Abstract
Effects of functional sweeteners on the development of the metabolic syndrome and atherosclerosis are unknown. The objective was to compare the effect of dietary carbohydrate in the form of sucrose (SUCR) to D-tagatose (TAG; an isomer of fructose currently used as a low-calorie sweetener) on body weight, blood cholesterol concentrations, hyperglycemia, and atherosclerosis in low-density lipoprotein receptor deficient (LDLr−/−) mice. LDLr−/− male and female mice were fed either standard murine diet or a diet enriched with TAG or SUCR as carbohydrate sources for 16 weeks. TAG and SUCR diets contained equivalent amounts (g/kg) of protein, fat, and carbohydrate. We measured food intake, body weight, adipocyte diameter, serum cholesterol and lipoprotein concentrations, and aortic atherosclerosis. Macrophage immunostaining and collagen content were examined in aortic root lesions. CONTROL and TAG-fed mice exhibited similar energy intake, body weights and blood glucose and insulin concentrations, but SUCR-fed mice exhibited increased energy intake and became obese and hyperglycemic. Adipocyte diameter increased in female SUCR-fed mice compared to TAG and CONTROL. Male and female SUCR-fed mice had increased serum cholesterol and triglyceride concentrations compared to TAG and CONTROL. Atherosclerosis was increased in SUCR-fed mice of both genders compared to TAG and CONTROL. Lesions from SUCR-fed mice exhibited pronounced macrophage immunostaining and reductions in collagen content compared to TAG and CONTROL mice. These results demonstrate that in comparison to sucrose, equivalent substitution of TAG as dietary carbohydrate does not result in the same extent of obesity, hyperglycemia, hyperlipidemia, and atherosclerosis.
INTRODUCTION
Obesity is at epidemic proportions in the United States, as two-thirds of the adult population is classified as either overweight or obese (as defined by a BMI; >25 kg/m2 or >30 kg/m2, respectively) (1). Numerous studies describe the link between obesity and diseases such as hypertension, dyslipidemia, and type 2 diabetes (2). The clustering of abdominal obesity with these diseases is defined as the metabolic syndrome (3). Importantly, the primary cause of death in obese patients with the metabolic syndrome is cardiovascular disease (4).
Since the majority of the US population is overweight or obese with an increased risk of cardiovascular disease, a significant portion of the population is striving to lose weight. In 2007, about a third of adults (36%) report having tried to lose weight in the past, and two in five (41%) were currently trying (5). A common practice to reduce caloric intake is to decrease or eliminate table sugar, or sucrose, whereas some dieters opt for use of alternative sweeteners. Alternative sweeteners provide zero (nonnutritive, or artificial sweeteners) or a reduced amount (nutritive sweeteners) of calories.
TAG, an isomer of fructose, is ~90% as sweet as sucrose and is used as a nutritive or low-calorie sweetener (6). TAG supplies 1.5 kcal/g of energy (as compared to 4 kcal/g from sucrose), in large part because TAG is incompletely absorbed by the small intestine (7). TAG was classified in 2001 as a Generally Recognized as Safe product by the United States Food and Drug Administration (7). Some approved uses of TAG include as a sweetener in diet beverages at concentrations up to 1%, light ice creams or yogurts at concentrations up to 3%, and regular or dietetic hard candies at levels up to 15% (8).
Animals or humans fed high-energy diets from fructose or sucrose exhibit an elevation in serum cholesterol because of an imbalance of very low-density lipoprotein (VLDL)-triglyceride production and clearance, which is directly influenced by fatty acid availability. Consumption of a high-carbohydrate diet, particularly fructose and sucrose, has been demonstrated to elevate circulating cholesterol levels in rodents (9), dogs (10), nonhuman primates (11), and humans (12). In low- density lipoprotein receptor deficient (LDLr−/−) mice fed a diet enriched in sucrose, serum LDL cholesterol concentrations and atherosclerosis were increased compared to mice fed an energy-matched diet enriched in saturated fatty acids (13). However, it has not been determined whether a nutritive or functional sweetener, such as TAG, would induce similar levels of atherosclerotic disease.
The purpose of this study was to define the effects of a diet enriched with TAG as the carbohydrate source on body weight, serum glucose and lipids, and atherosclerosis in LDLr−/− mice. In addition, we compared an equivalent gram basis of dietary TAG to sucrose as a carbohydrate source. We hypothesized that in comparison to sucrose, dietary TAG-enrichment would result in minimal changes in body weight, lipid metabolism, glucose, or atherosclerosis formation in atherosclerosis-susceptible LDLr−/− mice.
METHODS AND PROCEDURES
Mice and diets
LDLr−/− (backcrossed 10 times on a C57BL/6J background) male and female mice were bred in-house. All mice were maintained in a barrier facility and a 12-h light/dark cycle. Diets were designed (Harlan Teklad) with D-tagatose (TAG; TD05579) or sucrose (SUCR; TD97111) as the primary carbohydrate source, and contained an equivalent gram mass (g/kg of diet) of TAG and sucrose (Table 1). In addition, we matched (g/kg of diet) all other ingredients (coconut, olive oil, corn oil, casein, maltodextrin) between the SUCR and TAG diets. Due to differences in energy content between TAG (~1.5 kcal/g) and sucrose (4.0 kcal/g), the TAG-containing diet provided more calories from fat and protein (Table 1). Both diets contained equivalent amounts of the following (in g/kg): 10.0 Vitamin Mix Teklad (40060), 2.3 Choline Bitartrate, 0.04 Ethoxyquin (antioxidant), 36.8 Mineral Mix AIN-93G-MX (TD 94046), 4.2 Calcium Phosphate dibasic CaHPO4. At ~8 weeks of age, groups of male and female mice were placed on diets enriched with TAG (N = 6/gender) or sucrose (SUCR; N = 6/gender) for 16 weeks. We included groups of LDLr−/− mice fed standard murine diet (Teklad, TD2018, N = 5/gender) for comparison (Table 1). Before the 16-week feeding period, TAG and SUCR diets were introduced into the diet in a gradual manner over 3 weeks, using an increasing ratio of powdered TAG- or SUCR- containing diet to standard murine laboratory diet (TD2018). Food intake was measured for the first 4 weeks and body weight was monitored weekly. Food intake is expressed as average kcal/day, given the differences in energy density between the diets. Fasting (4 h) blood glucose concentrations were determined at the time of killing through tail vein puncture (Freestyle Flash glucometer; Abbott Diabetes Care, Alameda, CA).
Table 1.
Composition of control and experimental diets
| CONTROL | SUCR | TAG | |
|---|---|---|---|
| Energy (kcal/g) | 3.4 | 4.0 | 3.3 |
| Energy sources (% kcal) | |||
| Protein | 23.0 | 18.2 | 22.2 |
| Fat | 17.0 | 20.0 | 24.3 |
| Carbohydrate | 60.0 | 61.8 | 53.5 |
| Components (g/kg) | |||
| Sucrose | – | 291.9 | – |
| D-tagatose | – | – | 291.9 |
| Corn starch | – | 245.3 | 245.3 |
| Casein | – | 206.1 | 206.1 |
| Cellulose | – | 11.9 | 11.9 |
| Coconut oil | – | 32.5 | 32.5 |
| Olive oil | – | 33.4 | 33.4 |
| Corn oil | – | 20.6 | 20.6 |
At the study end point, mice were anesthetized (ketamine/xylazine, 100/10 mg/kg, IP.) and the left ventricle was punctured to obtain blood. After the right atrium was cut, mice were exsanguinated by perfusion through the left ventricle and tissues (liver, kidneys, spleen, epididymal fat, retroperitoneal fat, tibialis anterior muscle (14), and aortic tissue from the heart to ileal bifurcation) were dissected. Tissues were snap-frozen in liquid nitrogen and then stored at −80 °C; the heart and aorta were fixed overnight in 4% paraformaldehyde made with phosphate buffered solution, and then transferred to phosphate buffered solution for storage. Retroperitoneal adipose tissue, a visceral adipose depot, was stored in formalin and subsequently embedded in paraffin for examination of adipose morphology and macrophage immunostaining (see below). The wet weight of adipose tissues (epididymal fat, retroperitoneal fat) was normalized to body weight (%) as an index of adiposity.
Blood analyses
Total serum cholesterol and plasma triglyceride concentrations were determined on individual mice using enzymatic assay kits (Wako Pure Chemical, Richmond, VA). Lipoprotein distributions were evaluated in individual serum samples (50 μl) from four mice of each group after fractionation by size exclusion chromatography on a Superose six column. Sera samples chosen for lipoprotein analysis were within 5% of the mean sera cholesterol concentration for each group. Fractions were collected and cholesterol concentrations were determined using an enzymatic kit (Wako Pure Chemical, Richmond, VA). Plasma insulin levels were measured on individual mice using an ELISA kit (Mercodia Insulin ELISA; Mercodia AB, Sweden).
Quantification of atherosclerosis
Atherosclerosis was quantified by en face analysis of lesion surface area according to previously described methods (15). Briefly, aorta were cleaned of adventitial tissue, cut longitudinally along the entire length, and then pinned out on a wax surface. Digital images of the aortic arch and thoracic surface were captured with a Q Color 3 Olympus camera (Center Valley, PA). Atherosclerotic lesions were quantified by two independent observers using Image Pro Plus 5.1 (Media Cybernetics, Silver Spring, MD). Data are expressed as the percentage of the aortic arch covered with grossly discernable atherosclerotic lesions.
Immunocytochemical and morphological characterization of aortic root components and adipose tissue
Serial sections (5 μm) were generated from the aortic sinus to the ascending arch (700 μm) of the aortic root at the entry into the heart and mounted onto charged and precleaned microscope slides. In addition, sections from specific regions of the vessel (aortic sinus, orifices of coronary arteries, and the ascending aorta) were placed on barium fluoride windows for infrared microspectroscopy. Sections (5 μm) were also generated from paraffin-embedded visceral retroperitoneal adipose tissue. Slides containing aortic root and adipose tissue sections were analyzed by immunohistochemistry for the detection of macrophages using CD68 (primary antibody: 1:200 dilution; rat antimouse; Serotec, Kidlington, Oxford, UK) and F4/80 (primary antibody: 1:200 dilution; rat antimouse; Serotec), respectively. From each experimental group, four aortic root (50 μm apart) and four adipose tissue sections were analyzed for macrophage immunostaining. For both tissues, primary antisera were incubated at 40 °C for 15 min, followed by incubation with secondary biotinylated antibody under the same conditions (1:500 dilution; rabbit-antirat; Vector Laboratories, Burlingame, CA). Paraffin-embedded adipose tissue sections underwent antigen retrieval (steam; 10-min incubation in Antigen Unmasking Solution; Vector Laboratories, Burlingame, CA) before antibody incubations. A peroxidase-based ABC system and red chromagen AEC (both from Vector Laboratories) were used to identify the antigen-antibody reaction. A nonimmune serum was used as a control; nuclei were visualized by counterstaining with hematoxylin. Aortic root sections (two slides containing nine sections each and representing a distance of 80–100 μm) were also stained using Gomori Trichrome.
For characterization of adipocyte morphology, adipose tissue sections (two sections/gender/diet group) were deparaffinized and stained using hematoxylin and eosin. In four fields from each section, adipocyte number, diameter, and area were determined using Image Pro Plus 5.1.
Infrared microscopy
The infrared microspectrometer used at beamline U2b of the vacuum UV storage ring of the National Synchrotron Light Source at Brookhaven National Laboratory, Upton NY consisted of a Nic PLAN infrared microscope interfaced to a Nicolet Magna 860 infrared spectrometer (Thermo Electron, Madison, WI). A liquid nitrogen cooled 250 cm MCT detector with a frequency range of 50–4,000 cm−1 was used. Schwartzchild 32×_and 10×_all-reflecting mirror lenses were used for the objective and condenser, respectively. A remote projected image plane mask before the objective produced the apertures used for single point spectra or raster scan mapping via a digitally controlled motorized microscope stage. Spectra were recorded in transmission mode. A clear location on the infrared microscope slide (Delmar Ventures, San Diego, CA) was used to obtain a background spectrum.
Mapping was also accomplished from a globar source focal plane array instrument. The Perkin-Elmer Spotlight model 300 was used to obtain rectangular maps of select regions of the sections being examined. For focal plane array images, the 5.5 × 5.5 μm pixel size was used.
Calculations and statistics
Data are presented as the mean ± s.e.m. Data were analyzed using 1-way ANOVA, and tested for use of parametric or nonparametric post hoc analysis. If statistical differences existed between experimental groups, Tukey's test was utilized for post hoc analyses. Values of P < 0.05 were considered to be statistically significant. All statistical analyses were performed using GraphPad Prism 4.0 (San Diego, CA) or SigmaStat (SPSS, Chicago, IL), with exception of statistical analyses of the infrared microspectroscopy data.
Detailed statistical methods for analysis of infrared microscopy data can be found in Supplementary Material online.
RESULTS
Sucrose, but not TAG, increases body weight, adiposity, blood glucose, and insulin concentrations
In both genders, mice fed a diet enriched with sucrose exhibited a marked increase in body weight compared to TAG and CONTROL (P < 0.01 compared to TAG or CONTROL; Figure 1a,b, Table 2). In contrast, male and female mice fed a TAG-enriched diet exhibited a body weight similar to CONTROL (Figure 1a,b). Male and female SUCR mice exhibited elevations in energy intake (14.4 ± 0.6 and 12.8 ± 0.4), compared to CONTROL (9.4 ± 0.7 and 10.8 ± 0.4) and TAG-fed mice (10.9 ± 0.6 and 9.8 ± 0.4 kcal/day, Figure 1c,d; P < 0.001). Total adipose tissue mass (epidydimal + retroperitoneal adipose weight) was increased in both male and female SUCR-fed mice compared to TAG and CONTROL (P < 0.01 compared to TAG or CONTROL; Supplementary Figure S1e,f online). When normalized for total body weight, gonadal adipose tissue mass remained larger in all SUCR-fed mice compared to TAG and CONTROL (P < 0.001); this trend was also observed for retroperitoneal adipose adjusted to body weight in males but not females (Supplementary Figure S1a-d online). Representative sections of retroperitoneal adipose tissue illustrate increased adipocyte size in female SUCR-fed mice (Figure 2b) compared to TAG (Figure 2c) or CONTROL (Figure 2a), which was confirmed in quantitative measurements of adipocyte diameter (CONTROL, 30 ± 1; SUCR, 50 ± 1; TAG, 31 ± 1 μm, P < 0.05; Figure 2g and Table 2). Adipose tissue from male mice exhibited similar results (Figure 2g and Table 2). Macrophage positive F4/80 immunostaining was detected in adipose sections from SUCR mice (Figure 2e), but not in sections from CONTROL (Figure 2d) or TAG mice (Figure 2f). At the study end point, male SUCR-fed mice exhibited increased fasting blood glucose concentrations (267 ± 13 mg/dl) compared to CONTROL (207 ± 23 mg/dl) and TAG-fed mice (168 ± 5 mg/dl; P < 0.05). Female SUCR-fed mice exhibited increased fasting blood glucose concentrations (177 ± 6 mg/dl) compared to TAG (145 ± 11 mg/dl; P < 0.05), but not CONTROL mice (151 ± 8 mg/dl). Interestingly, fasting blood glucose concentrations were greater in male compared to female SUCR-fed mice. Plasma insulin concentrations were increased in male SUCR mice compared to TAG (P < 0.01), whereas plasma insulin concentrations of female diet groups were similar (Table 2).
Figure 1.
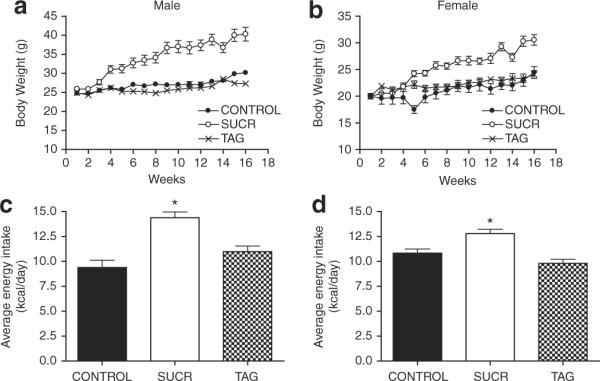
A diet containing sucrose, but not TAG, promotes the development of obesity in male and female LDLr−/− mice. (a) Male and (b) female SUCR-fed mice exhibit increased body weight compared to TAG and CONTROL mice. Beginning on week 7 in males and week 11 in females, body weight was greater in SUCR-fed mice compared to TAG and CONTROL. Data are mean ± s.e.m. from N = 5–6 mice/ gender. Accordingly, (c) male and (d) female SUCR-fed mice exhibit increased energy intake compared to both CONTROL and TAG mice (*P < 0.001). LDLr−/−, low-density lipoprotein receptor deficient.
Table 2.
Metabolic parameters of control and experimental mice
| Gender | Diet | Body weight (g) | Plasma insulin (uIU/ml) | Adipocyte diameter (μm) | Total plasma triglycerides (mg/dl) | Tibialis anterior (mg) |
|---|---|---|---|---|---|---|
| Male | CONTROL | 30 ± 0 | 17 ± 3 | 74 ± 3 | 110 ± 20 | 58 ± 4 |
| SUCR | 40 ± 2** | 28 ± 6* | 83 ± 3 | 822 ± 148** | 59 ± 3 | |
| TAG | 27 ± 0 | 13 ± 1 | 79 ± 3 | 162 ± 29 | 58 ± 2 | |
| Female | CONTROL | 24 ± 1 | 15 ± 1 | 30 ± 1 | 79 ± 16 | 35 ± 7 |
| SUCR | 31 ± 1** | 17 ± 2 | 50 ± 1** | 326 ± 37** | 43 ± 2 | |
| TAG | 24 ± 1 | 14 ± 1 | 31 ± 1 | 54 ± 8 | 42 ± 3 |
Data are mean ± s.e.m. from N = 5/6 mice/group.
P < 0.01 compared to TAG.
P < 0.01 compared to TAG and CONTROL.
Figure 2.
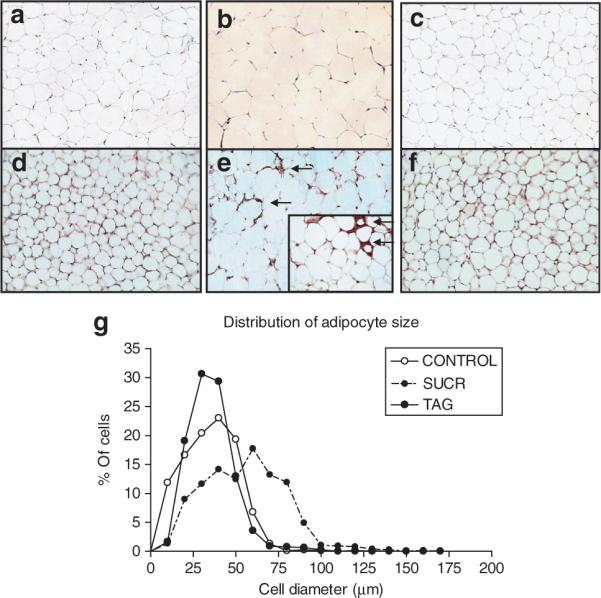
(a–c) Adipocyte diameter and (d–f) macrophage immunostaining are increased in retroperitoneal adipose tissue sections from SUCR-fed compared to TAG and CONTROL LDLr−/− mice. Representative adipose sections from (a) CONTROL, (b) SUCR, and (c) TAG-fed mice, demonstrating larger adipocytes in SUCR compared to TAG and CONTROL mice. F4/80 macrophage immunostaining was not evident in sections from (d) CONTROL or (f) TAG-fed mice (all images are ×20 magnification). In contrast, adipose tissue macrophages were observed in sections from SUCR-fed mice (e; arrows denote positively stained cells; inset is ×40). (g) Distribution of adipocyte size. LDLr−/−, low-density lipoprotein receptor deficient.
Sucrose increases serum lipids and atherosclerosis to a greater extent than TAG
Male and female SUCR-fed mice exhibited markedly increased total serum cholesterol concentrations (1,368 ± 98 and 912 ± 71, respectively) compared to TAG (519 ± 32 and 464 ± 40) and CONTROL mice (250 ± 6 and 184 ± 16 mg/dl; P < 0.001) of each gender (Figure 3a,b). In addition, total serum cholesterol concentrations in male and female TAG-fed mice were increased compared to CONTROL (Figure 3a,b; P < 0.05). Characterization of lipoprotein-cholesterol distributions revealed significantly elevated concentrations of VLDL- and LDL-cholesterol in male and female SUCR-fed compared to TAG or CONTROL mice, as quantified by area-under-the curve analysis (Figure 3c,d; P < 0.001 vs. TAG, CONTROL). In addition, TAG-fed mice exhibited increased VLDL- and LDL-cholesterol concentrations compared to CONTROL (Figure 3c,d; P < 0.05 vs. CONTROL).
Figure 3.
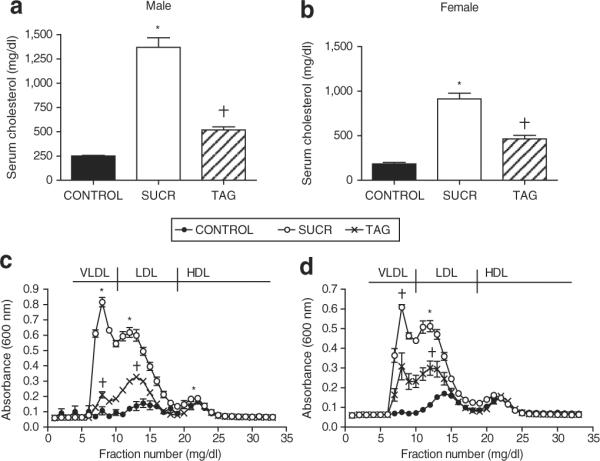
Total serum cholesterol concentrations and lipoprotein cholesterol concentrations are markedly increased in SUCR-fed compared to TAG and CONTROL LDLr−/− mice. Total serum cholesterol concentrations are increased in (a) male and (b) female SUCR-fed mice compared to TAG and CONTROL. (a) Male and (b) female TAG-fed mice exhibit increased total serum cholesterol concentrations compared to CONTROL. Lipoprotein cholesterol concentrations in (c) male and (d) female mice demonstrate a marked increase in VLDL/LDL cholesterol in SUCR-compared to TAG and CONTROL. However, VLDL and LDL cholesterol concentrations were increased in (c) male and (d) female TAG-fed mice compared to CONTROL. Data are mean ± s.e.m. from N = 5–6 mice/gender/diet group. *, Denotes significantly different from TAG and CONTROL; †, denotes significantly different from CONTROL, P < 0.05. HDL, high-density lipoprotein; LDL, low-density lipoprotein; LDLr−/−, LDL receptor deficient; VLDL, very LDL.
Elevations in serum cholesterol concentrations were associated with a striking increase in atherosclerotic lesion surface area in male and female SUCR-fed mice compared to TAG and CONTROL mice of each gender (Figure 4a,b; P < 0.001). In males, TAG-feeding did not significantly increase the extent of atherosclerosis. However, in females, TAG-fed mice exhibited increased atherosclerosis compared to CONTROL (Figure 4b; P < 0.05).
Figure 4.
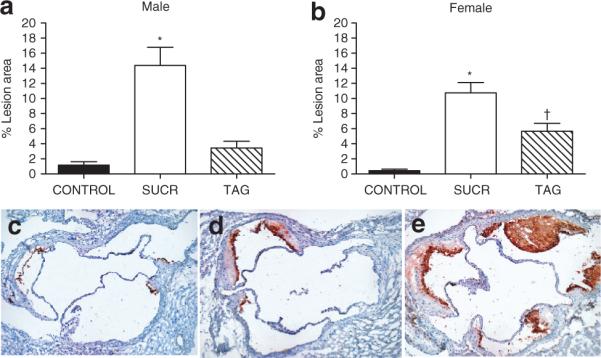
A diet containing sucrose results in marked increases in the extent of (a,b) atherosclerosis and (c–e) macrophage immunostaining in aortic roots from LDLr−/− mice. (a) Male and (b) female SUCR-fed animals exhibited a marked extent of atherosclerosis in the aortic arch compared to TAG and CONTROL. Female TAG-fed mice exhibited elevated atherosclerosis development compared to CONTROL. Data are mean ± s.e.m. from N = 5–6 mice/gender/diet group. *, Denotes significantly different from CONTROL and TAG-fed mice, P < 0.05. †, denotes significantly different from CONTROL, P < 0.05. c–e, Representative sections reveal pronounced macrophage immunostaining in the aortic root from (e) sucrose-fed mice compared to (d) TAG and (c) CONTROL. LDLr−/−, low-density lipoprotein receptor deficient.
Immunohistochemistry was used to assess the degree of macrophage infiltration in the aortic root of each group. CONTROL LDLr−/− mice did not exhibit positive macrophage immunostaining, as genetically modified hyperlipidemic mice do not readily develop atherosclerosis unless challenged with a high-energy diet (16). However, atherosclerotic lesions in aortic roots of SUCR-fed mice stained positive for macrophages (Figure 4e). Furthermore, the larger lesions in aortic roots of SUCR-fed mice were associated with more pronounced macrophage immunostaining compared to relatively smaller atherosclerotic lesions in TAG-fed mice.
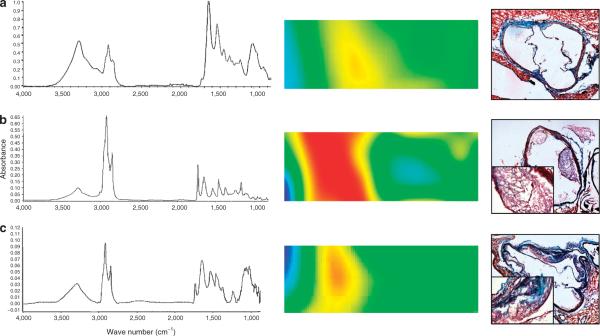
Infrared microspectrometry of lesions in the CONTROL mice showed the lowest concentration of lipid (Figure 5a, doublet between 2,800 and 3,000 cm−1). Atherosclerotic lesions of SUCR-fed mice had the largest concentration of lipids, while lesions in TAG-fed mice showed an intermediate lipid content. The broad peak at 1,100 cm−1 associated with collagen, which may strengthen fibrous caps in lipid-filled atheromas, is missing in the spectrum of lesions from SUCR-fed mice (Figure 5b). Gomori Trichrome staining for collagen illustrates an increase in collagen deposition (teal blue coloration in Figure 5c, right column) within atherosclerotic lesions of TAG compared to SUCR mice.
Figure 5.
Infrared microspectrometry of lesions in the mice. Left, average infrared spectrum of lesions from each group. Middle, infrared spectral image, with the intima oriented to the left, and the lumen in blue. The spectral image shows the relative lipid concentration in shades of red based on the peak doublet between 2,800 and 3,000 cm−1 (image size = 30 × 90 μm). Right, Gomori trichrome staining in aortic root sections illustrates increases in collagen deposition (teal blue color) in TAG compared to SUCR-fed mice. Inset is a higher magnification (×40) of the lesion area. (a) CONTROL mice showed the lowest concentration of vascular wall lipid (peak doublet between 2,800 and 3,000 cm−1). (b) Atherosclerotic lesions of SUCR-fed mice had by far the largest concentration of lipids, while lesions in the (c) TAG-fed mice showed an intermediate lipid content. The broad peak at 1,100 cm−1, associated with collagen, is missing in the spectrum of lesions from SUCR-fed mice.
Discussion
This study was designed to compare diets containing TAG and SUCR (as the primary carbohydrate source) to investigate their effects on aspects of the metabolic syndrome and atherosclerosis in LDLr−/− mice. A diet enriched in SUCR promoted increased energy intake, obesity, elevated blood glucose and insulin, increased total serum cholesterol concentrations, and markedly augmented atherosclerosis in LDLr−/− mice. In contrast, a diet containing an equivalent amount of TAG had no effect on body weight or the insulin levels. Although TAG-fed mice exhibited increased serum cholesterol and atherosclerosis compared to CONTROL, the extent of these changes were far less than those observed in sucrose-fed mice. Finally, aortic atherosclerotic lesions detected in TAG-fed mice were comprised of increased collagen, suggestive of a more stable plaque phenotype compared to SUCR-fed mice.
Results from this study demonstrate a sucrose-enriched diet promotes the development of obesity in LDLr−/− mice, while TAG did not promote substantial weight gain or enhanced adiposity. TAG, currently used as a nutritive or low-calorie sweetener, is incompletely absorbed from the lower digestive tract and remains in the colon to be fermented by resident bacteria. Incomplete absorption of TAG from the gastrointestinal tract may explain the prevention of weight gain by TAG-fed mice. Studies investigating the effect of TAG on food intake have yielded inconsistent results (17); however, our data suggest TAG consumption does not affect food intake. Palatable diets (in the form of high sugar or high fat) have been demonstrated to alter satiety signals and lend to hyperphagia in rodents (18). Accordingly, male and female SUCR mice exhibit increased energy intake per day compared to both CONTROL and TAG mice. The SUCR diet was more energy dense (4.0 kcal/g) compared to the TAG (3.3 kcal/g) or CONTROL diet (3.4 kcal/g). The combination of elevated food intake and increased energy density of the diet likely contributed to the development of obesity in SUCR mice.
Numerous studies have demonstrated the antihyperglycemic effects of TAG (reviewed in (6)). Specifically, Donner et al. (19) examined effects of TAG on the glycemic response of diabetic and nondiabetic subjects to a sucrose load. When diabetic subjects were administered TAG (75 g) before a glucose tolerance test, they exhibited a significantly blunted increase in blood glucose compared to control diabetic subjects; insulin levels were not affected by the TAG treatment. Our results are consistent with and extend previous findings by demonstrating that chronic consumption of a diet enriched in TAG, in contrast to sucrose, does not promote increased glucose or insulin levels. Collectively, these results suggest that TAG would assist with glycemic control. However, SUCR-fed mice exhibited elevated energy intake, body weight, and adiposity compared to TAG and CONTROL; thus, the direct effect of TAG on glucose regulation, independent of its effect to suppress weight gain, is difficult to distinguish in the current study. Importantly, TAG is currently in a placebo-controlled phase 3 clinical trial designed to evaluate its efficacy as an antidiabetic agent (6).
Oral administration of TAG (45 g/day over 3 divided doses) has been demonstrated to significantly elevate high-density lipoprotein cholesterol (30–41.7 mg/dl) in type 2 diabetics not taking medications for lipids (20). While we did not observe an effect of TAG to significantly increase high-density lipoprotein cholesterol in LDLr−/− mice, total serum cholesterol levels were decreased in both male and female TAG compared to SUCR mice. However, it should be noted that TAG fed mice were not totally protected against hypercholesterolemia or atherosclerosis, suggesting that metabolic use of TAG may contribute to some degree of elevated cholesterol precursor. Alternatively, modest increases in fat as an energy source between the TAG and CONTROL diet may have contributed to increased serum cholesterol and atherosclerosis in TAG-fed mice compared to CONTROL. Moreover, the decreased fiber content of both experimental diets, compared to the CONTROL diet, may have further increased susceptibility to atherosclerosis in TAG or SUCR mice. Once it has been absorbed into the blood stream, TAG is metabolized in the liver along the same pathway used in fructose metabolism, through phosphorylation by fructokinase to TAG-1-P. This metabolite, like fructose-1-P, is cleaved by aldolase to yield glyceraldehyde and dihydroxyacetone phosphate. While aldolase acts on both fructose-1-P and TAG-1-P as substrates, the rate of cleavage of TAG-1-P is 50% of that of fructose-1-P (21). Thus, TAG-1-P concentrations would increase, resulting in a stimulation of glucokinase activity and increased phosphorylation of glucose to glucose-6-P to activate glycogen synthase (22). The literature also suggests that TAG-1-P (similarly to fructose-1-P) inhibits glycogen phosphorylase (23), thereby limiting glycogen utilization. The net effects from TAG metabolism would be to enhance glycogen synthesis, and to diminish glycogen utilization. Thus, pyruvate generation from glycolysis would be limited, reducing acetyl CoA through the Krebs cycle as a precursor to cholesterol. These effects of TAG may have contributed to the observed reductions in serum cholesterol in TAG-compared to SUCR-fed mice.
Previous results demonstrate that irrespective of the mode of inducing obesity, adipocyte hypertrophy with obesity is associated with infiltration of macrophages into adipose tissue (24). Further, adipocyte size has been shown to correlate positively with the release of proinflammatory factors leptin, monocyte chemoattractant protein-1, and interleukin-6, among others (25). Indeed, obesity-associated inflammation is a link between obesity and increased risks for the development of type 2 diabetes, hypertension, and cardiovascular disease (24,26). Our results demonstrate that in SUCR- fed mice exhibiting hyper-cholesterolemia, obesity, hyperglycemia and atherosclerosis, macrophages were detected in adipose tissue. Localized and/ or systemic inflammation in SUCR mice may have contributed to glucose dysregulation or the development of atherosclerosis. In contrast, smaller adipocyte size in TAG-fed mice may have limited macrophage infiltration and inflammation, thereby decreasing aspects of the metabolic syndrome in TAG compared to SUCR-fed mice.
An important aspect of these studies is the measurement of atherosclerosis in mice with differing cholesterol concentrations from dietary carbohydrate manipulation. Interestingly, we found that male and female mice differed in their responsiveness to the development of atherosclerosis from SUCR and TAG-containing diets. In male mice TAG did not result in increased atherosclerosis compared to CONTROL; however, male mice fed a SUCR diet exhibited more extensive atherosclerosis compared to females. In contrast, female mice fed the TAG-containing diet exhibited an increase in atherosclerosis, but had a less pronounced atherosclerotic response to SUCR compared to males. Gender differences in effects of TAG on atherosclerosis may result from differences in serum cholesterol or the lipoprotein cholesterol profile. For example, female mice fed the TAG-diet exhibited an increased VLDL/LDL ratio compared to males. Interestingly, the elevation in VLDL cholesterol occurred in female TAG-fed mice despite a generally lower serum cholesterol concentration than that observed in male TAG-fed mice.
A novel finding in this study was a reduction in the collagen composition of atherosclerotic lesions among the diet groups. The increase in collagen content of atherosclerotic lesions from TAG-fed mice compared to SUCR may contribute to a more stable plaque phenotype (27,28). The mechanism of this effect is unclear. However, these results suggest that in addition to minimizing the extent of atherosclerosis from carbohydrate consumption, a diet enriched in TAG may favor a more stable plaque phenotype.
A drawback to use of TAG, like that of other poorly absorbed sugars, is that their consumption is associated with gastrointestinal distress. For instance, a 75 g dose of TAG (19) led to gastric distress in the form of diarrhea, nausea and/or flatulence in 100% of subjects. However, when lower doses of TAG (10–30 g) were administered, only 3 of 10 reported gastrointestinal symptoms. This side effect results from the poor absorption of TAG in the small intestines, resulting in a longer resident time and bacterial fermentation in the large intestine, with increased flatulence and fluid retention. Absorption studies involving TAG have shown a phase-in period for administration improves overall absorption of TAG, as indicated by increased fecal excretion of tagatose (25.7 compared to 1.7%) in unadapted vs. adapted rats (exposed to 100 g/kg of TAG in the diet for 4 weeks) (29). It will be important to determine whether gastrointestinal effects of TAG limit its tolerability in current phase 3 clinical studies.
In conclusion, in comparison to sucrose, a diet enriched in TAG as a carbohydrate source did not promote obesity or hyperglycemia, adipocyte hypertrophy, and resulted in a lesser extent of hypercholesterolemia and atherosclerosis. Future studies should investigate whether D-tagatose could be used in the management of obesity and its associated disorders of diabetes and coronary artery disease.
Supplementary Material
ACKNOWLEDGMENTS
Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract no. DE-AC02-98CH10886. We thank Spherix for the gift of D-tagatose.
Footnotes
SUPPLEMENTARY MATERIAL Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
DISCLOSURE R.A.L. serves as a member of the board of directors of Spherix.
REFERENCE
- 1.Egger G, Swinburn B. An “ecological” approach to the obesity pandemic. BMJ. 1997;315:477–480. doi: 10.1136/bmj.315.7106.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocchini AP. Obesity hypertension. Am J Hypertens. 2002;15(2 Pt 2):S50–S52. doi: 10.1016/s0895-7061(01)02299-3. [DOI] [PubMed] [Google Scholar]
- 3.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13–e18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 5.Serdula MK, Mokdad AH, Williamson DF, et al. Prevalence of attempting weight loss and strategies for controlling weight. JAMA. 1999;282:1353–1358. doi: 10.1001/jama.282.14.1353. [DOI] [PubMed] [Google Scholar]
- 6.Lu Y, Levin GV, Donner TW. Tagatose, a new antidiabetic and obesity control drug. Diabetes Obes Metab. 2008;10:109–134. doi: 10.1111/j.1463-1326.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- 7.Levin GV. Tagatose, the new GRAS sweetener and health product. J Med Food. 2002;5:23–36. doi: 10.1089/109662002753723197. [DOI] [PubMed] [Google Scholar]
- 8.Rulis AM. Agency response letter GRAS notice nr GRN 000078. 2001 US Food and Drug Administration: < http://www.cfsan.fda.gov/~rdb/opa-g078.html>.
- 9.Storlien LH, Oakes ND, Pan DA, Kusunoki M, Jenkins AB. Syndromes of insulin resistance in the rat. Inducement by diet and amelioration with benfluorex. Diabetes. 1993;42:457–462. doi: 10.2337/diab.42.3.457. [DOI] [PubMed] [Google Scholar]
- 10.Martinez FJ, Rizza RA, Romero JC. High-fructose feeding elicits insulin resistance, hyperinsulinism, and hypertension in normal mongrel dogs. Hypertension. 1994;23:456–463. doi: 10.1161/01.hyp.23.4.456. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan SR, Clevidence BA, Pargaonkar PS, Radhakrishnamurthy B, Berenson GS. Varied effects of dietary sucrose and cholesterol on serum lipids, lipoproteins and apolipoproteins in rhesus monkeys. Atherosclerosis. 1979;33:301–314. doi: 10.1016/0021-9150(79)90182-5. [DOI] [PubMed] [Google Scholar]
- 12.Swanson JE, Laine DC, Thomas W, Bantle JP. Metabolic effects of dietary fructose in healthy subjects. Am J Clin Nutr. 1992;55:851–856. doi: 10.1093/ajcn/55.4.851. [DOI] [PubMed] [Google Scholar]
- 13.Merkel M, Velez-Carrasco W, Hudgins LC, Breslow JL. Compared with saturated fatty acids, dietary monounsaturated fatty acids and carbohydrates increase atherosclerosis and VLDL cholesterol levels in LDL receptor-deficient, but not apolipoprotein E-deficient, mice. Proc Natl Acad Sci USA. 2001;98:13294–13299. doi: 10.1073/pnas.231490498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregorevic P, Allen JM, Minami E, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12:787–789. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henriques TA, Huang J, D'Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology. 2004;145:3866–3872. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- 16.Jawien J, Nastalek P, Korbut R. Mouse models of experimental atherosclerosis. J Physiol Pharmacol. 2004;55:503–517. [PubMed] [Google Scholar]
- 17.Buemann B, Toubro S, Raben A, Blundell J, Astrup A. The acute effect of D-tagatose on food intake in human subjects. Br J Nutr. 2000;84:227–231. [PubMed] [Google Scholar]
- 18.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 19.Donner TW, Wilber JF, Ostrowski D. D-tagatose, a novel hexose: acute effects on carbohydrate tolerance in subjects with and without type 2 diabetes. Diabetes Obes Metab. 1999;1:285–291. doi: 10.1046/j.1463-1326.1999.00039.x. [DOI] [PubMed] [Google Scholar]
- 20.Donner TW. The metabolic effects of dietary supplementation with D-tagatose in patients with type 2 diabetes. Diabetes. 2006;55(Suppl 1):A110, 461P. [Google Scholar]
- 21.Rognstad R. Gluconeogenesis from D-tagatose by isolated rat and hamster liver cells. FEBS Lett. 1975;52:292–294. doi: 10.1016/0014-5793(75)80828-3. [DOI] [PubMed] [Google Scholar]
- 22.Seoane J, Gomez-Foix AM, O'Doherty RM, et al. Glucose 6-phosphate produced by glucokinase, but not hexokinase I, promotes the activation of hepatic glycogen synthase. J Biol Chem. 1996;271:23756–23760. doi: 10.1074/jbc.271.39.23756. [DOI] [PubMed] [Google Scholar]
- 23.Gergely P, Toth B, Farkas I, Bot G. Effect of fructose 1-phosphate on the activation of liver glycogen synthase. Biochem J. 1985;232:133–137. doi: 10.1042/bj2320133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–372. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 28.Cullen P, Baetta R, Bellosta S, et al. Rupture of the atherosclerotic plaque: does a good animal model exist? Arterioscler Thromb Vasc Biol. 2003;23:535–542. doi: 10.1161/01.ATV.0000060200.73623.F8. [DOI] [PubMed] [Google Scholar]
- 29.Saunders JP, Zehner LR, Levin GV. Disposition of D-[U-14C]tagatose in the rat. Regul Toxicol Pharmacol. 1999;29(2 Pt 2):S46–S56. doi: 10.1006/rtph.1998.1251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.