Abstract
Pulmonary alveolar proteinosis (PAP) comprises a heterogenous group of diseases characterized by abnormal surfactant accumulation resulting in respiratory insufficiency, and defects in alveolar macrophage- and neutrophil-mediated host defense. Basic, clinical and translational research over the past two decades have raised PAP from obscurity, identifying the molecular pathogenesis in over 90% of cases as a spectrum of diseases involving the disruption of GM-CSF signaling. Autoimmune PAP represents the vast majority of cases and is caused by neutralizing GM-CSF autoantibodies. Genetic mutations that disrupt GM-CSF receptor signaling comprise a rare form of hereditary PAP. In both autoimmune and hereditary PAP, loss of GM-CSF signaling blocks the terminal differentiation of alveolar macrophages in the lungs impairing the ability of alveolar macrophages to catabolize surfactant and to perform many host defense functions. Secondary PAP occurs in a variety of clinical diseases that presumedly cause the syndrome by reducing the numbers or functions of alveolar macrophages, thereby impairing alveolar macrophage-mediated pulmonary surfactant clearance. A similar phenotype occurs in mice deficient in the production of GM-CSF or GM-CSF receptors. PAP and related research has uncovered a critical and emerging role for GM-CSF in the regulation of pulmonary surfactant homeostasis, lung host defense, and systemic immunity.
Keywords: GM-CSF, macrophage, neutrophil, innate immunity, proinflammatory cytokines, phagocytosis, antimicrobial killing, autoimmunity, surfactant homeostasis
INTRODUCTION
Pulmonary alveolar proteinosis (PAP) is a rare syndrome characterized by the accumulation of surfactant in pulmonary alveoli resulting in varying degrees of respiratory insufficiency, and myeloid cell dysfunction resulting in increased risk of infection [1; 2]. Pulmonary surfactant is a natural substance comprised of lipids and proteins that is produced in alveolar epithelial cells-type II (AEC-II) and is vital to lung structure and function. It acts at the alveolar air-liquid-tissue interface by reducing surface tension and preventing alveolar collapse. Surfactant also contributes to pulmonary host defense by opsonization of microbial pathogens and by direct microbial killing [3; 4]. The pathogenesis of PAP remained enigmatic for nearly four decades after its description in 1958 until genetically manipulated mice deficient in granulocyte/macrophage-stimulating factor (GM-CSF) were serendipitously found to develop a phenotype virtually identical to PAP in humans [5; 6]. Rapidly accelerated by this seminal observation, a flurry of research subsequently elucidated the molecular pathogenesis of several distinct clinical forms of PAP accounting for the vast majority of cases. Results also identified a critical role for GM-CSF in the regulation of surfactant homeostasis, alveolar macrophage maturation and function, lung host defense, and innate immunity. Notably, this regulation is remarkably similar in humans, non-human primates and mice. This review will focus on recent progress regarding the pathogenesis of PAP and regulation of myeloid cell immune functions by GM-CSF in humans and in animal models.
GM-CSF
GM-CSF is a 23 kDa cytokine produced by various cells that binds to heterologous cell-surface receptors comprised of a low-affinity GM-CSF-binding α glycoprotein and a non-binding, affinity-converting β glycoprotein [7]. Ligand binding causes the formation of dodecahedral complexes, each containing α, β and β-associated Janus kinase 2 chains. Autophosphorylation of the latter initiates signaling through multiple pathways including the signal transducer and activator of transcription-5 (STAT5) with pleiotropic effects on myeloid cells [8; 9; 10]. Studies in GM-CSF deficient mice demonstrate that GM-CSF is required to stimulate surfactant catabolism, numerous immune functions, and the terminal differentiation of alveolar macrophages [11; 12; 13; 14; 15; 16; 17; 18].
PULMONARY SURFACTANT: PRODUCTION, CLEARANCE, AND HOMEOSTASIS
Surfactant is comprised primarily of phospholipids [19; 20; 21] that contribute to formation of surface tension-lowering lipid layer. Ten percent of surfactant is protein, including surfactant proteins (SP-A, -B, -C, -D). SP-B binds to the phospholipid bilayer contributing to the organization and packaging of surfactant in lamellar bodies during biogenesis [20; 22; 23; 24; 25]. SP-C is embedded in the phospholipid bilayer and functions in the formation and maintenance of surfactant layers at the air-liquid interface [22; 23; 26]. SP-A and SP-D are hydrophilic proteins important in pulmonary host-defense that function by opsonizing and by directly killing various microbial pathogens [4; 27]. Maintenance of surfactant pool size is vital to lung function and is tightly regulated by the balanced production, secretion, reuptake, recycling and catabolism of surfactant within alveoli (Figure 1) [28; 29]. GM-CSF is a critical regulator of surfactant catabolism in alveolar macrophages but does not regulate surfactant uptake by these cells, or uptake, catabolism, or recycling of surfactant in AEC-II [30].
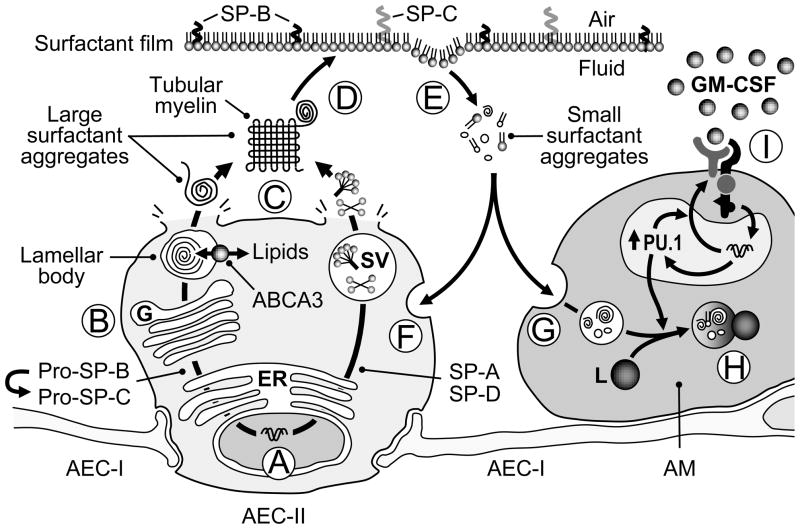
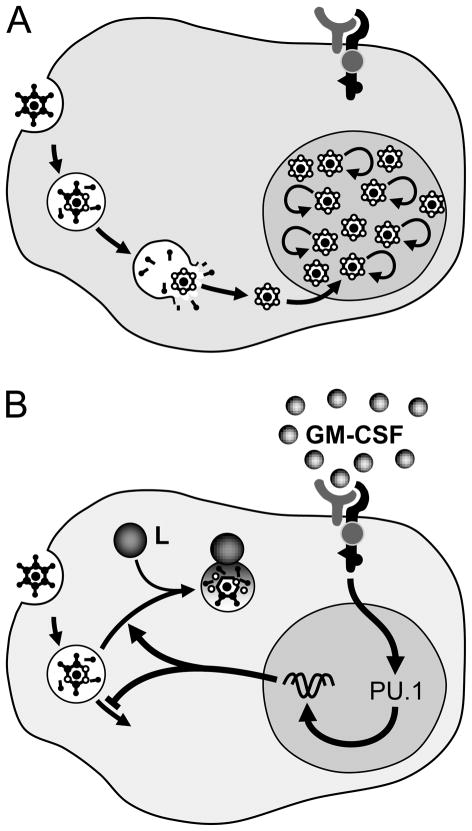
Figure 1. Schematic representation of surfactant homeostasis and regulation by GM-CSF.
Surfactant proteins A–D are synthesized in alveolar epithelial cells type II (AEC-II) (A) processed as they are transported through the endoplasmic reticulum and Golgi apparatus (B) into lamellar bodies (SP-B, SP-C) or secretory vesicles (SP-A, SP-D). Surfactant lipids are also synthesized in AEC-II and transported into lamellar bodies in part via the membrane lipid transporter ABCA3. Mature surfactant is secreted into the alveolar space via lamellar bodies and secretory vesicles (SV) where they form large surfactant aggregates, including tubular myelin (C). Tubular myelin comprises tightly packed ‘leaflets’ of surfactant that ‘unravel’ providing a source of surfactant phospholipids and proteins (D) forming surfactant mono- and multi-layers that reduce alveolar surface tension and prevent alveolar collapse.
Spent surfactant expelled from the monolayer as small surfactant aggregates (E) and approximately 70% is internalized by AEC-II (F) and 30% is internalized by alveolar macrophages (G). In AEC-II, half is recycled and half is catabolized. In alveolar macrophages, surfactant is translocated in endosomes that fuse with lysosomes (L) to form phagolysosomes where it is catabolized (H). Catabolism of surfactant lipids and proteins in alveolar macrophages requires stimulation by GM-CSF (I) via the transcription factor PU.1 by a mechanism that has not been defined.
DISORDERS OF SURFACTANT HOMEOSTASIS
Distinct clinical forms of PAP have been identified over the past two decades including autoimmune PAP caused by GM-CSF autoantibodies [31], hereditary PAP caused by GM-CSF receptor mutations [32; 33], and secondary PAP associated with various underlying clinical disorders presumed to cause the syndrome by reducing alveolar macrophage numbers or functions [34] (Figure 2). These disorders of surfactant accumulation are caused by reduced pulmonary clearance (not increased production), in most cases with minimal or no impact on alveolar architecture. The accumulated material comprises surfactant that is essentially normal in composition and function, as well as cellular debris and cytokines [35]. In each disorder, surfactant production, secretion, and reuptake are normal while surfactant catabolism in alveolar macrophages is impaired. Surfactant homeostasis is also disrupted in disorders of surfactant production (DSP), a distinct group of diseases caused by genetic mutations in the genes encoding SP-B, SP-C, or ABCA3 [36; 37; 38]. However, DSP are distinguished from PAP by the presence of surfactant dysfunction, parenchymal lung distortion, and their clinical course [37; 38; 39; 40]. The pathogenesis of each form of PAP will be discussed and that of DSP will be discussed to distinguish them from PAP.
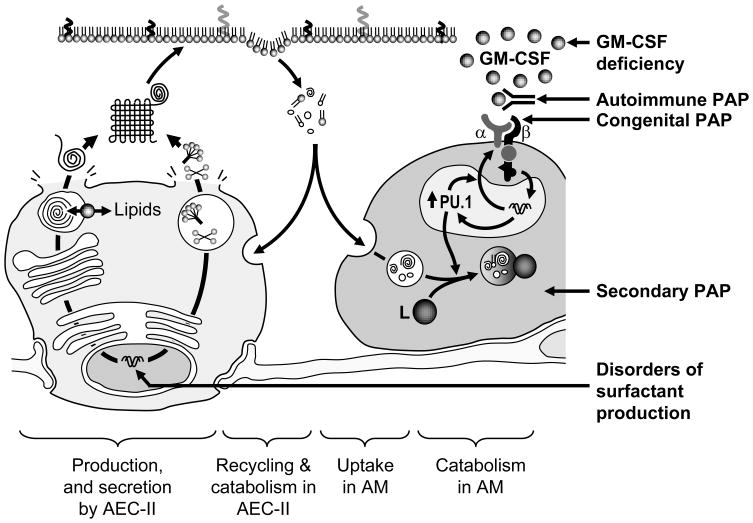
Figure 2. Disorders of homeostasis.
Surfactant homeostasis is achieved by the balanced production of surfactant in AEC-II and its recycling or catabolism in AEC-II and catabolism in alveolar macrophages. The PAP syndrome, characterized by accumulation of surfactant, can occur with disruption of GM-CSF signaling at the level of GM-CSF production as in GM-CSF deficiency in mice, or the presence of neutralizing GM-CSF autoantibodies in patients with autoimmune PAP, the homozygous presence of genetic mutations in either GM-CSF receptor α or β chains that disrupt GM-CSF signaling. In secondary PAP, the syndrome is presumed to be caused by a reduction in either the numbers or functions of alveolar macrophages caused by any one of various underlying clinical disorders. In contrast, disorders of surfactant production caused by mutations in the genes encoding SP-B, SP-C, and ABCA3 (and likely others) result in the production of biochemically and functionally abnormal surfactant that disrupts alveolar structure resulting in gross parenchymal lung distortion. See text for details.
PATHOGENESIS OF PAP DUE TO DISRUPTION OF GM-CSF SIGNALING
GM-CSF Deficient Mice
The first major clue regarding the pathogenesis of PAP was the discovery mice deficient in GM-CSF develop a lung phenotype [5; 6] that is biochemically, pathologically, physiologically, histologically, and ultrastructurally similar to human PAP. It begins at birth with development of foamy alveolar macrophages and progresses slowly over time to include a patchy distribution of lesions comprised of well-preserved alveoli filled with a fine granular, eosinophilic, periodic acid-Schiff positive material [5; 6]. The phenotype also comprises an accumulation of lymphocytes in a patchy peri-broncho-vascular distribution. The accumulated material was structurally, biochemically and functionally similar to normal surfactant [13]. Importantly, accumulation was due to reduced clearance (not increased production [5]) caused by reduced catabolism in alveolar macrophages, not AEC-II [12]. Increased levels of several cytokines (monocyte chemotactic protein 1 and macrophage-colony stimulating factor) serve as useful biomarkers of PAP [14; 41; 42; 43]. GM-CSF deficient mice have increased mortality from uncontrolled pulmonary and systemic infections [44] and increased susceptibility to a wide variety of microbial infections including Gram negative and Gram positive bacteria, malarial parasites, virus, and M. tuberculosis [44; 45; 46; 47; 48; 49].
The disruption of surfactant homeostasis, increased microbial susceptibility, and abnormal alveolar macrophage morphology and function in GM-CSF-deficient mice were corrected by replacement [50; 51; 52]. The effects of GM-CSF on surfactant homeostasis and alveolar macrophage function were found to be mediated primarily by PU.1, a transcription factor required for terminal differentiation, surfactant catabolism and multiple immune functions of alveolar macrophages [14].
GM-CSF Receptor β Deficient Mice
Mice deficient in the GM-CSF receptor β chain develop a lung phenotype similar to that of GM-CSF deficient mice [53]. GM-CSF levels are increased in serum and lung in these mice due to reduced clearance [32], a molecular observation of translational significance (see below) [54]. The disruption of surfactant homeostasis were corrected by bone marrow transplantation, demonstrating that the site of the molecular pathogenesis was cells of hematologic and not pulmonary origin, i.e., alveolar macrophages not pulmonary epithelial cells [55]
Autoimmune PAP in Humans
A second major clue regarding the pathogenesis of PAP was the strong association of high levels of neutralizing GM-CSF autoantibodies with idiopathic PAP [31], the most common clinical form representing 90% of cases [56]. This rare disease has a prevalence of 6–7 individuals per million in the general population, occurs in all ethnic groups and is approximately twice as common in males, presumably due to the association with smoking. Individuals usually present in the third to fourth decades of life with progressive dyspnea of insidious onset, diffuse bilateral lung infiltrates on radiological evaluation, and pulmonary pathology comprised of well-preserved alveoli filled with a granular, eosinophilic, periodic acid-Schiff (PAS)-positive material. Secondary infections can occur and account for 18% of the reported mortality in PAP [1], presumably due to myeloid cell defects (see below).
The GM-CSF autoantibodies are polyclonal, comprised primarily of IgG1 and IgG2, with only small amounts of IgG3 and IgG4 [57]. They recognize human GM-CSF with high avidity (20±7.5 pM) and high specificity [58] and are capable of neutralizing far more GM-CSF than is physiologically present [58]. Interestingly, GM-CSF autoantibodies have been consistently detected and comprise the major anti-cytokine activity in pharmaceutical immunoglobulin prepared from pooled serum from healthy individuals [59]. Further, GM-CSF autoantibodies were also present in healthy donors, albeit at levels (median [interquartile range] = 1.04 [0.63–1.72] μg/ml) [57] far lower than those present in PAP patients (59.8 [27.4–116.5] μg/ml). These results suggested the hypothesis that the risk of PAP is increased when GM-CSF autoantibody levels are increased above a critical threshold, which has been reported to be ~5 μg/ml [60] (Figure 3). The function of GM-CSF autoantibodies in healthy individuals is uncertain. However, the finding that GM-CSF levels in serum are higher than earlier reports suggested and that more than 99% of serum GM-CSF in healthy individuals and PAP patients exists in the form of immune complexes suggesting that GM-CSF autoantibodies may have a scavenging role [57].
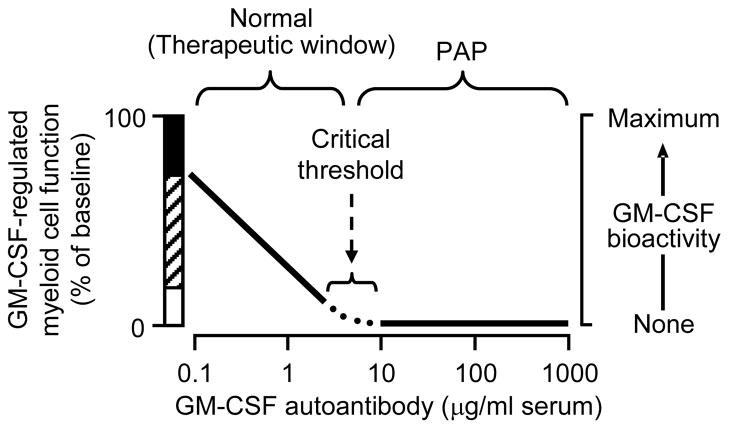
Figure 3. Relationship between GM-CSF autoantibody concentration, GM-CSF bioactivity, and GM-CSF dependent myeloid functions.
At GM-CSF autoantibody concentrations below the critical threshold, GM-CSF bioactivity and GM-CSF dependent functions (i.e., CD11b stimulation, neutrophil phagocytosis) vary inversely with autoantibody concentration. At and above the critical threshold, GM-CSF bioactivity and GM-CSF-dependent functions are reduced minimum values (i.e., alveolar macrophage catabolism of surfactant). The hatched region represents the inverse relationship; the black bar represents priming caused by supranormal GM-CSF levels present during infection or exogenous administration; the white bar represents residual, non-zero minimal values of some function (e.g., phagocytosis). The critical threshold concept provides an explanation of why a high level of GM-CSF autoantibody is virtually diagnostic of autoimmune PAP and yet autoantibody levels (which are generally well above the critical threshold value) do not reflect lung disease severity. Adapted from reference [57].
Reproduction of Autoimmune PAP in Healthy, Non-Human Primates
In the context of data from GM-CSF deficient mice, the strong association of neutralizing GM-CSF autoantibodies in patients with PAP is compelling evidence suggesting a potential role in pathogenesis. However, their presence in healthy individuals and pharmaceutical immunoglobulin prepared from healthy individuals was troubling. This problem was addressed in translational studies in which GM-CSF autoantibodies were isolated from a patient with PAP and injected into healthy, non-human primates [61]. Serum GM-CSF autoantibody levels were maintained above 40 μg/ml for up to ten months resulted in the reproduction of the biochemical, cellular, and histopathologic features of PAP. The first abnormality observed was the accumulation of surfactant within alveolar macrophages and was followed by progressive accumulation of intraalveolar surfactant. This provided a ‘first look’ at the initiation of PAP, since clinically, patients only present after surfactant has accumulated to sufficient levels to impair gas exchange (a late complication). These data confirm GM-CSF autoantibodies as the pathogenic driver of the common clinical form of PAP, suggesting it can be appropriately called autoimmune PAP.
Hereditary PAP
PAP associated with non-detection of GM-CSF receptor β chains on blood leukocytes was reported in three infants presenting with respiratory failure [62]. All had the typical radiological manifestations and two had histopathologic findings similar to GM-CSF receptor β chain deficient mice and autoimmune PAP. Altered GM-CSF signaling was demonstrated by ligand binding studies and progenitor clonogenic assays. CSF2RA mRNA and protein was detectable in all three patients. A point mutation within CSF2RB encoding the GM-CSF receptor β chain was detected in one patient, but was subsequently shown to be present in 6% of 184 multinational healthy individuals (NCPI single nucleotide polymorphism (SNP) Database ID rs1801122 (http://www.genecards.org)) and thus, was not pathogenic. Notwithstanding, this report suggested the GM-CSF receptor β chain was critical in surfactant homeostasis in humans and in the pathogenesis of hereditary PAP.
Hereditary PAP caused by abnormalities or absence of GM-CSF receptor α was reported in three children [32; 33]. One child [32] developed progressive dyspnea of insidious onset by 3–4 years and diffuse lung disease and respiratory insufficiency by 6 years. Lung histopathology was similar to autoimmune PAP and GM-CSF deficient mice. However, GM-CSF autoantibodies were absent and GM-CSF levels were elevated in the lungs and serum. The GM-CSF receptor β chain was present and had a normal nucleotide sequence. However, the α chain had a reduced molecular weight, abnormal glycosylation pattern, reduced GM-CSF binding, and impaired activation of STAT5 [32]. Karyotyping, genomic chromosomal hybridization, and high resolution SNP analysis revealed a 1.6 Mb deletion in the pseudo-autosomal region at Xp22.33 encompassing the maternal CSF2RA allele, and a point mutation (G196R) in the paternal CSF2RA allele that altered glycosylation of the GM-CSF receptor α peptide and markedly reduced GM-CSF binding. This child’s asymptomatic 8-year-old sister had increased serum GM-CSF and SP-D (both markers of PAP lung disease severity) and subsequent radiological and pulmonary function abnormalities demonstrated she had PAP. A 4 year old child developed PAP in association with Turner’s syndrome and loss of both CSF2RA alleles [33]. Subsequent screening of pediatric patients with unexplained PAP (i.e., who were negative for GM-CSF autoantibody) for an elevated serum GM-CSF level resulted in identification of 5 additional patients aged 3–11 years of age with PAP caused by various CSF2RA mutations (B.C.T., unpublished results). Together, these cases indicate that loss of GM-CSF receptor function causes hereditary PAP, but that additional factors determine the severity of lung disease in PAP.
PATHOGENESIS OF SECONDARY PAP
Secondary PAP is defined clinically as the occurrence of the PAP syndrome in an individual with an underlying disease known to be associated with the development of PAP [2]. The diagnosis is usually made based on the presence of typical radiological manifestations and lung histopathological findings. Secondary PAP has been reported in association with various diverse clinical disorders, including hematological disorders (myelodysplastic syndrome, leukemia, lymphoma, aplastic anemia, pharmacologically induced leukopenia, others), immunological diseases (severe combined immunodeficiency, monoclonal gammopathy, selective immunoglobulin A deficiency, others), lysinuric protein intolerance, and infections (Cytomegalovirus, Mycobacterium tuberculosis, Nocardia, Pneumocystis jiroveci, others) [34; 62; 63; 64; 65; 66; 67; 68; 69; 70; 71; 72; 73; 74; 75; 76; 77; 78; 79; 80]. It has also been reported in association with various toxic inhalation syndromes, including inhalation of inorganic dusts (silica, cement, titanium, and aluminum), organic dusts (sawdust, fertilizer, bakery flour, others), and fumes (chlorine, varnish, others) [81; 82; 83; 84; 85; 86; 87; 88]. In a national cross-sectional study of 248 adult individuals with PAP syndrome in Japan, secondary PAP accounted for 9.7% of the cases [56]. Accurate diagnosis of secondary PAP is important since its prognosis is worse than that of autoimmune PAP (Koh Nakata, personal communication).
The pathogenesis of secondary PAP is poorly understood and in some cases the strength of the disease-association is questionable. In one report, individuals with myelodysplasia who developed PAP had functional defects in GM-CSF signaling suggesting a pathogenesis based on disruption GM-CSF stimulation of alveolar macrophage-mediated surfactant clearance [89]. In other reports, correlation of the onset and resolution of PAP with the onset and resolution of severe reductions in myeloid cell numbers [68] suggested the pathogenesis was based on reduction of the numbers of alveolar macrophages reducing their capacity for removing surfactant from the lungs. Together, these results suggest that secondary PAP may be caused by an acquired loss of GM-CSF signaling, reduced alveolar macrophage numbers, or alveolar macrophage dysfunction.
Murine Models of Secondary PAP
Murine models of secondary PAP have been reported. Rats exposed to inhaled silica develop PAP [90] although the mechanism was not determined. PAP also develops in mice with severe combined immunodeficiency, again without determination of the mechanism involved [91]. A recent study reported that use of dichloromethylene diphosphonic acid-encapsulated liposomes to reduce alveolar macrophage numbers increased pulmonary surfactant levels [92]. Although limited, these data support the hypothesis that secondary PAP occurs as a consequence of disorders that reduce either the numbers or functions of alveolar macrophages.
PATHOGENESIS OF DISORDERS OF SURFACTANT PRODUCTION
Disorders of surfactant production are considered briefly here since they comprise disorders of surfactant homeostasis that are sometimes confused with PAP (Figure 2). These disorders differ widely in presentation, natural history, pathogenesis, prognosis and therapeutic options.
SP-B deficiency presents as unexplained acute respiratory failure in full-term neonates within minutes to hours after birth and is uniformly fatal without intervention with mechanical ventilation [36]. Alveolar development and structure are normal immediately prior to birth. Since SP-B is required for processing of pro-SP-C, mature SP-C is also reduced resulting in deficiency of both of hydrophobic proteins critical for lowering alveolar surface tension [22]. Consequently, adaptation to air-breathing immediately after birth results in alveolar collapse [39], distorting the alveolar anatomy. Thus, the clinical manifestations are a consequence of surfactant deficiency and dysfunction and not of excessive surfactant accumulation as in PAP. Function altering recessive mutations in the gene encoding ABCA3, an integral membrane lipid transporter located on the limiting membrane of lamellar vesicles in AEC-II, result in various clinical presentations ranging from respiratory failure and death in neonates to interstitial lung disease in adolescents [36; 37; 38; 39; 40]. Lamellar bodies are abnormal in appearance and result in biochemically abnormal surfactant that is dysfunctional [93].
Spontaneous and hereditary mutations in the gene encoding SP-C result in a poorly defined interstitial lung disease in children and adults that can cause respiratory failure and death. This disease results in gross distortion of lung structure due to widening of alveolar walls and extensive fibrosis.
Animal models of function-altering mutations in SFTPB (encoding SP-B), SFTPC (encoding SP-C), and ABCA3 have generally reproduced the corresponding human diseases faithfully [94; 95; 96].
In summary, disorders of surfactant production disrupt surfactant homeostasis by virtue of deficiency of one or more surfactant components that impairs surfactant function, resulting in, parenchymal distortion, fibrosis, and respiratory insufficiency. While a variable degree of accumulation of abnormal surfactant can occur, these disorders are distinguished from PAP based on differences in their clinical presentation, surfactant dysfunction, lung histopathology, natural history, and generally poorer prognosis [40].
THERAPY FOR DISORDERS OF SURFACTANT HOMEOSTASIS
Current standard therapy of autoimmune PAP is whole lung lavage, a procedure performed under general anesthesia in which one lung is mechanically ventilated while the other is repeatedly filled with saline with intensive chest percussion to emulsify the accumulated surfactant and then drained to physically remove it [97]. This is effective in nearly all patients although is required repeatedly in many due to continued surfactant accumulation [1]. It is effective in hereditary PAP[32] and, though less well studied, also in secondary PAP [98]. Advances in the pathogenesis spawned several new therapeutic approaches for autoimmune PAP. These include plasmapheresis [99], GM-CSF augmentation [100; 101; 102; 103; 104], and rituximab [105; 106]. All are aimed at restoring signaling by GM-CSF, the first by removing the GM-CSF-blocking autoantibody, the second by augmenting GM-CSF beyond the neutralizing capacity of the antibody, and the third by depleting autoantibody-producing B lymphocytes. All of these potential therapies are in current clinical testing. Bone marrow transplantation has been attempted for some forms of secondary PAP and hereditary PAP [33]. There is currently no specific therapy for DSP other than lung transplantation, which has been successful in SP-B deficiency.
THE EMERGING ROLE OF GM-CSF IN IMMUNE REGULATION
Secondary Infections In PAP
Microbial infections account for 18% of reported deaths attributable to PAP [1]. These infections occur at pulmonary and extrapulmonary sites, indicating that the predisposition to infection is systemic, rather than confined to the lungs [73; 107; 108; 109]. Although organisms common in community- and hospital-acquired infections have been reported in PAP (e.g., Streptococcus, Klebsiella, Hemophilus, Staphylococcus, Pseudomonas, Serratia, Proteus, Escherichia), more often reports have identified ‘unusual’ or opportunistic microbial pathogens (Mycobacteria, Aspergillus, Nocardia and others) [1]. GM-CSF deficient mice also have increased mortality from uncontrolled pulmonary and systemic infections and increased susceptibility to a wide variety of microbial pathogens including Gram positive and Gram negative bacteria (Streptococcus, Pseudomonas. aeruginosa, Listeria monocytogenes), fungi (Pneumocystis jiroveci), mycobacteria (M. Tuberculosis), malarial parasites (Plasmodium chabaudi), and virus (adenovirus) [44; 45; 46; 47; 48; 49]. The increased pulmonary infection risk in GM-CSF deficient mice can be rescued by expression of GM-CSF specifically in the lungs [47]. Interestingly, overexpression of GM-CSF in the lungs of mice reduced the susceptibility to bacterial lung infection beyond that of wild type mice resulting in mice that were ‘super-resistant’ to infection [47]. Together, studies in man and mice with disrupted GM-CSF signaling demonstrate that GM-CSF plays an important role in immunity through regulation of myeloid cell maturation and activation and the recognition, molecular signaling responses, phagocytosis, and clearance of microbial pathogens.
GM-CSF Regulation of Alveolar Macrophage Immune Functions
Alveolar macrophages from GM-CSF deficient mice have a number of functional and mechanistic defects, including impaired adhesion, expression of pathogen recognition- and other receptors, phagocytosis, reactive oxygen species generation, microbial killing, and pathogen-stimulated proinflammatory and chemokine secretion. These defects are rescued by expression of GM-CSF in the lungs of transgenic mice, demonstrating that the presence of GM-CSF in the lungs is required for normal alveolar macrophage immune functions [14]. Alveolar macrophages from GM-CSF deficient mice have reduced expression of PU.1, a macrophage ‘master’ transcription factor involved in macrophage differentiation [14; 17]. The level of GM-CSF in the lungs determines the level of PU.1 expression in alveolar macrophages [14]. Expression of PU.1 in alveolar macrophages from GM-CSF deficient mice rescues the alveolar macrophage innate immune functions [14]. The presence of elevated levels of GM-CSF autoantibodies in patients with autoimmune PAP or healthy non-human primates reduces PU.1 mRNA levels and innate immune functions of alveolar macrophages (97 and 98). GM-CSF augmentation therapy rescues PU.1 expression and innate immune functions in humans [110]. Together, these and other results strongly support the conclusion that GM-CSF, via PU.1, stimulates the terminal differentiation of alveolar macrophages in the lungs and for the acquisition of normal immune functions [14; 57; 61; 111].
GM-CSF has pleiotropic effects on myeloid cells, regulating survival, proliferation, differentiation, priming, and activation. Recent advances in our understanding of GM-CSF receptor structure and function now provide an explanation for this pleiotropy. The dose-dependent effects of GM-CSF are mediated in a mutually exclusive, reciprocal fashion through two β-chain residues: Ser585 at low GM-CSF levels and Tyr577 at high levels [112]. Thus, the GM-CSF receptor acts as a binary switch promoting cell survival and differentiation at low doses and proliferation and antimicrobial functions at high levels. GM-CSF bioactivity is at the center of the mechanism that regulates myeloid functions. Low levels of GM-CSF present in normal healthy tissues promote the terminal differentiation of tissue macrophages while high levels typically present at sites of inflammation promote macrophage priming and enhance the macrophage activation response.
GM-CSF is important in the regulation of molecular signaling pathways involved in the innate immune recognition of microbial pathogens. GM-CSF deficient mice have reduced local and systemic responses to lipopolysaccharide (LPS) exposure in vivo and in vitro [15]. Specifically, the LD50 of intravenous LPS administration is increased in GM-CSF deficient mice compared to strain-matched wild type mice [113]. Pulmonary administration also results in lower levels of LPS-stimulated proinflammatory cytokines (e.g., TNFα), chemokines (e.g., MIP-1), and pulmonary neutrophil recruitment in GM-CSF deficient mice compared to control mice [15]. Administration of neutralizing anti-GM-CSF antibodies to wild type mice also results in lower levels of LPS-stimulated molecular and cellular inflammation compared to controls [114]. These findings can be reproduced in vitro by exposure of isolated alveolar macrophages to LPS, which results in reduced expression of inflammatory cytokines and chemokines [15]. Further, the proinflammatory responses and mortality from experimental peritonitis or intraperitoneal LPS exposure were blunted in GM-CSF deficient mice compared to controls [115]. Normal responses were rescued by intraperitoneal instillation of wild type macrophages or macrophages from GM-CSF deficient mice retrovirally transduced to express PU.1. Since the pathways involved in the molecular recognition of LPS have been defined [116], the effects of GM-CSF on this innate response were studied in detail. The low levels of GM-CSF present physiologically were found to enable TLR-4 signaling in macrophages by stimulating expression of several components of this pathway (Figure 4). GM-CSF also regulates the expression of other pathogen associated molecular pattern (PAMP) receptors on macrophages including TLR2, mannose receptors, and scavenger receptors as well as receptors recognizing the Fc motif of ligand-bound immunoglobulin (FcγI, FcγII, and FcγIII) [14; 15; 16; 17; 18]. These results indicate that GM-CSF plays an important role in the response to infection by regulating the innate immune recognition and initial inflammatory signaling responses of macrophages to microbial infection.
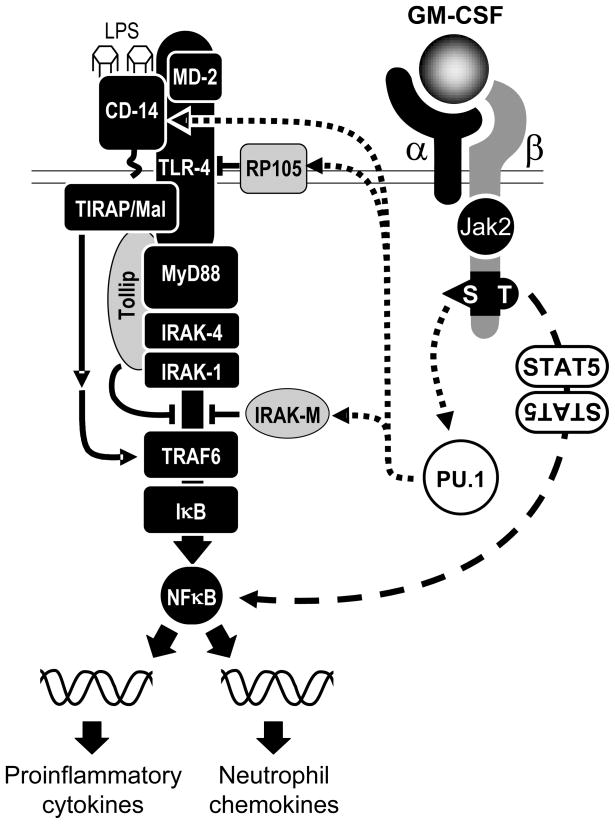
Figure 4. Schematic of proposed mechanism by which GM-CSF regulates LPS responses in macrophages.
GM-CSF initially binds to the low-affinity GM-CSF receptor α chain (α), followed by association with the affinity-enhancing β chain (β) and constitutively associated Janus kinase 2 (JAK 2) resulting in assembly of a dodecameric structure signaling complex.
At low concentrations of GM-CSF (<300 pg/ml), as are normally present in normal healthy tissues, signaling occurs through Ser585 of the β chain and results in myeloid cell survival and GM-CSF stimulated expression of some GM-CSF-dependent TLR-4 signaling pathway components, CD14, RP105, and IRAK-M (dotted lines); other components are not affected by GM-CSF. Both GM-CSF-dependent and GM-CSF-independent components are required for normal responses to LPS. At high concentrations of GM-CSF (>300 pg/ml), signaling via Ser585 is extinguished and signaling occurs exclusively via residue Tyr577, activating STAT5 signaling, which enhances NFκB activity and the LPS response. Adapted from reference [15].
GM-CSF also regulates molecular communications between innate and adaptive immune responses, e.g. interleukin-18 (IL-18)/interferon-γ (IFN-γ) axis. Normally, macrophages exposed to microbial pathogens (e.g. adenovirus) respond by secretion of IL-18, which in turn stimulates Th1 and NK cells to secrete IFNγ [16; 117]. IFNγ has pleiotropic effects on innate and adaptive immunity, for example, enhancement of Fcγ receptor expression, phagocytosis and other host defense functions of macrophages and T and B lymphocytes. In GM-CSF deficient mice, stimulation of IFNγ production by adenovirus-exposed macrophages was markedly reduced due to their reduced capacity for secretion of IL-18 and IL-12. GM-CSF deficient mice also have blunted responses in collagen-induced arthritis [118] and experimental allergic encephalitis [119], which has stimulated clinical interest in the potential use of GM-CSF autoantibodies to treat inflammatory and autoimmune diseases [120]. The molecular basis of such complex inflammatory responses involving macrophages is currently the subject of active investigation.
GM-CSF is also important in the capacity of macrophages to internalize a wide range of microbial pathogens and particulates. For example, macrophages from GM-CSF deficient mice have reduced phagocytic internalization of microbial pathogens [14; 16; 17]. Consistent with the reduction in Fc receptor levels in these mice, Fc receptor-mediated phagocytosis of latex microspheres by macrophages is reduced. However, this effect can not be completely explained by changes in Fc receptor expression since phagocytosis of unopsonized latex beads and zymosan is also impaired. Further, impaired uptake is more pronounced for larger (i.e. 2–10 μm) beads than for smaller (< 100 nm) beads and uptake of bacteria (~ 0.5 μm) is more severely affected than that of adenoviral virions (~100 Å diameter). Since macrophages internalize adenovirus by endocytosis, these results suggest GM-CSF may have a differential effect on phagocytosis and endocytosis. This hypothesis is supported by the observation that endocytosis of surfactant by alveolar macrophages is not impaired in GM-CSF deficient mice or patients with either autoimmune PAP or hereditary PAP. The phagocytic defect of alveolar macrophages in GM-CSF deficient mice was corrected in vivo by restoration of GM-CSF in the lungs [16] and in vitro by retroviral expression of PU.1 [14], demonstrating that GM-CSF, via PU.1, critically regulates phagocytosis of microbial pathogens by macrophages in mice. Phagocytosis of bacteria, zymosan and latex particles by alveolar macrophages from human PAP patients is similarly impaired [57]. While GM-CSF has marked effects on macrophage cytoskeletal organization and cell morphology, the precise mechanism(s) by which phagocytosis is regulated by GM-CSF is not known.
GM-CSF plays a critical role in the killing and pulmonary clearance of various extracellular and obligate intracellular microbial pathogens, including Streptococcus, Pseudomonas, Pneumocystis, Mycobacterium, and Adenovirus [14; 18; 45; 46; 47]. For each of these organisms, GM-CSF expression in the lungs is required to control the lung infection and to enable clearance. Further, increased levels of pulmonary GM-CSF in transgenic mice confer an increased capacity for pulmonary clearance by increasing both the numbers and intrinsic antimicrobial activity of alveolar macrophages. In isolated macrophages from GM-CSF deficient mice, defective killing of Gram negative and Gram positive bacteria are regulated by PU.1, independent of effects on phagocytosis [14]. Although the precise mechanism responsible is not known, GM-CSF regulates several important antimicrobial activities in macrophages including the production of reactive oxygen species (e.g., superoxide and hydrogen peroxide radicals), expression of antimicrobial enzymes (e.g., lysozyme, other proteases) and intracellular translocation to sites of destruction ([47], and unpublished observation). Adenoviral infection provides a useful example of the latter [18] (Figure 5).
Figure 5. GM-CSF, via PU.1, uncouples microbial uptake from infection.
A. Schematic of adenoviral infection pathway in normal epithelial cells and alveolar macrophages from GM-CSF deficient mice. Virions are internalized by receptor-mediated endocytosis and rapidly escape endosomal confinement via endosome-lysis, a mechanism dependent on both viral and host factors. Virions are then efficiently translocated to the nucleus where the nuclear injection of the viral genome results in viral replication and transduction of the cell. B. In alveolar macrophages from normal mice or from GM-CSF deficient mice after retroviral-transduction to restore PU.1 expression, adenoviral virions are rapidly internalized but cannot escape endosomal confinement, and undergo fusion with lysosomes forming phagolysosomes where virions are nearly completely destroyed without transduction of the cell. Ectopic expression of PU.1 in epithelial cells also blocks endosomal escape, and nuclear transduction and promotes viral clearance.
GM-CSF Regulation of Neutrophil Immune Functions
Prompted by the implication of a possible defect in systemic immunity in patients with autoimmune PAP and in GM-CSF deficient mice, functional defects were identified in circulating neutrophils from both [111]. The defects comprise impaired cellular adhesion, reactive oxygen species production, phagocytosis, and bacterial killing [111]. Notably, the differential pattern of impairment in these various functions varied widely among the different functions but was strikingly similar between the two species, suggesting that GM-CSF regulates neutrophil functions similarly in man and mice (see figure 1d of reference [111]). Importantly, the phagocytic defect was reproduced in healthy mice by injection of anti-mouse GM-CSF antibodies and in healthy non-human primates by injection of PAP patient-derived GM-CSF autoantibodies. The defect in cellular adhesion was partially explained by complete inhibition of the GM-CSF-stimulated increase in cell surface levels of the adhesion molecule CD11b in PAP patients and in GM-CSF autoantibody-injected non-human primates [61]. The defects in autoimmune PAP patients were associated with loss of GM-CSF receptor signaling as judged by the inability of GM-CSF to stimulate phosphorylation of the immediately downstream signaling molecule STAT5. However, the precise mechanisms underlying these abnormalities are currently unknown. Notwithstanding, disruption of GM-CSF signaling does not appear to affect neutrophil differentiation because patients with autoimmune PAP had normal PU.1 levels in their neutrophils, cell surface differentiation markers, morphology, and numbers of circulating neutrophils. Thus, GM-CSF plays a critical role in regulating the basal functional capacity of mature circulating neutrophils, providing a potential explanation for the increased systemic infection risk in PAP patients and GM-CSF deficient mice.
CONCLUSIONS
Disruption of GM-CSF signaling causes a syndrome of biochemical, cellular, physiological, and histopathologic abnormalities that are remarkably similar irrespective of the pathogenic mechanism (i.e., autoimmune or genetic), site of interruption (i.e., GM-CSF production, neutralization of GM-CSF bioactivity, or disruption of α or β receptor function), or species involved (human, non-human primates, or mice). The resulting syndrome is primarily one in which structurally normal alveoli become progressively filled with essentially normal surfactant, which can be successfully treated in the vast majority of human patients by whole lung lavage therapy. The pathogenesis of secondary PAP is poorly understood but may be caused by multiple mechanisms that reduce surfactant clearance capacity of the resident alveolar macrophage population. The actual association of various clinical disorders with an increased risk of secondary PAP is more certain for some but not all reported disorders. The utility of whole lung lavage therapy in secondary PAP is less well established but is clearly useful in some cases and should be considered. In marked contrast to PAP, disorders of surfactant production are characterized by production of structurally and functionally abnormal surfactant that fails to fulfill the important task of alveolar stabilization leading to gross parenchymal lung distortion. The resulting lung diseases range from acute respiratory failure with the first breath of life to insidious onset of interstitial lung disease and pulmonary fibrosis in adults. They actually comprise surfactant deficiencies rather than surfactant excess and do not respond to whole lung lavage therapy. Thus PAP and disorders of surfactant production are most appropriately considered as separate and distinct groups of diseases and not as part of a spectrum of the PAP syndrome. Our knowledge of the pathogenesis of these disorders has advanced tremendously. Notwithstanding, inconsistent use of multiple, overlapping terms for poorly defined groups of patients comprising various clinical subtypes of PAP and disorders of surfactant production is confusing and misleading. Further compounding this is the use of a classification system based on pathogenesis (autoimmune PAP, hereditary PAP caused by GM-CSF receptor mutations, SP-B deficiency) and also based on clinical description (secondary PAP). Future studies should focus to the pathogenic mechanisms of secondary PAP. Basic, clinical and translational PAP research over the past two decades have identified the critical role of GM-CSF in myeloid cell-mediated immunity, the implications of which extend far beyond this rare syndrome.
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute (HL0085453) and the National Center for Research Resources (to B.C.T).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med. 2002;166:215–35. doi: 10.1164/rccm.2109105. [DOI] [PubMed] [Google Scholar]
- 2.Trapnell BC, Whitsett JA, Nakata K. Pulmonary Alveolar Proteinosis. N Engl J Med. 2003;349:2527–39. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 3.McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest. 2002;109:707–12. doi: 10.1172/JCI15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright JR. Host defense functions of pulmonary surfactant. Biol Neonate. 2004;85:326–32. doi: 10.1159/000078172. [DOI] [PubMed] [Google Scholar]
- 5.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–6. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 6.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994;91:5592–6. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton JA, Anderson GP. GM-CSF Biology. Growth Factors. 2004;22:225–31. doi: 10.1080/08977190412331279881. [DOI] [PubMed] [Google Scholar]
- 8.Hercus TR, Thomas D, Guthridge MA, Ekert PG, King-Scott J, Parker MW, Lopez AF. The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood. 2009;114:1289–98. doi: 10.1182/blood-2008-12-164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen G, Hercus TR, McClure BJ, Stomski FC, Dottore M, Powell J, Ramshaw H, Woodcock JM, Xu Y, Guthridge M, McKinstry WJ, Lopez AF, Parker MW. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell. 2008;134:496–507. doi: 10.1016/j.cell.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 10.Guthridge MA, Powell JA, Barry EF, Stomski FC, McClure BJ, Ramshaw H, Felquer FA, Dottore M, Thomas DT, To B, Begley CG, Lopez AF. Growth factor pleiotropy is controlled by a receptor Tyr/Ser motif that acts as a binary switch. Embo J. 2006;25:479–89. doi: 10.1038/sj.emboj.7600948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida M, Ikegami M, Reed JA, Chroneos ZC, Whitsett JA. GM-CSF regulates surfacant Protein-A and lipid catabolism by alveolar macrohpages. Am J Physiol Lung Cell Mol Physiol. 2001;280:L379–L386. doi: 10.1152/ajplung.2001.280.3.L379. [DOI] [PubMed] [Google Scholar]
- 12.Ikegami M, Jobe AH, Huffman Reed JA, Whitsett JA. Surfactant metabolic consequences of overexpression of GM-CSF in the epithelium of GM-CSF-deficient mice. Am J Physiol. 1997;273:L709–14. doi: 10.1152/ajplung.1997.273.4.L709. [DOI] [PubMed] [Google Scholar]
- 13.Ikegami M, Ueda T, Hull W, Whitsett JA, Mulligan RC, Dranoff G, Jobe AH. Surfactant metabolism in transgenic mice after granulocyte macrophage-colony stimulating factor ablation. Am J Physiol. 1996;270:L650–8. doi: 10.1152/ajplung.1996.270.4.L650. [DOI] [PubMed] [Google Scholar]
- 14.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15:557–67. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 15.Berclaz PY, Carey B, Fillipi MD, Wernke-Dollries K, Geraci N, Cush S, Richardson T, Kitzmiller J, O’Connor M, Hermoyian C, Korfhagen T, Whitsett JA, Trapnell BC. GM-CSF regulates a PU.1-dependent transcriptional program determining the pulmonary response to LPS. Am J Respir Cell Mol Biol. 2007;36:114–21. doi: 10.1165/rcmb.2006-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berclaz PY, Shibata Y, Whitsett JA, Trapnell BC. GM-CSF, via PU.1, regulates alveolar macrophage Fcgamma R-mediated phagocytosis and the IL-18/IFN-gamma-mediated molecular connection between innate and adaptive immunity in the lung. Blood. 2002;100:4193–200. doi: 10.1182/blood-2002-04-1102. [DOI] [PubMed] [Google Scholar]
- 17.Berclaz PY, Zsengeller Z, Shibata Y, Otake K, Strasbaugh S, Whitsett JA, Trapnell BC. Endocytic Internalization of Adenovirus, Nonspecific Phagocytosis, and Cytoskeletal Organization Are Coordinately Regulated in Alveolar Macrophages by GM-CSF and PU.1. J Immunol. 2002;169:6332–42. doi: 10.4049/jimmunol.169.11.6332. [DOI] [PubMed] [Google Scholar]
- 18.Carey B, Staudt MK, Bonaminio D, van der Loo JC, Trapnell BC. PU.1 Redirects Adenovirus to Lysosomes in Alveolar Macrophages, Uncoupling Internalization from Infection. J Immunol. 2007;178:2440–7. doi: 10.4049/jimmunol.178.4.2440. [DOI] [PubMed] [Google Scholar]
- 19.Batenburg JJ. Surfactant phospholipids: synthesis and storage. Am J Physiol. 1992;262:L367–85. doi: 10.1152/ajplung.1992.262.4.L367. [DOI] [PubMed] [Google Scholar]
- 20.Serrano AG, Perez-Gil J. Protein-lipid interactions and surface activity in the pulmonary surfactant system. Chem Phys Lipids. 2006;141:105–18. doi: 10.1016/j.chemphyslip.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Veldhuizen R, Nag K, Orgeig S, Possmayer F. The role of lipids in pulmonary surfactant. Biochim Biophys Acta. 1998;1408:90–108. doi: 10.1016/s0925-4439(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 22.Weaver TE. Synthesis processing and secretion of surfactant proteins B and C. Biochim Biophys Acta. 1998;1408:173–9. doi: 10.1016/s0925-4439(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 23.Weaver TE, Conkright JJ. Function of surfactant proteins B and C. Annu Rev Physiol. 2001;63:555–78. doi: 10.1146/annurev.physiol.63.1.555. [DOI] [PubMed] [Google Scholar]
- 24.Whitsett JA, Nogee LM, Weaver TE, Horowitz AD. Human surfactant protein B: structure, function, regulation, and genetic disease. Physiol Rev. 1995;75:749–57. doi: 10.1152/physrev.1995.75.4.749. [DOI] [PubMed] [Google Scholar]
- 25.Hawgood S, Derrick M, Poulain F. Structure and properties of surfactant protein B. Biochim Biophys Acta. 1998;1408:150–60. doi: 10.1016/s0925-4439(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 26.Beers MF, Fisher AB. Surfactant protein C: a review of its unique properties and metabolism. Am J Physiol. 1992;263:L151–60. doi: 10.1152/ajplung.1992.263.2.L151. [DOI] [PubMed] [Google Scholar]
- 27.Kingma PS, Whitsett JA. In defense of the lung: surfactant protein A and surfactant protein D. Curr Opin Pharmacol. 2006;6:277–83. doi: 10.1016/j.coph.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Jobe AH, Ikegami M. Surfactant metabolism. Clin Perinatol. 1993;20:683–96. [PubMed] [Google Scholar]
- 29.Wright JR. Clearance and recycling of pulmonary surfactant. Am J Physiol. 1990;259:L1–12. doi: 10.1152/ajplung.1990.259.2.L1. [DOI] [PubMed] [Google Scholar]
- 30.Trapnell BC, Whitsett JA. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 31.Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y, Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–80. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki T, Sakagami T, Rubin BK, Nogee LM, Wood RE, Zimmerman SL, Smolarek T, Dishop MK, Wert SE, Whitsett JA, Grabowski G, Carey BC, Stevens CA, Van der Loo JCM, Trapnell BC. Familial Pulmonary Alveolar Proteinosis Caused by Mutations in CSF2RA. J Exp Med. 2008;205:2703–2710. doi: 10.1084/jem.20080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Moczygemba M, Doan ML, Elidemir O, Fan LL, Cheung SW, Lei JT, Moore JP, Tavana G, Lewis LR, Zhu Y, Muzny DM, Gibbs RA, Huston DP. Pulmonary alveolar proteinosis caused by deletion of the GM-CSFRalpha gene in the X chromosome pseudoautosomal region 1. J Exp Med. 2008;205:2711–6. doi: 10.1084/jem.20080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ladeb S, Fleury-Feith J, Escudier E, Tran Van Nhieu J, Bernaudin JF, Cordonnier C. Secondary alveolar proteinosis in cancer patients. Support Care Cancer. 1996;4:420–6. doi: 10.1007/BF01880639. [DOI] [PubMed] [Google Scholar]
- 35.Brasch F, Birzele J, Ochs M, Guttentag SH, Schoch OD, Boehler A, Beers MF, Muller KM, Hawgood S, Johnen G. Surfactant proteins in pulmonary alveolar proteinosis in adults. Eur Respir J. 2004;24:426–35. doi: 10.1183/09031936.04.00076403. [DOI] [PubMed] [Google Scholar]
- 36.Nogee LM. Alterations in SP-B and SP-C expression in neonatal lung disease. Annu Rev Physiol. 2004;66:601–23. doi: 10.1146/annurev.physiol.66.032102.134711. [DOI] [PubMed] [Google Scholar]
- 37.Nogee LM. Genetic mechanisms of surfactant deficiency. Biol Neonate. 2004;85:314–8. doi: 10.1159/000078171. [DOI] [PubMed] [Google Scholar]
- 38.Nogee LM. Genetics of pediatric interstitial lung disease. Curr Opin Pediatr. 2006;18:287–92. doi: 10.1097/01.mop.0000193310.22462.1f. [DOI] [PubMed] [Google Scholar]
- 39.Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347:2141–8. doi: 10.1056/NEJMra022387. [DOI] [PubMed] [Google Scholar]
- 40.Whitsett JA, Wert SE, Trapnell BC. Genetic disorders influencing lung formation and function at birth. Hum Mol Genet. 2004;13(Spec No 2):R207–15. doi: 10.1093/hmg/ddh252. [DOI] [PubMed] [Google Scholar]
- 41.Iyonaga K, Suga M, Yamamoto T, Ichiyasu H, Miyakawa H, Ando M. Elevated bronchoalveolar concentrations of MCP-1 in patients with pulmonary alveolar proteinosis. Eur Respir J. 1999;14:383–9. doi: 10.1034/j.1399-3003.1999.14b24.x. [DOI] [PubMed] [Google Scholar]
- 42.Paine R, 3rd, Morris SB, Jin H, Wilcoxen SE, Phare SM, Moore BB, Coffey MJ, Toews GB. Impaired functional activity of alveolar macrophages from GM-CSF- deficient mice. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1210–8. doi: 10.1152/ajplung.2001.281.5.L1210. [DOI] [PubMed] [Google Scholar]
- 43.Bonfield TL, Russell D, Burgess S, Malur A, Kavuru MS, Thomassen MJ. Autoantibodies against granulocyte macrophage colony-stimulating factor are diagnostic for pulmonary alveolar proteinosis. Am J Respir Cell Mol Biol. 2002;27:481–6. doi: 10.1165/rcmb.2002-0023OC. [DOI] [PubMed] [Google Scholar]
- 44.Seymour JF, Lieschke GJ, Grail D, Quilici C, Hodgson G, Dunn AR. Mice lacking both granulocyte colony-stimulating factor (CSF) and granulocyte-macrophage CSF have impaired reproductive capacity, perturbed neonatal granulopoiesis, lung disease, amyloidosis, and reduced long-term survival. Blood. 1997;90:3037–49. [PubMed] [Google Scholar]
- 45.Ballinger MN, Paine R, 3rd, Serezani CH, Aronoff DM, Choi ES, Standiford TJ, Toews GB, Moore BB. Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2006;34:766–74. doi: 10.1165/rcmb.2005-0246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez-Juarrero M, Hattle JM, Izzo A, Junqueira-Kipnis AP, Shim TS, Trapnell BC, Cooper AM, Orme IM. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol. 2005;77:914–22. doi: 10.1189/jlb.1204723. [DOI] [PubMed] [Google Scholar]
- 47.LeVine AM, Reed JA, Kurak KE, Cianciolo E, Whitsett JA. GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J Clin Invest. 1999;103:563–9. doi: 10.1172/JCI5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paine R, 3rd, Preston AM, Wilcoxen S, Jin H, Siu BB, Morris SB, Reed JA, Ross G, Whitsett JA, Beck JM. Granulocyte-macrophage colony-stimulating factor in the innate immune response to Pneumocystis carinii pneumonia in mice. J Immunol. 2000;164:2602–9. doi: 10.4049/jimmunol.164.5.2602. [DOI] [PubMed] [Google Scholar]
- 49.Riopel J, Tam M, Mohan K, Marino MW, Stevenson MM. Granulocyte-macrophage colony-stimulating factor-deficient mice have impaired resistance to blood-stage malaria. Infect Immun. 2001;69:129–36. doi: 10.1128/IAI.69.1.129-136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reed JA, Ikegami M, Cianciolo ER, Lu W, Cho PS, Hull W, Jobe AH, Whitsett JA. Aerosolized GM-CSF ameliorates pulmonary alveolar proteinosis in GM-CSF-deficient mice. Am J Physiol. 1999;276:L556–63. doi: 10.1152/ajplung.1999.276.4.L556. [DOI] [PubMed] [Google Scholar]
- 51.Zsengeller ZK, Reed JA, Bachurski CJ, LeVine AM, Forry-Schaudies S, Hirsch R, Whitsett JA. Adenovirus-mediated granulocyte-macrophage colony-stimulating factor improves lung pathology of pulmonary alveolar proteinosis in granulocyte-macrophage colony-stimulating factor-deficient mice. Hum Gene Ther. 1998;9:2101–9. doi: 10.1089/hum.1998.9.14-2101. [DOI] [PubMed] [Google Scholar]
- 52.Huffman JA, Hull WM, Dranoff G, Mulligan RC, Whitsett JA. Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J Clin Invest. 1996;97:649–55. doi: 10.1172/JCI118461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robb L, Drinkwater CC, Metcalf D, Li R, Kontgen F, Nicola NA, Begley CG. Hematopoietic and lung abnormalities in mice with a null mutation of the common beta subunit of the receptors for granulocyte-macrophage colony-stimulating factor and interleukins 3 and 5. Proc Natl Acad Sci U S A. 1995;92:9565–9. doi: 10.1073/pnas.92.21.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carey BC, Suzuki T, Uchida K, Sakagami T, Wood RE, Luisetti M, Rubin B, Kevill K, Trapnell BC. An Algorithm for Diagnosis of Familial Pulmonary Alveolar Proteinosis (PAP) Am J Respir Crit Care Med. 2009 in press. [Google Scholar]
- 55.Nishinakamura R, Wiler R, Dirksen U, Morikawa Y, Arai K, Miyajima A, Burdach S, Murray R. The pulmonary alveolar proteinosis in granulocyte macrophage colony-stimulating factor/interleukins 3/5 beta c receptor-deficient mice is reversed by bone marrow transplantation. J Exp Med. 1996;183:2657–62. doi: 10.1084/jem.183.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ichiwata T, Tanaka N, Yamaguchi E, Eda R, Oishi K, Tsuchihashi Y, Kaneko C, Nukiwa T, Krischer JP, Nakata K. Characteristics of a Large Cohort of Autoimmune Pulmonary Alveolar Proteinosis Patients in Japan. Am J Respir Crit Care Med. 2008;177:752–762. doi: 10.1164/rccm.200708-1271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uchida K, Nakata K, Suzuki T, Luisetti M, Watanabe M, Koch DE, Stevens CA, Beck DD, Denson LA, Carey BC, Keicho N, Krischer JP, Yamada U, Trapnell BC. Granulocyte/Macrophage Colony-Stimulating Factor Autoantibodies and Myeloid Cell in Healthy Individuals. Blood. 2009;113:2547–2556. doi: 10.1182/blood-2009-05-155689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uchida K, Nakata K, Trapnell BC, Terakawa T, Hamano E, Mikami A, Matsushita I, Seymour JF, Oh-Eda M, Ishige I, Eishi Y, Kitamura T, Yamada Y, Hanaoka K, Keicho N. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004;103:1089–98. doi: 10.1182/blood-2003-05-1565. [DOI] [PubMed] [Google Scholar]
- 59.Svenson M, Hansen MB, Ross C, Diamant M, Rieneck K, Nielsen H, Bendtzen K. Antibody to granulocyte-macrophage colony-stimulating factor is a dominant anti-cytokine activity in human IgG preparations. Blood. 1998;91:2054–61. [PubMed] [Google Scholar]
- 60.Sakagami T, Beck D, Suzuki T, Uchida K, Wood RE, Carey BC, Wert SE, Ikegami M, Whitsett JA, Keller G, Ryckman F, Brody A, Luisetti M, Natata K, Trapnell BC. Pulmonary Alveolar Proteinosis (PAP) Reproduced in Non-Human Primates. Am J Respir Crit Care Med. 2010 doi: 10.1164/rccm.201001-0008OC. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakagami T, Suzuki T, Uchida K, Carey BC, Wood RE, Wert S, Whitsett J, Luisetti M, Trapnell B. Human GM-CSF Autoantibodies Cause Pulmonary Alveolar Proteinosis. N Engl J Med. doi: 10.1056/NEJMc0904077. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dirksen U, Nishinakamura R, Groneck P, Hattenhorst U, Nogee L, Murray R, Burdach S. Human pulmonary alveolar proteinosis associated with a defect in GM-CSF/IL-3/IL-5 receptor common beta chain expression. J Clin Invest. 1997;100:2211–7. doi: 10.1172/JCI119758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breslow A, Snow P, Rosenberg MH. Pulmonary Alveolar Proteinosis and Chronic Lymphatic Leukemia. Med Ann Dist Columbia. 1965;34:209–12. [PubMed] [Google Scholar]
- 64.Carnovale R, Zornoza J, Goldman AM, Luna M. Pulmonary alveolar proteinosis: its association with hematologic malignancy and lymphoma. Radiology. 1977;122:303–6. doi: 10.1148/122.2.303. [DOI] [PubMed] [Google Scholar]
- 65.Eldar M, Shoenfeld Y, Zaizov R, Fogel R, Asherov J, Liban E, Pinkhas J. Pulmonary alveolar proteinosis associated with fanconi’s anemia. Respiration. 1979;38:177–9. doi: 10.1159/000194077. [DOI] [PubMed] [Google Scholar]
- 66.Green D, Dighe P, Ali NO, Katele GV. Pulmonary alveolar proteinosis complicating chronic myelogenous leukemia. Cancer. 1980;46:1763–6. doi: 10.1002/1097-0142(19801015)46:8<1763::aid-cncr2820460811>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 67.Loire R, Meunier P, Lenglet JP, Tabib A, Tolot F. [Waldenstrom’s disease with terminal pulmonary alveolar proteinosis] Lyon Med. 1971;226:319–23. [PubMed] [Google Scholar]
- 68.Pamuk GE, Turgut B, Vural O, Demir M, Hatipoglu O, Unlu E, Altaner S, Gerenli M, Cakir B. Pulmonary alveolar proteinosis in a patient with acute lymphoid leukemia regression after G-CSF therapy. Leuk Lymphoma. 2003;44:871–4. doi: 10.1080/1042819021000055093. [DOI] [PubMed] [Google Scholar]
- 69.Shoji N, Ito Y, Kimura Y, Nishimaki J, Kuriyama Y, Tauchi T, Yaguchi M, Payzulla D, Ebihara Y, Ohyashiki K. Pulmonary alveolar proteinosis as a terminal complication in myelodysplastic syndromes: a report of four cases detected on autopsy. Leuk Res. 2002;26:591–5. doi: 10.1016/s0145-2126(01)00178-3. [DOI] [PubMed] [Google Scholar]
- 70.Tammaa M, Nasser W, Zaman M, Hernandez AM. Respiratory failure in an 83-year-old man with multiple myeloma. Pulmonary alveolar proteinosis. Tenn Med. 2009;102:37–9. [PubMed] [Google Scholar]
- 71.Ganguli PC, Lynne-Davies P, Sproule BJ. Pulmonary alveolar proteinosis bronchiectasis and secondary amyloidosis: a case report. Can Med Assoc J. 1972;106:569. passim. [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia Rio F, Alvarez-Sala R, Caballero P, Prados C, Pino JM, Villamor J. Six cases of pulmonary alveolar proteinosis: presentation of unusual associations. Monaldi Arch Chest Dis. 1995;50:12–5. [PubMed] [Google Scholar]
- 73.Oerlemans WG, Jansen EN, Prevo RL, Eijsvogel MM. Primary cerebellar nocardiosis and alveolar proteinosis. Acta Neurol Scand. 1998;97:138–41. doi: 10.1111/j.1600-0404.1998.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 74.Patiroglu T, Akyildiz B, Patiroglu TE, Gulmez IY. Recurrent pulmonary alveolar proteinosis secondary to agammaglobulinemia. Pediatr Pulmonol. 2008;43:710–3. doi: 10.1002/ppul.20818. [DOI] [PubMed] [Google Scholar]
- 75.Pessach I, Walter J, Notarangelo LD. Recent advances in Primary Immunodeficiencies: identification of novel genetic defects and unanticipated phenotypes. Pediatr Res. 2009 doi: 10.1203/PDR.0b013e31819dbe1e. [DOI] [PubMed] [Google Scholar]
- 76.Ranchod M, Bissell M. Pulmonary alveolar proteinosis and cytomegalovirus infection. Arch Pathol Lab Med. 1979;103:139–42. [PubMed] [Google Scholar]
- 77.Ruben FL, Talamo TS. Secondary pulmonary alveolar proteinosis occurring in two patients with acquired immune deficiency syndrome. Am J Med. 1986;80:1187–90. doi: 10.1016/0002-9343(86)90683-2. [DOI] [PubMed] [Google Scholar]
- 78.Samuels MP, Warner JO. Pulmonary alveolar lipoproteinosis complicating juvenile dermatomyositis. Thorax. 1988;43:939–40. doi: 10.1136/thx.43.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tran Van Nhieu J, Vojtek AM, Bernaudin JF, Escudier E, Fleury-Feith J. Pulmonary alveolar proteinosis associated with Pneumocystis carinii. Ultrastructural identification in bronchoalveolar lavage in AIDS and immunocompromised non-AIDS patients. Chest. 1990;98:801–5. doi: 10.1378/chest.98.4.801. [DOI] [PubMed] [Google Scholar]
- 80.Witty LA, Tapson VF, Piantadosi CA. Isolation of mycobacteria in patients with pulmonary alveolar proteinosis. Medicine (Baltimore) 1994;73:103–9. doi: 10.1097/00005792-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Davidson JM, Macleod WM. Pulmonary alveolar proteinosis. Br J Dis Chest. 1969;63:13–28. doi: 10.1016/s0007-0971(69)80040-9. [DOI] [PubMed] [Google Scholar]
- 82.Dawkins SA, Gerhard H, Nevin M. Pulmonary alveolar proteinosis: a possible sequel of NO2 exposure. J Occup Med. 1991;33:638–41. [PubMed] [Google Scholar]
- 83.Humble S, Allan Tucker J, Boudreaux C, King JA, Snell K. Titanium particles identified by energy-dispersive X-ray microanalysis within the lungs of a painter at autopsy. Ultrastruct Pathol. 2003;27:127–9. doi: 10.1080/01913120309925. [DOI] [PubMed] [Google Scholar]
- 84.Keller CA, Frost A, Cagle PT, Abraham JL. Pulmonary alveolar proteinosis in a painter with elevated pulmonary concentrations of titanium. Chest. 1995;108:277–80. doi: 10.1378/chest.108.1.277. [DOI] [PubMed] [Google Scholar]
- 85.McCunney RJ, Godefroi R. Pulmonary alveolar proteinosis and cement dust: a case report. J Occup Med. 1989;31:233–7. doi: 10.1097/00043764-198903000-00008. [DOI] [PubMed] [Google Scholar]
- 86.Miller RR, Churg AM, Hutcheon M, Lom S. Pulmonary alveolar proteinosis and aluminum dust exposure. Am Rev Respir Dis. 1984;130:312–5. doi: 10.1164/arrd.1984.130.2.312. [DOI] [PubMed] [Google Scholar]
- 87.Rosen SG, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med. 1958;258:1123–42. doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- 88.Xipell JM, Ham KN, Price CG, Thomas DP. Acute silicoproteinosis. Thorax. 1977;32:104–11. doi: 10.1136/thx.32.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dirksen U, Hattenhorst U, Schneider P, Schroten H, Gobel U, Bocking A, Muller KM, Murray R, Burdach S. Defective expression of granulocyte-macrophage colony-stimulating factor/interleukin-3/interleukin-5 receptor common beta chain in children with acute myeloid leukemia associated with respiratory failure. Blood. 1998;92:1097–103. [PubMed] [Google Scholar]
- 90.Heppleston AG, Wright NA, Stewart JA. Experimental alveolar lipo-proteinosis following the inhalation of silica. J Pathol. 1970;101:293–307. doi: 10.1002/path.1711010402. [DOI] [PubMed] [Google Scholar]
- 91.Jennings VM, Dillehay DL, Webb SK, Brown LA. Pulmonary alveolar proteinosis in SCID mice. Am J Respir Cell Mol Biol. 1995;13:297–306. doi: 10.1165/ajrcmb.13.3.7654386. [DOI] [PubMed] [Google Scholar]
- 92.Forbes A, Pickell M, Foroughian M, Yao LJ, Lewis J, Veldhuizen R. Alveolar macrophage depletion is associated with increased surfactant pool sizes in adult rats. J Appl Physiol. 2007;103:637–45. doi: 10.1152/japplphysiol.00995.2006. [DOI] [PubMed] [Google Scholar]
- 93.Doan ML, Guillerman RP, Dishop MK, Nogee LM, Langston C, Mallory GB, Sockrider MM, Fan LL. Clinical, radiological and pathological features of ABCA3 mutations in children. Thorax. 2008;63:366–73. doi: 10.1136/thx.2007.083766. [DOI] [PubMed] [Google Scholar]
- 94.Fitzgerald ML, Xavier R, Haley KJ, Welti R, Goss JL, Brown CE, Zhuang DZ, Bell SA, Lu N, McKee M, Seed B, Freeman MW. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J Lipid Res. 2007;48:621–32. doi: 10.1194/jlr.M600449-JLR200. [DOI] [PubMed] [Google Scholar]
- 95.Clark JC, Wert SE, Bachurski CJ, Stahlman MT, Stripp BR, Weaver TE, Whitsett JA. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci U S A. 1995;92:7794–8. doi: 10.1073/pnas.92.17.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Glasser SW, Detmer EA, Ikegami M, Na CL, Stahlman MT, Whitsett JA. Pneumonitis and emphysema in sp-C gene targeted mice. J Biol Chem. 2003;278:14291–8. doi: 10.1074/jbc.M210909200. [DOI] [PubMed] [Google Scholar]
- 97.Trapnell BC, Nakata K, Kavuru MS. Pulmonary Alveolar Proteinosis Syndrome. In: Mason RJ, Broaddus VC, Martin TG, King T, Schraufnagel D, Murray JF, Nadel JA, editors. Murray and Nadel’s Textbook of Respiratory Medicine. Elsevier; Philadelphia: 2010. [Google Scholar]
- 98.Ceruti M, Rodi G, Stella GM, Adami A, Bolongaro A, Baritussio A, Pozzi E, Luisetti M. Successful whole lung lavage in pulmonary alveolar proteinosis secondary to lysinuric protein intolerance: a case report. Orphanet J Rare Dis. 2007;2:14. doi: 10.1186/1750-1172-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luisetti M, Rodi G, Campo I, Mariani F, Poszzi E, Trapnell BC. Plasmapheresis for treatment of pulmonary alveolar proteinosis. Eur Respir J. 2009;33:1–3. doi: 10.1183/09031936.00097508. [DOI] [PubMed] [Google Scholar]
- 100.Venkateshiah SB, Yan TD, Bonfield TL, Thomassen MJ, Meziane M, Czich C, Kavuru MS. An open-label trial of granulocyte macrophage colony stimulating factor therapy for moderate symptomatic pulmonary alveolar proteinosis. Chest. 2006;130:227–37. doi: 10.1378/chest.130.1.227. [DOI] [PubMed] [Google Scholar]
- 101.Seymour JF, Presneill JJ, Schoch OD, Downie GH, Moore PE, Doyle IR, Vincent JM, Nakata K, Kitamura T, Langton D, Pain MC, Dunn AR. Therapeutic efficacy of granulocyte-macrophage colony-stimulating factor in patients with idiopathic acquired alveolar proteinosis. Am J Respir Crit Care Med. 2001;163:524–31. doi: 10.1164/ajrccm.163.2.2003146. [DOI] [PubMed] [Google Scholar]
- 102.Kavuru MS, Sullivan EJ, Piccin R, Thomassen MJ, Stoller JK. Exogenous granulocyte-macrophage colony-stimulating factor administration for pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2000;161:1143–8. doi: 10.1164/ajrccm.161.4.9906044. [DOI] [PubMed] [Google Scholar]
- 103.Seymour JF, Dunn AR, Vincent JM, Presneill JJ, Pain MC. Efficacy of granulocyte-macrophage colony-stimulating factor in acquired alveolar proteinosis. N Engl J Med. 1996;335:1924–5. doi: 10.1056/NEJM199612193352513. [DOI] [PubMed] [Google Scholar]
- 104.Tazawa R, Trapnell BC, Inoue Y, Arai T, Takada T, Nasuhara Y, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ishii H, Yokoba M, Tanaka N, Yamaguchi E, Eda R, Tsuchihashi Y, Morimoto K, Akira M, Terada M, Otsuka J, Ebina M, Kaneko C, Nukiwa T, Krischer JP, Akazawa K, Nakata K. Inhaled Granulocyte/Macrophage-Colony Stimulating Factor as Therapy of Pulmonary Alveolar Proteinosis. Am J Respir Crit Care Med. 2010 doi: 10.1164/rccm.200906-0978OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dalrymple H, Malur A, Gagnon G, Marshall I, Kavuru MS, Barna B, Thomassen MJ. Rituximab Depletes B-Lymphocytes and Improves Symptoms in Pulmonary Alveolar Proteinosis. J Allergy Clin Immunol. 2008;121:S225. [Google Scholar]
- 106.Borie R, Debray MP, Laine C, Aubier M, Crestani B. Rituximab therapy in autoimmune pulmonary alveolar proteinosis. Eur Respir J. 2009;33:1503–6. doi: 10.1183/09031936.00160908. [DOI] [PubMed] [Google Scholar]
- 107.Supena R, Karlin D, Strate R, Cramer PG. Pulmonary alveolar proteinosis and Nocardia brain abscess. Report of a case. Arch Neurol. 1974;30:266–8. doi: 10.1001/archneur.1974.00490330074014. [DOI] [PubMed] [Google Scholar]
- 108.Walker DA, McMahon SM. Pulmonary alveolar proteinosis complicated by cerebral abscess: report of a case. J Am Osteopath Assoc. 1986;86:447–50. [PubMed] [Google Scholar]
- 109.Seymour JF. Extra-pulmonary aspects of acquired pulmonary alveolar proteinosis as predicted by granulocyte-macrophage colony-stimulating factor-deficient mice. Respirology. 2006;11(Suppl):S16–22. doi: 10.1111/j.1440-1843.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 110.Bonfield TL, Raychaudhuri B, Malur A, Abraham S, Trapnell BC, Kavuru MS, Thomassen MJ. PU.1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1132–6. doi: 10.1152/ajplung.00216.2003. [DOI] [PubMed] [Google Scholar]
- 111.Uchida K, Beck DC, Yamamoto T, Berclaz PY, Abe S, Staudt MK, Carey BC, Filippi MD, Wert SE, Denson LA, Puchalski JT, Hauck DM, Trapnell BC. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356:567–79. doi: 10.1056/NEJMoa062505. [DOI] [PubMed] [Google Scholar]
- 112.Guthridge MA, Barry EF, Felquer FA, McClure BJ, Stomski FC, Ramshaw H, Lopez AF. The phosphoserine-585-dependent pathway of the GM-CSF/IL-3/IL-5 receptors mediates hematopoietic cell survival through activation of NF-kappaB and induction of bcl-2. Blood. 2004;103:820–7. doi: 10.1182/blood-2003-06-1999. [DOI] [PubMed] [Google Scholar]
- 113.Basu S, Dunn AR, Marino MW, Savoia H, Hodgson G, Lieschke GJ, Cebon J. Increased tolerance to endotoxin by granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1997;159:1412–7. [PubMed] [Google Scholar]
- 114.Bozinovski S, Jones J, Beavitt SJ, Cook AD, Hamilton JA, Anderson GP. Innate immune responses to LPS in mouse lung are suppressed and reversed by neutralization of GM-CSF via repression of TLR-4. Am J Physiol Lung Cell Mol Physiol. 2004;286:L877–85. doi: 10.1152/ajplung.00275.2003. [DOI] [PubMed] [Google Scholar]
- 115.Spight D, Trapnell B, Zhao B, Berclaz P, Shanley TP. Granulocyte-macrophage-colony-stimulating factor-dependent peritoneal macrophage responses determine survival in experimentally induced peritonitis and sepsis in mice. Shock. 2008;30:434–42. doi: 10.1097/SHK.0b013e3181673543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 117.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–44. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 118.Campbell IK, Bendele A, Smith DA, Hamilton JA. Granulocyte-macrophage colony stimulating factor exacerbates collagen induced arthritis in mice. Ann Rheum Dis. 1997;56:364–8. doi: 10.1136/ard.56.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CC. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194:873–82. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–44. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]