Abstract
Teratoma formation is a critical obstacle to safe clinical translation of human embryonic stem (ES) cell-based therapies in the future. As current methods of isolation are unable to yield 100% pure population of differentiated cells from a pluripotent donor source, potential development of these tumors is a significant concern. Here we used non-invasive reporter gene imaging to investigate the relationship between human ES cell number and teratoma formation in a xenogenic model of ES cell transplantation. Human ES cells (H9 line) were stably transduced with a double fusion (DF) reporter construct containing firefly luciferase and enhanced green fluorescent protein (Fluc-eGFP) driven by a human ubiquitin promoter. Immunodeficient mice received intramyocardial (n = 35) or skeletal muscle (n = 35) injection of 1 × 102, 1 × 103, 1 × 104, 1 × 105 or 1 × 106 DF positive ES cells suspended in saline for myocardium and Matrigel for skeletal muscle. Cell survival and proliferation were monitored via bioluminescence imaging (BLI) for an 8 week period following transplantation. Mice negative for Fluc signal after 8 weeks were followed out to day 365 to confirm tumor absence. Significantly, in this study, a minimum of 1 × 105 ES cells in the myocardium and 1 × 104 cells in the skeletal muscle was observed to be requisite for teratoma development, suggesting that human ES cell number may be a critical factor in teratoma formation. Engraftment and tumor occurrence were also observed to be highly dependent on ES cell number. We anticipate these results should yield useful insights to the safe and reliable application of human ES cell derivatives in the clinic.
Keywords: molecular imaging, embryonic stem cell, tumorigenicity, teratoma, differentiation
Introduction
Embryonic stem (ES) cells are self-renewing pluripotent cells derived from the inner cell mass of a blastocyst.1 These cells can be differentiated into any cell type of the human body and represent a potentially ideal source of therapeutic donor populations for use in regenerative therapy. A critical barrier to the application of ES cells in human patients is teratoma formation. Teratomas are complex tumors caused by contamination of therapeutic cells by residual ES cells that escape the differentiation process. Because no current method of isolation can yield a 100% pure population of differentiated cells from a pluripotent donor source, development of these tumors is a significant concern.2,3 A recent case report of teratoma development in a child receiving fetal neural stem cell transplantation for treatment of ataxia telangiectasia highlights this risk.4 It is therefore imperative to improve our understanding of the tumorigenesis of ES cells and basic characteristics of teratoma formation. Importantly, the degree of purity required for safe administration of human ES cell derivatives and the possibility that teratoma development might depend on a critical threshold number of undifferentiated cells are essential questions which remain to be answered.
Results
In this study, we investigated the relationship between human ES cell number and kinetics of teratoma formation using bioluminescence imaging (BLI) in a xenogenic model of ES cell transplantation. BLI has been validated to be a reliable method of tracking mouse ES cell survival, migration and proliferation in living subjects.5 Mouse ES cell lines that stably express reporter constructs do not differ from normal cells in terms of cell viability, proliferation or capacity for differentiation.6 We chose the heart as a site for cell delivery because of its prominence as a target for regenerative therapies. Varying numbers of human ES cells ranging from 1 × 102 to 1 × 106 cells were delivered to the myocardial wall of the left ventricle of immunodeficient SCID mice and monitored longitudinally for tumor development for up to one year.
Stable transduction of human ES cells with double fusion reporter construct
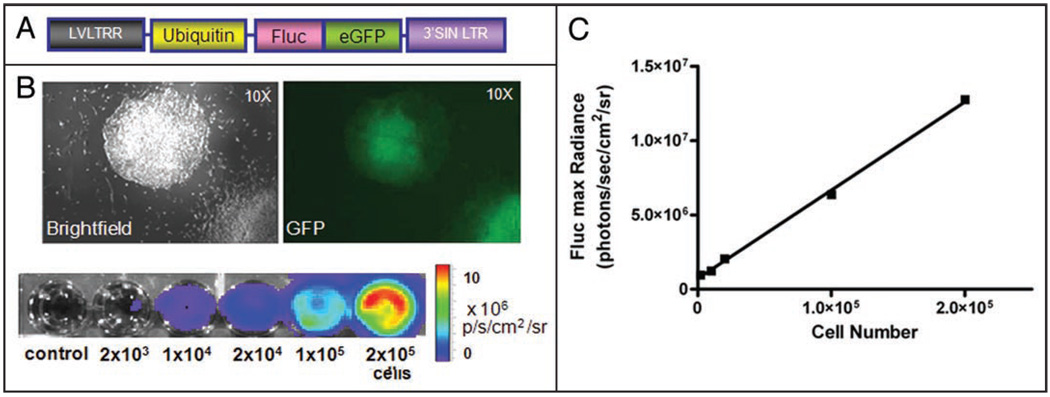
To track growth of human ES cell-derived teratomas, the federally approved H9 human ES cell line (Wicell, Madison, WI) was stably transduced with a self inactivating lentiviral vector carrying a human ubiquitin promoter driving firefly luciferase and enhanced green fluorescence protein (Fluc-eGFP) as previously described (Fig. 1A and B).7 Ex vivo culture assays confirmed that Fluc signal (max photons/sec/cm2/sr) correlated strongly with human ES cell number (r2 = 0.99, Fig. 1B and C).
Figure 1.
Characterization of stably transduced human ES cells with double fusion Fluc-eGFP reporter gene. (A) schema of the double fusion (DF) reporter gene with ubiquitin promoter driving Fluc and eGFP. (B) Stably transduced human ES cells have robust reporter gene expression as shown by fluorescence microscopy and BLI. (C) A strong correlation (r2 = 0.99) exists between BLI signals and human ES cell numbers.
Longitudinal monitoring of intramyocardial teratoma formation
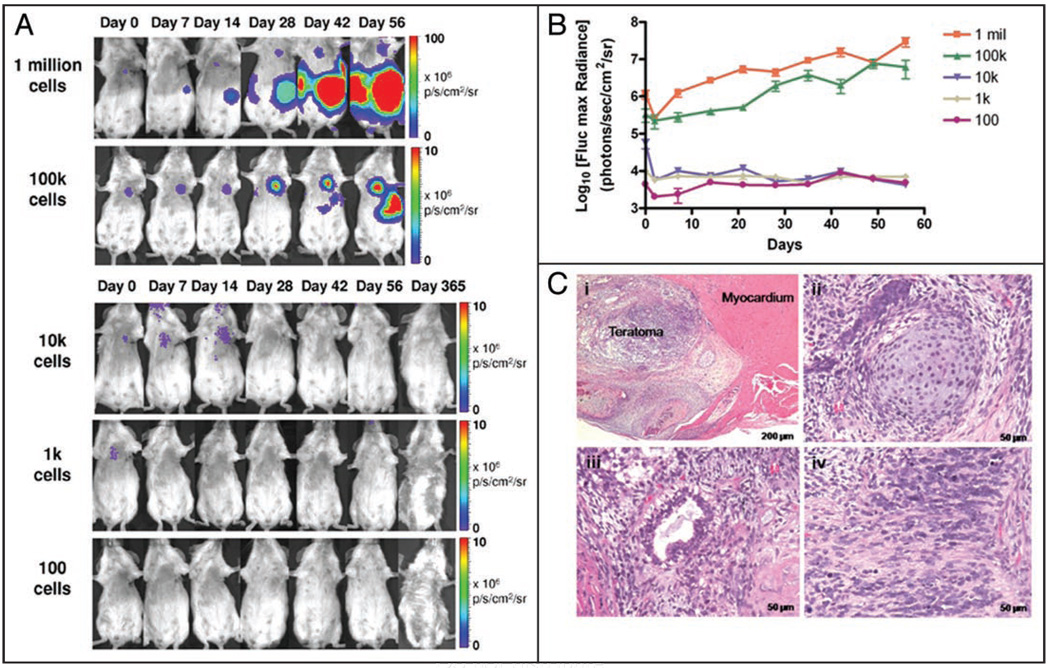
Immunodeficient adult SCID mice (n = 35) were divided into five groups (n = 7 each) and injected intramyocardially with 1 × 102, 1 × 103, 1 × 104, 1 × 105 or 1 × 106 Fluc-eGFP positive ES cells suspended in phosphate buffered saline (PBS). For visualization of cell survival and proliferation, animals were imaged longitudinally using a Xenogen In Vivo Imaging System (IVIS, Alameda, CA) for BLI (Fig. 2A). Imaging of cell survival and proliferation was conducted at days 0, 2, 7, 14, 21, 28, 35, 42, 49 and 56 following transplantation to calculate the kinetics of teratoma formation (Fig. 2B). At day 56, tumor-bearing animals were sacrificed, and teratomas were excised for histological staining with hematoxylin and eosin (H&E) (Fig. 2C). Mice that did not form tumors in the initial 8 weeks were imaged on a regular basis through day 365 post-injection, after which a subset of animals were sacrificed for full body necropsy to confirm the absence of tumor development during the study period.
Figure 2.
Potential for varying numbers of human ES cells to form teratomas following intramyocardial injection. (A) Different numbers of undifferentiated Fluc-eGFP positive human ES cells were injected intramyocardially into SCID mice. BLI reveals tumor development in animals transplanted with 1 × 106 or 1 × 105 cells but not in animals receiving 1 × 104 cells or less. (B) Quantitative analysis of the kinetics of teratoma development using BLI. (C) Representative H&E staining of intramyocardial teratoma via H&E: (i) low power view of intramyocardial teratoma, (ii) cartilage (mesoderm), (iii) mucinous glandular epithelium (endoderm) and (iv) neural tissue (ectoderm) confirm presence of cellular derivatives from all three germ layers.
Effects of cell number on teratoma formation
We found differences in cell number had a profound effect upon both the occurrence and rate of teratoma formation (Table 1, Fig. 2B). All mice receiving intramyocardial injection of 1 × 106 ES cells were observed to grow tumors by week 3–4, as evidenced by progressively robust BLI signals. Interestingly, teratomas in this group developed at both intra- and extra-cardiac locations as a result of extra-cardiac leakage and stem cell migration within a week of cell injection. Sites of ES cell migration included the kidney, liver, thoracic and abdominal cavities. Histological staining by H&E confirmed the presence of all three germ layers (Fig. 2C). Of the animals which received 1 × 105 human ES cells, only a minority (2 out of 7) developed tumors. In these animals, the rate of cell proliferation and tumor growth began more slowly than animals that received 1 × 106 cells but increased dramatically from week 4 onwards. By comparison, delivery of 1 × 104 or fewer cells did not result in teratoma formation in any of the animals. Several animals that received 1 × 104 cells (n = 3) exhibited short-term graft formation as evidenced by a faint BLI signal over the heart that declined slowly over several weeks. However, the signal was indistinguishable from background levels after 1 month and did not return to a detectable threshold during the remainder of the one-year study period. Full body necropsy and histological analysis following animal sacrifice did not reveal presence of teratoma within the myocardium or elsewhere within the animal. Similarly, administration of 1 × 103 cells resulted in signal detectable only for several days, and delivery of 1 × 102 cells produced no detectable signal even when images were acquired immediately following cell injection.
Table 1.
Effect of human ES cell number on teratoma formation
| ES cell dose | Site of injection | # Sites tested | # Tumors developed |
|---|---|---|---|
| 102 cells | myocardium | 7 | 0 |
| 103 cells | myocardium | 7 | 0 |
| 104 cells | myocardium | 7 | 0 |
| 105 cells | myocardium | 7 | 2 |
| 106 cells | myocardium | 7 | 7 |
| 102 cells | skeletal muscle | 7 | 0 |
| 103 cells | skeletal muscle | 7 | 0 |
| 104 cells | skeletal muscle | 7 | 5 |
| 105 cells | skeletal muscle | 7 | 7 |
| 106 cells | skeletal muscle | 7 | 7 |
Effect of human ES cell number upon teratoma formation in the myocardium and skeletal muscle. Higher numbers of cells result in elevated occurrence of tumor development both in the myocardium and skeletal muscle.
Longitudinal monitoring of teratoma formation in skeletal muscles
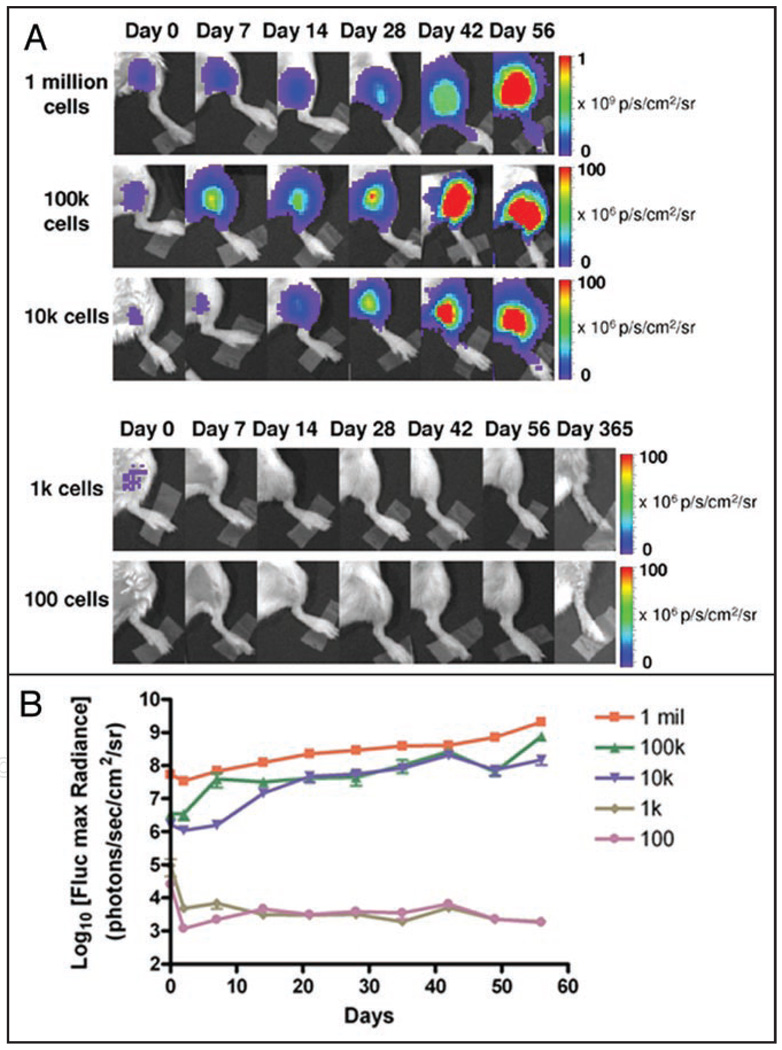
Given that many of the animals that developed intracardiac tumors also exhibited extra-cardiac signals within the first week of cellular transplantation into the myocardium, we concluded the heart may be characterized by a high level of cellular dispersal due to the rapid contraction of the murine heart and entry of cells into the left ventricular cavity. To test whether such cellular dispersal negatively impacted teratoma formation especially in animals that received lower numbers of cells, we delivered 1 × 102, 1 × 103, 1 × 104, 1 × 105 and 1 × 106 Fluc-eGFP positive human ES cells to an alternative anatomical site. To further promote anatomical localization, cells were suspended in 30 µl of Matrigel (BD Sciences, San Jose, CA) and delivered to the lower limbs of a second cohort of immunodeficient animals (n = 7 per group). Imaging was conducted using a Xenogen IVIS system on days 0, 2, 7, 14, 21, 28, 35, 42, 49 and 56 following transplantation. Delivery of cells to the hindlimbs using Matrigel resulted in higher occurrence and increased rate of teratoma formation as compared to intramyocardial injection of cells suspended in PBS (Table 1, Fig. 3). All animals that received 1 × 105 or 1 × 106 cells and the majority of animals (5 out of 7) that received 1 × 104 cells developed tumors by week 2–3 as observed by BLI (Fig. 3A and B). By comparison, animals that received 1 × 103 cells exhibited short-term grafts that lasted for only several days before loss of signal. Delivery of 1 × 102 cells did not result in detectable signal. Tumors that did form remained localized to the leg with no evidence of cellular migration. All mice that did not form teratomas within the first 8 weeks were monitored out to one year for tumor development after which the leg was dissected and confirmed to be tumor free.
Figure 3.
Potential for varying numbers of human ES cells to form teratomas following localization to skeletal muscles using Matrigel. (A) Different numbers of undifferentiated human ES cells were delivered to the lower limbs of SCID mice. Rates and occurrence of teratoma formation were higher compared to cell delivery to the myocardium. (B) Longitudinal monitoring of cell survival and tumor development in murine hindlimbs via BLI.
Discussion
Taken together, these results suggest that a critical threshold of undifferentiated human ES cells may be required for teratoma formation to occur. Theoretically, a single self-renewing undifferentiated ES cell should be sufficient to populate a tumor given the perfect microenvironment. However, various factors may prevent teratoma formation as seen with intramyocardial delivery of 1 × 104 or fewer cells in this study. Failure of cells to engraft, anoikis, hypoxic conditions following delivery, and residual levels of innate immunogeneicity in SCID animals may all impair the ability of cells to successfully engraft and proliferate.8,9 Most likely, the minimum number of human ES cells required to form a teratoma is not “set”; in our study, intramyocardial delivery of 1 × 105 cells resulted in tumor formation in some of the animals but not others. The heart also presents a unique environment for cell delivery because administered cells may be easily dispersed due to cardiac contractility, leakage into the thoracic cavity, and entry of cells into the systemic circulation.
Administration of higher numbers of cells may promote formation of stable grafts capable of withstanding adverse environmental conditions and cellular dispersal following delivery. We observed that stable graft formation as evidenced by robust and increasing BLI signal within the first two weeks following cell delivery inevitably led to tumor development in all animals without exception. By comparison, loss or decline of signal in the same period, as in animals that received 1 × 104 human ES cells in the heart or 1 × 103 cells in the skeletal muscle, did not lead to teratoma development. In animals that received 1 × 102 cells, BLI signal was not detected even immediately after cell injection Previous studies have estimated that a minimum of 500 Fluc positive cells are required for visualization by BLI in the heart,12 indicating that in mice negative for signal, delivered cells did not proliferate above this number for the study period of one year.
Depending on the site of delivery and method of cell administration, it is possible that even fewer numbers of ES cells could potentially lead to tumor formation. Several studies focusing on allogeneic models of mouse ES cell transplantation have shown that as few as 400–500 mouse ES cells can lead to teratoma formation in immunodeficient mice10 and between 50,000 and 100,000 cells are required for tumor formation in immunocompetent animals.11 This is the first study to systematically analyze the effects of human ES cell number upon teratoma formation. These results should provide valuable insights into the kinetics of teratoma development and help overcome one of the major hurdles for clinical translations of human ES cell-based therapy.
Materials and Methods
Culture of undifferentiated human ES cells
H9 hES cells (WiCell, Madison, WI) were cultured on a feeder layer of irradiated murine embryonic fibroblasts (MEFs) using hES cell culture medium consisting of 80% Dulbecco’s modified Eagle’s medium/F-12 (Invitrogen, Carlsbad, CA), 20% knock-out serum replacement (Invitrogen), 1 mM L-glutamine, 1% nonessential amino acids, 0.1 mM β-mercaptoethanol, and 8 ng/ml basic fibroblast growth factor (Invitrogen). Cells were passaged every 4–6 days using Collagenase IV (Invitrogen). Prior to transplantation, cells were moved to a Matrigel (BD Biosciences, San Jose, CA) coated plate and cultured for two passages under feeder free conditions using mTeSR-1 (Stemcell Technologies, Vancouver, Canada) to eliminate presence of MEFs.
Lentiviral transduction of human ES cells with a double-fusion reporter gene
ES cells of the H9 cell line were transduced with a self inactivating lentiviral vector carrying a human ubiquitin promoter driving firefly luciferase and enhanced green fluorescence protein (Fluc-eGFP) at a multiplicity of infection (MOI) of 10. After two rounds of cell sorting for eGFP by a Vantage SE cell sorter (Becton Dickinson Immunocytometry Systems, San Jose, CA), a population of human ES cells stably expressing the DF construct was isolated and expanded on feeder layers of MEFs as described above.
Preparation of varying numbers of human ES cells for cardiac and skeletal muscle transplantation
Human ES cells were harvested from feeder free Matrigel coated plates by treating cells with cell dissociation buffer (Invitrogen) for 10 minutes. Cells were counted using a hemocytometer and suspended in PBS at a concentration of 1 × 106 cells/30 µl PBS. Serial dilution was subsequently used to obtain 1 × 105, 1 × 100, 1×103 and 1 × 102 cells per 30 µl PBS. ES cells were kept on ice for <45 min for optimal viability prior to injection.
Cardiac and skeletal muscle transplantation of DF positive human ES cells
All animal study protocols were approved by the Stanford Animal Research Committee. Surgical procedures were performed on 8–10 week old female immunocompromised SCID beige mice (Charles River Laboratories, Inc.,) by a single experienced micro-surgeon. Briefly, animals were knocked down using 2% isoflurane. Mice were intubated, ventilated and anesthesia was maintained with inhaled isoflurane (1% to 2%). An aseptic left thoracotomy was performed and the pericardium was opened. Mice were subsequently injected intramyocardially with 1 × 106, 1 × 105, 1 × 104, 1 × 103 or 1 × 102 human ES cells suspended in 30 µl of PBS (n = 7 per group). For skeletal muscle injections, mice were knocked down and maintained on anesthesia using 1–2% isoflurane. Human ES cells were suspended in 30 µl of Matrigel and injected directly into the gastrocnemius muscles of recipient mice using a 28.5-gauge insulin syringe. Mice were divided into the following groups (n = 7 each): 1 × 106, 1 × 105, 1 × 104, 1 × 103 and 1 × 102 cells.
Optical bioluminescence imaging (BLI) of DF positive human ES cell transplanted animals
BLI was performed on all animals using a Xenogen IVIS system. All animals were imaged on days 0, 2, 7, 14, 21, 28, 49 and 56 following cell transplantation. The reporter probe D-Luciferin (375 mg/kg) was administered via intra-peritoneal injection 10 minutes prior to image acquisition, after which animals were imaged for 20 min using 1 second to 5 minute acquisition intervals repetitively for the study period. Region of interest (ROI) were drawn over the signals using the Igor image analysis software (Wavemetrics, Lake Oswego, OR). BLI signal was standardized for acquisition time and quantified in units of maximum photons per second per square centimeter per steridian (photons/sec/cm2/sr).
Postmortem immunohistochemical staining
Animals were sacrificed according to protocols approved by the Stanford Animal Research Committee after the duration of the study. Teratomas and major organs (heart, liver, kidney, spleen) were explanted and processed for H&E staining. For animals that received hindlimb injection, the gastrocnemius muscle was dissected, sectioned and stained with H&E. Slides were interpreted by an expert pathologist (A.J.C.).
Data analysis
Data are given as mean ± SEM. For statistical analysis, the two-tailed Student’s t-test was used. Differences were considered significant at p < 0.05.
Acknowledgements
This work was supported by fundings from CIRM RS1-00322 (J.C.W.), CIRM RC1-00151 (J.C.W.), NIH HL091453 (J.C.W.), and HHMI research fellowship (A.L.).
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Gepstein L. Derivation and potential applications of human embryonic stem cells. Circ Res. 2002;91:866–876. doi: 10.1161/01.res.0000041435.95082.84. [DOI] [PubMed] [Google Scholar]
- 3.Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, et al. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2008;3:3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Bogt KE, Swijnenburg RJ, Cao F, Wu JC. Molecular imaging of human embryonic stem cells: keeping an eye on differentiation, tumorigenicity and immunogenicity. Cell Cycle. 2006;5:2748–2752. doi: 10.4161/cc.5.23.3533. [DOI] [PubMed] [Google Scholar]
- 6.Wu JC, Cao F, Dutta S, Xie X, Kim E, Chungfat N, et al. Proteomic analysis of reporter genes for molecular imaging of transplanted embryonic stem cells. Proteomics. 2006;6:6234–6249. doi: 10.1002/pmic.200600150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, et al. In vivo visualization of embryonic stem cell survival, proliferation and migration after cardiac delivery. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drukker M, Katchman H, Katz G, Even-Tov Friedman S, Shezen E, Hornstein E, et al. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells. 2006;24:221–229. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- 9.Zvibel I, Smets F, Soriano H. Anoikis: roadblock to cell transplantation? Cell Transplant. 2002;11:621–630. doi: 10.3727/000000002783985404. [DOI] [PubMed] [Google Scholar]
- 10.Harkany T, Andäng M, Kingma HJ, Görcs TJ, Holmgren CD, Zilberter Y, et al. Region-specific generation of functional neurons from naive embryonic stem cells in adult brain. J Neurochem. 2004;88:1229–1239. doi: 10.1046/j.1471-4159.2003.02243.x. [DOI] [PubMed] [Google Scholar]
- 11.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 12.Sheikh AY, Lin SA, Cao F, Cao Y, van der Bogt KE, Chu P, et al. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25:2677–2684. doi: 10.1634/stemcells.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]