Abstract
Candida is the fourth most common cause of nosocomial bloodstream infections (BSI), being Candida albicans the most common species. This study evaluated the distribution of Candida spp isolates at a tertiary care medical center. The associated factors and outcome of patients with candidemia at the Puerto Rico Medical Center (PRMC) were evaluated. Laboratory data from May 2005 to April 2006 was reviewed. Blood cultures reported as positive for Candida spp were identified and records were reviewed. Two hundred and four blood cultures were reported with Candida spp, corresponding to 85 different episodes of candidemia in 82 patients: 3 patients presented more than one candidemia episode with two different Candida spp. In seventy-two percent (61/85) of candidemia episodes, the organism isolated was a non-albicans Candida, being C. parapsilosis the most common species isolated with 49% (42/85). Sixty five records were evaluated; of which 45 cases were reviewed (20 cases were excluded from the study due to incomplete information). The predominant factors identified were being on broad spectrum antibiotics 95.6% (43/45), central catheter placement 97.8% (44/45), mechanical ventilation 64.4% (29/45), and urinary catheter placement 73.3% (33/45). The mortality among the reviewed cases was 48.9% (22/45).
Background
Nosocomial bloodstream infections (BSI) are an important cause of morbidity and mortality in hospitalized patients. Candida infections account for the fourth most common pathogen in patients admitted to critical care units as evidenced in a multi-center study performed in 49 hospitals in the United States.1 A multicenter prospective observational study conducted at several tertiary care centers in the United States revealed that C. albicans was the most common Candida species isolated in BSI.2 Similar surveillance studies had reported an increasing trend of candidemias secondary to non-albicans Candida (NAC).3
NAC presents a therapeutic challenge in view of increased incidence of azole-resistance when compared to C. albicans species. C. glabrata and C. krusei are usually considered to be resistant to azoles.4 On the other hand, important virulence factors, such as adhesion and biofilm formation affecting indwelling devices, may be responsible for C. parapsilosis infections.5-7
The Puerto Rico Medical Center (PRMC) at San Juan, Puerto Rico, provides medical care to an underserved population with a wide variety of conditions. At our institution, which is a tertiary care hospital and a referral center for the Caribbean, the distribution of fungal BSI has not been documented. The aim of this study was to evaluate the distribution of Candida spp at our center. Additional information such as demographics, comorbidities, presence of artificial medical devices, and outcome were also obtained to evaluate associated risk factors present in our patients’ population.
Methods
This study is a retrospective review of positive blood cultures with Candida spp reported by the bacteriology division of patients admitted to the PRMC from May 2005 to April 2006. Identification was performed at the laboratory according to standard microbiological techniques. Records were reviewed in order to evaluate patients’ demographics, potential risk factors, and outcome. Candidemia was defined as the presence of at least one positive blood culture with Candida spp. Mortality was defined as death during hospitalization.
Statistical analysis was performed using the statistical package for the social science (SPSS version 16.0 for Windows). Summary statistics were performed using measures of central tendency and dispersion. Categorical variables were summarized using proportions and percentages. Pearson X2 and Fisher’s exact test statistics were employed to compare differences between survived and deceased patients.
Results
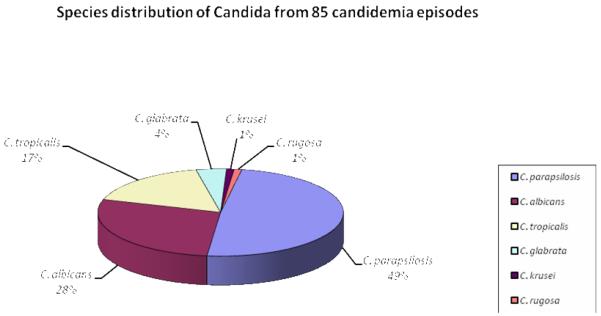
The frequency of different Candida species in the 82 patients with BSI is shown in Graphic 1. Two-hundred and four isolates were reported during the evaluated period, corresponding to 85 different episodes of candidemia in 82 patients. The evaluated isolates corresponding to 85 candidemia episodes were identified as: 42 (49%) C. parapsilosis, 24 (28%), C. albicans, 14 (17%) C. tropicalis, 3 (4%) C. glabrata, 1 (1%) C. krusei, and 1 (1%) C. rugosa. Three patients presented two different Candida spp isolated during hospitalization representing different candidemia episodes: one patient presented with C. parapsilosis and C. tropicalis; and the other two patients presented with C. tropicalis and C. albicans.
Graphic 1.
Species distribution from 85 cases of candidemia from May 2005 to April 2006
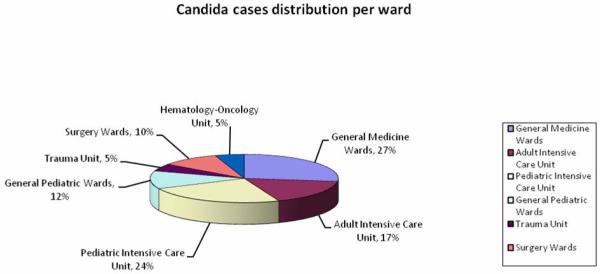
The distribution of the identified Candida cases is shown in Graphic 2; 22 were from general medicine (27%), 14 from the adult intensive care unit (17%), 20 from the pediatric intensive care unit (24%), 10 from general pediatrics (12%), 4 from the trauma unit (5%), 8 from surgery units (10%) (orthopedic, neurosurgery, and general surgery), and 4 from the hematology-oncology unit (5%). Overall, 41% of the patients belonged to an intensive care unit; including the three patients with two different Candida spp identified (1 in adult intensive care unit and 2 in pediatric intensive care unit).
Graphic 2.
Distribution of the 82 patients with candidemia divided per ward
Sixty-five records from the identified cases were evaluated. Forty-five cases were analyzed and the remaining twenty records were excluded from the study due to incomplete information. Demographics at the time of diagnosis were 16 females (35.6%) and 29 males (64.4%). Our population consisted of 34 adults (75.6%) with a mean (s.d) age of 55.2 (15.5); and 11 pediatric patients (24.4%) of which 9 were premature infants. Review of potential predisposing factors revealed that 97.8% of the cases had central venous catheters, 73.3% had a urinary catheter, 64.4% required mechanical ventilation, and 95.6% had received broad spectrum antibiotics. The average number of antibiotics used prior to the development of candidemia was 3 (range 0 to 7) in the survival group and 4 (range from 0 to 8) in the deceased group. The mortality was 48.9%. Other evaluated factors included past medical history, such as history of malignancy (18%), diabetes mellitus (22.2%), steroid administration (15.6%), HIV (8.9%), recent trauma (11.1%), abdominal surgery (17.8%), recent hospitalization (31.1%), total parenteral nutrition (46.7%), and hemodialysis (22.2%).
A comparison of clinical characteristics between the patients who survived (n=23) and those who died (n=22) is summarized in Table 1. Statistical analysis of the most common identifiable risk factors revealed that there exists a positive correlation between mortality of candidemia and early onset during admission (p=0.048). When analyzed by age groups (infants and adults), the same mortality was observed in this study. In the infant group, it was seen that 10 out of 11 patients (90.9%) had a strong evidence of TPN being a risk factor for candidemia and mortality (p=0.001). The presence of a urinary catheter (p=0.06) and mechanical ventilation (p=0.08) suggests an increased risk for mortality, but more data would be needed to verify this conclusion. From the analysis, there was insufficient evidence to conclude that there is a relationship between mortality and hemodialysis (p=0.13). Despite the presence of central catheters in 97.8% of the patients in this study, statistical analysis failed to establish a correlation between this risk factor and mortality (p=0.99).
Table 1.
Demographical data, risk factors, and mortality obtained from record review of patients with systemic candidiasis
| Cases Reviewed (N) | Total (%) 45 |
Survived (%) 23 (51.1) |
Deceased (%) 22 (48.9) |
p-value |
|---|---|---|---|---|
| Adults | 34 (75.6) | 17 (50) | 17 (50) | 0.99 |
|
| ||||
| Pediatric | 11 (24.4) | 6 (54.5) | 5 (45.5) | 0.55 |
|
| ||||
| Female | 16 (35.6) | 6 (37.5) | 10 (62.5) | 0.16 |
|
| ||||
| Male | 29 (64.4) | 17 (58.6) | 12 (41.4) | 0.25 |
|
| ||||
| Previous Antibiotic Administration | 43 (95.6) | 22 (51.2) | 21 (48.8) | 0.99 |
|
| ||||
| Cancer/ Hematologic malignancy | 8 (17.8) | 3 (37.5) | 5 (62.5) | 0.38 |
|
| ||||
| Diabetes mellitus | 10 (22.2) | 4 (40) | 6 (60) | 0.42 |
|
| ||||
| HIV | 4 (8.9) | 2 (50) | 2 (50) | 0.99 |
|
| ||||
| Steroid administration | 7 (15.6) | 4 (57.1) | 3 (42.9) | 0.78 |
|
| ||||
| Recent Trauma | 5 (11.1) | 2 (40) | 3 (60) | 0.60 |
|
| ||||
| Surgical intervention | ||||
|
| ||||
| Orthopedic | 4 (8.9) | 1 (25) | 3 (75) | 0.27 |
| Abdominal | 8 (17.8) | 5 (62.5) | 3 (37.5) | 0.43 |
| Pelvic | 1 (2.2) | 1 (100) | 0 (0) | 0.99 |
| Neurosurgical | 5 (11.1) | 4 (80) | 1 (20) | 0.20 |
|
| ||||
| Recent hospitalization | 14 (31.1) | 4 (28.6) | 10 (71.4) | 0.048 |
|
| ||||
| Total Parenteral Nutrition | 21 (46.7) | 12 (57.1) | 9 (42.9) | 0.46 |
|
| ||||
| Central Catheter | 44 (97.8) | 22 (50) | 22 (50) | 0.99 |
|
| ||||
| Urinary Catheter | 33 (73.3) | 14 (42.4) | 19 (57.6) | 0.06 |
|
| ||||
| Mechanical Ventilation | 29 (64.4) | 12 (41.4) | 17 (58.6) | 0.08 |
|
| ||||
| Hemodialysis | 10 (22.2) | 3 (30) | 7 (70) | 0.13 |
Conclusions
The incidence of Candida BSI has considerably increased since the early 1980’s.8 Candidemia is the fourth most common source of BSI and represents 8-15% of all nosocomial sepsis in USA.1 The incidence of Candida related BSI in Puerto Rico has not been studied.
The PRMC is a supra-tertiary institution providing services to an underserved population and is also a referral center for medical, pediatric, gynecologic, and surgical patients, including its subspecialties. It is also the leading trauma center in the Caribbean. Our center provides medical care to hematologic malignancy patients and has the only leukemia/bone marrow transplant unit available in the Island. The severity of illness present in a considerable amount of patients, especially those admitted to Medicine wards and intensive care units, could be a contributing factor to the high incidence of candidemia observed in these groups (27% in the Medicine wards and 41% in intensive care units). In contrast to current data, which presents C. albicans as the primary pathogen causing fungemia across the world, C. parapsilosis was identified as the leading species at our center. To our knowledge, this finding has only been reported by another center in Osaka, Japan.9 Several studies have suggested an increased incidence of C. parapsilosis in Latin America, which might in part explain our findings.5,7,10
Antifungal prophylaxis and empirical therapy is commonly used in patients with acute myelogenous leukemia receiving standard chemotherapy leading to neutropenia, in allogeneic bone marrow transplant, and in high risk autologous bone marrow transplant.4 This approach to the hematologic malignancy patient, which is also practiced at our institution, might explain the low incidence of candidemia in this group (5% of the samples studied). Taking into consideration that C. albicans, which was the only species isolated in this group, is repeatedly reported in literature as being susceptible to fluconazole 7,8, the use of this medication as preemptive or prophylactic therapy should be adequate for our patients and could be considered as the first choice antifungal agent when therapy for candidemia is required at our center.
The risk factors identified for the development of candidemia in our study were similar to those described in the literature. 6-8,11-16 The majority of patients had a central venous catheter (97.8%), a urinary catheter (73.3%), was on mechanical ventilation 7,8,11-13 (64.4%) or had received broad spectrum antibiotics (95.6%). C. parapsilosis is an exogenous pathogen that may be found on the skin and is known to form biofilms on catheters and other medical devices. Infections with this organism have been associated with hyperalimentation, poor catheter care, and breaks in infection control practices.6,7 Further studies regarding infection control techniques at our institution need to be considered in order to determine if these factors influenced our findings.
The mortality in our study was 48.9%, which correlates with the range reported in literature of 40-60%.1,11,17-20 Evaluation of risk factors in our population leads to suggest that the presence of a urinary catheter or exposure to mechanical ventilation, which are usually present in critically ill patients with guarded prognosis, contribute independently to a fatality outcome. Statistical analysis failed to confirm an association between central catheter placement and mortality. This finding may be explained by the fact that 97.8% of the patients studied had a central catheter, making the group of patients without central catheter too small for comparison.
In summary, species distribution of Candida BSI at the PRMC was found to differ from the one published in literature. C. parapsilopsis was the most common species encountered, in contrast to C. albicans at other similar settings. This finding may be accounted for by the frequent use of central venous catheters (97.8% of the evaluated cases) and recent reports of changing Candida epidemiology in Latin America.
The mortality of patients with candidemia and the prevention of this infection seem to be greatly influenced by knowledge of local epidemiology and risk factors. Further investigation studies regarding aseptic techniques, care of inserted medical devices, removal of unnecessary catheters, and avoidance of unnecessary antibiotic administration should be designed to determine if these factors could be contributing to the rate of candidemia at our institution. Candida species susceptibility testing, which was not being performed on a regular basis at the PRMC at the moment of this investigation, is currently available. A prospective investigation should be designed to integrate susceptibility testing to our study and to confirm the role of fluconazole as first line agent.
Acknowledgement
This publication was possible by Grant Number P20 RR11126 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Parts of this manuscript were presented at the local ACP chapter competition, at the IDSA 2007 meeting that took place in San Diego, California, and at the University of Puerto Rico Medical Sciences Campus- XXVII Research and Education Annual Forum 2007.
References
- 1.Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial Bloodstream Infections in United States Hospitals: A Three Year Analysis. Clin Infect Dis. 1999;29:239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 2.Pappas PG, Rex JH, Lee J, et al. A Prospective Observational Study of Candidemia: Epidemiology, Therapy, and Influences on Mortality in Hospitalized Adult and Pediatric Patients. Infect Dis. 2003;37:634–644. doi: 10.1086/376906. [DOI] [PubMed] [Google Scholar]
- 3.Diekema DJ, Messer SA, Brueggemann AB, Coffman SL, Doern GV, Herwaldt LA, Pfaller MA. Epidemiology of Candidemia: 3- Year Results from Emerging Infections and the Epidemiology of Iowa Organism Study. J Clin Microbiol. 2002;40(4):1298–1302. doi: 10.1128/JCM.40.4.1298-1302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, Walsh TJ, Edwards JE. Guidelines for the Treatment of Candidiasis. Clin Infect Dis. 2004;38:161–189. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn DM, Mukherjee PK, Clark TA, et al. Candida parapsilosis characterization in an Outbreak Setting. Emerg Infec Dis. 2004;10(4):1074–1080. doi: 10.3201/eid1006.030873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojic EM, Daroiche RO. Candida Infections of Medical Devices. Clin Microbiol Rev. 2004;17(2):255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfaller MA, Diekman DJ. Epidemiology of Invasive Candidiasis: a Persistent Public Health Problem. Clinl Microbiol Rev. 2007;20(1):133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fridkin SK, Jarvis WR. Epidemiology of Nosocomial Fungal Infections. Clin Microbiol Rev. 1999;9(4):499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura T, Takahashi H. Epidemiological study of Candida infections in blood: susceptibilities of Candida spp. to antifungal agents, and clinical features associated with the candidemia. J Infect Chemother. 2006;12(3):132–138. doi: 10.1007/s10156-006-0438-y. [DOI] [PubMed] [Google Scholar]
- 10.Pfaller MA, Jones RN, Doern GV, et al. Bloodstream Infections due to Candida Species: SENTRY Antimicrobial Surveillance Program in North America and Latin America, 1997-1998. Antimicrob Agents Chemother. 2000;44(3):747–751. doi: 10.1128/aac.44.3.747-751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viudes A, Peman J, Ubeda P, Lopez-Ribot JL, Gobernado M. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur J Clin Microbiol Infect Dis. 2002;21(11):767–774. doi: 10.1007/s10096-002-0822-1. [DOI] [PubMed] [Google Scholar]
- 12.Ostrosky-Zeichner L. New approaches to the risk of Candida in the intensive care unit. Curr Opin Infect Dis. 2003;16(6):533–7. doi: 10.1097/00001432-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Peres-Bota D, Rodriguez-Villalobos H, Dimopoulos G, Melot C, Vincent JL. Potential risk factors for infection with Candida spp. in critically ill patients. Clin Microbiol Infect. 2004;10(6):550–555. doi: 10.1111/j.1469-0691.2004.00873.x. [DOI] [PubMed] [Google Scholar]
- 14.Diekeman DJ, Pfaller MA. Nosocomial Candidemia: An Ounce of Prevention is Better than a Pound of Cure. Infect Control Hosp Epidemiol. 2004;25(8):624–626. doi: 10.1086/502451. [DOI] [PubMed] [Google Scholar]
- 15.Almirante B, Rodriguez D, Park BJ, et al. Epidemiology and Predictors of Mortality in Cases of Candida Bloodstream Infection: Results from Population-Based Surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2005;43(4):1829–1835. doi: 10.1128/JCM.43.4.1829-1835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YR, Lin LC, Young TG, Liu CE, Chen CH, Tsay RW. Risk factors for candidemia-related mortality at a medical center in central Taiwan. J Microbiol Immunol Infect. 2006;39:155–161. [PubMed] [Google Scholar]
- 17.Alonso-Valle J, Acha O, García-Palomo JD, Fariñas- Álvarez C, Fernandez-Mazarrasa C, Fariñas MC. Candidemia in a Tertiary Care Hospital: Epidemiology and Factors Influencing Mortality. Eur J Clin Microbiol Infect Dis. 2004;22(4):254–257. doi: 10.1007/s10096-003-0890-x. [DOI] [PubMed] [Google Scholar]
- 18.Nolla J, Sitges A, Leon-Gil C, Martinez J, Leon-Regidor MA, Ibanez P, Torres JM, Study Group of Fungal Infection in the ICU Candidemia in non-neutropenic critically ill patients: análisis of prognostic factors and assessment of systemic antifungal therapy. Intensive Care Med. 1997;23(1):23–30. doi: 10.1007/s001340050286. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Abraham R, Keller N, Teodorovitch N, Barzilai A, Harel R, Barzilay Z, Paret G. Predictors of adverse outcome from candidal infection in a tertiary care hospital. J Infection. 2004;49(4):317–323. doi: 10.1016/j.jinf.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Peman J, Canton E, Gobernado M, Spanish ECMM Working Group on Candidemia Epidemiology and antifungal susceptibility of Candida species isolated from blood: results of a 2-year multicentre study in Spain. J Clin Microbiol Infect Dis. 2004;24(1):23–30. doi: 10.1007/s10096-004-1267-5. [DOI] [PubMed] [Google Scholar]