Abstract
New neurons formed in the adult brain are incorporated into existing circuits. However, the number of new neurons recruited into a given brain region varies widely depending on the experience of the animal. An emerging general principle is that recruitment and early neuronal survival may be correlated with activity or use of the brain region. Here we show that use-dependent neuronal survival also occurs in the higher order auditory processing region of the songbird caudomedial nidopallium (NCM). We suggest that retention of young neurons may in part be influenced by use of the system without an increased demand for learning or behavioral plasticity.
Keywords: caudomedial nidopallium, NCM, songbird, zebra finch, adult neurogenesis, auditory cortex, behavior, deaf, sensory deprivation
INTRODUCTION
In the adult vertebrate, particular neural populations are continually replaced throughout adulthood whereas other neuron types presumably persist more or less for the lifespan of the organism [12]. In some brain regions, the number of incoming neurons compensates for the number of "replaceable" neurons that die [23, 24, 36, 51]. In other systems, or under different conditions, neuron addition need not balance neuron death [51, 58]. Regulatory mechanisms underlying neuronal incorporation are not well understood, although evidence increasingly suggests that functional activity of the circuitry that incorporates new neurons may contribute to the survival of young incoming neurons. For instance, hippocampal-dependent learning is correlated with an increase in the incorporation and survival of new neurons in the dentate gyrus of the hippocampus [17, 54, 64]. In addition, olfactory deprivation results in diminished new neuron incorporation of the granule cells of the olfactory bulb [11, 30, 41, 65] and olfactory enrichment results in increased survival of these neurons [49]. Use of a brain region and lifespan of newly recruited neurons also appear to be correlated in the songbird nucleus HVC, part of the motor pathway for song production. Singing is a behavioral measure of the firing activity of HVC neurons that project to the premotor Robust Nucleus of the Archistriatum (RA) [18] and singing rate is correlated with increased survival of new HVC-RA projection neurons [4, 26]. Singing also upregulates brain-derived neurotrophic factor (BDNF), which may mediate the survival of young HVC-RA neurons [26, 47].
Here we further test the relationship between use of a brain region and the survival of new neurons within that brain region using the avian caudomedial nidopallium (NCM). NCM is a target of subdivisions of the primary auditory region Field L, which receives thalamic input from the auditory nucleus ovoidalis. NCM contains at least 2 subtypes of GABAergic neurons, based on calbindin expression and soma size, as well as excitatory neurons [44, 45]. It is not known which neuron types are replaceable or whether new neuron incorporation is balanced by neuron death. Activity in NCM corresponds specifically to perceptual processing and memory of conspecific vocalizations, as measured by electrophysiology [8, 9, 42, 59], expression of the immediate early gene zenk [6, 7, 20, 48] and behavioral recognition tasks [16].
New neuron survival in NCM is influenced by the bird's experience as well as neuron age and position within NCM. Birds that are introduced into large social groups of unfamiliar individuals maintain more young neurons than birds that are placed with a single other individual or housed alone [1, 5, 27]. Although there are numerous potentially relevant variables associated with complex social housing, a compelling difference between the housing groups in these studies was the amount of exposure to novel conspecific songs [2], suggesting use-based retention of young neurons in NCM.
To determine whether the survival of young neurons in NCM is influenced by NCM usage in the absence of exposure to novel songs, we compared NCM neuron incorporation between hearing and deafened adult male zebra finches kept in stable social groups. We found a striking decrease in 1 month old neurons incorporated into the NCM of deaf birds compared to hearing birds, which was concentrated in the medial region of NCM. These findings are consistent with the idea that use of NCM may be a key component in regulating young neuron survival in this population.
MATERIALS AND METHODS
Experimental design
All procedures were approved by the Queens College Institutional Animal Care and Use Committee. Sixteen male zebra finches were raised in individual family cages in our breeding colony until birds reached adulthood (>90 days old). At this time, young adults were assigned to 1 of 2 large group cages (8 per cage), matched for age, with brothers split between the 2 groups to balance potential genetic differences in neurogenesis [21].
The 2 group cages were housed together in a room without other zebra finches, separated visually and acoustically by sound attenuation chambers throughout the experiment. In this way, we controlled for social complexity, prevented social change, and prevented hearing birds from exposure to novel songs. This was done as a first step toward assessing whether the use of NCM, in the absence of demands for new learning, impacts new neuron survival.
At 4–5 months of age, the birds received intramuscular injections of bromodeoxyuridine (BrdU; 80 μl of a 10 mg/ml solution in 0.1 M tris buffered saline, pH= 7.4; Boehringer Mannheim, Indianapolis, IN) 3 times/day for 4 consecutive days to label mitotically active cells. Two to three days after the last BrdU injection, experimental birds were deafened by bilateral surgical removal of the cochlea [25]. Deafening was done after cell birth dating so that we could attribute any effects on new neurons to post mitotic events. Birds were anesthetized with ketamine (0.03–0.05 mg ketamine/g weight of bird) and xylazine (0.06 mg xylazine/g). A skin incision was made caudal to the external auditory meatus and muscle was retracted. A patch of outer skull was removed with fine forceps and a hole was bored into the bony cochlear casing thereby exposing the cochlea. The cochlea was then removed with a small wire hook and the hole sealed with cyanoacrylate. The muscles were replaced and skin sutured. Control birds received sham surgeries in which the skull patch was removed but the cochlea was not exposed. After recovery, birds were placed back in their respective preoperative cages.
Histology
Twenty eight days after deafening (30–31 days after the final BrdU injection), birds were deeply anesthetized and perfused transcardially with 0.1M PBS followed by 4% paraformaldehyde. The brains were post fixed for 1 h in 4% paraformaldehyde, rinsed in PBS for 3 hrs, and then dehydrated in increasing concentrations of ethanol and embedded in polyethylene glycol (PEG; Polysciences, Warrington, PA). Six-μm sagittal sections were cut on a rotary microtome. Care was taken to orient the brain with the midline parallel to the blade edge. The first complete section through the telencephalon was saved and subsequently every eighth section was mounted onto Superfrost + + slides, air dried overnight, and stored at −20º C. Three series of tissue, each series offset by 1 section, were produced and all series were processed for immunocytochemistry.
Immunocytochemistry
To label BrdU and Hu, sections were brought to room temperature in PBS, then exposed to citrate buffer at 95°C for 10 min, followed by a 5 min wash in phosphate buffer (PB), 3 min in 2.5% pepsin in 0.1N HCl at 37°C, and three 3 min washes in PB. Sections were then blocked with 10% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) and 0.3% Triton X-100 in PB for 1 hr at room temperature, followed by overnight exposure to sheep anti-BrdU (12.5 μg/ml at 4°C; Capralogics, Hardwick, MA). After three 10 min PB washes, sections were processed with an avidin–biotin blocking kit (Vector Laboratories, Burlingame, CA), followed by a 2 hr incubation in biotin-conjugated donkey anti-sheep IgG (1:200, Chemicon International, Temecula, CA). After three 10 min PB washes in the dark, streptavidin conjugated to Alexa 488 (1.25 μg/ml, Molecular Probes, Eugene, OR) was applied for 1 hr in the dark for visualization of BrdU. This was followed by three 10 min washes in PB, 1 hr in blocking solution in the dark, and overnight exposure to mouse anti-Hu primary antibody (10 μg/ml in blocking solution) (Hu MAB16A11, Molecular Probes) at 4°C. After three 10 min PB washes at room temperature in the dark, tissue was exposed to donkey anti-mouse IgG conjugated to Cy-3 (6.25 μg/ml, Jackson ImmunoResearch) for 1 hr in the dark to visualize labeling of the cytoplasmic neuron-specific Hu protein. Sections were then washed, dehydrated in ethanols, immersed briefly in xylenes, and cover slipped with Krystalon.
Mapping NCM
Data were collected without knowledge of bird identity. Area measurements and cell counts were performed using a computer-yoked fluorescence microscope and mapping software (Olympus BX51; Lucivid microprojection, Neurolucida, MicroBrightField, Inc., Colchester, VT). The caudoventral boundary of NCM was defined by the caudal and ventral edges of the brain and/or the lateral ventricle [32]. The rostral border of NCM in medial sections was defined by the caudal medial mesopallium (CMM, previously called caudal medial hyperstriatum ventrale, CMHV). In lateral sections, the rostral border of NCM was determined in relation to the Field L Complex subdivision L2, which was identified using dark field optics and appeared as a diffuse bright band of densely packed cells rich in neuropil [14]. The rostral border of NCM was traced conservatively approximately 300μm caudal to the Field L subdivison L2 to avoid the intervening subdivision L, which cannot be identified by differences in optical density that are apparent with dark field microscopy.
NCM Subregions
The contours of NCM were outlined in 20 parasagittal sections from approximately 170–986 μm lateral to the midline. Within this medial-lateral range, brain sections were divided into medial (170–410 μm) and lateral (746–986 μm) subregions prior to cell counting to determine whether there was a difference in new neuron incorporation between these regions (Figure 1A). We based our divisions on patterns of zenk expression in response to playback of novel songs which shows highest levels in NCM from the midline to approximately 500 μm lateral to the midline. More laterally, zenk is still expressed, but is less dense, and less consistently distributed [34]. Although there is no discrete cytoarchitechtural lateral boundary for NCM, studies of connectivity, electrophysiology, and zenk expression suggest that functionally, NCM is likely contained within 1.2 mm of the midline (Mello, pers. comm.; comparative summary of NCM sampling regions provided by [60]).
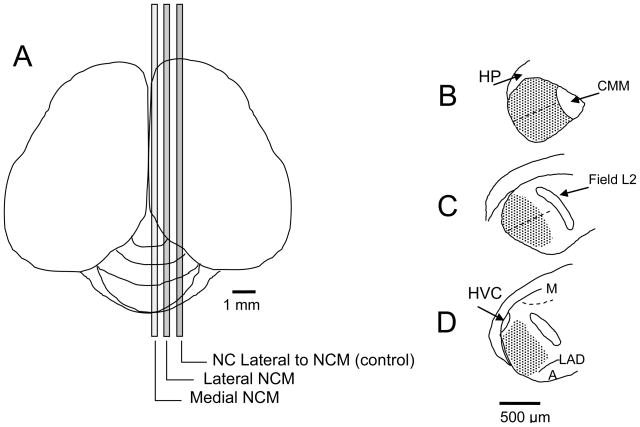
Figure 1.
Labeled cells were compared in medial and lateral divisions of NCM, each containing 240 μm of tissue along the medial-lateral axis, separated by 336μm (A). A representative section in each division is shown as an outline drawn in Neurolucida using Lucivid: (B) Medial (~170–410 μm from midline), (C) Lateral (~746–986 μm from midline), (D) NC lateral to NCM (~1322–1562 from midline). Each tracing in NCM was divided into dorsal and ventral regions, approximately parallel to the dorsal and ventral edges of the brain and perpendicular to the caudal edge (dashed lines). Stippled areas indicate traced regions in which cells were counted. Anatomical landmarks: hippocampus (HP), caudal medial mesopallium (CMM), mesopallium (M), dorsal arcopallial lamina (LAD) arcopallium (A). Nucleus HVC and Field L2 are described in the text.
After tracing the contours of NCM, each tracing was divided into dorsal and ventral regions using Neurolucida (Figure 1B–D). Labeled cells in 10 sections were counted per medial-lateral division per bird. We compared labeled cell numbers between medial and lateral NCM, and between dorsal and ventral NCM.
Mapping control regions: nidopallium lateral to NCM, hippocampus, medial striatum caudal to Area X
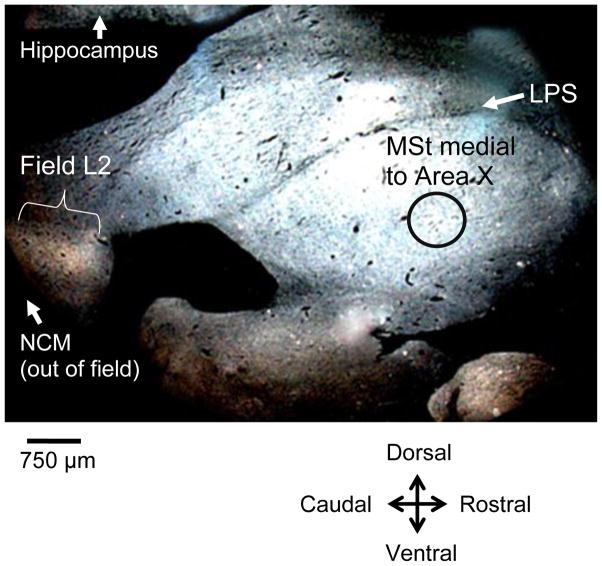
To test for specificity of potential effects of deafening on neuron incorporation, we also counted new neurons in caudal nidopallium (NC) lateral to NCM, the hippocampus, and a region of the medial striatum (MSt, previously lobus parolfactorius) medial and caudal to Area X. These areas were selected because they are regions of high neuron incorporation yet are outside of the song system, which may be functionally affected by deafening. We traced 10 sections of the control region in NC lateral to NCM, within the range of about 1322–1562 μm from the midline. In tracing NC, we maintained an approximate continuation of our NCM tracings by using the caudal and ventral edge of the brain, and/or the lateral ventricle as the caudoventral boundary of our control NC region. The dorsal boundary of NC was defined by the lateral ventricle, the ventral boundary of lateral NC was the dorsal arcopallial lamina (LAD), and the rostral boundary was traced approximately 300 μm caudal to the Field L subdivison L2. We then traced 10 sections of the hippocampus distributed approximately 500 μm-1250 μm lateral to the midline, roughly corresponding to sagittal plates 2–5 in the stereotaxic atlas of the zebra finch brain [38]. Hippocampal areas were bounded by the lateral ventricle and caudal and dorsal edge of the brain. The rostral boundary of the hippocampus was drawn 1 mm from the caudal extent of the brain. To sample a region of the medial striatum medial and caudal to Area X, we outlined a circular template 0.75 mm in diameter and placed this on 10 sections distributed approximately 200μm - 986 μm lateral from the midline, corresponding roughly to sagittal plates 1–6 in the stereotaxic atlas of the zebra finch brain [38]. The template was placed ventral to the pallio-subpallialis lamina (LPS) (Figure 2).
Figure 2.
Darkfield rostroventral section showing the circular template (0.75 mm in diameter, black circle outline in figure) used to sample a control region of medial striatum (MSt) on 10 sections distributed approximately 200 μm-986 μm lateral from the midline. The template was placed ventral to the pallio-subpallialis lamina (LPS) and caudal to the area where Area X would emerge in more lateral sections. Field L is seen as a light band in the caudal region of the brain. NCM is caudal to Field L.
Cell Quantification
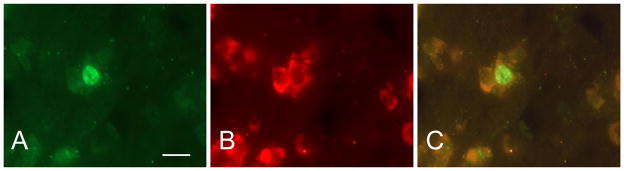
BrdU-labeled cells were identified with a fluorescein isothiocyanate (FITC) filter and Hu-labeled cells were identified with a rhodamine filter. Double-labeled cells were identified by alternating between these two filters and also using a dual FITC-rhodamine filter (Figure 3).
Figure 3.
BrdU-labeled cells were identified with a fluorescein isothiocyanate (FITC) filter (A) and Hu-labeled cells were identified with a rhodamine filter (B). Double-labeled cells were identified by alternating between these two filters and also using a dual FITC-rhodamine filter (C). Scale bar = 20 μm
Volume sampled
Areas were multiplied by section thickness and summed to calculate the volumes of regions sampled. Cell densities per mm3 were calculated by dividing the number of cells in each label category (BrdU+/Hu+ and BrdU+/Hu-) by the volume sampled.
Statistical analysis
We tested differences between hearing and deaf groups using a 2 factor ANOVA with repeated measures of the 3 NCM/NC regions in the medial-lateral dimension. A 2 factor ANOVA was also conducted with the repeated measures of 2 NCM regions in the dorsal-ventral dimension. Post-hoc Tukey's tests were used for pair wise comparisons. Differences between hearing and deaf groups in control regions were analyzed with ANOVA. Data are shown as means + SEM.
RESULTS
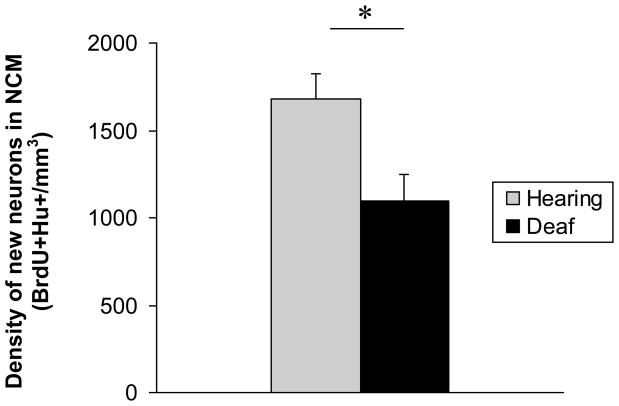
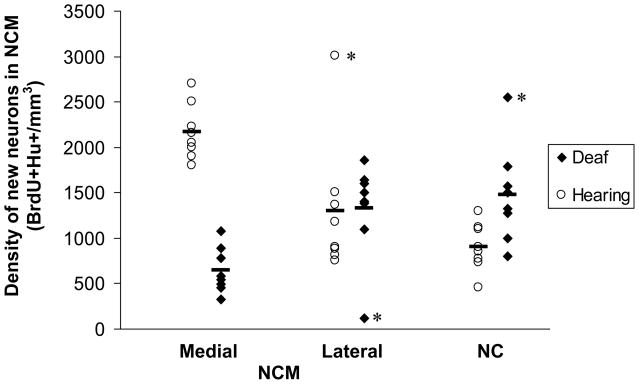
We found an overall difference in the density of new neurons in NCM (BrdU+Hu+ cells per mm3) between hearing and deaf birds (Figure 4, 2-factor ANOVA, main effect of treatment (deafening); F=39.4, p<0.0001). Because our tracings and cell counts in control NC were designed to be compared directly to medial and lateral NCM, we compared these 3 regions simultaneously using a 2-factor ANOVA. There was no difference in new neuron density among medial NCM, lateral NCM, and NC regions when birds in both hearing and deaf groups were combined (main effect of medial-lateral position; F=0.74, p=0.486). However, there was a significant interaction between treatment and medial-lateral position, indicating an effect of deafening within particular regions along the medial-lateral axis (F 18.72, p<0.001). Tukey's post-tests identified significant differences between hearing and deaf birds in the density of new neurons in medial NCM, but not lateral NCM or NC (Figure 5, medial NCM t = 6.422, p<0.05; lateral NCM t=0.127, p>0.05; NC t= 2.428, p>0.05).
Figure 4.
Deaf birds had a significantly lower density of new neurons in NCM than hearing birds.
Figure 5.
Deafening resulted in a significant decrease in new neuron density in medial NCM compared with hearing birds. There was no difference between deaf and hearing birds in new neuron density in lateral NCM or in caudomedial nidopallium (NC) lateral to NCM. Within the hearing group, the density of new neurons in the medial NCM was significantly higher than that in both the lateral NCM and NC. There was no difference in new neuron density between lateral NCM and NC in the hearing birds. Within the deaf group, new neuron density between the medial and lateral divisions was significantly different. Neither medial NCM nor lateral NCM were different from NC in the deaf birds. Removal of the 3 outliers (asterisks) did not change the results. Bars indicate means for each region within a treatment group, n=8 for each region within a treatment group.
We then examined differences in new neuron density across regions within the hearing birds and found significantly higher new neuron densities in medial NCM compared with lateral NCM and NC. There was no difference in new neuron density between lateral NCM and NC (Tukey's post-test, medial NCM v. lateral NCM t=3.68, p<0.05; medial NCM v. NC t=5.33, p<0.05; lateral NCM v. NC t=1.67, p>0.05). Comparisons across medial-lateral regions within the deaf group identified a significantly lower new neuron density in medial NCM compared with NC (Tukey's post-test, t=3.52, p<0.05). New neuron density in lateral NCM did not differ from that of medial NCM or NC (Tukey's post-test, lateral v. medial, t=2.88, p>0.05; lateral v. NC, t=0.63, p>0.05).
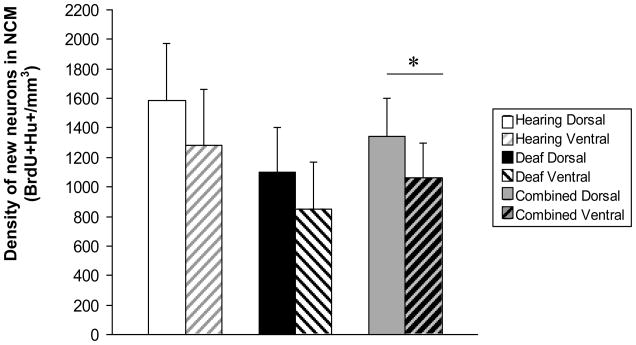
There was a significantly higher density of new neurons in dorsal NCM compared with ventral NCM when deaf and hearing birds were combined, (Figure 6, main effect of region, F=9.38, p=0.220). There was no interaction between treatment group and region, indicating that deafening did not differentially decrease dorsal or ventral neuron density (F=0.06, p=0.81). Within the small age range of birds used, there was no correlation between bird age and new neuron density in either the deaf or hearing groups (p>0.05). There was no difference in BrdU+Hu- cell counts between hearing and deaf birds overall, with all regions combined (mean density mm3 ± SEM, Hearing = 300±91.5, Deaf = 291±50; p>0.05).
Figure 6.
We found a significantly higher density of new neurons in dorsal NCM compared with ventral NCM when all birds were combined. The differences between dorsal and ventral NCM new neuron densities were not significant in either control or experimental groups considered separately, although the trend was similar in both groups.
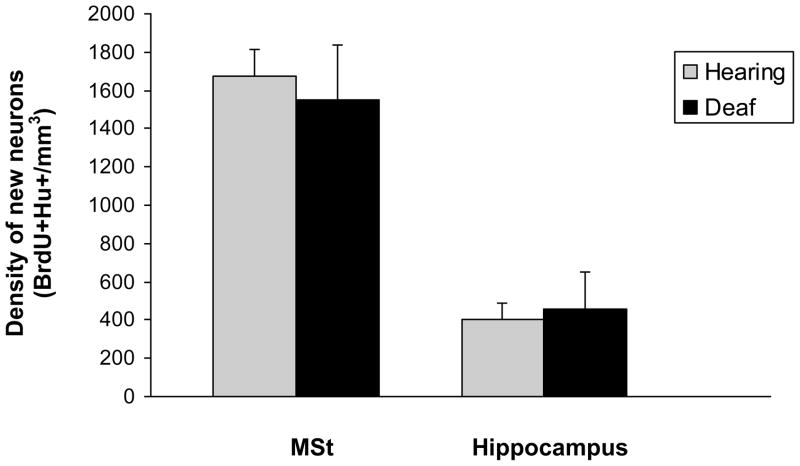
We did not find a difference in new neuron density between hearing and deaf birds in the hippocampus or medial striatum (Figure 7, p>0.05). There was also no difference between BrdU+Hu- cells between hearing and deaf birds in the hippocampus or medial striatum (p>0.05).
Figure 7.
There was no difference in new neuron density in the medial striatum (MSt) or hippocampus between hearing and deaf birds.
DISCUSSION
We found that blocking auditory input into NCM in adulthood decreased new neuron survival in NCM in a place-dependent manner. In normally hearing birds, the incorporation of new neurons was not homogeneous throughout our sampling of NCM. The highest density of new neurons per mm3 was seen in medial NCM, which was significantly greater than new neuron incorporation in lateral NCM and our control NC region outside of NCM. Surgical deafening by cochlea removal resulted in a significant decrease in new neuron incorporation when both medial and lateral regions of NCM were combined. However, this effect seemed to be due to a large and significant decrease in new neurons in medial NCM of deaf birds as there was no statistical difference between deaf and hearing birds in lateral NCM when examined separately in post-hoc analyses. Within the deafened group, neuron incorporation in medial NCM was significantly lower than in lateral NCM -- the reverse pattern from that of the hearing birds. Neuron incorporation in lateral NCM of deaf birds did not differ significantly from either medial NCM or NC. We also compared new neuron density between dorsal and ventral NCM. We found a significantly greater density of new neurons in dorsal NCM than in ventral NCM when hearing and deaf birds were combined. When examined separately, both hearing and deaf birds showed a similar trend for higher density of new neurons in dorsal NCM than ventral NCM and there was no evidence that deafening differentially affected dorsal or ventral neuron incorporation. We found no effect of deafening on new neuron incorporation in the control regions of the hippocampus or medial striatum caudal and medial to the song system nucleus Area X. These results suggest that the observed decrease in new neurons in NCM was not due to a general effect of deafening such as a systemic change in testosterone or neuronal growth factor such as BDNF [46, 47]. The data are consistent with the hypothesis that decreased functional activity in a higher order sensory region resulted in decreased survival of new neurons formed in the adult brain. While differential rates of neuronal incorporation in different regions of NCM have not yet been associated with functional specializations, the findings underscore the observation by Barnea et al. [5] and Adar et al. [1], that precise locations within brain regions should be considered in assessing the role of experience-dependent neuronal recruitment.
It is intriguing that the pattern of neuron addition across subdivisions of NCM in our control animals mirrors reports of differential levels of zenk expression in response to acoustic stimuli. The zenk response to conspecific songs is consistently more robust in medial and dorsal NCM, regions of higher neuron incorporation, than in lateral and ventral NCM (e.g., 15, 50, 60]. Zenk expression has been suggested to be possible indicator of cellular learning and memory [32] and thus it is tempting to speculate there may be a functional correlation between a substrate rich in young neurons and the encoding of new songs. Moreover, zenk selectivity in medial NCM, but not lateral NCM, is modulated by estradiol [50], which may subserve seasonal plasticity in females’ selectivity for male song [31]. In addition, playback of a bird’s tutor song results in a significant correlation between the density of zenk-positive cells in lateral NCM, but not medial NCM, and the percentage of song elements a bird has copied from his tutor [60]. This again suggests that differential neuron addition may be related to functional subdomains in NCM. In this case, decreased neuron addition in lateral NCM may be potentially associated with the long-term acoustic memory of the tutor’s song, and perhaps decreased neuron incorporation in this region is related to the stability of a long-term memory.
NCM is part of the auditory pathway
Bilateral cochlea extraction removes afferent innervation of the spiral ganglion cells which project to the cochlear nucleus. Neurons of the cochlear nucleus send information to the midbrain dorsal lateral nucleus of the mesencephalon (MLd) by direct projections and also indirectly via the superior olive and lateral lemniscus. From here, the MLd projects to the ovoidalis nucleus of the thalamus which relays input to the primary auditory area Field L in the nidopallium, which is composed of 4 interconnected subregions [61, 63, 66]. Two of these subregions, Field L2a and L3, send axons to the NCM. NCM also receives input from the auditory region of the caudal medial mesopallium (CMM) which is interconnected with Field L, and receives a direct projection from the shell of the ovoidalis, which bypasses Field L [63]. Surgical deafening therefore may impact NCM function not only by blocking direct ascending information but also via numerous interconnected pathways between NCM and Field L as well as the caudal medial and lateral mesopallium (CMM and CLM) auditory processing regions. Projections from NCM are less well understood and a direct pathway from NCM into the song system has not been identified [61].
NCM functions in conspecific song processing
Immediate early gene (IEG) expression and electrophysiological recordings have shown that NCM neurons respond differentially to familiar and novel conspecific songs, suggesting that NCM may play a role in song discrimination [8, 32, 33]. IEG expression is greatest for songs with higher behavioral salience [34] and this has been confirmed by fMRI data [62]. NCM neurons also display experience-dependent plasticity, supporting the idea that NCM may function in learning and remembering conspecific songs [8, 9, 33, 55]. NCM neurons of adults have been shown to retain a long-term memory of song learned from tutors during juvenile song development [6, 7, 16, 42]. To our knowledge, electrophysiological recordings in deaf NCM have not been published. Therefore, we cannot speculate on neuronal activity in deaf birds, although deafening blocks NCM from functional auditory processing.
Experience-dependent and independent neuron incorporation in NCM
Neuron incorporation in NCM has been shown to be influenced by conditions of social housing. Adult male zebra finches placed in a large social group had more new neurons in NC (not restricted to medial NC) than birds housed singly or in male-female pairs [1, 5, 27]. This effect was not driven by increased singing in large groups and Adar et al. [2] suggested that new neuron survival in NCM may reflect auditory exposure to conspecific songs. Moreover, a higher number of novel cage mates promoted the survival of younger (1 month old) neurons while less social novelty promoted the survival of older neurons (3 month old). Adar et al. [1] proposed that differential survival of young and old neurons may correspond to information load and demand for plasticity placed on NCM. They suggested that perhaps the task of learning new songs led to preferential retention of recently incorporated neurons, whereas a social context encouraging the retention of familiar songs led to the preservation of older neurons. Younger neurons have been shown to display membrane properties that differ from those of older neurons and which result in a lower threshold for the induction of long-term potentiation and synaptic plasticity [37, 52]. The idea that younger neurons may be required for plasticity that exceeds the range or type provided by older neurons was proposed by Nottebohm [39, 40] and remains a strong hypothesis in our understanding of neuron replacement across systems [54].
Our findings are consistent with this idea, and yet add the observation that retention of young neurons also occurs simply in the presence of NCM activity without the requirement of learning new information. Here we are assuming that the hearing birds, housed in a constant social and auditory environment, did not learn or form new memories of novel songs. However, a type of learning may have occurred in the act of maintaining song memories of familiar individuals, perhaps in a manner analogous to the proposal of engrainment of the song motor pattern with use of the song motor pathway [28]. Nevertheless, whereas young neurons may be permissive for learning new information and may provide a substrate for increased plasticity, an immediate demand for learning new information does not seem to be a requirement for neuron incorporation and early survival -- at least not in the population of neurons we identified. In this case, young neurons may serve to preserve a healthy neuronal population, or accurate firing patterns, in order to maintain preexisting information.
We were surprised at the high rate of new neuron incorporation even in deaf birds, with presumably no functional NCM activity. This suggests that to some extent, there may exist an endogenous program for neuronal incorporation in the absence of experience-based survival. In addition to this (perhaps baseline) rate of incorporation, increased use of NCM in hearing birds resulted in increased incorporation.
Several scenarios are consistent with our results. We may be seeing decreased neuron incorporation in deaf birds because older neurons are living longer in the absence of activity, precluding opportunities for new neuron incorporation [51]. Alternatively, decreased neuron incorporation may reflect a shorter lifespan of young neurons which require activity to survive regardless of the behavior of older neurons. In addition, both of these very different potential outcomes of activity deprivation may act in combination and the effect of activity deprivation on neuronal lifespan may depend on the age of the neuron in a manner consistent with Adar et al. [1]. Perhaps activity promotes the survival of young neurons while shortening the lifespan of older neurons by contributing to their deterioration. Finally, there may be decreased survival in both young and older neurons in the absence of auditory activity. If so, NCM total neuron number would be diminishing in deaf birds as has been shown in the mammalian olfactory bulb following olfactory deprivation [30].
Activity-based survival of new neurons may be a general property of adult neuronal incorporation
A correlation between use of the circuitry (and/or use of newly incorporated neurons) and new neuron survival, independent of novel information processing, is also evident in the song motor pathway. The rate of song production in adults was found to be positively correlated with survival of new neurons in HVC of the song motor pathway [4, 26]. Activity-dependent survival of new neurons occurs in the mammalian hippocampus as well [17, 56, 57]. However, the distinction between hippocampal activity and hippocampal-dependent learning is unclear [3, 10, 22, 54]. A more informative comparison may be with neuron survival in the mammalian main olfactory bulb. This is the first relay in the olfactory sensory pathway and receives projections from the olfactory sensory neurons in the olfactory epithelium, which undergo neuronal turnover as well. Incoming neuroblasts differentiate into GABAergic granular and periglomerular interneurons. Unilateral olfactory deprivation via nares occlusion [11, 19, 30, 65], unilateral axotomy of the olfactory receptor neurons [29], and transgenic anosmic mice [41] have been used to demonstrate decreased incorporation of granule neurons following olfactory sensory deprivation. Although consistent with a use-based model of neuronal incorporation, these studies of olfactory bulb neuron survival did not directly control for novel sensory experiences which may have stimulated learning. A specific test of usage-dependent neuron incorporation independent of learning was conducted by systemic administration of diazepam, which reduced overall neuronal activity [13]. Two weeks of treatment resulted in a significant decrease in olfactory granule cells between ages 14–28 days post cell birth dating [65]. Thus in the olfactory bulb, neural activity seems to contribute to new neuron survival. Neuronal activity has also been shown to prolong neuronal survival during development and in vitro [35, 53] although the mechanism by which this occurs has yet to be identified.
SUMMARY
Here we show that sensory deprivation of auditory information by bilateral cochlea removal in adulthood results in decreased survival of new neurons in the higher order avian auditory region of the caudomedial nidopallium (NCM). In order to assess the role of the use of a brain region while minimizing the potential for learning novel information, we compared new neuron incorporation between deaf birds and hearing birds that were prevented from exposure to novel songs. Consistent with other findings of use-dependent incorporation and neuron survival, we suggest that the number of new neurons incorporated into the system increases with increasing activity of the local circuitry, perhaps including new neurons. In addition, we found relatively high levels of neuron incorporation in deaf birds, suggesting a baseline source of potentially experience-independent new neurons. Thus while newly recruited neurons may be permissive for increased plasticity, an immediate demand for learning novel information, or exposure to novel experience, is not a requirement for the retention and early survival of new neurons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adar A, Nottebohm F, Barnea A. The relationship between nature of social change, age, and position of new neurons and their survival in adult zebra finch brain. J Neurosci. 2008;28:5394–5400. doi: 10.1523/JNEUROSCI.5706-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adar A, Lotem A, Barnea A. The effect of social environment on singing behavior in the zebra finch (Taeniopygia guttata) and its implication for neuronal recruitment. Behav Brain Res. 2008;187:178–184. doi: 10.1016/j.bbr.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Borda B, Nottebohm F. Gonads and singing play separate, additive roles in new neuron recruitment in adult canary brain. J Neurosci. 2002;22:8684–90. doi: 10.1523/JNEUROSCI.22-19-08684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnea A, Mishal A, Nottebohm F. Social and spatial changes induce multiple survival regimes for new neurons in two regions of the adult brain: An anatomical representation of time? Behav Brain Res. 2006;167:63–74. doi: 10.1016/j.bbr.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Bolhuis JJ, Hetebrij E, Den Boer-Visser AM, De Groot JH, Zijlstra GG. Localized immediate early gene expression related to the strength of song learning in socially reared zebra finches. Eur J Neurosci. 2001;13:2165–2170. doi: 10.1046/j.0953-816x.2001.01588.x. [DOI] [PubMed] [Google Scholar]
- 7.Bolhuis JJ, Zijlstra FF, Den Boer-Visser AM, Van Der Zee EA. Localized neuronal activation in the zebra finch brain is related to the strength of song learning. Proc Natl Acad Sci USA. 2000;97:2282–2285. doi: 10.1073/pnas.030539097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc Natl Acad Sci USA. 1995;92:3406–3410. doi: 10.1073/pnas.92.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chew SJ, Vicario DS, Nottebohm F. A large-capacity memory system that recognizes the calls and songs of individual birds. Proc Natl Acad Sci USA. 1996;93:1950–1955. doi: 10.1073/pnas.93.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clelland CD, Choi M, Romberg C, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corotto FS, Henegar JR, Maruniak JA. Odor deprivation leads to reduced neurogenesis and reduced neuronal survival in the olfactory bulb of the adult mouse. Neuroscience. 1994;61:739–744. doi: 10.1016/0306-4522(94)90397-2. [DOI] [PubMed] [Google Scholar]
- 12.Doetsch F, Scharff C. Challenges for brain repair: insights from adult neurogenesis in birds and mammals. Brain Behav Evol. 2001;58:306–322. doi: 10.1159/000057572. [DOI] [PubMed] [Google Scholar]
- 13.Eintrei C, Sokoloff L, Smith CB. Effects of diazepam and ketamine administered individually or in combination on regional rates of glucose utilization in rat brain. Br J Anaesth. 1999;82:596–602. doi: 10.1093/bja/82.4.596. [DOI] [PubMed] [Google Scholar]
- 14.Fortune ES, Margoliash D. Cytoarchitectonic organization and morphology of cells of the field L complex in male zebra finches (Taenopygia guttata) J Comp Neuro. 1992;325:388–404. doi: 10.1002/cne.903250306. [DOI] [PubMed] [Google Scholar]
- 15.Gentner TQ, Hulse SH, Duffy D, Ball GF. Response biases in auditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. J Neurobiol. 2001;46:48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Gobes SH, Bolhuis JJ. Birdsong memory: a neural dissociation between song recognition and production. Current Biol. 2007;17:789–793. doi: 10.1016/j.cub.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 17.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;3:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 18.Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- 19.Henegar JR, Maruniak JA. Quantification of the effects of long-term unilateral naris closure on the olfactory bulbs of adult mice. Brain Res. 1991;568:230–234. doi: 10.1016/0006-8993(91)91402-m. [DOI] [PubMed] [Google Scholar]
- 20.Huesmann GR, Clayton DF. Dynamic role of postsynaptic caspase-3 and BIRC4 in zebra finch song-response habituation. Neuron. 2006;52:1061–1072. doi: 10.1016/j.neuron.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurley P, Pytte C, Kirn JR. Nest of origin predicts adult neuron addition rates in the vocal control system of the zebra finch. Brain Behav Evol. 2008;71:263–70. doi: 10.1159/000127046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kempermann G, Kuh HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 23.Kirn JR, Nottebohm F. Direct evidence for loss and replacement of projection neurons in adult canary brain. J Neurosci. 1993;13:1654–63. doi: 10.1523/JNEUROSCI.13-04-01654.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirn J, O'Loughlin B, Kasparian S, Nottebohm F. Cell death and neuronal recruitment in the high vocal center of adult male canaries are temporally related to changes in song. Proc Natl Acad Sci U S A. 1994;16:7844–7848. doi: 10.1073/pnas.91.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol. 1965;22:770–783. [PubMed] [Google Scholar]
- 26.Li XC, Jarvis ED, Alvarez-Borda B, Lim DA, Nottebohm F. A relationship between behavior, neurotrophin expression, and new neuron survival. Proc Natl Acad Sci U S A. 2000;97:8584–8589. doi: 10.1073/pnas.140222497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipkind D, Nottebohm F, Rado R, Barnea A. Social change affects the survival of new neurons in the forebrain of adult songbirds. Behav Brain Res. 2002;133:31–43. doi: 10.1016/s0166-4328(01)00416-8. [DOI] [PubMed] [Google Scholar]
- 28.Lombardino AJ, Nottebohm F. Age at deafening affects the stability of learned song in adult male zebra finches. J Neurosci. 2000;20:5054–5064. doi: 10.1523/JNEUROSCI.20-13-05054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandairon N, Jourdan F, Didier A. Deprivation of sensory inputs to the olfactory bulb up-regulates cell death and proliferation in the subventricular zone of adult mice. Neurosci. 2003;119:507–516. doi: 10.1016/s0306-4522(03)00172-6. [DOI] [PubMed] [Google Scholar]
- 30.Mandairon N, Sacquet J, Jourdan F, Didier A. Long-term fate and distribution of newborn cells in the adult mouse olfactory bulb: Influences of olfactory deprivation. Neurosci. 2006;141:443–451. doi: 10.1016/j.neuroscience.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 31.Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23:1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- 32.Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mello CV, Velho TA, Pinaud R. Song-induced gene expression: a window on song auditory processing and perception. Ann N Y Acad Sci. 2004;1016:263–81. doi: 10.1196/annals.1298.021. [DOI] [PubMed] [Google Scholar]
- 34.Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A. 1992;89:6818–22. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mennerick S, Zorumski CF. Neural activity and survival in the developing nervous system. Mol Neurobiol. 2000;22:41–54. doi: 10.1385/MN:22:1-3:041. [DOI] [PubMed] [Google Scholar]
- 36.Ninkovic J, Mori T, Götz M. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci. 2007;27:10906–10911. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nissant A, Bardy C, Katagiri H, Murray K, Lledo PM. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat Neurosci. 2009;6:728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- 38.Nixdorf-Bergweiler BE, Bishof HJ., A sterotaxic atlas of the brain of the zebra finch Copyright © 2007 Barbara E. Nixdorf-Bergweiler and Hans-Joachim Bischof.
- 39.Nottebohm F. From bird song to neurogenesis. Sci Am. 1989;260:74–79. doi: 10.1038/scientificamerican0289-74. [DOI] [PubMed] [Google Scholar]
- 40.Nottebohm F. Neuronal replacement in adult brain. Brain Res Bull. 2002;57:737–749. doi: 10.1016/s0361-9230(02)00750-5. [DOI] [PubMed] [Google Scholar]
- 41.Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6101–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci USA. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinaud R, Terleph TA. A songbird forebrain area potentially involved in auditory discrimination and memory formation. J Biosci. 2008;33:145–55. doi: 10.1007/s12038-008-0030-y. [DOI] [PubMed] [Google Scholar]
- 44.Pinaud R, Fortes AF, Lovell P, Mello CV. Calbindin-positive neurons reveal a sexual dimorphism within the songbird analogue of the mammalian auditory cortex. J Neurobio. 2006;66:182–195. doi: 10.1002/neu.20211. [DOI] [PubMed] [Google Scholar]
- 45.Pinaud R, Velho TA, Jeong JK, Tremere LA, Leăo RM, Gersdorff H, Mello C. GABAergic neurons participate in the brain's response to birdsong auditory stimulation. Eur J Neurosci. 2004;20:1318–1330. doi: 10.1111/j.1460-9568.2004.03585.x. [DOI] [PubMed] [Google Scholar]
- 46.Rasika S, Nottebohm F, Alvarez-Buylla A. Testosterone increases the recruitment and/or survival of new high vocal center neurons in adult female canaries. Proc Natl Acad Sci USA. 1994;91:7854–8. doi: 10.1073/pnas.91.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 48.Ribeiro S, Cecchi GA, Magnasco MO, Mello CV. Toward a song code: evidence for a syllabic representation in the canary brain. Neuron. 1998;21:359–371. doi: 10.1016/s0896-6273(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 49.Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–89. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanford SE, Lange HS, Maney DL. Topography of estradiol-modulated genomic responses in the songbird auditory forebrain. Devel Neurobiol. 2010;70:73–86. doi: 10.1002/dneu.20757. [DOI] [PubMed] [Google Scholar]
- 51.Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron. 2000;25:481–492. doi: 10.1016/s0896-6273(00)80910-1. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 53.Schonfeld-Dado E, Segal M. Activity-dependent survival of neurons in culture: a model of slow neurodegeneration. J Neural Transm. 2009;116:1363–1369. doi: 10.1007/s00702-009-0256-3. [DOI] [PubMed] [Google Scholar]
- 54.Shors T. From stem cells to grandmother cells: how neurogenesis relates to learning and memory. Cell Stem Cell. 2008;3:253–258. doi: 10.1016/j.stem.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Stripling R, Volman SF, Clayton DF. Response modulation in the zebra finch neostriatum: relationship to nuclear gene regulation. J Neurosci. 1997;17:3883–93. doi: 10.1523/JNEUROSCI.17-10-03883.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 57.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tekumalla PK, Tontonoz M, Hesla MA, Kirn JR. Effects of excess thyroid hormone on cell death, cell proliferation, and new neuron incorporation in the adult zebra finch telencephalon. J Neurobiol. 2002;51:323–341. doi: 10.1002/neu.10053. [DOI] [PubMed] [Google Scholar]
- 59.Terleph TA, Mello CV, Vicario DS. Species differences in auditory processing dynamics in songbird auditory telencephalon. J Neurobio. 2007;67:1498–1510. doi: 10.1002/dneu.20524. [DOI] [PubMed] [Google Scholar]
- 60.Terpstra NJ, Bolhuis JJ, den Boer-Visser AM. An analysis of the neural representation of birdsong memory. J Neurosci. 2004;24:4971–4977. doi: 10.1523/JNEUROSCI.0570-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Theunissen FE, Noopur A, Shaevitz S, Woolley SMN, Fremouw T, Hauber ME. Neuroscience of Birdsong. Cambridge University Press; 2008. Song selectivity and the songbird brain; pp. 157–173. [Google Scholar]
- 62.Van Meir V, Boumans T, De Groof G, Van Audekerke J, Smolders A, Scheunders P, Sijbers J, Verhoye M, Balthazart J, Van der Linden A. Spatiotemporal properties of the BOLD response in the songbirds' auditory circuit during a variety of listening tasks. Neuroimage. 2005;25:1242–1255. doi: 10.1016/j.neuroimage.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 63.Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 64.Waddell J, Shors TJ. Neurogenesis, learning and associative strength. Eur J Neurosci. 2008;27:3020–8. doi: 10.1111/j.1460-9568.2008.06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamaguchi M, Mori K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc Natl Acad Sci USA. 2005;102:9697–9702. doi: 10.1073/pnas.0406082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zaretsky MD, Konishi M. Tonotopic organization in the avian telencephalon. Brain Research. 1976;111:167–171. doi: 10.1016/0006-8993(76)91058-1. [DOI] [PubMed] [Google Scholar]