Abstract
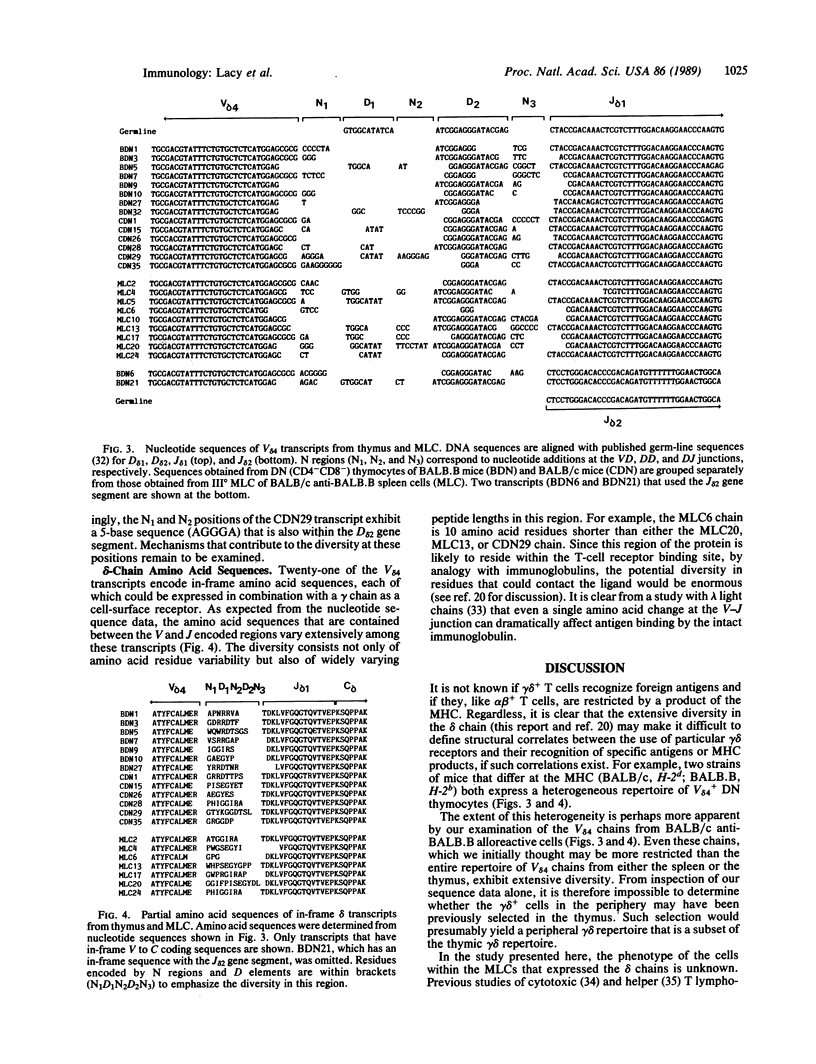
A small percentage (approximately 5%) of the cells in the adult thymus expresses a heterodimeric receptor, gamma delta, that exhibits extensive clonal diversity. The specificity and function of these cells are unclear. Furthermore, it is not known if their role in the immune system is primarily one that operates within the thymus during the selection of the T-cell repertoire or if they function primarily in an antigen-recognition capacity in the peripheral lymphoid system. To examine if gamma delta+ T cells in the periphery are as diverse as those in the thymus, we used the polymerase chain reaction to amplify delta-chain transcripts from polyclonal populations of thymic and splenic lymphocytes (the latter were derived from allogeneic mixed lymphocyte cultures). The nucleotide sequences of delta chains from the spleen, like those from the thymus, were all different. Most of the diversity was present in the region between the variable (V) and joining (J) gene segments and was generated through the use of the two known diversity (D) elements, D delta 1 and D delta 2, and by the addition or deletion of bases at the V delta D delta 1, D delta 1D delta 2, and D delta 2J delta junctions. The extensive gamma delta repertoire among peripheral cells suggests that they have the potential to recognize an array of ligands that could be as diverse as those recognized by alpha beta+ cells. The amplification strategy described here can be used to analyze rapidly the diversity exhibited by any of the members of the immunoglobulin-like gene families that undergo rearrangement.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. P., McIntyre B. W., Bloch D. Tumor-specific antigen of murine T-lymphoma defined with monoclonal antibody. J Immunol. 1982 Nov;129(5):2293–2300. [PubMed] [Google Scholar]
- Asarnow D. M., Kuziel W. A., Bonyhadi M., Tigelaar R. E., Tucker P. W., Allison J. P. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988 Dec 2;55(5):837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Azuma T., Igras V., Reilly E. B., Eisen H. N. Diversity at the variable-joining region boundary of lambda light chains has a pronounced effect on immunoglobulin ligand-binding activity. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6139–6143. doi: 10.1073/pnas.81.19.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank I., DePinho R. A., Brenner M. B., Cassimeris J., Alt F. W., Chess L. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature. 1986 Jul 10;322(6075):179–181. doi: 10.1038/322179a0. [DOI] [PubMed] [Google Scholar]
- Bluestone J. A., Pardoll D., Sharrow S. O., Fowlkes B. J. Characterization of murine thymocytes with CD3-associated T-cell receptor structures. Nature. 1987 Mar 5;326(6108):82–84. doi: 10.1038/326082a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Dialynas D. P., Strominger J. L., Smith J. A., Owen F. L., Seidman J. G., Ip S., Rosen F., Krangel M. S. Identification of a putative second T-cell receptor. Nature. 1986 Jul 10;322(6075):145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Riberdy J., Ang S. L., Seidman J. G., Devlin P., Krangel M. S. Two forms of the T-cell receptor gamma protein found on peripheral blood cytotoxic T lymphocytes. Nature. 1987 Feb 19;325(6106):689–694. doi: 10.1038/325689a0. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Iwashima M., Kaplan K. B., Elliott J. F., Davis M. M. A new T-cell receptor gene located within the alpha locus and expressed early in T-cell differentiation. 1987 Jun 25-Jul 1Nature. 327(6124):677–682. doi: 10.1038/327677a0. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Iwashima M., Wettstein D. A., Kaplan K. B., Elliott J. F., Born W., Davis M. M. T-cell receptor delta gene rearrangements in early thymocytes. Nature. 1987 Dec 24;330(6150):722–727. doi: 10.1038/330722a0. [DOI] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Dembić Z., Haas W., Weiss S., McCubrey J., Kiefer H., von Boehmer H., Steinmetz M. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986 Mar 20;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Elliott J. F., Rock E. P., Patten P. A., Davis M. M., Chien Y. H. The adult T-cell receptor delta-chain is diverse and distinct from that of fetal thymocytes. Nature. 1988 Feb 18;331(6157):627–631. doi: 10.1038/331627a0. [DOI] [PubMed] [Google Scholar]
- Garman R. D., Doherty P. J., Raulet D. H. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell. 1986 Jun 6;45(5):733–742. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- Haskins K., Kubo R., White J., Pigeon M., Kappler J., Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med. 1983 Apr 1;157(4):1149–1169. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S., Satyanarayana K., Devlin P., Band H., McLean J., Strominger J. L., Brenner M. B., Krangel M. S. Extensive junctional diversity of rearranged human T cell receptor delta genes. Science. 1988 Jun 10;240(4858):1541–1544. doi: 10.1126/science.3259726. [DOI] [PubMed] [Google Scholar]
- Hayday A. C., Saito H., Gillies S. D., Kranz D. M., Tanigawa G., Eisen H. N., Tonegawa S. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985 Feb;40(2):259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Heilig J. S., Glimcher L. H., Kranz D. M., Clayton L. K., Greenstein J. L., Saito H., Maxam A. M., Burakoff S. J., Eisen H. N., Tonegawa S. Expression of the T-cell-specific gamma gene is unnecessary in T cells recognizing class II MHC determinants. Nature. 1985 Sep 5;317(6032):68–70. doi: 10.1038/317068a0. [DOI] [PubMed] [Google Scholar]
- Heilig J. S., Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. 1986 Aug 28-Sep 3Nature. 322(6082):836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- Heilig J. S., Tonegawa S. T-cell gamma gene is allelically but not isotypically excluded and is not required in known functional T-cell subsets. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8070–8074. doi: 10.1073/pnas.84.22.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B., Mjolsness S., Janeway C., Jr, Hayday A. C. Transcripts of functionally rearranged gamma genes in primary T cells of adult immunocompetent mice. Nature. 1986 Oct 16;323(6089):635–638. doi: 10.1038/323635a0. [DOI] [PubMed] [Google Scholar]
- Kranz D. M., Saito H., Heller M., Takagaki Y., Haas W., Eisen H. N., Tonegawa S. Limited diversity of the rearranged T-cell gamma gene. 1985 Feb 28-Mar 6Nature. 313(6005):752–755. doi: 10.1038/313752a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Federspiel N. A., Ruitenberg J. J., Phillips J. H., Allison J. P., Littman D., Weiss A. The T cell antigen receptor complex expressed on normal peripheral blood CD4-, CD8- T lymphocytes. A CD3-associated disulfide-linked gamma chain heterodimer. J Exp Med. 1987 Apr 1;165(4):1076–1094. doi: 10.1084/jem.165.4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis L. A., Cron R., Bluestone J. A. Major histocompatibility complex-linked specificity of gamma delta receptor-bearing T lymphocytes. Nature. 1987 Nov 19;330(6145):262–264. doi: 10.1038/330262a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. M., Fowlkes B. J., Bluestone J. A., Kruisbeek A., Maloy W. L., Coligan J. E., Schwartz R. H. Differential expression of two distinct T-cell receptors during thymocyte development. Nature. 1987 Mar 5;326(6108):79–81. doi: 10.1038/326079a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Gottlieb P. D., Bevan M. J. Fractionation of lymphocyte populations with monoclonal antibodies specific for LYT-2.2 and LYT-3.1. J Immunol. 1980 Sep;125(3):1136–1143. [PubMed] [Google Scholar]
- Reilly E. B., Kranz D. M., Tonegawa S., Eisen H. N. A functional gamma gene formed from known gamma-gene segments is not necessary for antigen-specific responses of murine cytotoxic T lymphocytes. 1986 Jun 26-Jul 2Nature. 321(6073):878–880. doi: 10.1038/321878a0. [DOI] [PubMed] [Google Scholar]
- Roth M. E., Lacy M. J., McNeil L. K., Kranz D. M. Selection of variable-joining region combinations in the alpha chain of the T cell receptor. Science. 1988 Sep 9;241(4871):1354–1358. doi: 10.1126/science.2970673. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. 1984 Jun 28-Jul 4Nature. 309(5971):757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- Saito T., Weiss A., Miller J., Norcross M. A., Germain R. N. Specific antigen-Ia activation of transfected human T cells expressing murine Ti alpha beta-human T3 receptor complexes. Nature. 1987 Jan 8;325(7000):125–130. doi: 10.1038/325125a0. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]