Abstract
Sex steroids are essential for spermatogenesis; however, normal intratesticular concentrations of these hormones in man have not been extensively studied. To improve our understanding of intratesticular hormone concentrations, we performed bilateral testicular aspirations in a group of normal men, determined sex steroid concentrations within each testis, and compared these levels to serum hormone concentrations. Ten healthy human subjects aged 20–49 underwent bilateral testicular aspirations. Intratesticular hormone concentrations of testosterone, dihydrotestosterone (DHT), and estradiol were measured using liquid chromatography–tandem mass spectrometry. Intratesticular testosterone concentrations ranged from 119 to 1251 ng/mL, with a mean of 635 ± 368 ng/mL. Intratesticular estradiol ranged from 0.41 to 3.9 ng/mL, with a mean of 2.4 ± 1.3 ng/mL. Intratesticular DHT ranged from 1.1 to 7.9 ng/mL, with a mean of 3.5 ± 3.2 ng/mL. Intratesticular testosterone and estradiol concentrations correlated highly with serum luteinizing hormone (LH; r = 0.87 and r = 0.70 respectively, P < .01). Intratesticular testosterone correlated highly with serum testosterone. Moreover, a significant correlation between the right and left testes was observed for testosterone (r = 0.82, P = .003), but not for estradiol or DHT. Intratesticular hormone concentrations can be safely assessed by testicular aspiration. Intratesticular testosterone and estradiol correlate highly with serum LH concentrations, and variation in serum LH accounts for most of the variation in intratesticular testosterone among men. In addition, intratesticular testosterone is highly correlated between testes in a given individual. Direct measurement of intratesticular testosterone will improve our understanding of the relationship between intratesticular sex steroids and spermatogenesis, and may have implications for the development of male hormonal contraception.
Keywords: Contraception, hormone, infertility, testis
Intratesticular androgens have a critical role in supporting spermatogenesis. In rodents, reductions of up to 75% of the intratesticular testosterone (IT-T) concentration are still compatible with normal levels of spermatogenesis; however, below that level sperm maturation is not detected (Ahmad et al, 1973; Cunningham and Huckins, 1979; Zirkin et al, 1989). In men, whether a minimum concentration of intratesticular steroids is necessary to support spermatogenesis has not been determined. Residual androgens in the testis, despite a paucity of luteinizing hormone (LH), appear to play a role in supporting spermatogenesis in some mouse models (Zhang et al, 2004). It has been proposed that such gonadotropin-independent intratesticular steroidogenesis could play a role in supporting residual spermatogenesis in men who do not achieve azoospermia during male contraceptive treatment (Anderson et al, 1996; McLachlan et al, 2002; Jarow and Zirkin, 2005; Amory et al, 2006). A greater understanding of the normal intratesticular hormonal milieu and the relationship between intratesticular hormone levels and gonadotropins in normal, healthy men could facilitate such investigations.
Understanding the hormonal requirements necessary to support spermatogenesis in men has been difficult. Until recently, methods for measuring intratesticular hormone concentrations in men required testis tissue obtained by testicular biopsy or at the time of orchidectomy (Morse et al, 1973; Takahashi et al, 1982; Marie et al, 2001). These methods are considered to be too invasive for assessment of normal, healthy men; therefore, prior studies were in infertile men. In 2001, Jarow et al demonstrated that fine-needle tissue aspiration of the testes could be used to quantify intratesticular hormone concentrations. This procedure used to obtain intratesticular fluid for quantification of steroids by radioimmunoassay was further refined with measurement of testicular aspiration fluid steroid hormone levels by mass spectrometry (Zhao et al, 2004); however, comparison with contemporaneously measured serum hormone concentrations and between the testes in a given individual was performed only for testosterone. In this study, we performed testicular aspirations in a group of normal men to better understand the relationship between intratesticular concentrations of testosterone and its active metabolites, as well as the relationship among intratesticular sex steroids, circulating gonadotropins (LH and follicle-stimulating hormone [FSH]), and sex steroid levels in normal men.
Materials and Methods
Subjects
Healthy men aged 18–50 years old were recruited for this study. Subjects had to have a normal general medical and reproductive history and physical examination, including a testicular exam with measurements of testis volume by Prader orchidometer, normal serum gonadotropins and testosterone levels, and normal seminal fluid analysis based on the World Health Organization criteria with sperm concentration >20 million/mL, >50% motility, and >15% normal morphology (World Health Organization, 1999). Exclusion criteria included poor general health; abnormal blood test results; active alcohol or drug abuse; history of testicular or scrotal surgery; infertility; chronic pain syndrome; use of steroids, testosterone, or medications that might interfere with androgen metabolism, including ketoconazole and glucocorticoids; known bleeding disorder; or use of medications that may affect bleeding time (such as ongoing aspirin or warfarin use).
Prior to the aspiration procedure, each subject’s vital signs were taken and a blood sample was drawn for assessment of serum hormone levels (15–20 minutes prior to testicular aspiration). After local anesthesia administration at the spermatic cord with 1% buffered lidocaine, a 19-gauge needle was used to perform bilateral testicular aspirations as previously described (Jarow et al, 2001; Coviello et al, 2005). Subjects were evaluated 1 week following the procedure, and 1 month later a follow-up semen analysis was performed.
The institutional review board of the University of Washington approved this study protocol prior to study initiation (National Clinical Trial 00756561). Informed consent was obtained from all subjects prior to screening evaluation.
Measurements
Testicular fluid samples were placed immediately on ice and centrifuged at 300 × g. Supernatant fluid was stored at −70°C. We measured right and left testicular fluid samples for IT-T, intratesticular dihydrotestosterone (IT-DHT), and intratesticular estradiol (IT-E2) by liquid chromatography–tandem mass spectrometry (LC/MS/MS) on a Waters Aquity UPLC coupled with a Micromass Premiere-XE tandem quadrupole mass spectrometer (Waters Corp, Milford, Massachusetts) as described previously (Kalhorn et al, 2007). For IT-E2, human serum samples (100 μL) or intratesticular fluid samples were diluted with water to a final volume of 0.5 mL. The samples were left at room temperature for 1 hour, at which time 4.0 mL t-butyldimethyl ether (TBDME) was added. The tubes were sealed, extracted on a horizontal shaker, centrifuged, and flash frozen on dry ice. The top organic phase was decanted into a conical screw-top tube and evaporated to dryness under nitrogen. Additional TBDME (150 μL) was added, the tubes vortexed, and the solvent again removed under nitrogen. The residue was dissolved in 40 μL 100 mM pH 10.5 sodium carbonate followed by the addition of 40 μL 1.0 mg/mL dansyl chloride in acetonitrile. The tubes were sealed and heated at 60°C for 5 minutes. The tubes were centrifuged and the supernatant removed for analysis. Samples were analyzed in triplicate and injected twice.
The lower limit of quantification for all 3 sex steroid assays was 0.04 pg/mL. The intra-assay and interassay coefficients of variation for testosterone were 3.5% and 7.7% respectively, and for dihydrotestosterone (DHT) they were 3.5% and 6.3%. The intra-assay coefficient of variation for estradiol was 14% using human serum. Serum LH and FSH were quantified by immunofluorometric assay (Page et al, 2007). The intra-assay coefficient of variation for LH was 5.6%, and the interassay coefficient was 13.9%. For FSH, the intra-assay and interassay coefficients of variation were 3.0% and 5.0% respectively. All samples for all subjects were batched and measured in 1 assay.
Statistical Analysis
Because of nonnormal distributions, all hormone concentrations were natural log–transformed prior to analysis using parametric statistics. Correlations between serum hormone levels and intratesticular hormones, and between testosterone and other steroid hormones, were performed using the Pearson technique. Statistical analyses were performed using STATA version 10.0 (Stata Corporation, College Park, Texas). For all analyses, a P value of <.05 was considered significant.
Results
Thirteen men were screened for the study and 10 met inclusion criteria and completed all study procedures. Of the 3 men who were excluded from the study, 2 were excluded for abnormal semen parameters and 1 was excluded for abnormal liver function tests. Testicular aspiration samples ranged in volume from 0.5 to 50 μL, with a mean volume of 8.5 μL. All subjects tolerated the procedure well, with pain during the procedure ranging from 1 to 5 on a 10-point scale. There were no serious adverse events. On follow-up exam, 2 out of 10 patients had mild bruising noted at the site of the lidocaine injection bilaterally.
Subject characteristics are shown in Table 1. Six subjects were Caucasian, 2 Asian, 1 African-American, and 1 native Hawaiian. The range of IT-T was 119–1251 ng/mL, with a median (interquartile [IQ] range) of 486 (429–897) ng/mL. The range of IT-DHT was 1.1–7.9 ng/mL, with a median (IQ range) of 3.7 (1.1–4.7) ng/mL. The range of IT-E2 was 0.4–3.9 ng/mL, with a median (IQ range) of 2.7 (1.3–2.4) ng/mL (Table 2). Serum testosterone measured at the time of the testicular aspiration ranged from 1.4 to 8.2 ng/mL, with a median (IQ range) of 3.0 (2.3–3.9) ng/mL. Serum DHT ranged from 82 to 525 pg/mL, with a median (IQ range) of 200 (120–240) pg/mL. Serum estradiol ranged from 14 to 33 pg/mL, with a median (IQ range) of 25 (19–29) pg/mL.
Table 1.
Subject characteristics (n = 10)
| Characteristic | Median (IQ Range) |
|---|---|
| Age, y | 23 (21–32) |
| BMI, kg/m2 | 25.9 (23.4–28.7) |
| Sperm concentration, millions/mL | 75 (28–133) |
| LH, IU/L | 3.7 (2.1–5.4) |
| FSH, IU/L | 1.8 (1.4–2.5) |
| Serum testosterone, ng/mL | 3.0 (2.3–3.9) |
| Serum DHT, ng/mL | 0.20 (0.12–0.24) |
| Serum E2, ng/mL | 0.025 (0.019–0.029) |
| Testis volume—right, mL | 25 (20–25) |
| Testis volume—left, mL | 25 (15–25) |
Abbreviations: BMI, body mass index; DHT, dihydrotestosterone; E2, estradiol; FSH, follicle-stimulating hormone; IQ, interquartile; LH, luteinizing hormone.
Table 2.
Intratesticular hormone concentrations (average between right and left testis for each subject) and serum hormone concentrations in 10 normal men (all values are median [IQ range])
| Hormone | Intratesticular Concentration, ng/mL | Serum Concentration, ng/mL | Ratio of Intratesticular to Serum Concentration |
|---|---|---|---|
| Testosterone | 486 (429–897) | 3.0 (2.3–3.9) | 181 (128–233) |
| DHT | 3.7 (1.1–4.7) | 0.20 (0.12–0.24) | 15 (9–24) |
| E2 | 2.7 (1.3–2.4) | 0.025 (0.019–0.029) | 88 (68–141) |
Abbreviations: DHT, dihydrotestosterone; E2, estradiol; IQ, interquartile.
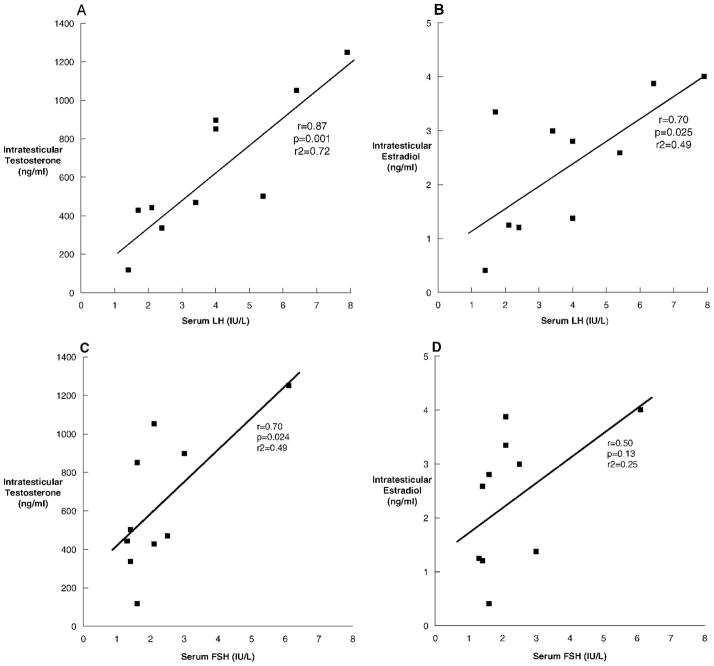
To investigate the relationship between serum gonadotropins and intratesticular steroid concentrations that could contribute to the wide variability in intratesticular hormone levels observed, especially in testosterone, we looked for correlations between gonadotropins and intratesticular hormones. Serum LH, drawn approximately 15–20 minutes prior to the testicular aspiration, correlated strongly with IT-T (r = 0.87, P = .001; Figure 1A) and IT-E2 (r = 0.70, P = .025; Figure 1B), but not with IT-DHT (r = 0.25, P = .5; data not shown). Serum FSH also correlated with IT-T (r = 0.70, P = .024; Figure 1C), but not with IT-E2 (r = 0.50, P = .13; Figure 1D) or IT-DHT (r = 0.09, P = .82; data not shown).
Figure 1.
The relationships between serum luteinizing hormone (LH) and intratesticular testosterone (A), LH and intratesticular estradiol (B), follicle-stimulating hormone (FSH) and intratesticular testosterone (C), and FSH and intratesticular estradiol (D) in 10 normal men undergoing testicular aspiration.
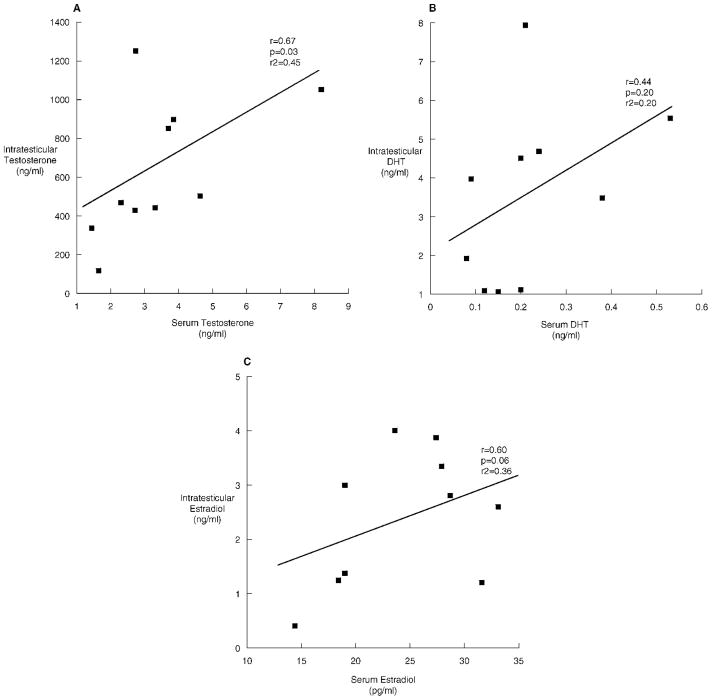
We also evaluated the relationship between intratesticular and serum steroid concentrations. IT-T was significantly correlated with serum testosterone (r = 0.67, P = .03; Figure 2A). However, IT-DHT did not correlate with serum DHT (r = 0.44, P = .20; Figure 2B). IT-E2 displayed a borderline correlation with serum estradiol (r = 0.60, P = .06; Figure 2C). The ratio of intratesticular to serum hormone concentrations is presented in Table 2.
Figure 2.
The relationships between intratesticular testosterone and serum testosterone (A), intratesticular dihydrotestosterone (DHT) and serum DHT (B), and intratesticular estradiol and serum estradiol (C) in 10 normal men undergoing testicular aspiration.
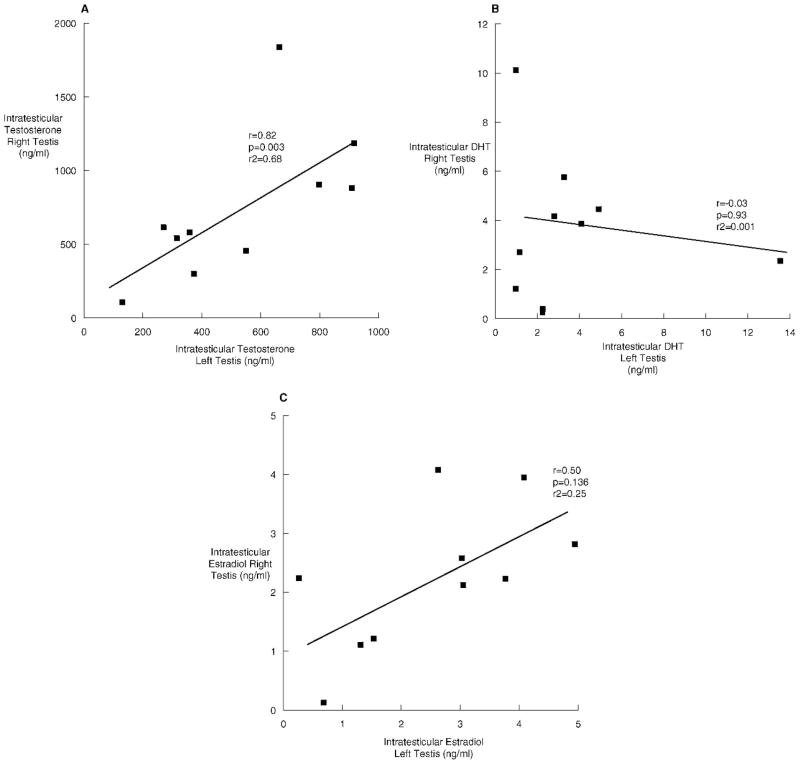
When examining the relationship between IT-T and its metabolites, we discovered that IT-T concentrations correlated highly with IT-E2 (r = 0.79, P < .01), but not with IT-DHT (r = 0.57, P = .83). IT-T concentrations were highly correlated between the right and left testes (r = 0.82, P = .003; Figure 3A). There was no correlation between IT-DHT in the right and left testes (r = −0.03, P = .93; Figure 3B). There was a weak, nonsignificant correlation for IT-E2 between the right and left testes (r = 0.50, P = .14; Figure 3C). Serum testosterone correlated with serum DHT (r = 0.63, P = .05), but not serum estradiol (r = 0.31, P = .40).
Figure 3.
The relationships between right and left testes of intratesticular testosterone (A), intratesticular dihydrotestosterone (B), and intratesticular estradiol (C) in 10 normal men undergoing testicular aspiration.
Discussion
We have used intratesticular fine-needle aspiration coupled with liquid chromatography–tandem mass spectrometry to determine the intratesticular concentrations of testosterone, DHT, and estradiol in healthy normal men. We found a strong correlation between LH and both IT-T and IT-E2, as well as a strong correlation between IT-T and IT-E2, though not between IT-T and IT-DHT. We found that serum T also correlates strongly with IT-T, though it does not with serum E2 and IT-E2. Interestingly, we found no correlation between serum DHT and IT-DHT. We also found a strong correlation for IT-T between the 2 testes.
Our results regarding IT-T concentrations are comparable to previous reports using this technique (Zhao et al, 2004) and add to the small body of data in this regard. We used the same minimally invasive percutaneous testicular aspiration technique under local anesthesia to obtain intratesticular fluid. However, we measured simultaneous serum steroid hormone concentrations in addition to intratesticular fluid. We also used a LC/MS/MS assay, which allows for a more sensitive and specific assay, in comparison to most previous data using radioimmunoassay (Jarow et al, 2001; McLachlan et al, 2002; Coviello et al, 2004; Matthiesson et al, 2005). Earlier reports of intratesticular concentrations used extracts from testis tissue obtained by biopsy or at orchidectomy, but evaluated primarily infertile men or men with prostate cancer, and used general anesthesia, which can alter hormone concentrations (Morse et al, 1973; Takahashi et al, 1982; Marie et al, 2001). Although our IT-T concentrations measured by LC/MS/MS are similar to those reported by Zhao et al (2004), our IT-E2 and IT-DHT values are somewhat lower. Increased sample size in future studies may narrow this apparent discrepancy, although we cannot rule out that differences in assay methodology might contribute to these differences.
Although the range of IT-T levels was quite broad, this variation seems to be explained by variation in LH and may reflect a pulsatile concentration of IT-T, similar to pulsatility in serum testosterone levels (Baker et al, 1975). The strong correlations between serum LH and IT-T and between serum LH and estradiol illustrate that IT-T and estradiol likely vary with LH pulses. Further evidence for this comes from the observation that such a relationship between IT-T and gonadotropins does not exist in men who have undergone prolonged gonadotropin suppression using exogenous testosterone in combination with a progestin (Page et al, 2007).
The significant correlations between IT-T and serum FSH, and between IT-E2 and serum FSH, were partially due to the presence of 1 subject with the highest IT-T and FSH. When this subject was omitted from these analyses, the correlations were no longer significant, implying that these correlations may be due to chance. In any case, it is clear that this relationship is not as strong as that between IT-T and LH and IT-E2 and LH. This is likely due to the known stimulatory role of LH on testosterone biosynthesis in Leydig cells, whereas FSH is not known to play a role in this process. It seems possible that the observed correlations are more likely due to cosecretion of FSH and LH from the pituitary rather than any direct effect of FSH on IT-T. Similarly, the significant correlation between serum T and IT-T is due to the presence of a signal outlier (not the same subject as omitted above) because removal of this subject reduced the P value from .03 to .07. This further illustrates the need for additional study of these relationships in a larger sample of men.
We also examined the relationship between intratesticular and contemporaneous serum hormone concentrations. Intratesticular hormone concentrations are significantly higher than serum concentrations, as previously shown in both mice and humans (Turner et al, 1984; Jarow et al, 2001). Interestingly, the intratesticular to serum ratios of testosterone and estradiol are nearly 200 and 100 respectively, but serum DHT is only about 15 times higher than IT-DHT. This suggests that DHT is formed primarily at peripheral sites rather than within the testes. This is compatible with studies of 5α-reductase expression, which demonstrate high levels of expression in the skin, gut, kidney, and prostate and only modest expression in the male reproductive tract (Thigpen et al, 1993). Indeed, DHT may not be critical for spermatogenesis because chronic inhibition of 5α-reductase has a minor impact on sperm concentrations in most men (Amory et al, 2007) and does not appear to augment sperm suppression when added to other male hormonal contraceptive agents (Kinniburgh et al, 2001).
Intratesticular concentrations of testosterone are highly correlated between the right and left testes. The correlation for estradiol between the 2 testes did not quite reach statistical significance, likely because of our small sample size. Interestingly, there was no correlation between DHT levels in the right and left testes in this small sample of normal men. Many factors could contribute to this finding, including possible differential geographic expression of the enzyme 5α-reductase that metabolizes testosterone into DHT (Mahony et al, 1998). Blood flow in the right and left testes is also known to be unequal because of variations in the anatomy of the blood supply to each testis (Fritjofsson et al, 1969), which might theoretically impact nontesticular-derived sources of DHT within each testicle.
There are several weaknesses with our study. In particular, the small sample size prevents us from having adequate statistical significance to define normative ranges for intratesticular hormone concentrations. However, the strengths of this study include our ability to use a highly sensitive LC/MS/MS assay in normal men and the use of a minimally invasive technique that allows the subjects to avoid general anesthesia, which can alter serum steroid concentrations. Future studies using the same assay will allow us to develop a larger cohort of normal men from whom to calculate a normal range for intratesticular hormones. Serum hormone concentrations also fluctuate with circadian rhythms (Plymate et al, 1989), yet we did not attempt to time our aspirations with identified LH pulses, which may also alter intratesticular hormone concentrations.
Measurement of intratesticular steroid hormone concentrations will provide essential information for future studies of the hormonal regulation of spermatogenesis. Our findings have implications for the treatment of male infertility and male contraceptive development. For example, the relationship between intratesticular hormones and infertility has not been explored systematically in large numbers of infertile compared with normal men, despite the known central contribution of testosterone to spermatogenesis. The use of the technique presented here, which allows for repeat assessment of intratesticular hormones in an individual, combined with sensitive assay techniques using LC/MS/MS, will allow us to explore the relationship between intratesticular steroid hormone concentrations and spermatogenesis in future cross-sectional and interventional studies. These findings also have implications for the development of male hormonal contraception. Male hormonal contraception uses exogenously administered androgens and progestins to suppress hypothalamic release of gonadotropin-releasing hormone and pituitary release of gonadotropins (LH and FSH). This suppresses endogenous production of testosterone, and subsequently spermatogenesis, while providing systemic androgens to maintain activity at peripheral sites and prevent symptomatic hypogonadism. Although rates of azoospermia with use of both androgens and progestin reach 90%–95% (Page et al, 2008), we have little understanding of why the remaining 5%–10% of men fail to achieve azoospermia. Animal models suggest that gonadotropin-independent androgen production may support residual spermatogenesis in the setting of gonadotropin ablation (Zhang et al, 2004). Alternatively, high-dose exogenous androgens used in such regimens could diffuse into the testes and support spermatogenesis. These hypotheses have been difficult to test in humans, but could be examined using our technique of testicular aspiration in men during trials of experimental male hormonal contraceptives.
In summary, our study has confirmed and extended earlier work demonstrating that testosterone, DHT, and estradiol can be measured by a minimally invasive, percutaneous, fine-needle aspiration technique of the testes, and that these concentrations are much higher than those in the serum. In addition, we have demonstrated that serum LH correlates very strongly with IT-T and estradiol, accounting for some of the wide range of normal values and providing evidence for the importance of gonadotropins in the regulation of intratesticular steroid concentration. A strong correlation also exists between testosterone concentrations in the right and left testes, indicating that a unilateral aspiration technique is likely sufficient to determine IT-T and estradiol concentrations. Future studies will include larger populations to establish a normal hormone range for intratesticular hormone concentrations. This paradigm, coupled with hormone manipulation, will allow us to identify the critical relationships and thresholds for intratesticular hormones to support spermatogenesis in men.
Acknowledgments
We thank Ms Iris Nielsen (Research Study Coordinator) and Ms Marilyn Busher (Study Nurse) for their excellent assistance with this study, as well as our study volunteers without whom this research would not be possible. We would also like to acknowledge the dedication of Mr Thomas F. Kalhorn, who unexpectedly passed away during preparation of this manuscript.
Supported by the National Institute of Child Health and Human Development through cooperative agreements U54-HD-12629 and U54 HD-42454 as part of the specialized Cooperative Centers Program in Reproductive Research and the Cooperative Contraceptive Research Centers Program. M.Y.R. is supported by Training in Reproductive Biology grant 5 T32 HD007453. A.M.M. is supported by the Department of Veterans Affairs. J.K.A. is supported, in part, by the National Institute of Child Health and Human Development, through grant K23 HD45386. S.T.P. is supported by the National Institute of Aging, a Division of the National Institute of Health, by grant K23 AG027238.
References
- Ahmad N, Haltmeyer GC, Eik-Nes KB. Maintenance of spermatogenesis in rats with intratesticular implants containing testosterone or dihydrotestosterone (DHT) Biol Reprod. 1973;8:411–419. doi: 10.1093/biolreprod/8.4.411. [DOI] [PubMed] [Google Scholar]
- Amory JK, Page ST, Bremner WJ. Oral testosterone in oil: pharmacokinetic effects of 5alpha reduction by finasteride or dutasteride and food intake in men. J Androl. 2006;27:72–78. doi: 10.2164/jandrol.05058. [DOI] [PubMed] [Google Scholar]
- Amory JK, Wang C, Swerdloff RS, Anawalt BD, Matsumoto AM, Bremner WJ, Walker SE, Haberer LJ, Clark RV. The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrinol Metab. 2007;92:1659–1665. doi: 10.1210/jc.2006-2203. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Wallace AM, Wu FC. Comparison between testosterone enanthate-induced azoospermia and oligozoospermia in a male contraceptive study. III. Higher 5 alpha-reductase activity in oligozoospermic men administered supraphysiological doses of testosterone. J Clin Endocrinol Metab. 1996;81:902–908. doi: 10.1210/jcem.81.3.8772548. [DOI] [PubMed] [Google Scholar]
- Baker HW, Santen RJ, Burger HG, De Kretser DM, Hudson B, Pepperell RJ, Bardin CW. Rhythms in the secretion of gonadotropins and gonadal steroids. J Steroid Biochem. 1975;6:793–801. doi: 10.1016/0022-4731(75)90069-2. [DOI] [PubMed] [Google Scholar]
- Coviello AD, Bremner WJ, Matsumoto AM, Herbst KL, Amory JK, Anawalt BD, Yan X, Brown TR, Wright WW, Zirkin BR, Jarow JP. Intratesticular testosterone concentrations comparable with serum levels are not sufficient to maintain normal sperm production in men receiving a hormonal contraceptive regimen. J Androl. 2004;25:931–938. doi: 10.1002/j.1939-4640.2004.tb03164.x. [DOI] [PubMed] [Google Scholar]
- Coviello AD, Matsumoto AM, Bremner WJ, Herbst KL, Amory JK, Anawalt BD, Sutton PR, Wright WW, Brown TR, Yan X, Zirkin BR, Jarow JP. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab. 2005;90:2595–2602. doi: 10.1210/jc.2004-0802. [DOI] [PubMed] [Google Scholar]
- Cunningham GR, Huckins C. Persistence of complete spermatogenesis in the presence of low intratesticular concentrations of testosterone. Endocrinology. 1979;105:177–186. doi: 10.1210/endo-105-1-177. [DOI] [PubMed] [Google Scholar]
- Fritjofsson A, Persson JE, Pettersson S. Testicular blood flow in man measured with xenon-133. Scand J Urol Nephrol. 1969;3:276–280. doi: 10.3109/00365596909135416. [DOI] [PubMed] [Google Scholar]
- Jarow JP, Chen H, Rosner TW, Trentacoste S, Zirkin BR. Assessment of the androgen environment within the human testis: minimally invasive method to obtain intratesticular fluid. J Androl. 2001;22:640–645. [PubMed] [Google Scholar]
- Jarow JP, Zirkin BR. The androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann N Y Acad Sci. 2005;1061:208–220. doi: 10.1196/annals.1336.023. [DOI] [PubMed] [Google Scholar]
- Kalhorn TF, Page ST, Howald WN, Mostaghel EA, Nelson PS. Analysis of testosterone and dihydrotestosterone from biological fluids as the oxime derivatives using high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:3200–3206. doi: 10.1002/rcm.3205. [DOI] [PubMed] [Google Scholar]
- Kinniburgh D, Anderson RA, Baird DT. Suppression of spermatogenesis with desogestrel and testosterone pellets is not enhanced by addition of finasteride. J Androl. 2001;22:88–95. [PubMed] [Google Scholar]
- Mahony MC, Swanlund DJ, Billeter M, Roberts KP, Pryor JL. Regional distribution of 5alpha–11reductase type 1 and type 2 mRNA along the human epididymis. Fertil Steril. 1998;69:1116–1121. doi: 10.1016/s0015-0282(98)00094-6. [DOI] [PubMed] [Google Scholar]
- Marie E, Galeraud-Denis I, Carreau S. Increased testicular steroid concentrations in patients with idiopathic infertility and normal FSH levels. Arch Androl. 2001;47:177–184. doi: 10.1080/014850101753145870. [DOI] [PubMed] [Google Scholar]
- Matthiesson KL, Stanton PG, O’Donnell L, Meachem SJ, Amory JK, Berger R, Bremner WJ, McLachlan RI. Effects of testosterone and levonorgestrel combined with a 5alpha-reductase inhibitor or gonadotropin-releasing hormone antagonist on spermatogenesis and intratesticular steroid levels in normal men. J Clin Endocrinol Metab. 2005;90:5647–5655. doi: 10.1210/jc.2005-0639. [DOI] [PubMed] [Google Scholar]
- McLachlan RI, O’Donnell L, Stanton PG, Balourdos G, Frydenberg M, de Kretser DM, Robertson DM. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J Clin Endocrinol Metab. 2002;87:546–556. doi: 10.1210/jcem.87.2.8231. [DOI] [PubMed] [Google Scholar]
- Morse HC, Horike N, Rowley MJ, Heller CG. Testosterone concentrations in testes of normal men: effects of testosterone propionate administration. J Clin Endocrinol Metab. 1973;37:882–886. doi: 10.1210/jcem-37-6-882. [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bremner WJ. Advances in male contraception. Endocr Rev. 2008;29:465–493. doi: 10.1210/er.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ST, Kalhorn TF, Bremner WJ, Anawalt BD, Matsumoto AM, Amory JK. Intratesticular androgens and spermatogenesis during severe gonadotropin suppression induced by male hormonal contraceptive treatment. J Androl. 2007;28:734–741. doi: 10.2164/jandrol.107.002790. [DOI] [PubMed] [Google Scholar]
- Plymate SR, Tenover JS, Bremner WJ. Circadian variation in testosterone, sex hormone-binding globulin, and calculated non-sex hormone-binding globulin bound testosterone in healthy young and elderly men. J Androl. 1989;10:366–371. doi: 10.1002/j.1939-4640.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Takahashi J, Higashi Y, LaNasa JA, Winters SJ, Oshima H, Troen P. Studies of the human testis. XVII. Gonadotropin regulation of intratesticular testosterone and estradiol in infertile men. J Clin Endocrinol Metab. 1982;55:1073–1080. doi: 10.1210/jcem-55-6-1073. [DOI] [PubMed] [Google Scholar]
- Thigpen AE, Silver RI, Guileyardo JM, Casey ML, McConnell JD, Russell DW. Tissue distribution and ontogeny of steroid 5 alpha-reductase isozyme expression. J Clin Invest. 1993;92:903–910. doi: 10.1172/JCI116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TT, Jones CE, Howards SS, Ewing LL, Zegeye B, Gunsalus GL. On the androgen microenvironment of maturing spermatozoa. Endocrinology. 1984;115:1925–1932. doi: 10.1210/endo-115-5-1925. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. Cambridge, United Kingdom: Cambridge University Press; 1999. [Google Scholar]
- Zhang FP, Pakarainen T, Zhu F, Poutanen M, Huhtaniemi I. Molecular characterization of postnatal development of testicular steroidogenesis in luteinizing hormone receptor knockout mice. Endocrinology. 2004;145:1453–1463. doi: 10.1210/en.2003-1049. [DOI] [PubMed] [Google Scholar]
- Zhao M, Baker SD, Yan X, Zhao Y, Wright WW, Zirkin BR, Jarow JP. Simultaneous determination of steroid composition of human testicular fluid using liquid chromatography tandem mass spectrometry. Steroids. 2004;69:721–726. doi: 10.1016/j.steroids.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Santulli R, Awoniyi CA, Ewing LL. Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology. 1989;124:3043–3049. doi: 10.1210/endo-124-6-3043. [DOI] [PubMed] [Google Scholar]