Abstract
Rationale
Several lines of evidence support a role for the endogenous opioid system in mediating behaviors associated with drug dependence. Specifically, recent findings suggest that the kappa-opioid receptor (KOR) may play a role in aspects of nicotine dependence, which contribute to relapse and continued tobacco smoking.
Objective
The objective of this study is to determine the involvement of the KOR in the initial behavioral responses of nicotine, nicotine reward, and nicotine withdrawal using the highly selective KOR antagonist JDTic. JDTic doses of 1, 4, 8, or 16 mg/kg were administered subcutaneously (s.c.) 18 h prior to nicotine treatment.
Results
JDTic dose-dependently blocked acute nicotine-induced antinociception in the tail-flick but not the hot-plate test and did not significantly attenuate morphine’s antinociceptive effect in either the tail-flick or hot-plate test. Furthermore, JDTic (8 and 16 mg/kg, s.c.) failed to block the expression of nicotine reward as measured by the conditioned place preference model. In contrast, JDTic and the KOR antagonist norBNI attenuated the expression of both the physical (somatic signs and hyperalgesia) and affective (anxiety-related behavior and conditioned place aversion) nicotine withdrawal signs.
Conclusions
Our findings clearly show that the KOR is involved in mediating the withdrawal aspects of nicotine dependence. The results from this study suggest that blockade of the KOR by selective KOR antagonists may be useful smoking cessation pharmacotherapies.
Keywords: Nicotinic acetylcholine receptors, Nicotine dependence, Kappa-opioid receptors, JDTic, Nicotine withdrawal
Introduction
Nicotine use and dependence continues to be a worldwide health problem. In 2008, an estimated 70.9 million Americans, aged 12 or older, were current (past month) users of a tobacco product. This represents 28.4% of the population in that age range. In addition, 59.8 million persons (23.9% of the population) were current cigarette smokers, 13.1 million (5.3%) smoked cigars, 8.7 million (3.5%) used smokeless tobacco, and 1.9 million (0.8%) smoked tobacco in pipes (SAMHSA 2009). Nicotine, a natural alkaloid of tobacco, is largely responsible for initiation and maintenance of tobacco dependence. Smokers typically experience considerable withdrawal symptoms during quitting attempts and are highly susceptible to relapse (Jarvis 2004). Withdrawal symptoms include anxiety, anger, difficulty in concentrating, sleep disturbance, and weight gain (Hughes and Hatsukami 1986). Even though many cigarette smokers report a desire to quit smoking, few are successful. Consequently, there is a great need for pharmacotherapies to aid smokers who desire to quit. While nicotinic acetylcholine receptors (nAChRs) are the primary site of action for nicotine, a growing number of studies suggest a role for the endogenous opioid system in mediating different behavioral responses to nicotine. Indeed, mice lacking the opioid peptide precursor preproenkephalin gene show reduced antinociception, reward, and dopamine extra-cellular levels in the nucleus accumbens (NAcc) after nicotine treatment (Berrendero et al. 2005). Similarly, nicotine-induced antinociception, reward, and physical nicotine dependence were reduced in mu-opioid receptor (MOR) knockout mice (Berrendero et al. 2002). The nonselective opioid antagonist naloxone precipitates somatic withdrawal signs in nicotine-dependent rats (Malin et al. 1993), and reports show that acute and chronic nicotine administration alters synthesis and release of endorphins (Jensen et al. 1990; Marty et al. 1985) and enkephalins (Isola et al. 2002; Wewers et al. 1999). Recently, an increase in the functional activity of MOR was observed in the spinal cord of mice tolerant to nicotine-induced antinociception (Galeote et al. 2006).
In contrast to MOR stimulation, which displays reinforcing properties, activation of the dynorphin/kappa-opioid receptor (KOR) system functions as a negative reinforcer, producing aversion, dysphoria, anhedonia, depression, and stress responses in humans and rodents (Bals-Kubik et al. 1993; Carlezon et al. 2006; Mague et al. 2003; Pfeiffer et al. 1986; Shirayama et al. 2004; Todtenkopf et al. 2004; Zimmer et al. 2001). Indeed, various studies suggest a role for the KOR in nicotine dependence behaviors. KOR antagonists have antidepressant-like effects (Beardsley et al. 2005; Mague et al. 2003; Shirayama et al. 2004) and can block the prodepressive-like effects produced by KOR agonists (Todtenkopf et al. 2004). The KOR was also found to modulate the acute and tolerant responses to nicotine-induced spinal antinociception, and KOR density was decreased in the spinal cord of nicotine-tolerant mice (Galeote et al. 2008). Further, KOR receptor ligands block acute nicotine-induced locomotor stimulation in rats (Hahn et al. 2000). While acute nicotine increased both prodynorphin and dynorphin mRNA in the striatum (Isola et al. 2009), dynorphin mRNA was decreased after chronic nicotine and nicotine withdrawal even though the increase in prodynorphin was still present (Isola et al. 2008). Taken together, these studies support a role for the dynorphin/KOR system in nicotine dependence.
The current study extends the available information to further assess the role of the KOR system in nicotine dependence behaviors that contribute to relapse and promote continued tobacco use using the KOR antagonist JDTic. JDTic has unique structural features compared to other commonly used KOR antagonists such as norBNI (Thomas et al. 2003). JDTic was found to be more potent than norBNI as a KOR antagonists in the [35S]GTPγS in vitro efficacy assay (Thomas et al. 2003) and was also more potent than norBNI as an antagonist of U50,488-induced diuresis and the forced-swim test that is characteristic of antidepressants (Beardsley et al. 2005; Carroll et al. 2004). The unique structure of JDTic combined with its potent and selective KOR activity makes it highly useful for further characterization of the role of the KOR in nicotine dependence. In the current study, JDTic was used to determine the role of the KOR in several behavioral aspects of nicotine dependence. Specifically, we measured nicotine-induced antinociception and hypothermia after a single injection of nicotine, nicotine reward using the conditioned place preference (CPP) paradigm, and physical (somatic signs and hyperalgesia) and affective (anxiety-related behavior and conditioned place aversion (CPA)) nicotine withdrawal signs in mice.
Methods
Animals
Male Institute for Cancer Research mice purchased from Jackson Laboratories (Bar Harbor, ME, USA) were housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care-approved animal care facility with food and water available ad libitum. The rooms were on a 12-h light/dark cycle (lights on at 7:00 A.M.). Mice were 8–10 weeks of age and weighed approximately 20–25 g at the start of all the experiments. All experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University and in accordance with the National Institutes of Health Guide for Animal Care and Use.
Drugs
(−)-Nicotine hydrogen tartrate salt [(−)-1-methyl-2-(3-pyridyl)pyrrolidine (+)-bitartrate salt] was purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Morphine sulfate [morphine hemi[sulfate pentahydrate]] was supplied by the National Institute on Drug Abuse (Washington, DC, USA). JDTic [(3R)-7-hydroxy-N-((1S)-1-[[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl]-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide] synthesized as previously described (Thomas et al. 2003) and norBNI (norbinaltorphimine dihydrochloride) were generous gifts from the Research Triangle Institute (Research Triangle Park, NC, USA). The doses of JDTic and norBNI used in our study are within the range of doses used to assess in vivo KOR effects as reported in the literature (Beardsley et al. 2005; Carroll et al. 2005; Galeote et al. 2008; Knoll et al. 2007). All drugs were dissolved in a physiological saline solution (0.9% sodium chloride) and injected subcutaneously (s.c.) at a volume of 10 mL/kg body weight. All doses are expressed as the free base of the drug.
Acute nicotine assessment
Naïve mice were injected s.c. with JDTic (1, 4, 8, or 16 mg/kg) 18 h prior to nicotine (2.5 mg/kg, s.c.). Due to JDTic’s very long duration of action (Carroll et al. 2004), an 18-h preinjection was chosen for the studies. Antinociception using the tail-flick and hot-plate tests was measured 5 min after nicotine injection or 20 min after morphine (8 mg/kg, s.c.), and changes in body temperature were measured 30 min after injection. To confirm an absence of mu antagonist effects by JDTic in these studies, JDTic (16 mg/kg) was also administered 1, 6, 18, and 24 h before morphine (8 mg/kg, s.c.) in the tail-flick test, and antinociception was measured 20 min after morphine.
Tail-flick test
Spinal antinociception was assessed by the tail-flick method of D’Amour and Smith (1941). Mice were lightly restrained, while a radiant heat source was directed onto the upper portion of the tail. A control response (2–4 s) was determined for each mouse 10 min before treatment, and test latency was determined after drug administration. The apparatus has an automatic cutoff of 10 s to minimize tissue damage. Antinociceptive response for the tail-flick test was expressed as the mean and (±)SEM of the maximum latency after drug treatment.
Hot-plate test
Supraspinal antinociception was assessed using the hot-plate test. Mice were placed into a 10-cm wide glass cylinder on a hot plate (Thermojust Apparatus, Columbus, OH, USA) as a measure of supraspinal antinociception. The hot plate is a rectangular heated surface surrounded by plexiglass and maintained at 55°C. The device is connected to a manually operated timer that records the amount of time the mouse spends on the heated surface before showing signs of nociception (e.g., jumping and paw licks). A control response (8–12 s) was determined for each mouse 10 min before treatment, and test latency was determined after drug administration. The timer has an automatic cut-off of 40 s to avoid tissue damage. Antinociceptive response for the hot-plate test was expressed as the mean and (±)SEM of the maximum latency after drug treatment.
Body temperature
Rectal temperature was measured by a thermistor probe (inserted 24 mm) and digital thermometer (YSI Inc., Yellow Springs, OH, USA). Readings were taken just before and at 30 min after nicotine injection. The difference in rectal temperature before and after treatment was calculated for each mouse. The ambient temperature of the laboratory varied from 21°C to 24°C from day to day.
Nicotine CPP assessment
An unbiased CPP paradigm was utilized in this study as described in Kota et al. (2007). Briefly, place-conditioning chambers consisted of two distinct compartments separated by a smaller intermediate compartment with openings that allowed access to either side of the chamber. On day1, animals were confined to the intermediate compartment for a 5-min habituation period, then allowed to move freely between compartments for 15 min. Time spent in each compartment was recorded. These data were used to separate the animals into groups of approximately equal bias. Mice that showed a clear preference for one side were removed from the study. Two mice were removed from the study based on this criteria. Days2–4 were the conditioning days during which the saline group received saline in both compartments, and drug groups received nicotine (0.5 mg/kg, s.c.) in one compartment and saline in the opposite compartment. Drug-paired compartments were randomized among all groups. On the evening of day4, after the evening conditioning session, mice received an injection of JDTic (8 or 16 mg/kg, s.c.) 18 h prior to test day. Day5 was the drug-free test day, and the procedure was the same as day1. Activity counts and time spent on each side were recorded via photosensors using Med Associates interface and software. Data were expressed as time spent on drug-paired side minus time spent on saline-paired side. A positive number indicated a preference for the drug-paired side, whereas a negative number indicated an aversion to the drug-paired side. A number at or near zero indicated no preference for either side.
Chronic nicotine administration protocol
Mice were anesthetized with sodium pentobarbital (45 mg/kg, i.p.) and implanted with Alzet osmotic mini pumps [model 1007D (7 days) Durect Corporation, Cupertino, CA, USA] filled with (−)-nicotine or saline solution as described in Jackson et al. (2008). The concentration of nicotine was adjusted according to animal weight and mini pump flow rate. For withdrawal studies, mice received 36 mg/kg/day for 7 days. For CPA studies, animals were implanted with 28-day mini pumps containing 36 mg/kg/day nicotine.
Nicotine withdrawal assessment
Withdrawal studies were conducted as previously described in Jackson et al. (2008). In brief, mini pumps were removed on the evening of day7 under ether anesthesia. Mice were allowed to recover for 1 h then were injected with vehicle, JDTic (8 mg/kg, s.c.), or norBNI (10 mg/kg, s.c.). Testing was initiated on day8, approximately 18–24 h after mini pump removal and 18 h after JDTic or norBNI treatment. The mice were first evaluated for 5 min in the plus maze test for anxiety-related behavior, followed by a 20-min observation of somatic signs measured as paw and body tremors, head shakes, backing, jumps, curls, and ptosis. Hyperalgesia was evaluated immediately following the somatic sign observation period. The specific testing sequence was chosen based on our prior studies showing that this order of testing reduced within-group variability and produced the most consistent results.
Nicotine CPA
The CPA protocol was conducted over the course of 4 days in a biased fashion as described in Jackson et al. (2009). In brief, mice were infused with saline or nicotine for 14 days prior to initiation of CPA testing to induce tolerance. Infusion continued throughout the duration of testing. Day1 of CPA testing was the pre-preference day where mice were placed in the gray center compartment for a 5-min habituation period, followed by a 15-min test period to determine baseline responses. The pre-preference score was used to pair each mouse with mecamylamine (3.5 mg/kg) to its initially preferred compartment. On days2 and 3 of CPA testing, all mice received injections of saline in the morning and were immediately confined to their non-preferred compartment for 30 min. No less than 4 h later, mice received an injection of mecamylamine and were immediately confined to their preferred compartment for 30 min. On the evening of day3, after the last conditioning session, mice received an injection of JDTic (16 mg/kg, s.c.) or norBNI (10 mg/kg, s.c.) 18 h prior to test day. Day4 was the drug-free test day, and the procedure was the same as day1. Activity counts and time spent on each side were recorded via photosensors using Med Associates interface and software. A post-preference score was determined for each mouse. Aversion was counted as mice spending less time in their initially preferred compartment on test day when compared to time spent in the same compartment prior to drug conditioning.
Statistical analysis
Statistical analyses of behavioral studies were performed using one-way analysis of variance test with treatment as the between subject factor. p values <0.05 were considered to be statistically significant. Significant results were further analyzed using the Neuman–Keuls post hoc test.
Results
Effect of JDTic on nicotine-induced hypothermia and antinociception
Mice were injected with nicotine (2.5 mg/kg, s.c.) after pretreatment with JDTic or its vehicle and tested later for changes in body temperature and thermal nociception. Antinociception was measured 5 min after nicotine injection using the tail-flick and hot-plate tests, and body temperature was assessed 30 min after nicotine injection. Figure 1a–c shows that there were significant effects of treatment on response latencies in the tail-flick test [F(6,35)= 8.235, p<0.0001] and hot-plate test [F(4,25) =26.19, p< 0.0001] and on body temperature [F(4,25) =211.01, p< 0.0001]. Post hoc testing indicated that nicotine alone produced a significant antinociceptive effect in the tail-flick and hot-plate tests and a significant reduction in body temperature. JDTic dose-dependently blocked the antinociceptive response of nicotine in the tail-flick test (Fig. 1a) but had no effect in the hot-plate assay or body temperature assessments at any dose tested (Fig. 1b, c). The highest dose of JDTic (16 mg/kg, s.c.) did not produce any significant response in saline mice in any of the behaviors measured.
Fig. 1.
Effects of JDTic on nicotine-induced hypothermia and antinociception. A single injection of nicotine (2.5 mg/kg, s.c.) induced a significant antinociception in the (a) tail-flick and (b) hot-plate tests, and (c) a decrease in body temperature in mice. a Nicotine-induced spinal antinociception measured using the tail-flick test was dose-dependently blocked by 18-h pretreatment with JDTic. JDTic had no significant effect in the hot-plate or body-temperature tests, and the highest dose used did not produce significant behavioral effects in saline treated mice. Each point represents the mean±SEM of six mice per group. Asterisk denotes p<0.05 vs. the saline and JDTic control groups and vs. nicotine-JDTic 16 mg/kg group for the tail-flick test (a)
To ensure that the JDTic doses and time course used in our experiments did not block MOR, mice were pretreated with the highest dose of JDTic used (16 mg/kg, s.c.) at 1, 6, 18, and 24 h prior to morphine (8 mg/kg, s.c.) treatment in the tail-flick test. Results are shown in Table 1. Treatment with JDTic had no significant effect on morphine-induced antinociception at any time tested [F(4,29) =0.13, p<0.96], suggesting that the observed effects are specific to the KOR at the doses and pretreatment times used.
Table 1.
JDTic does not block the acute antinociceptive effects of morphine in mice
| Treatment | Δ Tail-flick latency (mean±SEM) |
|---|---|
| Saline (1 h)-morphine | 8.9±0.7 |
| JDTic (1 h)-morphine | 8.9±0.1 |
| JDTic (6 h)-morphine | 8.8±0.8 |
| JDTic (19 h)-morphine | 8.8±0.8 |
| JDTic (24 h)-morphine | 8.0±1.3 |
Pretreatment with JDTic (16 mg/kg, s.c.) at 1, 6, 18, and 24 h prior to morphine (8 mg/kg, s.c.) has no significant effect of antinociception, as measured by the tail-flick test, suggesting an effect specific to kappa-opioid receptors. Data are expressed as the mean and (±)SEM of the maximum latency after drug treatment. Each group contained six mice. The vehicle control baseline=2.7±0.1 s [F(4,29)=0.13, p<0.96]
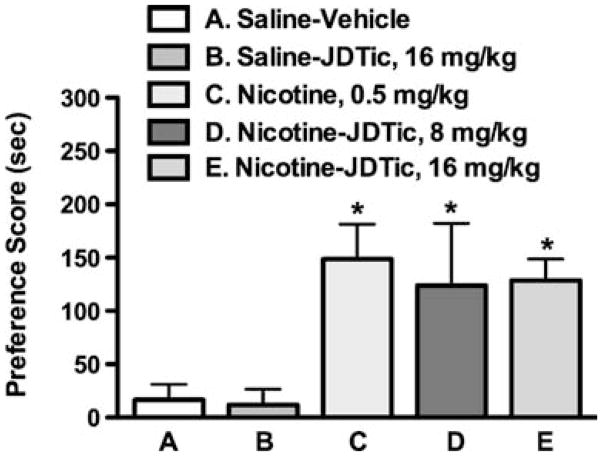
Effect of JDTic on the rewarding effects of nicotine
A place-conditioning procedure was used to assess the effects of JDTic on expression of a CPP associated with nicotine. Mice were initially exposed to conditioning sessions with saline or nicotine, and then treated with JDTic prior to testing. Figure 2 shows that there was a significant effect of treatment on expression of CPP [F(4,29) =8.333, p <0.0001]. Post hoc tests indicated that as previously reported by our laboratory (Walters et al. 2006), mice conditioned with nicotine alone (0.5 mg/kg, s.c.) displayed a robust and significant CPP. Pretreatment with JDTic (8 or 16 mg/kg, s.c.) did not significantly alter the expression of nicotine CPP conditioned with 0.5 mg/kg nicotine. JDTic did not produce a significant response in mice conditioned with saline.
Fig. 2.
Effects of JDTic on the expression of nicotine reward in mice. Nicotine (0.5 mg/kg, s.c.) induced a significant conditioned place preference (CPP) in mice. Eighteen-hour pretreatment with JDTic (8 or 16 mg/kg) had no effect on expression of nicotine CPP in mice conditioned with 0.5 mg/kg nicotine. Each point represents the mean± SEM of eight mice per group. Asterisk denotes p<0.05 vs. the saline and JDTic control groups
Physical and affective nicotine withdrawal signs are attenuated by KOR antagonists
Anxiety-related behavior (affective), somatic signs, and hyperalgesia (physical) were measured in mice following 18–24 h withdrawal from chronic nicotine and treatment with either JDTic or norBNI or their vehicles. Figure 3a–c (JDTic-treated groups) and Fig. 4a–c (norBNI-treated groups) show that there were significant effects of treatment on anxiety-related responses in the plus maze ([F(3,24)= 3.198, p<0.05] for JDTic; [F(3,28)=8.215, p<0.005] for norBNI), expression of somatic signs ([F(3,24)=74.006, p<0.0001] for JDTic; [F(3,28)=37.097, p<0.0001] for norBNI) and response latencies in the hot-plate test ([F(3,24)=7.817, p< 0.005] for JDTic; [F(3,28)=4.535, p<0.005] for norBNI) (Figs. 3 and 4). Post hoc testing indicated that nicotine withdrawal alone significantly increased anxiety-related behavior in the plus maze, increased expression of somatic withdrawal signs, and decreased response latencies in the hot-plate test. Eighteen-hour pretreatment with JDTic (8 mg/kg, s.c.) or norBNI (10 mg/kg, s.c.) significantly blocked all of these nicotine withdrawal signs. Kappa antagonist-treated mice exhibited a loss of anxiety-related behavior, attenuation of somatic signs, and an increased latency on the hot plate. To ensure that the results in the plus maze were not due to changes in locomotor activity, the average total number of arm crosses was analyzed for each group. There were no significant between group differences in this measure (Tables 2 and 3). The doses of JDTic and norBNI used in this assessment did not significantly affect behavioral responses in saline-infused mice in any withdrawal test and did not precipitate significant nicotine withdrawal signs in nicotine-dependent mice at 1, 8, or 18 h after administration (data not shown).
Fig. 3.
Physical and somatic nicotine withdrawal are blocked by pretreatment with JDTic. Mice chronically infused with nicotine for 7 days (36 mg/kg/day) were withdrawn from nicotine for 18–24 h. A significant (a) anxiety-related response, (b) increase in somatic withdrawal signs, and (c) hyperalgesia response were observed in nicotine-withdrawn mice. Eighteen-hour pretreatment with JDTic (8 mg/kg, s.c.) significantly attenuated expression of both the physical and affective nicotine withdrawal responses in mice. Each point represents the mean±SEM of six to eight mice per group. Asterisk denotes p<0.05 vs. the saline and JDTic control groups, and vs. nicotine-JDTic group. MP mini pump
Fig. 4.
Physical and somatic nicotine withdrawal are blocked by pretreatment with norBNI. Mice were spontaneously withdrawn from nicotine (18–24 h) and treated with norBNI 18 h prior to testing. Results show that expression of (a) the anxiety-related response, (b) the increase in somatic signs, and (c) the hyperalgesia response were blocked by pretreatment with norBNI. Each point represents the mean±SEM of six to eight mice per group. Asterisk denotes p<0.05 vs. the saline and norBNI control groups, and vs. nicotine-norBNI group
Table 2.
JDTic does not significantly alter the average number of arm crosses in the plus maze assessment
| Treatment | Average number of arm crosses±SEM |
|---|---|
| Saline MP-vehicle | 5.7±1.4 |
| Saline MP-JDTic | 5.0±0.9 |
| Nicotine MP-vehicle | 4.0±0.8 |
| Nicotine MP-JDTic | 4.3±0.8 |
Nicotine-withdrawn mice were treated with vehicle or JDTic (8 mg/kg, s.c.), and the total number of crosses between the open and closed arms of the plus maze was counted. Numbers are presented as the total average number of arm crosses ±SEM for six to eight mice per group MP mini pump
Table 3.
norBNI does not significantly alter the average number of arm crosses in the plus maze assessment
| Treatment group | Average number of arm crosses±SEM |
|---|---|
| Saline MP-vehicle | 4.5±1.2 |
| Saline MP-norBNI | 5.0±1.9 |
| Nicotine MP-vehicle | 3.6±0.9 |
| Nicotine MP-norBNI | 3.9±0.5 |
Nicotine-withdrawn mice were treated with vehicle or norBNI (10 mg/kg, s.c.), and the total number of crosses between the open and closed arms of the plus maze was counted. Numbers are presented as the total average number of arm crosses±SEM for six to eight mice per group MP mini pump
Expression of nicotine withdrawal aversion is blocked by pretreatment with KOR antagonists
A place-conditioning procedure was used to measure effects of kappa antagonists on expression of a CPA associated with nicotine withdrawal. Mice receiving chronic infusions of nicotine or saline via a minipump were exposed to conditioning sessions with mecamylamine or its vehicle, and JDTic or norBNI was administered 18 h prior to testing. Figure 5 shows that there was a significant effect of treatment on CPA [F(5,56)=3.779; p<0.05]. Post hoc testing indicated that mecamylamine treatment alone (3.5 mg/kg, s.c.) resulted in a significant CPA in chronic nicotine-exposed mice pretreated with vehicle. Pretreatment with JDTic (16 mg/kg, s.c.) or norBNI (10 mg/kg, s.c.) 18 h prior to the test day blocked expression of the mecamylamine-induced CPA. The doses of JDTic and norBNI used did not produce significant responses in saline-infused mice.
Fig. 5.

Expression of nicotine withdrawal aversion is blocked by kappa-opioid receptor antagonists. Mecamylamine (3.5 mg/kg, s.c.) precipitated a significant conditioned place aversion in chronic nicotine-infused mice. Expression of aversion was blocked by an 18-h pretreatment with the kappa-opioid antagonists, JDTic (16 mg/kg, s.c.), and norBNI (10 mg/kg, s.c.). Each point represents the mean±SEM of ten to 11 mice per group. Asterisk denotes p<0.05 vs. saline groups and nicotine-JDTic and nicotine-norBNI groups; sal saline, nic nicotine, mec mecamylamine
Discussion
Dynorphin is an opioid peptide derived from the prodynorphin precursor and is the endogenous ligand for the KOR (Chavkin et al. 1982). Activation of the dynorphin/KOR system produces aversive dysphoric-like effects in animals and humans (Land et al. 2008; Pfeiffer et al. 1986; Shippenberg et al. 2007). The activation of the dynorphin system in the NAcc stimulates a cascade of events leading to cAMP response-element binding protein phosphorylation and subsequent alteration in gene expression. This activation contributes to the dysphoria associated with cocaine and other drug dependence and also mediates the dysphoric component of stress (Land et al. 2008; McLaughlin and Chavkin 2003). Blockade of the dynorphin activity using the KOR antagonist norBNI or prodynorphin gene disruption blocked stress-induced reinstatement of cocaine-induced CPP in mice (McLaughlin and Chavkin 2003) and blocked stress-induced reinstatement of cocaine-seeking behavior in rats (Beardsley et al. 2005).
The current study suggests the involvement of the KOR in mediating some behavioral responses to nicotine. Pretreatment with the KOR antagonist JDTic dose-dependently reduced the acute nicotine-induced antinociceptive response in the tail-flick test, attenuated both physical and affective nicotine withdrawal signs in mice, and blocked the expression of nicotine withdrawal aversion but failed to alter nicotine’s effects in the CPP test.
JDTic dose-dependently blocked the antinociceptive response induced by a single injection of nicotine in the tail-flick test, but had no effect on nicotine’s antinociceptive action in the hot-plate test or nicotine-induced hypothermia. The effects of JDTic were specific to KOR since the antagonist failed to block morphine-induced analgesia using the same experimental conditions. Furthermore, lack of significant effects of JDTic on nicotine-induced hypothermia suggests that KORs are not involved in mediating this acute nicotine response. JDTic was reported to possess a very high selectivity ratio of KOR/delta receptors in both binding and functional blockade (over 1,000; Thomas et al. 2003). These results suggest that JDTic’s blockade of nicotine’s effects in the tail-flick test selectively implicates KOR. The blockade by JDTic is also consistent with previous studies showing that norBNI effectively attenuated acute nicotine-induced antinociception in the tail-immersion response and (Galeote et al. 2008) the tail-flick test (Campbell et al. 2007). While it is difficult to clearly delineate the site of mechanisms involved in the tail-flick and hot-plate tests, our data suggest a spinal mechanism for the effects of JDTic on nicotine antinociception. Indeed, previous reports showed that the spinal cord is an important site of action for nicotine in the tail-flick test (Christensen and Smith 1990; Damaj 2000; Damaj et al. 1998). Additionally, both nAChRs and KORs are distributed in the dorsal horn of the spinal cord (Galeote et al. 2008; Mansour et al. 1995). Overall, these results suggest that the KOR system may contribute to the spinal nicotinic antinociceptive effects as measured in the tail-flick test.
The role of the KOR was also examined in nicotine reward using the CPP paradigm. JDTic had no effect on the expression of nicotine reward in mice conditioned with 0.5 mg/kg. The results are consistent with the report that prodynorphin knockout mice did not differ from wild-type mice in a CPP paradigm using the same conditioning dose of nicotine (Galeote et al. 2009).
Evaluation of the role of the KOR in nicotine withdrawal revealed that physical (somatic signs and hyperalgesia) and affective (anxiety-related behavior and CPA) nicotine withdrawal signs were attenuated by pretreatment with the selective KOR antagonist JDTic as well as by pretreatment with the KOR antagonist norBNI. In agreement with our in vivo data, in vitro studies show altered levels of dynorphin and prodynorphin mRNA in the striatum following nicotine withdrawal, suggesting that heightened dynorphinergic tone is involved, in part, in the expression of negative affective states experienced during nicotine withdrawal (Isola et al. 2008). It is interesting that JDTic blocked the expression of all of the somatic and affective signs of withdrawal that we measured since these signs implicate various neuronal pathways and nAChRs subtypes (Jackson et al. 2008). Our data does not certainly argue for a common pathway or mechanism for nicotine withdrawal signs, but recent reports with bupropion point to the central role of dopaminergic mechanisms. Indeed, bupropion, a dopamine uptake inhibitor used as a smoking cessation agent, blocked the expression and development of somatic and affective signs of nicotine withdrawal (Paterson 2009). Previous studies have shown that extracellular levels of dopamine in the NAcc are reduced in adult rats experiencing withdrawal (Markou 2008). Since the dynorphin/KOR system maintains a tonic activity to inhibit dopamine transmission in these brain regions (Shippenberg et al. 2007), it is possible that the withdrawal signs and dysphoric state associated with nicotine withdrawal are regulated by a dynorphin/KOR system through dopaminergic mechanisms. Interestingly, JDTic was recently reported to decrease the number of somatic withdrawal signs in morphine-dependent rats (Carroll et al. 2005). Overall, our results further support the involvement of the dynorphin/KOR system in mediating the expression of nicotine withdrawal signs.
In summary, this study suggests a role for the KOR system in the negative aspects of nicotine dependence, which contributes to relapse and continued tobacco use. Using the highly selective KOR antagonist JDTic, our results clearly support a role for the dynorphin/KOR system in acute nicotine-induced behaviors and physical and affective nicotine withdrawal. Collectively, these results suggest that selective KOR antagonists may be beneficial as smoking cessation pharmacotherapies.
Acknowledgments
The authors wish to thank Cindy Evans and Tie Han for their technical contributions to this study. This work was supported by National Institute on Drug Abuse grants DA12610, DA005274, NS070715, and DA009045.
References
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Kieffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. J Neurosci. 2002;22:10935–10940. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Mendizabal V, Robledo P, Galeote L, Bilkei-Gorzo A, Zimmer A, Maldonado R. Nicotine-induced antinociception, rewarding effects, and physical dependence are decreased in mice lacking the preproenkephalin gene. J Neurosci. 2005;25:1103–1112. doi: 10.1523/JNEUROSCI.3008-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell VC, Taylor RE, Tizabi Y. Effects of selective opioid receptor antagonists on alcohol-induced and nicotine-induced antinociception. Alcohol Clin Exp Res. 2007;31:1435–1440. doi: 10.1111/j.1530-0277.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, Pollard GT, Aceto MD, Harris LS. Pharmacological properties of JDTic: A novel κ-opioid receptor antagonist. Eur J Pharmacol. 2004;501:111–119. doi: 10.1016/j.ejphar.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Harris LS, Aceto MD. Effects of JDTic, a selective kappa-opioid receptor antagonist, on the development and expression of physical dependence on morphine using a rat continuous-infusion model. Eur J Pharmacol. 2005;524:89–94. doi: 10.1016/j.ejphar.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Christensen MK, Smith DF. Antinociceptive effects of the stereoisomers of nicotine given intrathecally in spinal rats. J Neural Transm Gen Sect. 1990;80:189–194. doi: 10.1007/BF01245120. [DOI] [PubMed] [Google Scholar]
- Damaj MI. The involvement of spinal Ca(2+)/calmodulin-protein kinase II in nicotine-induced antinociception in mice. Eur J Pharmacol. 2000;404:103–110. doi: 10.1016/s0014-2999(00)00579-3. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Fei-Yin M, Dukat M, Glassco W, Glennon RA, Martin BR. Antinociceptive responses to nicotinic acetylcholine receptor ligands after systemic and intrathecal administration in mice. J Pharmacol Exp Ther. 1998;284:1058–1065. [PubMed] [Google Scholar]
- D’Amour FE, Smith DL. 74A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Galeote L, Kieffer BL, Maldonado R, Berrendero F. Mu-opioid receptors are involved in the tolerance to nicotine antinociception. J Neurochem. 2006;97:416–423. doi: 10.1111/j.1471-4159.2006.03751.x. [DOI] [PubMed] [Google Scholar]
- Galeote L, Maldonado R, Berrendero F. Involvement of kappa/dynorphin system in the development of tolerance to nicotine-induced antinociception. J Neurochem. 2008;105:1358–1368. doi: 10.1111/j.1471-4159.2008.05247.x. [DOI] [PubMed] [Google Scholar]
- Galeote L, Berrendero F, Bura SA, Zimmer A, Maldonado R. Prodynorphin gene disruption increases the sensitivity to nicotine self-administration in mice. Int J Neuropsychopharmacol. 2009;12:615–625. doi: 10.1017/S1461145708009450. [DOI] [PubMed] [Google Scholar]
- Hahn B, Stolerman IP, Shoaib M. Kappa-opioid receptor modulation of nicotine-induced behaviour. Neuropharmacology. 2000;39:2848–2855. doi: 10.1016/s0028-3908(00)00119-2. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Isola R, Zhang H, Duchemin AM, Tejwani GA, Neff NH, Hadjiconstantinou M. Met-enkephalin and preproenkephalin mRNA changes in the striatum of the nicotine abstinence mouse. Neurosci Lett. 2002;325:67–71. doi: 10.1016/s0304-3940(02)00240-9. [DOI] [PubMed] [Google Scholar]
- Isola R, Zhang H, Tejwani GA, Neff NH, Hadjiconstantinou M. Dynorphin and prodynorphin mRNA changes in the striatum during nicotine withdrawal. Synapse. 2008;62:448–455. doi: 10.1002/syn.20515. [DOI] [PubMed] [Google Scholar]
- Isola R, Zhang H, Tejwani GA, Neff NH, Hadjiconstantinou M. Acute nicotine changes dynorphin and prodynorphin mRNA in the striatum. Psychopharmacology (Berl) 2009;201:507–516. doi: 10.1007/s00213-008-1315-4. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Kota DH, Martin BR, Damaj MI. The role of various nicotinic receptor subunits and factors influencing nicotine conditioned place aversion. Neuropharmacology. 2009;56:970–974. doi: 10.1016/j.neuropharm.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MJ. Why people smoke. Brit J Med. 2004;328:277–279. doi: 10.1136/bmj.328.7434.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RA, Gilbert DG, Meliska CJ, Landrum TA, Szary AB. Characterization of a dose-response curve for nicotine-induced conditioned taste aversion in rats: relationship to elevation of plasma beta-endorphin concentration. Behav Neural Biol. 1990;53:428–440. doi: 10.1016/0163-1047(90)90310-3. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of κ-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Carter VA, Cunningham JS, Wilson OB. Naloxone precipitates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl) 1993;112:339–342. doi: 10.1007/BF02244930. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Markou A. Review. Neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty MA, Erwin VG, Cornell K, Zgombick JM. Effects of nicotine on beta-endorphin, alpha MSH, and ACTH secretion by isolated perfused mouse brains and pituitary glands, in vitro. Pharmacol Biochem Behav. 1985;22:317–325. doi: 10.1016/0091-3057(85)90397-1. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Chavkin C. Stress-induced activation of endogenous kappa opioid systems potentiates cocaine response International Narcotics Research Conference; Perpignan, France; 2003. Abstract 119. [Google Scholar]
- Paterson NE. Behavioural and pharmacological mechanisms of bupropion’s anti-smoking effects: recent preclinical and clinical insights. Eur J Pharmacol. 2009;603:1–11. doi: 10.1016/j.ejphar.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- SAMHSA (Substance Abuse and Mental Health Services Administration) Results from the 2008 National Survey on Drug Use and Health. Department of Health and Human Services; Washington, DC: 2009. [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Atkinson RN, Vinson NA, Catanzaro JL, Perretta CL, Fix SE, Mascarella SW, Rothman RB, Xu H, Dersch CM, Cantrell BE, Zimmerman DM, Carroll FI. Identification of (3R)-7-hydroxy-N-((1S)-1-[[(3R, 4R)-4-(3-hydroxyphenyl)-3, 4-dimethyl-1-piperidinyl]methyl]-2-methylpropyl)-1, 2, 3, 4-tetrahydro-3-isoquinolinecarboxamide as a novel potent and selective opioid kappa receptor antagonist. J Med Chem. 2003;46:3127–3137. doi: 10.1021/jm030094y. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Wewers ME, Dhatt RK, Snively TA, Tejwani GA. The effect of chronic administration of nicotine on antinociception, opioid receptor binding and met-enkelphalin levels in rats. Brain Res. 1999;822:107–113. doi: 10.1016/s0006-8993(99)01095-1. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Valjent E, Konig M, Zimmer AM, Robledo P, Hahn H, Valverde O, Maldonado R. Absence of Δ-9-tetrahydrocannabinol dysphoric effects in dynorphin-deficient mice. J Neurosci. 2001;21:9499–9505. doi: 10.1523/JNEUROSCI.21-23-09499.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]