slices of the 3-D reconstruction were serially served at 4.5° intervals to the delineator's workstation (Figure 1A).
Figure 1. 3-D delineation within the colorized, stacked-section, 3-D ONH reconstruction of a single ONH.
(A) A total of 40 serial digital radial sagittal slices, each 7 voxels thick, are served to the delineator at 4.5° intervals. (B) A representative digital sagittal slice, showing the all 13 marks, which are 3-D delineated using linked, simultaneous, colocalization of the sagittal slice (shown) and the transverse section image through the delineated point (C). (D) Representative 3-D point cloud showing all delineated points for a normal monkey ONH, relative to the posterior serial section image (vitreous top, orbital optic nerve bottom,).
Within each sagittal section image, the delineator marked seven structures: the lamina cribrosa, sclera, neural boundary, Bruch's membrane, internal limiting membrane, central retinal vessels, and subarachnoid space; and six pairs of neural canal landmark points: the Neural Canal Opening (NCO) which in the monkey is most often the innermost extension of Bruch's membrane opening (BMO), the anterior scleral canal opening (ASCO), the anterior laminar insertion (ALI), the posterior laminar insertion (PLI), the posterior scleral canal opening (PSCO), and the anterior end of the subarachnoid space (ASAS) (Figure 1B). While marking in the sagittal section view window, the delineator simultaneously viewed a slaved window showing the cursor's location within a digital transverse section image (Figure 1C). The 3-D Cartesian coordinates and category for each mark were saved, generating a 3-D point cloud that represented each of the marked structures (Figure 1D).
Three experienced delineators performed all of the delineations in this study. Both eyes of each animal were delineated by a single delineator. Delineators were not masked to the IOP status of each eye, however, they were effectively unaware of the IOP status during delineation because the file name of each volume within the delineation software did not contain that information. Upon completion of delineation of both eyes of each monkey, the marks were checked for accuracy by two authors with extensive experience (HY and CFB) and without knowledge of the IOP status.
The inter- and intra-delineator variability and reproducibility of our delineation technique has previously been characterized in a series of reports 24-26. Briefly, inter-delineator variability for each parameter was assessed by having five delineators (only 2 delineators for our cupping parameters 25) delineate all ONH landmark points within the normal and early glaucoma eyes of three monkeys. Intra-delineator reproducibility was assessed by having two of the five delineators (only one delineator for our cupping parameters) delineate all landmarks of both eyes of one monkey on two additional occasions at least 2 weeks apart. The inter- and intra-delineator variability data from the three pairs of monkeys (one eye is normal and the contralateral eye is early-glaucoma) of our previous reports are summarized in Table 2.
Table 2.
Overall Parameter by Monkey
| Parameters |
Monkey 1 |
Monkey 2 |
Monkey 3 |
Monkey 4 |
Monkey 5 |
Monkey 6 |
PIDmax# | Delineation Varibility
! |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neural Canal Architecture (μm) | Low IOP | H-L $ | Low IOP | H-L | Low IOP | H-L | Low IOP | H-L | Low IOP | H-L | Low IOP | H-L | Inter-D | Intra-D1 | Intra-D2 | |
|
NCO Offset
|
509 | 1 | 586 | 19 | 663 | 16 | 501 | −2 | 633 | 26 | NA | NA | 9 | 21 | 5 | 4 |
|
ASCO Offset
|
644 | −20 | 676 | 12 | 720 | 14 | 584 | −2 | 657 | 9 | 562 | 58 | 16 | 10 | 10 | 12 |
|
ALI Offset
|
645 | −20 | 675 | 13 | 720 | 14 | 585 | 1 | 654 | 10 | 569 | 62 | 16 | 8 | 5 | 18 |
|
PLI Offset
|
806 | −21 | 797 | 31 | 828 | 13 | 702 | −3 | 754 | 25 | 641 | 100 | 18 | 18 | 17 | 6 |
|
PSCO Offset
|
820 | −15 | 825 | 23 | 877 | −3 | 735 | −5 | 801 | 11 | 658 | 128 | 22 | 24 | 12 | 7 |
|
ASAS Offset
|
951 | −30 | 928 | 11 | 967 | 25 | 888 | −16 | 875 | 28 | 781 | 113 | 18 | 14 | 15 | 20 |
|
ASCO Depth
|
21 | 7 | 54 | −4 | 59 | −15 | 55 | −2 | 40 | −7 | 61 | −7 | 15 | 6 | 8 | 5 |
|
ALI Depth
|
22 | 6 | 54 | −4 | 61 | −16 | 57 | 4 | 40 | −5 | 70 | −1 | 20 | 14 | 6 | 4 |
|
PLI Depth
|
96 | −5 | 146 | −28 | 142 | −11 | 144 | −7 | 147 | 1 | 210 | −55 | 20 | 13 | 19 | 2 |
|
PSCO Depth
|
98 | 0 | 159 | −38 | 179 | −13 | 158 | −13 | 200 | −26 | 224 | −47 | 27 | 23 | 12 | 1 |
|
ASAS Depth
|
118 | 2 | 179 | −54 | 182 | 5 | 167 | −25 | 207 | −22 | 235 | −50 | 25 | 14 | 9 | 6 |
|
ONH Connective Tissue (μm)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lamina Cribrosa Position
|
−97 | −8 | −110 | 6 | −131 | 11 | −134 | −6 | −70 | −7 | −126 | 5 | 16 | 18 | 3 | 1 |
|
Lamina Cribrosa Thickness
|
90 | −2 | 125 | −13 | 133 | −13 | 128 | −12 | 129 | −11 | 150 | −33 | 16 | 16 | 26 | 15 |
|
Peripapillary Scleral Position
|
2 | 1 | −26 | 22 | −22 | 12 | −28 | 14 | −39 | 22 | −49 | 17 | 18 | 13 | 7 | 8 |
|
Peripapillary Scleral Thickness
|
115 | 15 | 157 | −16 | 152 | 11 | 149 | −7 | 173 | 8 | 196 | −39 | 14 | 38 | 5 | 10 |
|
Sclera Flange Thickness
|
58 | 0 | 77 | −9 | 94 | 1 | 81 | 4 | 86 | 12 | NA | NA | 5 | 14 | 12 | 11 |
|
ONH Laminar Cupping
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Post-NCO Total Prelaminar Volume Percent Change((mm3) * | 0.126 | 7.7% | 0.145 | 3.2% | 0.223 | −8.3% | 0.145 | 10.6% | 0.106 | 16.5% | 0.122 | 18.0% | 28.4% | 11% | NA | 2% |
Bold: Significant different (P<0.05) by ANOVA.
Grey: Between Eye Difference magnitude achieved statistical significant difference and exceeded the PID maximum (see Methods).
Difference between high and low IOP eye.
Statistical Analysis is not applicable for this parameter. Percent differences between high and low IOP eyes for this parameter are reported.
PIDmax values as previously reported28. Note that in most instances the PIDmax values, which represent how different the parameter is between the two eyes of 6 bilaterally normal monkeys, exceed the full range of the previously reported inter- and intra-delineator varibility24-26.
Data were previously reported24-26 inter-delineator (Inter-D) varibility data is the range of varibility in micrometers between five 5 delineators and intra-delineator varibility for two delineators (Intra-D1 and Intra-D2) is the range of varibility in micrometers for each delineator on n=3 delineation days.
Clinical Alignment of 3-D ONH Reconstructions
For each ONH, a reconstruction of the central retinal vessels was performed and three-dimensionally overlaid onto a pre-sacrifice photo using the qualitative match of the ONH and retinal vessels. Once preliminarily aligned (using the vessels only), the vessels and NCO points were co-visualized to assess the relationship of the optic disc margin in the photo to the delineated NCO points. A final 3-D adjustment was then performed to match NCO to the disc margin while maintaining vessel alignment.
NCO Reference Plane For each 3-D ONH reconstruction, a least-squares ellipse was fit to the 80 NCO points (which in this case are the end of Bruch's membrane) 24, 49 creating an NCO reference plane (Figure 2C)24. The centroid of the NCO ellipse established the center point for all measurements. All quantification of offset, depth, position, and post-NCO total prelaminar volume were made relative to this plane (Figure 2C-2G).
Quantification This report includes overall and regional quantification of neural canal offset and depth, lamina cribrosa position and thickness, scleral flange thickness, peripapillary scleral position and thickness, and post-NCO total prelaminar volume. The definitions and calculation methods of these parameters have been described in detail in our previous reports24-26, and are briefly summarized as follows (Figure 2). Neural canal landmark Depth is the anterior-to-posterior distance of each marked point to the NCO zero reference plane; and Offset is the distance of each mark, projected onto the NCO reference plane, from the NCO centroid. Laminar Position is the shortest distance from each delineated anterior laminar surface point to the NCO reference plane. Laminar Thickness was calculated at each delineated anterior laminar surface point as the shortest distance to the posterior laminar surface along a vector normal to the anterior laminar surface (fitted into continuous surface to generate the normal vector).
Peripapillary Scleral Position and Thickness was calculated in the same way as laminar position and thickness. Scleral Flange Thickness is defined as the distance from the anterior scleral flange surface to the neural canal boundary surface, measured at each delineated anterior scleral flange surface point along a vector parallel to the PSCO normal vector. Post-NCO Total Prelaminar Volume is designed to detect deformation of the anterior neural canal connective tissue in a single parameter, and is defined to be the total volume (tissue and non-tissue) beneath the NCO reference plane, above the lamina cribrosa and within the neural canal wall.
Overall and Regional Inter-eye Differences by Monkey
Overall and regional parameter values were calculated for both eyes of each monkey as outlined in Figure 3.
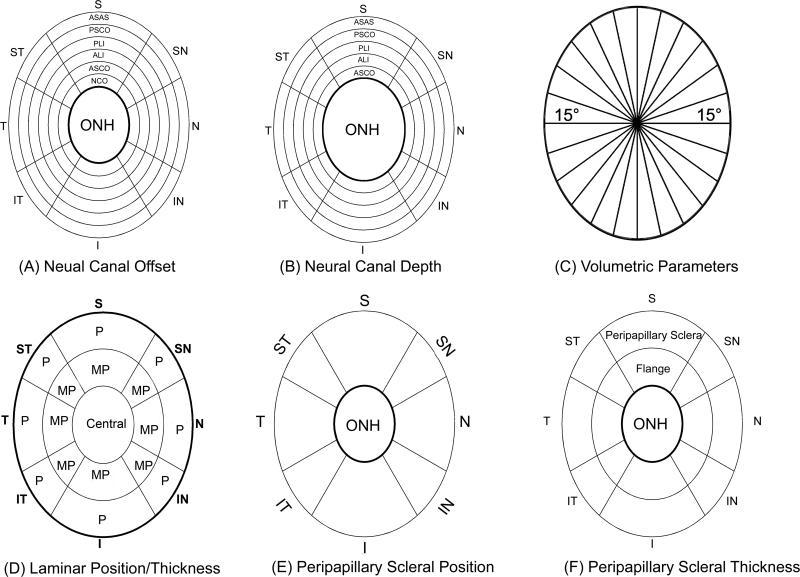
Figure 3. Parameter Regionalization (right eye configuration).
Neural canal offset (A) and depth data (B) for each neural canal landmark were pooled for eight anatomic regions, superior (S), superonasal (SN), nasal (N), inferonasal (IN), inferior (I), inferotemporal (IT), temporal (T), and superotemporal (ST). The S, N, I, and T regions contained all marks within 60° sections of the ONH centered about the S-I and N-T clinical axes, and the SN, IN, IT, and ST regions contained all marks in 30° radial sections of the ONH centered about the SN-IT and IN-ST axes. Concentric rings represent the different neural canal landmarks from its internal entrance NCO to its external exit PSCO as shown in the superior region of (A) and (B). Neural canal Depth measurements start with the ASCO rather than NCO. (C) Using the center of NCO, 12 radial sections perpendicular to NCO zero reference plane divided the volumetric parameters into 24, 15° radial regions. Then regional volumes were projected onto NCO zero reference plane, color coded by region and overlaid onto a standard ellipse. (D) Within the lamina, position and thickness data were pooled into 17 regions according to the three radial regions (central; MP, middle periphery; P, periphery) and eight quadrants same as in (A) and (B). (E) Peripapillary sclera position data were pooled into 8 regions (inner boundary starting from ASCO ellipse (dark black line) to an ellipse whose size is 1.62 times of the ASCO ellipse size (F) Peripapillary scleral thickness data were pooled into 16 regions two radial regions (F, flange thickness, covered area from ASCO to PSCO; And peripapillary sclera region, inner boundary starting from PSCO to an ellipse whose size is 1.62 times of the ASCO ellipse size).
Overall and Regional Schematic Data Maps by Monkey
Schematic depictions of overall (Figure 4) and regional (Figure 5) differences were created without regard for statistical significance for each monkey by overlaying the overall mean measurements for each eye (Figure 4) or for the superior and inferior regions (left Figure 5) and nasal temporal regions (right Figure 5) of each eye.
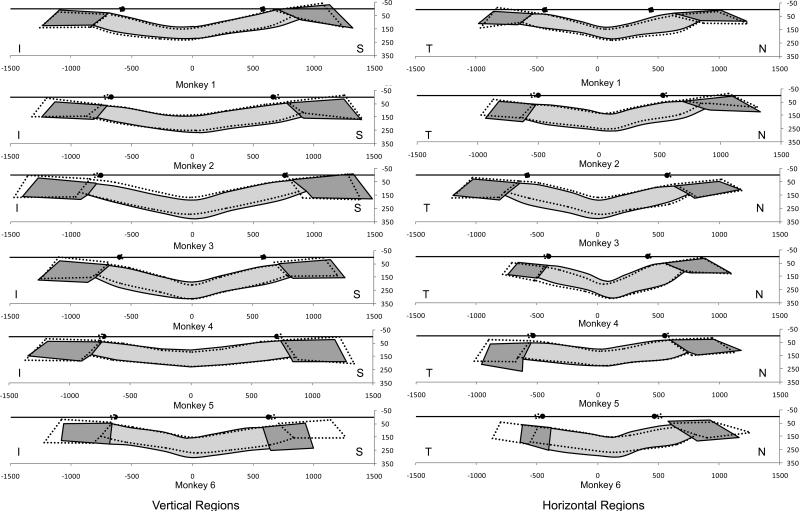
Figure 4. Schematic Representation of the overall deformation data for the low (solid colors) and high (dotted lines) IOP ONH of each monkey as reported in Table 2.
Qualitative differences between the 6 normal eyes include laminar surface curvature (relatively flat monkey 1, relatively curved monkeys 3, 4); laminar and peripapillary thickness (relatively thin monkey 1, relatively thick monkey 6); and neural canal opening size and obliqueness (small and less oblique monkey 6, large and more oblique monkey 3). Inter-eye differences following acute IOP elevation are greatest in Monkeys 5 and 6 and include radial expansion of the scleral portion of the neural canal posteriorly, accompanied by axial thinning of posterior canal, thinning of the lamina without substantial posterior deformation and posterior bowing of the peripapillary sclera. These changes were minimally and variably present in the other four eyes. There is no qualitative relationship between the geometry, IOP magnitude and degree of deformation in the high IOP eyes.
Figure 5. Schematic representation of the vertical and horizontal regional deformation data for the low (solid colors) and high (dotted lines) IOP ONH of each monkey.
Mean data from the superior and inferior (left) and nasal and temporal (right regions of both eyes of each animal are schematically overlaid as central vertical (left) and horizontal (right) sections. Net regional canal expansion can now be seen in monkeys 2, 3, 5 and 6 (though in monkey 1, there is contraction of the superior canal). Small overall posterior bowing of the peripapillary sclera are present in most animals. Small anterior and posterior deformations of the lamina cribrosa accompanied by laminar thinning are also present in most high IOP eyes. The canal, laminar and peripapillary sclera deformations are not symmetrical to the center of the NCO in some animals due to true asymmetric deformation and asymmetric neural canal architecture within the two eyes of an animal. While the inter-eye differences we report are accurate, they are a likely combination of true connective tissue deformation plus some reference plane induced artifacts in the subset of high IOP eyes in which true connective tissue deformation led to shifts and/or tilts in the position of the reference plane relative to the structures being measured. All data are plotted in right eye configuration.
Statistical Analyses
For each ONH, 80 measurements of the following parameters were made: offset and depth of the ASCO, ALI, PLI, PSCO, and ASAS. Measurement of laminar position and thickness, scleral flange thickness, and peripapillary scleral position and thickness were made at each delineation point after surfaces were fit to the anterior and posterior laminar and scleral points. Therefore for all non-volumetric parameters at least n=40 measurements were made for each ONH, and a factorial analysis of variance (ANOVA) was performed to assess the effect of region and IOP (IOP 10 vs IOP 30 or IOP 10 vs IOP 45) on each parameter between the two eyes of each monkey. Statistically significant differences between regions, IOPs, and region-by-IOP combinations required an overall significant F test followed by t tests using p values corrected for multiple comparisons50. Because our volumetric parameters do not lend themselves to multiple measures within individual eyes, a statistical assessment of intra-eye differences for Post-NCO total prelaminar volume for each monkey was not possible.
Statistically Significant and EPIDmax Differences
Statistically significant differences were determined by an ANOVA as outlined above. We additionally defined EPIDmax differences to be those statistically significant differences that exceeded the physiologic inter-eye difference (PID) maximum (the maximum inter-eye difference) within 6 bilaterally normal monkeys in a previous report28. Within this construct, we considered statistically significant differences between the high and low IOP eye of each monkey to be those that exceeded the combination of the variability of our 3-D Histomorphometric quantification method and the true biological variation of each parameter within a region. We considered EPIDmax differences to be those statistically significant differences that were more likely to be treatment effects, i.e. represent a biological response to acute pressure elevation rather than the difference between the two eyes of a normal monkey. For each volumetric parameter, we compared the inter-eye difference for each animal to the PID maximum difference for that parameter.
Difference, Change and Deformation Terminology
We measured post-mortem inter-eye differences for each parameter for each monkey. We emphasize EPIDmax inter-eye differences as those statistically significant differences that most likely represent acute IOP induced change in the high IOP eye of each monkey. We thus refer to EPIDmax inter-eye differences as “change” or “EPIDmax change”.
We further grouped our parameters into “position-change” and “thickness-change” parameters to better recognize the colocalized behavior of these two forms of deformation. To describe position-change we employed the following terminology as it relates to EPIDmax differences in our measurement parameters: Neural canal radial expansion was defined to be an EPIDmax increase, neural canal radial contraction an EPIDmax decrease in neural canal offset, lamina cribrosa anterior deformation was defined to be an EPIDmax increase (more anterior or inward), and lamina cribrosa posterior deformation an EPIDmax decrease (more posterior or outward) in anterior lamina cribrosa position relative to the NCO reference plane.
Peripapillary scleral posterior bowing was defined to be a posterior (outward) deformation of the peripapillary sclera relative to the more peripheral sclera which manifests as an EPIDmax increase in the position of the peripapillary sclera. In this scenario, because the peripapillary sclera carries the ONH with it as it moves outward, it assumes a final position that is anterior to the NCO reference plane. Post-NCO total prelaminar volume expansion was defined to be an EPIDmax volumetric increase andpost-NCO total prelaminar volume contraction an EPIDmax volumetric decrease in the space confined below the NCO reference plane, within the neural canal wall and above the anterior laminar surface. As such, this parameter volumetrically combines the individual deformations of the neural canal wall and lamina cribrosa relative to the NCO reference plane.
To describe thickness-change, we defined neural canal axial thickening to be an EPIDmax increase, and neural canal axial thinning an EPIDmax decrease in neural canal landmark depth. We defined laminar and peripapillary scleral thickening and thinning as EPIDmax increases or decreases in the thickness measurements for each tissue.
Results
Descriptive data
Descriptive data for the six normal monkeys and the histomorphometric optic disc size for each eye are reported in Table 1. Five rhesus monkeys and one cynomolgus monkey, aged from 5 to 14 years, were used for this study. Mean IOP under ketamine/xylazine anesthesia varied from 4 to 12 mmHg among both eyes of all six monkeys. Ultrasonic axial length measurements prior to sacrifice (Model A1500; Sonomed, Lake Success, NY) ranged from 18.29 - 21.39 mm.
Histomorphometric vertical disc size (measured at NCO) ranged from 1214 μm to 1641 μm, and histomorphometric horizontal disc size ranged from 864 μm to 1143 μm. Histomorphometric optic disc area ranged from 0.801 to 1.473 mm2 (based on an ellipse fit to 80 delineated NCO points – see methods).
Overall Data for Each Parameter by Monkey
Overall IOP-10 eye data along with the statistically significant and EPIDmax differences for the high IOP eye for each animal are reported in Table 2. Schematic plots of the overall data for both eyes of each monkey are presented in Figure 4.
Within the schematic plots of Figure 4, qualitative differences between the 6 normal eyes include laminar surface curvature (relatively flat in monkey 5 and relatively curved in monkeys 1 and 4); laminar and peripapillary thickness (relatively thin in monkey 1 and relatively thick in monkey 6); and neural canal opening size and obliqueness (small and less oblique in monkey 6, and large and more oblique monkey 3). Overall, the lamina cribrosa did not deform appreciably in the high IOP eyes of any of these monkeys compared to their contralateral IOP-10 eye. Inter-eye differences following acute IOP elevation are greatest in monkeys 5 and 6 and include expansion and thinning of the posterior neural canal, accompanied by thinning of the lamina without substantial posterior deformation and posterior bowing of the peripapillary sclera.
Within the overall data in Table 2, very little change achieved EPIDmax differences and therefore we emphasize the regional (below) rather than the overall results.
Regional Data for each Parameter by Monkey
Superimposed, averaged, central vertical and horizontal data for both eyes of each animal are schematically depicted in Figure 5. Statistically significant and EPIDmax regional differences for each parameter and for each monkey are reported in Figure 6. While the overall deformations depicted in Figure 4 are minimal, the schematics in Figure 5 qualitatively establish that regional alterations were more substantial. When the regional inter-eye difference data for each position and thickness parameter are arranged by monkey (Figure 6) three patterns of change are apparent. Monkey 1 demonstrates minimal regional position or thickness change. Monkeys 2, 3 and 4 demonstrate non-colocalized regional thinning and peripapillary scleral bowing. Monkeys 5 and 6 demonstrate colocalized regional thinning and position changes. These three patterns of early IOP-induced alterations, and the fact that each includes minimal laminar position change, are the principal findings of this report.
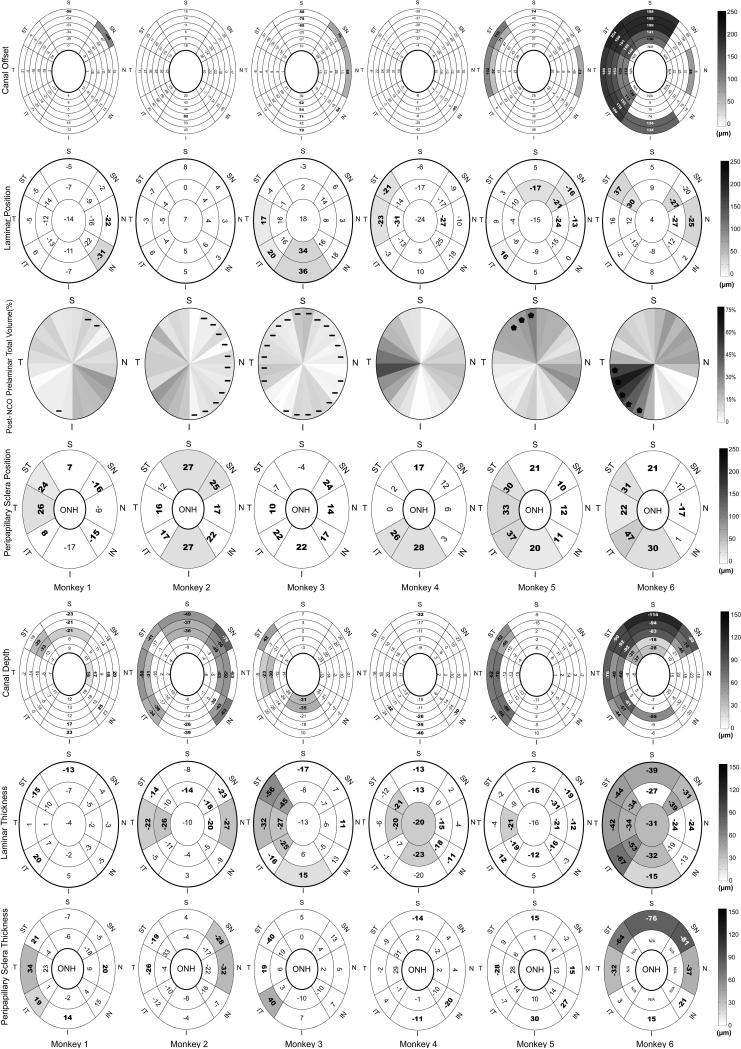
Figure 6. Regional Parameter Difference Maps for the high IOP eyes by money (in column) and by parameter (in row).
Theses maps demonstrate regional colocalization of statistically significant (bold - P< 0.05, ANOVA) and EPIDmax (shaded – see methods)28 differences within the high IOP eye of each animal. Values are the magnitude of change in the high IOP eye relative to its contralateral low IOP control. Positive/negative values in the colored regions are defined for each parameter as follows: canal offset – radial expansion/radial contraction of the canal; lamina cribrosa position – anterior (inward) movement/posterior (outward) of the anterior laminar surface; post-NCO total prelaminar volume – expansion/contraction of the space beneath the NCO reference plane, above the lamina and within the neural canal wall; For post-BMO total prelaminar volume, regional differences that exceeded PID maximum differences are marked by stars and regions with negative volume change (contractions) are marked with negative sign; Positive peripapillary scleral position – anterior (inward) shift of the peripapillary sclera relative to the NCO reference plane which most likely reflects posterior (outward) bowing of the peripapillary sclera and NCO reference plane (together) relative to the more peripheral sclera; canal depth - axial thickening/axial thinning of the canal; lamina cribrosa thickness – thickening/thinning; peripapillary scleral thickness – thickening/thinning of the peripapillary sclera; All data are plotted in right eye configuration. S, superior; SN, superonasal; N, nasal; IN, inferonasal; I, inferior; IT, inferotemporal; T, temporal; ST, superotemporal. See Figure 3 for a detailed description of regionalization.
Minimal detectable change
While the high IOP eye of monkey 1 demonstrated overall peripapillary scleral thickening (Table 2) and a region of colocalized neural canal thinning and peripapillary scleral posterior bowing and thickening (Figure 6), these changes were of minimal magnitude and extent.
Non-colocalized regional thinning, canal expansion and peripapillary scleral bowing
Monkeys 2, 3 and 4 demonstrate EPIDmax thinning of the neural canal and lamina and non-colocalized peripapillary scleral bowing.
Colocalized regional thinning and position changes
Monkeys 5 and 6 demonstrate enough regional position and thickness change so that these phenomena colocalize. The phenomenon of colocalization is not precise, but is best seen in the regions of EPIDmax post-NCO total prelaminar volume expansion that broadly colocalize with regional neural canal expansion and thinning as well as peripapillary scleral bowing in each eye.
Effect of Magnitude and Duration of IOP Elevation
There was no evidence for increased deformation in the IOP-45 (monkeys 1-3) compared to the IOP-30 (monkeys 4-6) eyes after 15 minutes of acute IOP elevation, either overall or regionally. However, the most extensive deformation clearly occurred within the one animal (monkey 6) in which the acute IOP elevation to 30 mmHg was held for 30 rather than 15 minutes.
Discussion
The purpose of this study was to three-dimensionally quantify the laminar, neural canal wall and peripapillary scleral components of ONH connective tissue deformation following 15 minutes of acute IOP elevation in 5 monkeys (30 minutes in monkey 6). The principal findings of this report are as follows. First, in all animals, the lamina deformed minimally in the high IOP eye, and was not only posterior but in some cases anterior. Second, all deformations (both position and thickness changes) were regional and specific to individual eyes. Third, the regional deformation within the six high IOP eyes spanned the following spectrum: minimal detectable change in monkey 1; regional thinning accompanied by non-colocalized peripapillary scleral bowing in monkeys 2, 3 and 4; and colocalized regional thickness and position changes in monkey 5 (superior temporally and temporally) and monkey 6 (inferior temporally and temporally). Fourth, by far the greatest deformation occurred in the one eye subjected to 30 minutes of elevated IOP. Fifth, neither the magnitude of IOP elevation nor the architecture of the ONH connective tissue appeared to relate to the magnitude of deformation.
These findings should be viewed in the following context. First, the lack of substantial laminar deformation in these animals is compatible with our previous study19, that of Levy and Crapps22 in which the period of IOP elevation was short, and recent studies in living human eyes using SD-OCT by Agoumi (Y. Agoumi et al. IOVS 2009;49:ARVO E-Abstract 4898). These findings are also consistent with predictions made by computational models done by Sigal et al. 51. Using eye-specific models, Sigal and colleagues observed that an acute increase in IOP led to peripapillary sclera bowing and lateral displacements of the lamina (mostly in the periphery) while the central lamina remained stationary or displaced slightly anteriorly, depending on the eye. The study, however, did not address the differences in ONH architecture that led to the variations in response to IOP.
Our deformations are less than those reported by Yan, et al 21 in which IOP was increased for 24 hours. Laminar and scleral canal wall deformations are likely to be viscoelastic23, 52, meaning the tissue reaches full deformation only after a given period of time, so longer IOP elevations may induce larger deformations. Although ex-vivo studies of human eyes suggest that an equilibration time of 15 minutes is sufficient to obtain a stable deformation of the vitreo-retinal interface 53, it is possible that the deformations in this study may have been more substantial had the period of IOP elevation been extended to hours or days. Based on our previous studies of connective architecture in monkey eyes19, 48 and recent parameterized finite element modeling of human51, 54-56 and monkey (Sigal IA, et al. IOVS 2008;49:ARVO E-Abstract 3668, Roberts MD, et al. IOVS 2008;49:ARVO E-Abstract 3669) ONHs, we believe that the manner in which the lamina cribrosa and/or scleral canal deform following a given IOP elevation is determined by the level and duration of IOP elevation and the structural stiffness of each tissue.
The structural stiffness of a tissue is the combination of its architecture (the quantity and distribution of load-bearing tissue) and its material properties (the stiffness or compliance of the tissue), both of which contribute to a structure's ability to withstand deformation under applied load 57. The results obtained in this work suggest that the structural stiffness of the lamina cribrosa and peripapillary sclera interact (Figure 7), which is consistent with our previous report 48 and predictions made with computational models57,65. For analysis it is useful to separate the IOP-induced deformation of the ONH tissues into scleral canal expansion or contraction, laminar and scleral canal thinning, and anterior or posterior laminar and peripapillary sclera deformation. Within this lamina-sclera dynamic, the structural stiffness of the peripapillary sclera largely governs whether the scleral canal expands following an increase in IOP. When the structural stiffness of the sclera is relatively low, an increase in IOP would produce an expansion of the canal, pulling on the lamina – an indirect effect of IOP on the lamina itself. When, instead, the structural stiffness of the sclera is relatively high, an increase in IOP produces a much more modest expansion of the canal. In this case the lamina cribrosa is not pulled taut, and is left to resist the direct force of IOP with its structural stiffness alone, deforming posteriorly. We believe that in most eyes the situation would not be at either of the extremes described above, but rather some combination of the two cases. It should be noted that even in the setting of minimal anterior or posterior laminar deformation, substantial tensile strain (local deformation) within the laminar beams may be induced by the expanding scleral canal 51.
Second, the fact that all deformations (both position and thickness changes) were regional and specific to each eye is best appreciated within the regional data of Figure 6. In saying that the deformations were regional, we mean that the EPIDmax deformations were focal (only one region) or regional (at least two regions), or they were greatest within a single region when diffuse (monkey 6 only). By individual-eye specific, we mean that the regions of greatest deformation were not consistent within these six high IOP eyes. We believe our study is the first to establish that early ONH connective tissue deformation following acute IOP elevation is regional in nature and is likely to be individual-eye specific.
Third, we have observed three patterns of acute ONH connective tissue deformation within these 6 animals: 1) minimal deformation; 2) non-colocalized regional thinning and peripapillary scleral bowing; and 3) colocalized position and thickness change. To determine if the observed deformations are portions of a continuum of ONH connective tissue deformation will require the development of spectral domain optical coherence tomography49, 58, 59 (Y. Agoumi et al. IOVS 2009;49:ARVO E-Abstract 4898) or second harmonic imaging60 to capture these same ONH landmarks at multiple time points following acute IOP elevation in living human and monkey eyes. Regardless of whether these patterns are part of a continuous spectrum of connective tissue deformation, our data strongly suggest that laminar and neural canal thinning, neural canal expansion, and peripapillary sclera bowing are early responses to acute IOP elevation in the monkey eye and that, within the limits of our experimental techniques, these phenomena colocalize within those eyes demonstrating the greatest deformation.
Fourth, by far the greatest deformation occurred in the one eye subjected to 30 minutes of elevated IOP even though pressure was only raised to 30 mmHg. This animal, which was among the younger animals, but not the youngest, had the thickest lamina and peripapillary sclera and the smallest scleral canal (Table 2 and Figures 4 and 5). It could be that the duration of IOP exposure may be an important determinant of acute ONH connective tissue deformation. Several investigators have reported substantially increased ONH connective tissue deformations following the prolonged application of load21, 61, 62. It is possible that the additional duration of IOP elevation and time dependant (or viscoelastic) effects alone, account for the magnitude of deformation in monkey 6. However, without more eyes exposed to longer IOP elevations and/or a series of individual eyes imaged at multiple time points following acute IOP elevation, the data from this one animal do not prove such a viscoelastic effect.
It is also possible that monkey 6 may simply have had a much more compliant lamina and sclera than the other animals. In our previous histologic study of ONH connective tissue compliance19, none of the normal monkeys achieved this magnitude of deformation, though some experienced up to 80 minutes of IOP elevation. This, along with the fact that this animal appears to have the thickest lamina and sclera and smallest scleral canal (Table 2 and Figures 4 and 5), suggests that the material properties of both the lamina and sclera in this eye are substantially less stiff than the other 5 high IOP eyes. Estimates of these material properties within engineering finite element models63, 64 will be the subject of a future report.
Fifth, neither the magnitude of IOP elevation nor the tissue-level architecture of the ONH connective tissue closely correlates to the magnitude of connective tissue deformation. While we believe that there should be consistent biomechanical determinants of ONH connective tissue behavior, these relationships are not obvious from the histomorphometric measurements in this report. Elucidating these relationships is one of the goals of ongoing finite element modeling54,64,65.
Our findings are limited by the following considerations. While we recently improved our 3-D histomorphometric methodologies to acquire data using a 1.5 × 1.5 × 1.5 μm voxels28, all of the reconstructions for this report were acquired with our older 2.5 × 2.5 × 3.0 μm voxel technique. Most of the differences we report substantially exceed the resolution of these 3-D reconstructions and we have previously demonstrated good reproducibility of our delineation techniques at this resolution24-26. In addition, there is tissue shrinkage effects from both fixation and embedding associated with this technique, but comparisons between the two eyes of each monkey should be valid since all eyes were treated identically.
For this study, we chose acute IOP elevations of 15-minute duration for two reasons. First, our previous ONH surface compliance testing had suggested that the majority of ONH surface deformation during 60 minutes of acute IOP elevation occurred during the first 15 minutes of elevated IOP66, 67. Second, perfusion fixation is done under deep pentobarbital anesthesia, which can lower systemic blood pressure. In previous animals, we had seen evidence of poor perfusion of the central retinal vessels in eyes that were perfusion fixed after 30 to 80 minutes of high IOP. However, even in the 5 high IOP eyes of this report using our old sacrifice protocol, blood pressures were low at the time of perfusion and we saw evidence of residual blood within the retinal and posterior ciliary circulation that suggested less than ideal fixative perfusion. This residual blood may also have been due to the fact that the fixative perfusion pressure may not have been high enough (3-4 psi) to push the fixative fluid into the small arteries of the ONH due to the flow limiting nature of the old perfusion system.
While all eyes sat at their set IOP for 60 minutes after perfusion, it is possible that our data may underestimate the magnitude of acute IOP induced deformation of ONH connective tissue if fixation was less than ideal. However, monkey 1 was sacrificed using our most current protocol, which ensures a fixative perfusion pressure of 80 mmHg as measured by direct real-time pressure monitoring via brachial artery cannulation. We did not see larger connective tissue deformations in this monkey, which suggests that the small deformations we report for the other monkeys are not artifactual.
Our study may also underestimate the laminar component of ONH connective tissue deformation following acute IOP elevation if cerebrospinal fluid (CSF) pressure became elevated during the period of perfusion, and retrolaminar tissue pressure was inadvertently elevated as a result 68. We did not monitor the CSF pressure in these experiments; however, we think this phenomenon is unlikely for the following reasons. First, as outlined earlier, BP during perfusion was probably low in the last five eyes and was not greater than 80 mm Hg in the first. Although the relationship between BP change and CSF pressure change in an animal that is undergoing fixation with glutaraldehyde has not been characterized, the fact that BP was low in most eyes suggests that CSF pressure was also likely low. Second, we qualitatively compared the size of the anterior-most portion of the subarachnoid space within the 3D histomorphometric reconstructions of the other 5 monkeys (perfusion fixed at low BP) to the first monkey and found no obvious difference. This suggested that the volume of the CSF space was not altered by the higher perfusion BP in the first animal. Finally, even if CSF pressure slowly climbed during the period of perfusion fixation, this process would have occurred at the same time the connective tissues were being fixed into a position determined by the previous 15 minutes of IOP elevation. We doubt that a slow CSF rise would alter the effects of glutaraldehyde being delivered to the tissues via the blood vessels.
However, a qualitative inspection of the subarachnoid space in all of our perfusion fixed and immersion fixed tissues 19, 20, 24-26, 28 suggests that the subarachnoid space is expanded in the perfusion fixed eyes. While the collapsed (immersion fixed) vs expanded (perfusion fixed) subarachnoid space may only represent the effects of physiologic CSF pressure (in the perfusion fixed eyes), elevated CSF pressure in the animals of this study affecting the laminar (but not the scleral) deformations we report remains a possibility, and may require further investigation.
It is possible that trephinating a 6-mm-diameter piece of the ONH and peripapillary sclera allowed residual stresses within the sclera to be relieved. The resultant post-fixation tissue warping, if present, could induce non-IOP-related alterations in tissue position that would confound our measurements. However, these effects would most likely occur in the peripheral sclera closest to the trephine edge, and therefore are unlikely to affect the measurements within the ONH and peripapillary sclera. In addition, we feel that a large degree of tissue warping in the setting of months of glutaraldehyde fixation is unlikely.
All of our measurements were made relative to a plane based on the delineated NCO points (which are the end of Bruch's membrane in most monkeys)24. Any collapse of the Border Tissue of Elshnig or compression of the choroid either from the period of high IOP or from post-trephination tissue warping, could conceivably alter these points leading to a posterior shift or tilt of the reference plane in the high IOP eye, which would induce anterior movement or tilting of the other structures relative to its contralateral normal. In the case of reference plane tilt, the effect would be greatest in the periphery and least near the point of inflection. In the case of a posterior shift, the effect would be similar in both central and peripheral measurements.
We believe that the measurements we report for both eyes of each animal are accurate based on the previously reported reproducibility of our delineation method and the voxel dimensions within our reconstructions24-26. We do not believe that important shifts in the reference plane occurred as a result of focal alterations in Border Tissue of Elschnig architecture or choroidal compression within the high IOP eyes for the following reasons. First, overall thinning of the anterior neural canal opening (ASCO and ALI) achieved statistical (but not EPIDmax) significance in monkey 3 only. Regional EPIDmax thinning of the anterior neural canal was only present in monkey 3 and 6. A small tilting effect can also be seen within the regional cross section schematics for monkeys 3 and 6 (Figure 5), wherein the high IOP ONH seems slightly rotated relative to the low IOP ONH.
The lack of substantial anterior scleral canal thinning in all 6 animals is important because it suggests that little posterior deformation of NCO relative to the ONH and peripapillary scleral landmarks we studied (as might happen if the choroid was compressed, or the Border Tissue of Elschnig was deformed by the acute IOP elevations) is present in any of the 6 high IOP eyes. The fact that the regional EPIDmax posterior canal thinning detected in Monkeys 1, 2, 5 and 6 greatly exceed the anterior canal thinning within their same regions (significant or otherwise) strongly suggests that the detected posterior canal thinning in these animals are not measurement artifact.
Second, we closely examined the 0°, 30°, 60°, 90°, 120° and 150° digital section images from both eyes of each animal and found no qualitative evidence of choroidal compression in the high IOP eyes. In fact, in all monkeys the choroid is substantially expanded even in the face of the elevated IOP in the high IOP eye.
Our study includes 5 rhesus and one cynomolgous monkey which may confound our results. While there may be species differences in normal monkey ONH connective tissue architecture and material properties that could influence their response to acute IOP elevation19, 20, 69, we doubt these are important in our study for the following reasons. First, by qualitative comparison the normal eye measurements of monkey 4 (cynomolgous) fall within the range determined by the 5 rhesus animals for each parameter except for NCO offset and laminar position where they are only minimally out of that range (Table2). Second, the overall behavior in the high IOP of this animal is also within, rather than at one extreme of the five rhesus animals (Figure 4 and 6).
We did not perform a formal assessment of reproducibility on the eyes in this study and the small differences we report may be within the variability of our measurements. However, we think this is unlikely for most parameters for the following reasons. First, the intra- and inter-delineator reproducibility of our parameters (Table 2) has been extensively characterized in a series of previous reports24-26. Second, close inspection of these data suggest that the range of intra-delineator variability for most parameters is less than the PID maximum value for each parameter28 that was used as the criteria for EPIDmax change. Thus, we believe that for most parameters, our EPIDmax difference, when present, represents change beyond the variability due to delineation, had it been directly assessed in these eyes.
Finally, we choose to order our monkeys (1-6) based on the regional change in the parameter Post NCO – Total Prelaminar Volume (Table 2 and Figure 6) and the degree to which this co-localized with EPIDmax changes in other parameters. Using these criteria, monkeys 5 and 6 demonstrate the greatest amount of co-localized deformation. At present, there is no precise way to order the monkeys deformation and this choice may be arbitrary, as by other overall and regional measures other monkeys may exceed Monkey 5. Our choice of this parameter reflects our underlying belief that there is a progression of the individual forms of deformation within these tissues that leads to their co-localization. At present this is a hypothesis which needs to be studied in living eyes using in-vivo measures of ONH connective tissue deformation as mentioned above.
Our results have the following implications. First, while the lamina demonstrated little anterior or posterior deformation it did demonstrate regional thinning, in some cases colocalized, with the greatest magnitude of canal expansion (monkey 6, Figure 6). This point is important because it emphasizes the difference between overall deformation of a biological structure (the lamina cribrosa) and local deformation (strain) within its component elements. Expansion of the scleral canal and the associated laminar thinning should result in expansion of the laminar pores and thinning and elongation of laminar beams. The effects on the contained laminar capillaries, astrocytes, and adjacent axons, as well as the transfer of nutrients between them may be substantial. Thinning of the lamina cribrosa also increases the steepness of the translaminar pressure gradient ((IOP – retrolaminar tissue pressure)/laminar thickness), which may have separate implications on astrocyte physiology and retinal ganglion cell axoplasmic transport and flow15, 16, 68, 70-75. Characterization of alterations in laminar beam microarchitecture27, 63 (Grimm J, et al. Burgoyne CF, IOVS 2007;48:ARVO E-Abstract 3295) in these eyes, and the stresses and strains contained therein76 (Kodiyalam S, et al.IOVS 2005;46: ARVO E-Abstract 1267), will also be the subject of future reports.
Second, while peripapillary scleral bowing and scleral canal expansion are likely to be important determinants of lamina cribrosa biomechanical behavior, their direct and indirect effect on axonal susceptibility within the ONH remains unclear. For a given perfusion pressure, bowing and thinning of the peripapillary sclera and laminar beams should influence blood flow within the contained laminar capillaries and posterior ciliary arteries, but these effects have yet to be determined.
Finally, our EPIDmax criteria for post-mortem change detection may be conservative. Within the regional data, in many instances statistically significant differences did not achieve EPIDmax criteria and were not considered as change. We believe the trends that are present in our data are important indicators of early viscoelastic deformation. This prediction is currently under study using in vivo SD-OCT imaging of the neural canal, lamina cribrosa and peripapillary sclera after 30, 60, 90 and 120 minutes of IOP elevation in very young and very old monkey eyes (Burgoyne CF, et al. IOVS 2008;49:ARVO E-Abstract 3655).
Acknowledgments
The authors gratefully acknowledge the following individuals for their assistance with this study. Jonathon Grimm and Juan Reynaud for their assistance with software for volumetric and thickness quantification, Galen Williams and Erica Dyrud for their help with delineation, Pris Zhou and Anthony Bellezza, PhD for their direction of animal testing, Joanne Couchman for her assistance with manuscript preparation and Stuart Gardiner for his statistical consultation.
Supported in part by USPHS grants R01EY011610 (CFB) from the National Eye Institute, National Institutes of Health, Bethesda, Maryland; a grant from the American Health Assistance Foundation, Rockville, Maryland (CFB); a grant from The Whitaker Foundation, Arlington, Virginia (CFB); a Career Development Award (CFB); The Legacy Good Samaritan Foundation, Portland, Oregon; and the Sears Trust for Biomedical Research, Mexico, Missouri.
References
- 1.Asai T, Katsumori N, Mizokami K. [Retinal ganglion cell damage in human glaucoma. 2. Studies on damage pattern]. Nippon Ganka Gakkai Zasshi. 1987;91:1204–1213. [PubMed] [Google Scholar]
- 2.Garcia-Valenzuela E, Shareef S, Walsh J, Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61:33–44. doi: 10.1016/s0014-4835(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest. Ophthalmol. Vis. Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- 4.Weber AJ, Kaufman PL, Hubbard WC. Morphology of single ganglion cells in the glaucomatous primate retina. Invest. Ophthalmol. Vis. Sci. 1998;39:2304–2320. [PubMed] [Google Scholar]
- 5.Quigley HA, McKinnon SJ, Zack DJ, Pease ME, Kerrigan-Baumrind LA, Kerrigan DF, Mitchell RS. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest. Ophthalmol. Vis. Sci. 2000;41:3460–3466. [PubMed] [Google Scholar]
- 6.Quigley HA. Ganglion cell death in glaucoma: pathology recapitulates ontogeny. Aust N Z J Ophthalmol. 1995;23:85–91. doi: 10.1111/j.1442-9071.1995.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 7.Wygnanski T, Desatnik H, Quigley HA, Glovinsky Y. Comparison of ganglion cell loss and cone loss in experimental glaucoma. Am J Ophthalmol. 1995;120:184–189. doi: 10.1016/s0002-9394(14)72606-6. [DOI] [PubMed] [Google Scholar]
- 8.Panda S, Jonas JB. Decreased photoreceptor count in human eyes with secondary angle- closure glaucoma. Invest. Ophthalmol. Vis. Sci. 1992;33:2532–2536. [PubMed] [Google Scholar]
- 9.Kendell KR, Quigley HA, Kerrigan LA, Pease ME, Quigley EN. Primary open-angle glaucoma is not associated with photoreceptor loss. Invest. Ophthalmol. Vis. Sci. 1995;36:200–205. [PubMed] [Google Scholar]
- 10.Nork TM, Ver Hoeve JN, Poulsen GL, Nickells RW, Davis MD, Weber AJ, Vaegan, Sarks SH, Lemley HL, Millecchia LL. Swelling and loss of photoreceptors in chronic human and experimental glaucomas. Arch Ophthalmol. 2000;118:235–245. doi: 10.1001/archopht.118.2.235. [DOI] [PubMed] [Google Scholar]
- 11.Janssen P, Naskar R, Moore S, Thanos S, Thiel HJ. Evidence for glaucoma-induced horizontal cell alterations in the human retina. Ger J Ophthalmol. 1996;5:378–385. [PubMed] [Google Scholar]
- 12.Yucel YH, Zhang Q, Gupta N, Kaufman PL, Weinreb RN. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol. 2000;118:378–384. doi: 10.1001/archopht.118.3.378. [DOI] [PubMed] [Google Scholar]
- 13.Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Atrophy of relay neurons in magno- and parvocellular layers in the lateral geniculate nucleus in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2001;42:3216–3222. [PubMed] [Google Scholar]
- 14.Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003;22:465–481. doi: 10.1016/s1350-9462(03)00026-0. [DOI] [PubMed] [Google Scholar]
- 15.Gaasterland D, Tanishima T, Kuwabara T. Axoplasmic flow during chronic experimental glaucoma. 1. Light and electron microscopic studies of the monkey optic nervehead during development of glaucomatous cupping. Invest. Ophthalmol. Vis. Sci. 1978;17:838–846. [PubMed] [Google Scholar]
- 16.Minckler DS, Bunt AH, Johanson GW. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Invest. Ophthalmol. Vis. Sci. 1977;16:426–441. [PubMed] [Google Scholar]
- 17.Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–649. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- 18.Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979;86:1803–1830. doi: 10.1016/s0161-6420(79)35338-6. [DOI] [PubMed] [Google Scholar]
- 19.Bellezza AJ, Rintalan CJ, Thompson HW, Downs JC, Hart RT, Burgoyne CF. Deformation of the lamina cribrosa and anterior scleral canal wall in early experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2003;44:623–637. doi: 10.1167/iovs.01-1282. [DOI] [PubMed] [Google Scholar]
- 20.Burgoyne CF, Downs JC, Bellezza AJ, Hart RT. Three-dimensional reconstruction of normal and early glaucoma monkey optic nerve head connective tissues. Invest. Ophthalmol. Vis. Sci. 2004;45:4388–4399. doi: 10.1167/iovs.04-0022. [DOI] [PubMed] [Google Scholar]
- 21.Yan DB, Coloma FM, Metheetrairut A, Trope GE, Heathcote JG, Ethier CR. Deformation of the lamina cribrosa by elevated intraocular pressure. Br J Ophthalmol. 1994;78:643–648. doi: 10.1136/bjo.78.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy NS, Crapps EE. Displacement of optic nerve head in response to short-term intraocular pressure elevation in human eyes. Arch Ophthalmol. 1984;102:782–786. doi: 10.1001/archopht.1984.01040030630037. [DOI] [PubMed] [Google Scholar]
- 23.Downs JC, Suh JK, Thomas KA, Bellezza AJ, Hart RT, Burgoyne CF. Viscoelastic material properties of the peripapillary sclera in normal and early-glaucoma monkey eyes. Invest. Ophthalmol. Vis. Sci. 2005;46:540–546. doi: 10.1167/iovs.04-0114. [DOI] [PubMed] [Google Scholar]
- 24.Downs JC, Yang H, Girkin C, Sakata L, Bellezza AJ, Thompson H, Burgoyne CF. 3-D Histomorphometry of the Normal and Early Glaucomatous Monkey Optic Nerve Head: Neural Canal and Subarachnoid Space Architecture. Invest. Ophthalmol. Vis. Sci. 2007;48:3195–3208. doi: 10.1167/iovs.07-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Downs JC, Bellezza AJ, Thompson H, Burgoyne CF. 3-D Histomorphometry of the Normal and Early Glaucomatous Monkey Optic Nerve Head: Prelaminar Neural Tissues and Cupping. Invest. Ophthalmol. Vis. Sci. 2007;48:5068–5084. doi: 10.1167/iovs.07-0790. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Downs JC, Girkin C, Sakata L, Bellezza A, Thompson H, Burgoyne CF. 3-D Histomorphometry of the Normal and Early Glaucomatous Monkey Optic Nerve Head: Lamina Cribrosa and Peripapillary Scleral Position and Thickness. Invest. Ophthalmol. Vis. Sci. 2007;48:4597–4607. doi: 10.1167/iovs.07-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts MD, Grau V, Grimm J, Reynaud J, Bellezza A, Burgoyne CF, Downs JC. Remodeling of the Connective Tissue Microarchitecture of the Lamina Cribrosa in Early Experimental Glaucoma. Invest. Ophthalmol. Vis. Sci. 2009;50:681–690. doi: 10.1167/iovs.08-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Downs JC, Burgoyne CF. Physiologic intereye differences in monkey optic nerve head architecture and their relation to changes in early experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2009;50:224–234. doi: 10.1167/iovs.08-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson EC, Morrison JC, Farrell S, Deppmeier L, Moore CG, McGinty MR. The effect of chronically elevated intraocular pressure on the rat optic nerve head extracellular matrix. Exp Eye Res. 1996;62:663–674. doi: 10.1006/exer.1996.0077. [DOI] [PubMed] [Google Scholar]
- 30.Johnson EC, Deppmeier LM, Wentzien SK, Hsu I, Morrison JC. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest. Ophthalmol. Vis. Sci. 2000;41:431–442. [PubMed] [Google Scholar]
- 31.Cepurna WO, Kayton RJ, Johnson EC, Morrison JC. Age related optic nerve axonal loss in adult Brown Norway rats. Exp Eye Res. 2005;80:877–884. doi: 10.1016/j.exer.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, Mahesh N, Porciatti V, Whitmore AV, Masland RH, John SW. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24:39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Varela HJ, Hernandez MR. Astrocyte responses in human optic nerve head with primary open-angle glaucoma. J Glaucoma. 1997;6:303–313. [PubMed] [Google Scholar]
- 35.Hernandez MR, Pena JD, Selvidge JA, Salvador-Silva M, Yang P. Hydrostatic pressure stimulates synthesis of elastin in cultured optic nerve head astrocytes. Glia. 2000;32:122–136. doi: 10.1002/1098-1136(200011)32:2<122::aid-glia20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19:297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- 37.Agapova OA, Yang P, Wang WH, Lane DA, Clark AF, Weinstein BI, Hernandez MR. Altered expression of 3 alpha-hydroxysteroid dehydrogenases in human glaucomatous optic nerve head astrocytes. Neurobiol Dis. 2003;14:63–73. doi: 10.1016/s0969-9961(03)00101-3. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez MR, Agapova OA, Yang P, Salvador-Silva M, Ricard CS, Aoi S. Differential gene expression in astrocytes from human normal and glaucomatous optic nerve head analyzed by cDNA microarray. Glia. 2002;38:45–64. doi: 10.1002/glia.10051. [DOI] [PubMed] [Google Scholar]
- 39.Brooks DE, Kallberg ME, Cannon RL, Komaromy AM, Ollivier FJ, Malakhova OE, Dawson WW, Sherwood MB, Kuekuerichkina EE, Lambrou GN. Functional and structural analysis of the visual system in the rhesus monkey model of optic nerve head ischemia. Invest. Ophthalmol. Vis. Sci. 2004;45:1830–1840. doi: 10.1167/iovs.03-0950. [DOI] [PubMed] [Google Scholar]
- 40.Cioffi GA, Sullivan P. The effect of chronic ischemia on the primate optic nerve. Eur J Ophthalmol. 1999;9(Suppl 1):S34–36. doi: 10.1177/112067219900901S12. [DOI] [PubMed] [Google Scholar]
- 41.Cioffi GA, Wang L, Fortune B, Cull G, Dong J, Bui B, Van Buskirk EM. Chronic ischemia induces regional axonal damage in experimental primate optic neuropathy. Arch Ophthalmol. 2004;122:1517–1525. doi: 10.1001/archopht.122.10.1517. [DOI] [PubMed] [Google Scholar]
- 42.Orgul S, Cioffi GA, Wilson DJ, Bacon DR, Van Buskirk EM. An endothelin-1 induced model of optic nerve ischemia in the rabbit. Invest. Ophthalmol. Vis. Sci. 1996;37:1860–1869. [PubMed] [Google Scholar]
- 43.Investigators A. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 44.Kass MA, Heuer DK, Higginbotham EJM, Johnson CA, Keltner JL, Miller JP, Parrish RK, II, Wilson MR, Gordon MOP. The Ocular Hypertension Treatment Study(A Randomized Trial Determines that Topical Ocular Hypotensive Medication Delays or Prevents the Onset of Primary Open-Angle Glaucoma). Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. MD. MD. PhD. MD. MD. MD. [DOI] [PubMed] [Google Scholar]
- 45.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 46.Anderson DR, Drance SM, Schulzer M. Factors that predict the benefit of lowering intraocular pressure in normal tension glaucoma. Am J Ophthalmol. 2003;136:820–829. doi: 10.1016/s0002-9394(03)00478-1. [DOI] [PubMed] [Google Scholar]
- 47.Albon J, Purslow PP, Karwatowski WS, Easty DL. Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol. 2000;84:318–323. doi: 10.1136/bjo.84.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellezza AJ, Rintalan CJ, Thompson HW, Downs JC, Hart RT, Burgoyne CF. Anterior scleral canal geometry in pressurised (IOP 10) and non-pressurised (IOP 0) normal monkey eyes. Br J Ophthalmol. 2003;87:1284–1290. doi: 10.1136/bjo.87.10.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strouthidis NG, Yang H, Fortune B, Downs JC, Burgoyne CF. Detection of optic nerve head neural canal opening within histomorphometric and spectral domain optical coherence tomography data sets. Invest Ophthalmol Vis Sci. 2009;50:214–223. doi: 10.1167/iovs.08-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edwards D, Berry JJ. The efficiency of simulation-based multiple comparisons. Biometrics. 1987;43:913–928. [PubMed] [Google Scholar]
- 51.Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Modeling individual-specific human optic nerve head biomechanics. Part I: IOP-induced deformations and influence of geometry. Biomech Model Mechanobiol. 2008 doi: 10.1007/s10237-008-0120-7. [DOI] [PubMed] [Google Scholar]
- 52.Downs JC, Suh JK, Thomas KA, Bellezza AJ, Burgoyne CF, Hart RT. Viscoelastic characterization of peripapillary sclera: material properties by quadrant in rabbit and monkey eyes. J Biomech Eng. 2003;125:124–131. doi: 10.1115/1.1536930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Reconstruction of human optic nerve heads for finite element modeling. Technol Health Care. 2005;13:313–329. [PubMed] [Google Scholar]
- 54.Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest. Ophthalmol. Vis. Sci. 2005;46:4189–4199. doi: 10.1167/iovs.05-0541. [DOI] [PubMed] [Google Scholar]
- 55.Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Finite element modeling of optic nerve head biomechanics. Invest. Ophthalmol. Vis. Sci. 2004;45:4378–4387. doi: 10.1167/iovs.04-0133. [DOI] [PubMed] [Google Scholar]
- 56.Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Modeling individual-specific human optic nerve head biomechanics. Part II: influence of material properties. Biomech Model Mechanobiol. 2008 doi: 10.1007/s10237-008-0119-0. [DOI] [PubMed] [Google Scholar]
- 57.Girard MJ, Downs JC, Bottlang M, Burgoyne CF, Suh JK. Peripapillary and Posterior Scleral Mechanics-Part II: Experimental and Inverse Finite Element Characterization. J Biomech Eng. 2009;131:051012. doi: 10.1115/1.3113683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srinivasan VJ, Adler DC, Chen Y, Gorczynska I, Huber R, Duker J, Schuman JS, Fujimoto JG. Ultrahigh-speed Optical Coherence Tomography for Three-Dimensional and En Face Imaging of the Retina and Optic Nerve Head. Invest. Ophthalmol. Vis. Sci. 2008;49:5103–5110. doi: 10.1167/iovs.08-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kagemann L, Ishikawa H, Wollstein G, Brennen PM, Townsend KA, Gabriele ML, Schuman JS. Ultrahigh-resolution spectral domain optical coherence tomography imaging of the lamina cribrosa. Ophthalmic Surg Lasers Imaging. 2008;39:S126–131. doi: 10.3928/15428877-20080715-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown DJ, Morishige N, Neekhra A, Minckler DS, Jester JV. Application of second harmonic imaging microscopy to assess structural changes in optic nerve head structure ex vivo. J Biomed Opt. 2007;12:024029. doi: 10.1117/1.2717540. [DOI] [PubMed] [Google Scholar]
- 61.Greene PR, McMahon TA. Scleral creep vs. temperature and pressure in vitro. Exp Eye Res. 1979;29:527–537. doi: 10.1016/0014-4835(79)90153-2. [DOI] [PubMed] [Google Scholar]
- 62.Phillips JR, Khalaj M, McBrien NA. Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest. Ophthalmol. Vis. Sci. 2000;41:2028–2034. [PubMed] [Google Scholar]
- 63.Roberts MD, Hart RT, Liang Y, Bellezza AJ, Burgoyne CF, Downs JC. Continuum-level finite element modeling of the optic nerve head using a fabric tensor based description of the lamina cribrosa.. ASME Summer Bioengineering Conference.; Keystone, CO: American Society of Mechanical Engineers. 2007. [Google Scholar]
- 64.Roberts MD, Liang Y, A.Sigal I, Grimm J, Reynaud J, Bellezza A, Claude FB, Downs JC. Effect of Laminar Material Properties within Continuum Finite Element Models of the Lamina Cribrosa in Bilaterally Normal Monkeys. Invest. Ophthalmol. Vis. Sci. 2009 Submitted. [Google Scholar]
- 65.Burgoyne CF, Quigley HA, Thompson HW, Vitale S, Varma R. Measurement of optic disc compliance by digitized image analysis in the normal monkey eye. Ophthalmology. 1995;102:1790–1799. doi: 10.1016/s0161-6420(95)30792-0. [DOI] [PubMed] [Google Scholar]
- 66.Burgoyne CF, Quigley HA, Thompson HW, Vitale S, Varma R. Early changes in optic disc compliance and surface position in experimental glaucoma. Ophthalmology. 1995;102:1800–1809. doi: 10.1016/s0161-6420(95)30791-9. [DOI] [PubMed] [Google Scholar]
- 67.Morgan WH, Yu DY, Cooper RL, Alder VA, Cringle SJ, Constable IJ. The influence of cerebrospinal fluid pressure on the lamina cribrosa tissue pressure gradient. Invest. Ophthalmol. Vis. Sci. 1995;36:1163–1172. [PubMed] [Google Scholar]
- 68.Downs JC, Blidner RA, Bellezza AJ, Thompson HW, Hart RT, Burgoyne CF. Peripapillary scleral thickness in perfusion-fixed normal monkey eyes. Invest. Ophthalmol. Vis. Sci. 2002;43:2229–2235. [PMC free article] [PubMed] [Google Scholar]
- 69.Jonas JB, Berenshtein E, Holbach L. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest. Ophthalmol. Vis. Sci. 2003;44:5189–5195. doi: 10.1167/iovs.03-0174. [DOI] [PubMed] [Google Scholar]
- 70.Morgan WH, Yu DY, Balaratnasingam C. The role of cerebrospinal fluid pressure in glaucoma pathophysiology: the dark side of the optic disc. J Glaucoma. 2008;17:408–413. doi: 10.1097/IJG.0b013e31815c5f7c. [DOI] [PubMed] [Google Scholar]
- 71.Berdahl JP, Allingham RR, Johnson DH. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology. 2008;115:763–768. doi: 10.1016/j.ophtha.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 72.Hollander H, Makarov F, Stefani FH, Stone J. Evidence of constriction of optic nerve axons at the lamina cribrosa in the normotensive eye in humans and other mammals. Ophthalmic Res. 1995;27:296–309. doi: 10.1159/000267739. [DOI] [PubMed] [Google Scholar]
- 73.Quigley H, Anderson DR. The dynamics and location of axonal transport blockade by acute intraocular pressure elevation in primate optic nerve. Invest. Ophthalmol. Vis. Sci. 1976;15:606–616. [PubMed] [Google Scholar]
- 74.Quigley HA, Anderson DR. Distribution of axonal transport blockade by acute intraocular pressure elevation in the primate optic nerve head. Invest. Ophthalmol. Vis. Sci. 1977;16:640–644. [PubMed] [Google Scholar]
- 75.Downs JC, Roberts MD, Burgoyne CF, Hart RT. Finite Element Modeling of the Lamina Cribrosa Microarchitecture in the Normal and Early Glaucoma Monkey Optic Nerve Head.. ASME Summer Bioengineering Conference.; Keystone, CO: American Society of Mechanical Engineers. 2007. [Google Scholar]