Abstract
Previous research indicated that male rats exhibited anxiety-like behavior following withdrawal from chronic ethanol exposure. This behavior, as well as other symptoms of withdrawal such as seizure susceptibility, may be sensitized in male rats repeatedly withdrawn from chronic ethanol exposure. Because there are sex differences in some alcohol effects, the present study explored whether male and female rats would respond differently to a course of repeated ethanol withdrawals. Similarly aged male and female rats were exposed to a control liquid diet or a diet containing ethanol (7% w/v). Ethanol-exposed rats had only one 5-day cycle of exposure or three cycles, with 2 days of control diet (CD) between cycles. At 5 h after the final ethanol was removed, the rats were placed as same-sexed pairs in the social interaction test; approximately half of the rats were tested later in the elevated plus maze. Rats withdrawn from ethanol after three cycles exhibited reduced the time spent in social interaction and general activity in the social interaction test and reduced the open and closed arm entries in the elevated plus maze. There were no sex differences in these effects. However, male rats exhibited a small anxiety-like response after one cycle of 5 days’ exposure to ethanol and female rats did not. Thus, there are no sex differences in the three-cycle multiple-withdrawal paradigm, but there may be differences after briefer exposures.
Keywords: Ethanol withdrawal, Repeated cycles, Sex, Social interaction test, Elevated plus maze
1. Introduction
It is becoming increasingly evident that repeated cycles of exposure to ethanol may lead to increased behavioral and physiological responses to ethanol withdrawal. After Ballenger and Post (1978) first called attention to the phenomenon, several investigators documented the changes in seizure susceptibility (Becker et al., 1997, 1998; McCown and Breese, 1990; Veatch and Gonzalez, 1996, 1999, 2000). There have also been reports that human subjects who have experienced multiple detoxifications may have increased craving for ethanol and a greater risk of relapse (Brown et al., 1988; Malcolm et al., 2000a,b). More recently, our laboratory has focused on anxiety-like behavior in ethanol-withdrawn rats (Knapp et al., 2004; Overstreet et al., 2002). For example, there is a greater reduction in social interaction behavior in rats repeatedly cycled through chronic ethanol exposure and withdrawal over 15 days compared with animals that were exposed to a single cycle or to similar amounts of 4.5% ethanol administered in a continuous (noncycled) fashion over 15 days (Overstreet et al., 2002). Moreover, these effects can be ameliorated by drugs interacting with serotonin (Overstreet et al., 2003) or corticotropin-releasing hormone (Overstreet et al., 2004) or be potentiated by exposure to restraint stress (Breese et al., 2004).
Virtually none of the above studies commented upon whether female humans or rats reacted to these protocols with similar responses to those of males. Because there are well-documented sex differences in the effects of ethanol in rodents (Devaud et al., 2003), as well as gender differences in anxiety disorders in humans (Lewinsohn et al., 1998; Pigott, 1999), the current paper addresses the issue of whether male and female rats exhibit similar degrees of anxiety-like behavior following withdrawal from three cycles of exposure to ethanol. The similar anxiety-like responses observed in the male and female rats in this paper parallel the findings of a recent report using a single cycle of similar duration to assay seizure susceptibility (Devaud and Chadda, 2001).
2. Methods
2.1. Animals
Male and female Sprague–Dawley rats (Charles-River, Raleigh, NC) were obtained at about 6 weeks of age and housed in groups of three or four for several days to adapt to the local conditions (light–dark cycle of 12:12, with lights on between 0900 and 2100). Then, the rats were housed individually for the remainder of the experiments, with food and fluids presented as described below.
2.2. Ethanol and control diets
Following the short period of adaptation, all rats were placed on nutritionally complete liquid diets similar with those used previously in this laboratory (e.g., Frye et al., 1983; Moy et al., 1997, 2000; Overstreet et al., 2002). Briefly, the diet was a lactalbumin/dextrose-based, nutritionally complete diet (with concentrations of vitamins, minerals, and other nutrients derived from ICN Research Diets). Dextrose calories in the control diet (CD) were equated with ethanol calories in the ethanol diet (ED). After 3 days on the CD, approximately 50% of the rats were placed on a diet containing ethanol (7% w/v) for three cycles of 5 days, interspersed with 2 days of CD.
A modified pair-feeding design was used in all of the diet studies. The rats maintained on the CD were given a volume of diet equivalent to the average volume consumed the previous day by the rats maintained on the ED. The rats were weighed at weekly intervals, and volumes of diet were adjusted to insure that the within-sex groups (across diet type) had similar body weights and increased similarly over time. Ethanol intake (g/kg/day) was comparable over the course of a single cycle (11.08 + 0.35 vs. 10.98 + 0.19 g/kg/day) but was slightly higher in females experiencing three cycles (12.47 ± 0.42 vs. 11.11 ± 0.29 g/kg/day). In general, behavioral assessments were conducted after 15 or 16 days of exposure to the ED between 5 and 6 h after the removal of the ethanol at 600. This time point was selected on the basis of previous observations of anxiety-like behavior in our laboratory (e.g., Knapp et al., 1998; Moy et al., 1997, 2000; Overstreet et al., 2002).
2.3. Social interaction test
Social interaction has been repeatedly validated as an index of anxiety-related behavior (see File and Seth, 2003) because it (social interaction) is decreased following anxiety-provoking stimuli, such as bright lights or exposure to cat odor (File, 1980; File and Hyde, 1978), after the administration of anxiogenic drugs (e.g., Battacharya et al., 1997; File and Lister, 1984; Guy and Gardner, 1985) or following withdrawal from drugs of abuse, including alcohol (Andrews et al., 1997; File et al., 1989, 1993; Irvine et al., 2001; Kampov-Polevoy et al., 2000). Conversely, social interaction can be increased by prior exposure to the test arena (File, 1980; File and Hyde, 1978) or the administration of anxiolytic drugs at doses that have little effect on locomotor activity (Barnes et al., 1990; File, 1980; Lightowler et al., 1994).
A modification of the standard social interaction test was used to reduce the number of animals needed for experiments (see Overstreet et al., 2002). In the present studies, pairs of rats with the same treatment were placed in the arena, and the social interactions initiated by each member of the pair were recorded, thereby requiring fewer rats. This procedure has been validated by previous studies in our laboratory (Overstreet et al., 2002, 2003).
Experienced observers who were blind to the experimental condition carried out the social interaction test in a square open field (60 × 60 cm, with sixteen 15 × 15 cm squares marked out on the floor). The rats were unfamiliar with the open field, and the lighting conditions were low to generate an intermediate level of anxiety-related behavior. Rat pairs were matched on the basis of body weights and treatment conditions and were placed simultaneously in the open field. During the 5-min session, line crosses (by two forepaws) and time spent in social interaction (grooming, sniffing, and following) were scored individually for each rat (Kampov-Polevoy et al., 2000; Overstreet et al., 2002, 2003). The number of rats used in this task was 23 per group.
2.4. Elevated plus maze
The elevated plus maze consists of two enclosed and two open arms (File et al., 1999; Gonzalez et al., 1998; Kampov-Polevoy et al., 2000). The apparatus was made of black Perspex and consisted of two closed and two open arms opposite each other, with a central arena of 10 × 10 cm. The arms were elevated 50 cm off the ground. The rat was placed in the apparatus, with the head in the central arena, and the following measures were recorded: number of entries into the closed arms by the whole rat (measure of activity), number of entries into the open arms (measure of anxiety), and time spent in the open arms by at least two forepaws (measure of anxiety). One rat from each of the pairs tested in the social interaction test was tested in the elevated plus maze within 5 min of completing the test (File et al., 1999; Gonzalez et al., 1998). Thus, the number of rats tested in this apparatus was 9 or 10 per group.
2.5. Single withdrawal
This experiment compared the anxiety responses in the social interaction test of four treatment groups: The elevated plus maze was not employed in this study because the two tasks provided equivalent results for the repeated withdrawal study. Male or female rats were exposed to CD throughout the study (CD-M and CD-F) or 7% ED for one cycle of 5 days (ED-M and ED-F). Between 5 and 6 h after the removal of ethanol at 0600, the rats were tested as pairs in the social interaction test, as described above.
2.6. Data analysis
Statistical analyses were carried out using the GBStat software package. The data were analyzed by two-way ANOVAs, with sex and ethanol exposure as the two main effects. If the interaction effects were statistically significant, post hoc analyses were performed using Tukey’s protected t tests. The social interaction test has a measure that is reflective of the general activity level of the rats (line crosses) as well as a measure that is reflective of the anxiety state (time spent in social interaction). Similarly, closed-arm entries in the elevated plus maze is a measure reflective of activity, and open-arm entries and time are measures reflective of anxiety. Therefore, these analyses determined whether a selective effect of ethanol withdrawal was observed on time spent in social interaction or in the open arms, the measures of anxiety-like behavior.
3. Results
3.1. Repeated withdrawals
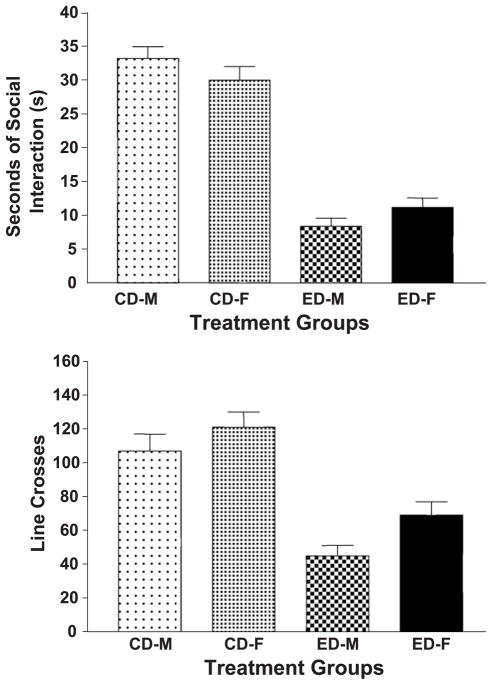
The effects of three cycles of 5 days’ exposure to 7% ethanol on measures in the social interaction test during ethanol withdrawal are illustrated in Fig. 1. There is a substantial decrease in the time spent in social interaction, as shown in the upper panel of Fig. 1 [ F(1,89) = 107.2, P < .0001]. There were no significant sex effects [ F(1,89 = 0.01, NS], but there was a nearly significant interaction effect [ F(1,89) = 3.52, P=.06]. This latter result probably resulted from the male rats having a slightly higher score in the CD group and a slightly lower score in the ED group (Fig. 1, upper panel). However, it should be emphasized that there are no significant sex differences in either the CD or ED groups.
Fig. 1.
Social interaction behavior in male and female rats after exposure to CD or three cycles of 5 days of 7% ED. Pairs of rats with the same treatment and sex were tested in the arena for 5 min approximately 5 h after the ethanol was removed. The data represent the mean sec ± S.E.M. (upper panel) or line crosses (lower panel) for the 23 rats in each group. CD: control diet; ED: ethanol diet; M: male; F: female.
The effects of ethanol withdrawal on line crosses, the measure of locomotor activity, were also quite substantial [ F(1,89) = 50.6, P < .0001], as illustrated in the lower panel of Fig. 1. There were also substantial sex differences [ F(1,89) = 5.77, P < .01], as both the CD and ED females were more active than their male counterparts were (Fig. 1, lower panel). However, there was no significant interaction effect [ F(1,89)= 0.34, NS]. Because both measures were substantially decreased by ethanol withdrawal, an analysis of covariance was conducted, with line crosses as the covariate. There was still a significant difference in the scores for social interaction [ F(3,107)= 34.60, P < .0001]. Thus, after correction for activity, there are still differences in the anxiety measure due to ethanol exposure. Male and female rats do not differ on this measure.
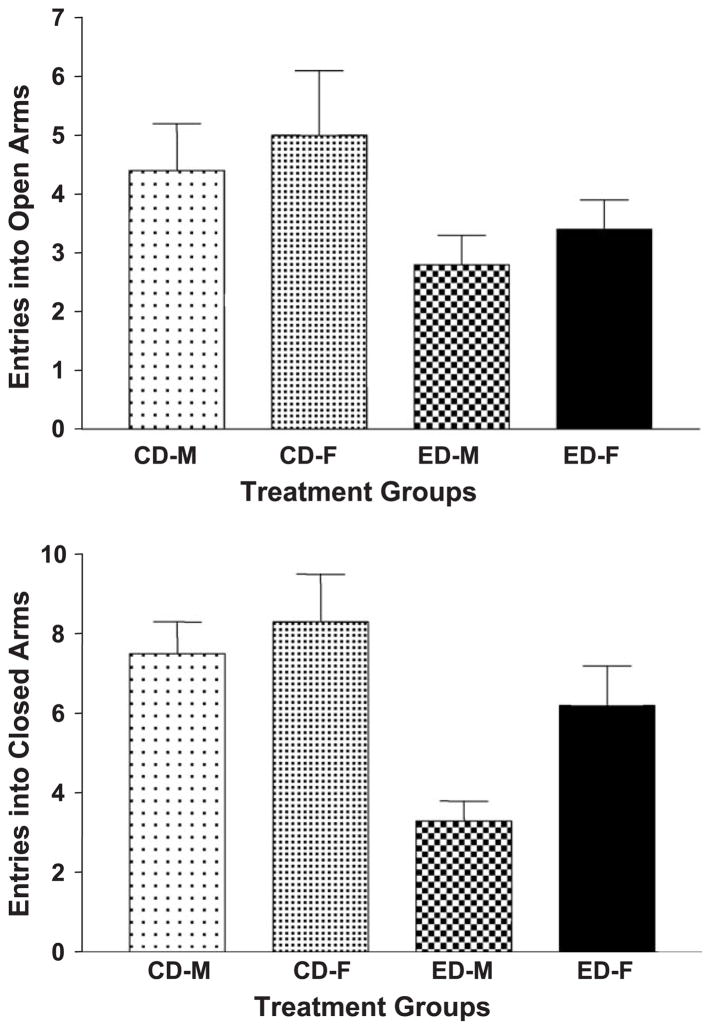
The data for arm entries in the elevated plus maze are illustrated in Fig. 2. There was a decrease in open-arm entries in the ethanol-withdrawn rats [ F(1,34) = 5.08, P=.03], but there were no sex [ F(1,34) = 0.79, NS] or interaction [ F(1,34) = 0.01, NS] effects (Fig. 2, upper panel). The results for time spent in the open arms revealed a similar pattern, with a significant treatment effect [ F(1,34) = 26.8, P < .0001] but no significant sex [ F(1,34) = 1.38, NS] or interaction [ F(1,34) = 0.01, NS] effects (data not shown). The pattern of effects of closed-arm entries was somewhat different. There was still a significant treatment effect [ F(1,34) = 11.98, P < .001], but the sex effect was also significant [ F(1,34) = 3.94, P < .05], while the interaction effect [ F(1,34) = 1.33, NS] was not (Fig. 2, lower panel). Note that the sex effect is associated with the females of both CD and ED groups being higher than their male counterparts.
Fig. 2.
Elevated plus maze behavior in male and female rats after exposure to control diet or three cycles of 5 days of 7% ED. Rats were tested in the maze for 5 min approximately 5 h after the ethanol was removed, immediately after completing the social interaction test. The data represent the mean number + S.E.M. of open (upper panel) or closed (lower panel) arm entries for 9 or 10 rats per group. CD: control diet; ED: ethanol diet; M: male; F: female.
Thus, female and male rats do not exhibit different anxiety-like behavior following repeated withdrawals from ethanol. The trend for females to be more active than males are was seen in both tasks, but the two sexes exhibited similar decreases in activity following ethanol withdrawal.
3.2. Single withdrawal
The effects of one cycle of 5 days’ exposure to ethanol on the social interaction test are illustrated in Fig. 3. There were highly significant treatment effects [ F(1,35) = 41.6, P < .0001], but no significant sex effects [ F(1,35) = 1.15, NS]. However, there was a significant Treatment × Sex interaction effect [ F(1,35) = 11.7, P < .001]. Thus, male rats given the CD spent the most time in social interaction, while male rats given ED spent the least time (Fig. 3, upper panel). In other words, male rats exposed to the one cycle of ED exhibited an anxiety-like response, and female rats did not. Despite this anxiety-like response in the male rats after one cycle of ethanol exposure, the time spent in social interaction is still about twice of that of the rats exposed to repeated cycles (compare upper panels of Figs. 1 and 3).
Fig. 3.
Social interaction behavior in male and female rats after exposure to CD or one cycle of 5 days of 7% ED. Pairs of rats with the same treatment and sex were tested in the arena for 5 min approximately 5 h after the ethanol was removed. The data represent the mean sec ± S.E.M. (upper panel) or line crosses (lower panel) for 23 rats in each group. Bars with different letters indicate significance group differences, P < .05, Tukey’s protected t test. CD: control diet; ED: ethanol diet; M: male; F: female.
The pattern of effects for line crosses was different from that for social interaction. There was a significant effect of treatment [ F(1,35) = 44.0, P < .0001], with both male and female rats exhibiting large reductions. Neither the sex [ F(1,35) = 0.03, NS] nor the interaction [ F(1,35) = 0.01] effects were significant. Thus, male and female rats exposed to one cycle of 5 days’ ethanol exposure exhibit similar reductions in activity. Indeed, these reductions appear to be as large after one cycle as after three cycles (compare lower panels of Figs. 1 and 3).
4. Discussion
This study of the consequences of multiple ethanol withdrawals on male and female rats has revealed a number of differences and similarities between the sexes that complement and extend previously published studies. The fact that female rats in the repeated-cycle protocol exhibited more line crosses and closed-arm entries is consistent with many previous studies suggesting that female rats are more active than male rats (Archer, 1973). The lack of difference in line crosses in the one-cycle study may be related to the fact that the rats were approximately 2 weeks younger when tested. It is well known that adolescent male rats are more active than adult male rats. Against this background of sex differences in activity measures, it should be stressed that there is little evidence of sex differences in the measures of anxiety-like behavior, such as time spent in social interaction and entries into the open arms. This finding confirms the view expressed by others that measures of activity and anxiety-like behavior in these two tasks are independent (Fernandes et al., 1999; File, 1980; File and Seth, 2003; Overstreet et al., 2002, 2003).
The fact that the male and female rats exposed to the three-cycle protocol exhibited similar reductions in anxiety-like behavior following ethanol withdrawal is reminiscent of a previously published report on a different phenotype. Although anxiety-like behavior and seizures likely involve quite different neurochemical and neuroanatomical substrates, Devaud and Chadda (2001) used bicuculline-induced seizures as an index of ethanol dependence and concluded that male and female rats exhibited similar reductions in seizure thresholds after 14 days of exposure to ethanol. The alcohol-preferring P rat, which drinks substantial amounts of ethanol voluntarily, was shown to exhibit both a reduction in seizure threshold to bicuculline and reduced time spent in social interaction after 6 weeks of voluntary drinking, but not earlier (Kampov-Polevoy et al., 2000).
In contrast, it appears that the male rat exhibits a mild anxiety-like response after just 5 days of exposure to ethanol and the female rat does not. Previous studies in our laboratory have found that rats exposed to one cycle of ethanol exposure exhibit small and nonsignificant reductions in social interaction behavior (Overstreet et al., 2002). The significant finding here is partially a consequence of the higher social interaction behavior of the rats maintained on CD. Nevertheless, this greater anxiety-like response of the male rats after a short course of alcohol exposure is consistent with another report in the literature. Devaud and Chadda (2001) reported that male rats exhibited a reduction in seizure thresholds after just 3 days of exposure to ethanol, but female rats did not. Further exploration of the time course of chronic ethanol-induced changes in anxiety-like behavior may complete the picture of sex differences.
Additional studies of the recovery of these withdrawal-induced behaviors may also elucidate sex differences. For example, Devaud and Chadda (2001) reported that female rats recovered sooner from ethanol exposure than did male rats, as reflected by changes in seizure susceptibility. Previous work from our laboratory established that the anxiety-like behavior of male rats recovered between 24 and 48 h after withdrawal from chronic ethanol exposure (Overstreet et al., 2002). It might also be possible to search for latent changes by reexposing animals to ethanol at various times after ethanol has been removed, as previously reported (Overstreet et al., 2002; Breese et al., 2004). Such a strategy allowed us to conclude that male rats subjected to 3 cycles of ethanol exposure could be induced (reinstated) to exhibit robust withdrawal-induced anxiety-like behavior following an additional single 5-day treatment cycle for up to 32 days after ethanol was last removed (Overstreet et al., 2002). However, it was not the intent of the present investigation to examine such latent adaptation with reinstatement procedures. Nonetheless, the present investigation demonstrates that male and female rats exhibit similar anxiety-like behavioral responses when withdrawn from ethanol that was given in three cycles of 5-day duration.
Although behaviors of the rats exposed to one or three cycles of ethanol exposure were not directly compared by statistical analysis, it is clear that the rats subjected to repeated withdrawals spent less time in social interaction (Figs. 1 and 3). Therefore, this study compares favorably with previous work showing a greater reduction in social interaction behavior in rats repeatedly withdrawn from ethanol (Overstreet et al., 2002; Breese et al., 2004).
Although the blood levels of ethanol were not recorded in this study, it is unlikely that they would have clarified the small sex differences that we observed after a single 5-day exposure to ethanol. Previous work showed that blood levels of ethanol were similar in rats treated continuously with 4.5% ED for 15 days or subjected to three cycles of 5 days’ exposure, although they exhibited different anxiety-like responses (Overstreet et al., 2002). We and others have shown that the ethanol blood level is essentially zero at 5 or 6 h into withdrawal, when the behavioral tests are conducted (Devaud and Chadda, 2001; Overstreet et al., 2002). Similarly, the slightly elevated ethanol intake in the female rats is unlikely to be of much consequence, as we have previously shown that rats repeatedly withdrawn from 4.5% ED exhibit an anxiety-like behavior in the social interaction test although their alcohol intake is significantly lower than that of rats exposed to 7% ethanol (Overstreet et al., 2002).
It also is worthy of note that the ethanol-withdrawn male and female rats exhibited comparable magnitudes of change in activity measures relative to controls, regardless of whether they were exposed to one or three cycles of ED. These findings provide further evidence for the independence of the measures of anxiety and activity, as claimed by others (e.g., File, 1980; File and Seth, 2003; Overstreet et al., 2002, 2003). In any case, when an analysis of covariance was carried out using line crosses in the social interaction test, there was still a significant difference in the time spent in social interaction.
In conclusion, male and female rats exhibit similar magnitudes of anxiety-like responses after repeated exposures to ethanol, but not after briefer exposure. Reduction in activity in ethanol-withdrawn rats is observed, regardless of sex or length of ethanol exposure.
Acknowledgments
This work was supported by the National Institute of Alcoholism and Alcohol Abuse (AA00243, AA11605, AA14284, and AA00214). We wish to thank Matthew Crist for technical support. We also acknowledge support from NSF.
References
- Andrews N, File SE, Fernandes C, Gonzalez LE, Barnes NM. Evidence that the median raphe nucleus–dorsal hippocampal pathway mediates diazepam withdrawal-induced anxiety. Psychopharmacology. 1997;130:228–34. doi: 10.1007/s002130050233. [DOI] [PubMed] [Google Scholar]
- Archer J. Tests for emotionality in rats and mice. A review Anim Behav. 1973;21:205–35. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Costall B, Kelly ME, Onaivi ES, Naylor RH. Ketotifen and its analogues reduce aversive responding in the rodent. Pharmacol Biochem Behav. 1990;37:785–93. doi: 10.1016/0091-3057(90)90564-x. [DOI] [PubMed] [Google Scholar]
- Battacharya SK, Satyan KS, Chakraharti A. Anxiogenic action of caffeine: an experimental study in rats. J Psychopharmacol. 1997;11:219–24. doi: 10.1177/026988119701100304. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados H, Weathersby RT. Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997;14:319–26. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Becker HC, Veatch LM, Diaz-Granados JL. Repeated ethanol withdrawal experience selectively alters sensitivity to different chemoconvulsant drugs in mice. Psychopharmacology. 1998;139:145–53. doi: 10.1007/s002130050699. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–82. doi: 10.1038/sj.npp.1300282. [Sep 3 (Electronic publication ahead of print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ME, Anton RF, Malcolm R, Ballenger JC. Alcoholic detoxification and withdrawal seizures: clinical support for a kindling hypothesis. Biol Psychiatry. 1988;23:414–507. doi: 10.1016/0006-3223(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol, Clin Exp Res. 2001;25:1689–96. [PubMed] [Google Scholar]
- Devaud LL, Alele P, Chadda R. Sex differences in the central nervous system actions of ethanol. Crit Rev Neurobiol. 2003;15(1–2):41–59. doi: 10.1615/critrevneurobiol.v15.i1.20. [DOI] [PubMed] [Google Scholar]
- Fernandes C, Gonzalez MI, Wilson CA, File SE. Factor analysis shows that female rat behavior is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol Biochem Behav. 1999;64:731–8. doi: 10.1016/s0091-3057(99)00139-2. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–38. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Lister RG. Do the reductions in social interaction produced by picrotoxin and pentylenetetrazole indicate anxiogenic actions? Neuropharmacology. 1984;23:793–6. doi: 10.1016/0028-3908(84)90113-8. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- File SE, Baldwin HA, Hitchcot PK. Flumazenil but not nitrendipine reverses the increased anxiety during ethanol withdrawal in the rat. Psychopharmacology. 1989;98:262–4. doi: 10.1007/BF00444702. [DOI] [PubMed] [Google Scholar]
- File SE, Andrews N, al-Farhan M. Anxiogenic responses of rats on withdrawal from chronic ethanol treatment: effects of tianeptine. Alcohol Alcohol. 1993;28:281–6. [PubMed] [Google Scholar]
- File SE, Ouagazzal AM, Gonzalez LE, Overstreet DH. Chronic fluoxetine in tests of anxiety in rat lines selectively bred for differential 5-HT1A receptor function. Pharmacol Biochem Behav. 1999;62:695–701. doi: 10.1016/s0091-3057(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Differential sensitivity of ethanol withdrawal signs in the rat to γ-aminobutyric acid (GABA) mimetics: blockade of audiogenic seizures but not forelimb tremors. J Pharmacol Exp Ther. 1983;226:720–5. [PubMed] [Google Scholar]
- Gonzalez LE, File SE, Overstreet DH. Selectively bred lines of rat differ in social interaction and hippocampal 5-HT1A receptor function: a link between anxiety and depression? Pharmacol Biochem Behav. 1998;59:787–92. doi: 10.1016/s0091-3057(97)00525-x. [DOI] [PubMed] [Google Scholar]
- Guy AP, Gardner CR. Pharmacological characterisation of a modified social interaction model of anxiety in the rat. Neuropsychobiology. 1985;13:194–200. doi: 10.1159/000118187. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Bagnalasta M, Marcon C, Motta C, Tessari M, File SE, et al. Nicotine self-administration and withdrawal: modulation of anxiety in the social interaction test in rats. Psychopharmacology. 2001;153:315–20. doi: 10.1007/s002130000586. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol, Clin Exp Res. 2000;24:278–84. [PubMed] [Google Scholar]
- Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol, Clin Exp Res. 1998;22:481–93. [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal anxiety in rats. Alcohol. 2004;32:101–11. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Gotlib IH, Lewinsohn M, Seeley JR, Allen NE. Gender differences in anxiety disorders and anxiety symptoms in adolescents. J Abnorm Psychology. 1998;107:109–17. doi: 10.1037//0021-843x.107.1.109. [DOI] [PubMed] [Google Scholar]
- Lightowler S, Kennett GA, Williamson U, Blackburn TP, Tulloch IF. Anxiolytic-like effect of paroxetine in the rat social interaction test. Pharmacol Biochem Behav. 1994;49:281–5. doi: 10.1016/0091-3057(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Herron JE, Anton RF, Roberts J, Moore J. Recurrent detoxification may elevate alcohol craving as measured by the obsessive compulsive drinking scale. Alcohol. 2000a;20:181–5. doi: 10.1016/s0741-8329(99)00073-7. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Roberts J-S, Wang W, Myrick H, Anton RF. Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification. Alcohol. 2000b;22:159–64. doi: 10.1016/s0741-8329(00)00114-2. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol ‘‘kindles’’ inferior collicular seizure activity: evidence for kindling of seizures associated with alcoholism. Alcohol, Clin Exp Res. 1990;14:394–9. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Criswell HE, Breese GR. Flumazenil blockade of anxiety following ethanol withdrawal in rats. Psychopharmacology. 1997;131:354–60. doi: 10.1007/s002130050303. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Duncan GE, Breese GR. Enhanced ultrasonic vocalization and Fos protein expression following ethanol withdrawal: effects of flumazenil. Psychopharmacology. 2000;152:208–15. doi: 10.1007/s002130000507. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decreases in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol, Clin Exp Res. 2002;26:1259–69. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2C antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacology. 2003;167:344–52. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–13. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott TA. Gender differences in the epidemiology and treatment of anxiety disorders. J Clin Psychiatry. 1999;60(Suppl 18):4–15. [PubMed] [Google Scholar]
- Veatch LM, Gonzalez LP. Repeated ethanol withdrawal produces site-dependent increases in EEG spiking. Alcohol, Clin Exp Res. 1996;20:262–7. doi: 10.1111/j.1530-0277.1996.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Veatch JM, Gonzalez LP. Repeated ethanol withdrawal delays development of focal seizures in hippocampal kindling. Alcohol, Clin Exp Res. 1999;23:1145–50. [PubMed] [Google Scholar]
- Veatch JM, Gonzalez LP. Nifedipine alleviates alterations in hippocampal kindling after repeated ethanol withdrawal. Alcohol, Clin Exp Res. 2000;24:484–91. [PubMed] [Google Scholar]